Supplemental Digital Content is available in the text
Keywords: hepatic metastasis, interleukin-6, pancreatic ductal adenocarcinoma, survival
Abstract
Several reports showed that interleukin-6 (IL-6) or -8 (IL-8) might be useful inflammatory biomarkers for pancreatic ductal adenocarcinoma (PDAC), although these clinical impact is still open to debate. The aim of this study was to elucidate whether serum levels of IL-6 and IL-8 at diagnosis could predict the tumor progression pattern of PDAC, especially in extensive hepatic metastasis.
According to the tumor burden of hepatic metastasis at the last follow-up, tumor progression pattern was defined as follows: no or limited (unilobar involvement and 5 or less in the within liver, limited group) and extensive hepatic metastasis (bilobar or more than 5, progressed group). Fifty-three PDAC patients with initially no or limited hepatic metastasis were enrolled retrospectively.
Around 42 (79.2%) were included in the limited and 11 (20.8%) in the progressed group. The median serum level of IL-6 in the progressed group was elevated significantly compared with the limited group. However, the median serum level of IL-8 was not. Furthermore, multivariate analysis revealed that the elevated serum level of IL-6 was an independent risk factor for progression to extensive hepatic metastasis (odds ratio 1.928, 95% confidence interval 1.131–3.365, P = 0.019), but IL-8 was not. However, higher IL-6 did not predict shorter survival.
High serum IL-6 can be an independent risk factor for progression to extensive hepatic metastasis in PDAC patients.
1. Introduction
The crucial role of chronic inflammation in tumor development is particularly recognized in the context of pancreatic ductal adenocarcinoma (PDAC).[1,2] Although the roles of interleukin-6 (IL-6) and interleukin-8 (IL-8) in cancer biology are still unclear, it has been suggested that IL-6 or IL-8 promote cell proliferation, migration, invasion, and angiogenesis.[3–6] Chronic inflammation can lead to production of cytokines that upregulate proinflammatory cytokines, such as IL-6 and IL-8, and affects progression of PDAC.[1–6]
PDAC spreads extensively to various organs, especially the liver.[7] Several studies showed that the presence of hepatic metastasis is an independent adverse prognostic factor in patients with metastatic PDAC.[8–10] Recently, 1 autopsy study for patterns of failure in patients with PDAC suggested that it is classified to limited versus extensive metastasis according to the tumor burden in the metastatic setting.[11] Some studies showed that increased IL-6 or IL-8 serum level is associated with the presence of hepatic metastasis and shorter survival in patients with PDAC.[12–16]
We aimed to elucidate whether serum IL-6 and IL-8 could predict the tumor progression pattern of PDAC, especially regarding extensive hepatic metastasis and eventually long-term prognosis.
2. Patients and methods
2.1. Patients
From 2010 to 2011, a total of 82 patients with PDAC were identified in Seoul National University Bundang hospital. We retrospectively studied 53 consecutive PDAC patients with initially no or limited hepatic metastasis (unilobar involvement and 5 or less in the within liver), excluding 29 patients who already had initially extensive hepatic metastasis (bilobar or more than 5). According to the tumor burden of hepatic metastasis at the last follow-up, the tumor progression pattern was defined as follows: no or limited (unilobar involvement and 5 or less in the within liver, limited group) and extensive hepatic metastasis (bilobar or more than 5, progressed group). This study protocol was reviewed and approved by the institutional review board of Seoul National University Bundang Hospital (IRB No.: B-1003/096-005).
2.2. Methods
The serum samples stored at –70°C until further evaluation. Four samples in each group were selected for target cytokines screening, and the level of 8 cytokines (granulocyte/macrophage colony-stimulating factor, interferon-γ, tumor necrosis factor-α, IL-2, IL-4, IL-6, IL-8, and IL-10) were measured using the Luminex Bead-based Multiplex Assay (R&D system, MN). Among 8 cytokines, the serum levels of the IL-6 and IL-8 were significant difference between limited and extensive hepatic metastasis (Supplementary Fig. 1). After target cytokines validation, measurement of serum IL-6 level, IL-8 level, C-reactive protein (CRP), neutrophil-lymphocyte ratio (NLR), and tumor marker carbohydrate antigen 19–9 (CA19–9) were performed at the time of initial evaluation. Serum IL-6 and IL-8 level were normally log distributed within both groups; hence, values were transformed to the natural logarithm (Ln). High IL-6 and IL-8 were defined as more than the median values, respectively. The high CA19-9 level was defined as more than 1000 U/mL.[17,18] Overall survival was defined as the time interval from diagnosis to death.
2.3. Statistical analysis
Kaplan–Meier analysis was used to generate survival curves and calculate median survival times which were compared by the log-rank test. The analysis of the risk factors for death was performed by the univariate Cox proportional hazard regression model. Risk factors were expressed as the hazard ratio. The analysis of the risk factors for progression to extensive hepatic metastasis was performed by logistic regression analysis. Risk factors were expressed as odds ratios. Among the clinical variables included in univariate analysis, those with a 2-sided P-value of less than 0.05 were chosen for multivariate analysis with stepwise selection. A comparison among the 2 subgroups was carried out using Student's t-test, the Mann–Whitney, or Pearson's correlation test for continuous variables, whereas the chi-square test was used to compare noncontinuous variables. A 2-sided P-value of less than 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS statistics 21.0 software for Windows (IBM Corporation, Armonk, NY).
3. Results
3.1. Patient characteristics
A total of 53 consecutive patients with initially no or limited hepatic metastasis of PDAC were enrolled in this study. Baseline patient characteristics are shown in Table 1. According to the progression pattern, 42 (79.2%) patients were included in the limited group and 11 (20.8%) patients in the progressed group. The median patient age at diagnosis was 66 years, and 31 (58.5%) patients were men. Regarding the tumor progression pattern and the serum concentration of inflammatory cytokines, the median serum level of Ln IL-6 of the progressed group was significantly greater than that of the limited group (2.0 vs 1.4, P = 0.025) (Fig. 1A). However, there was no significant correlation between the median serum level of Ln IL-8 and tumor progression pattern (3.2 vs 3.5, P = 0.978) (Fig. 1B). According to the progression pattern, the initial disease status was shown in Supplementary Fig. 2. Median follow-up of survivors showed 43 of 53 patients died (32 of 42 patients with the limited group, and 11 of 11 progressed groups). The causes of death in the limited group patients were disease progression in 22 (52.4%), infection in 8 (19.0%), and 1 each (2.4%) due to tumor bleeding and cardiovascular event. Most of the patients in the progressed group died because of disease progression (9, 81.8%), and there was 1 case (9.1%) each due to infection and cardiovascular event.
Table 1.
Patient characteristics.
Figure 1.
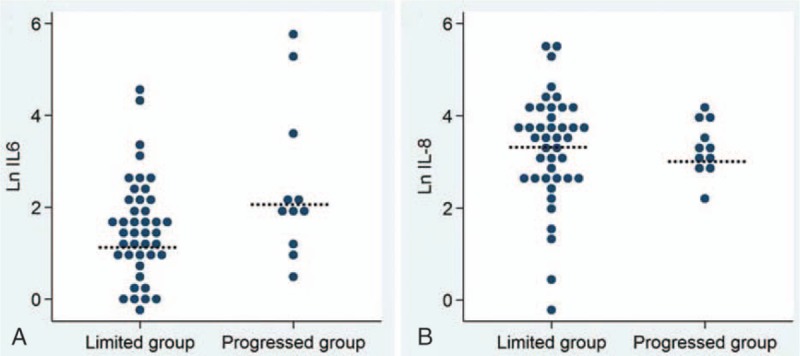
(A) The serum level of the Ln IL-6 in the limited and progressed group. The median serum level of Ln IL-6 of the progressed group was significantly greater than that of the limited group (2.0 vs 1.4, P = 0.025). (B) The serum level of the Ln IL-8 in the limited and progressed group. There was no significant correlation between the median serum level of Ln IL-8 and the tumor progression pattern (3.2 vs 3.5, P = 0.978). Ln = natural logarithm.
3.2. Correlation of inflammatory cytokines with CRP and NLR
The serum level of Ln IL-6 showed a significant direct correlation with the serum level of CRP and NLR (R = 0.395, P = 0.007; R = 0.326, P = 0.017), but there was no significant correlation between the serum level of Ln IL-8 and the serum level of CRP and NLR (R = 0.206, P = 0.203; R = 0.022, P = 0.881) (Fig. 2).
Figure 2.
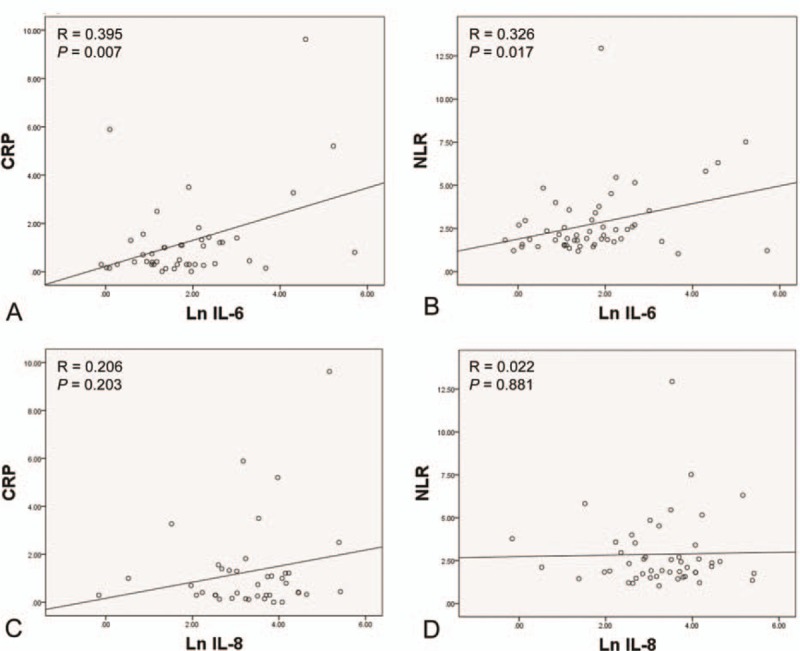
(A) The correlation between the serum level of Ln IL-6 and CRP. (B) The correlation between the serum level of Ln IL-6 and NLR. (C) The correlation between the serum level of Ln IL-8 and CRP. (D) The correlation between the serum level of Ln IL-8 and NLR. Ln = natural logarithm, IL = interleukin, CRP = C-reactive protein, NLR = neutrophil-lymphocyte ratio.
3.3. Risk factors for progression to extensive hepatic metastasis
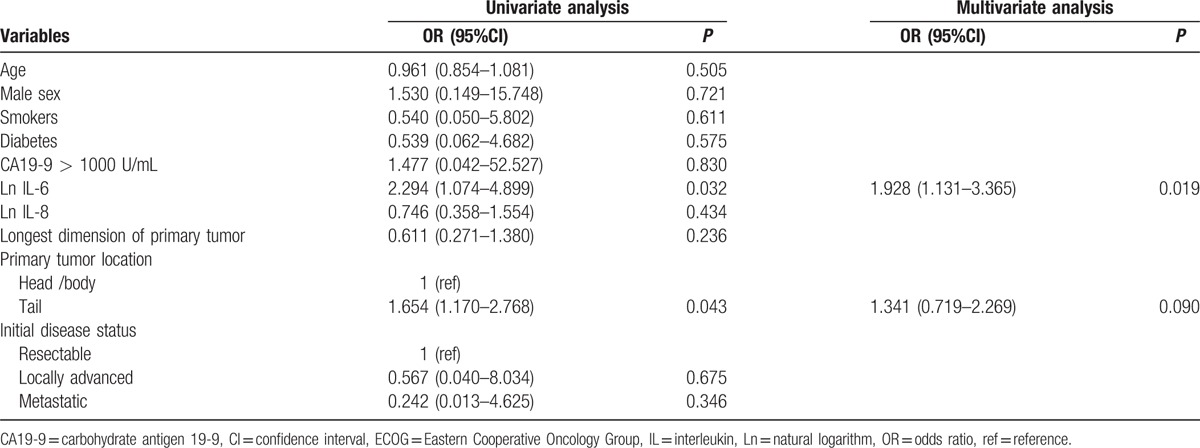
By univariate logistic regression analysis, location of the primary tumor in the tail of the pancreas and serum Ln IL-6 level were significantly associated with the tumor burden of hepatic metastasis at the last follow-up. Among these, a higher serum Ln IL-6 level was significantly associated with a large tumor burden of hepatic metastasis by multivariate logistic regression analysis (odds ratio 1.928, 95% confidence interval [CI] 1.131–3.365, P = 0.019). However, the serum Ln IL-8 level was not significantly associated with the tumor burden of hepatic metastasis (odds ratio 0.746, 95% CI 0.358–1.554, P = 0.434) (Table 2).
Table 2.
Risk factors for progression to extensive hepatic metastasis.
3.4. Risk factors for overall survival
The median overall survival for this study population was 10.0 months (95% CI, 6.5–13.5). According to the tumor progression pattern, the median overall survival of patients in the limited group and progressed group were 9.9 months (95% CI, 4.0–16.0) and 10.0 months (95% CI, 8.7–11.4), respectively. Analysis of the overall survival by univariate Cox regression analysis showed higher age, poor ECOG performance status, high CA19-9, higher longest dimension of the primary tumor, and initially locally advanced or metastatic cancer were significantly associated with shorter survival (Table 3). However, the survival curve according to the median serum values of IL-6 and IL-8 showed no significant difference in relationship to overall survival, respectively (P = 0.660, P = 0.915; Fig. 3).
Table 3.
Risk factors for overall survival.
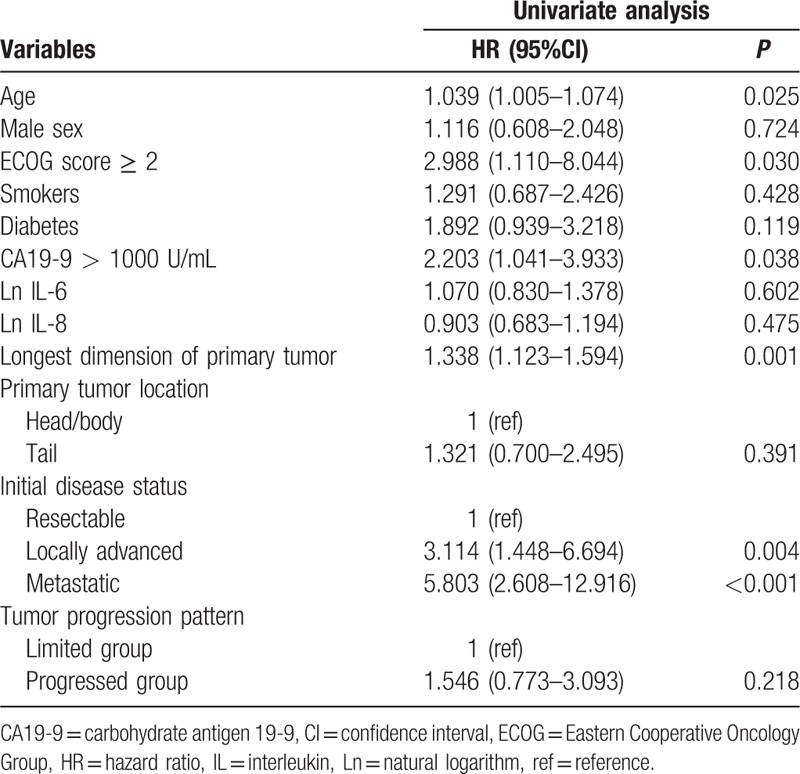
Figure 3.
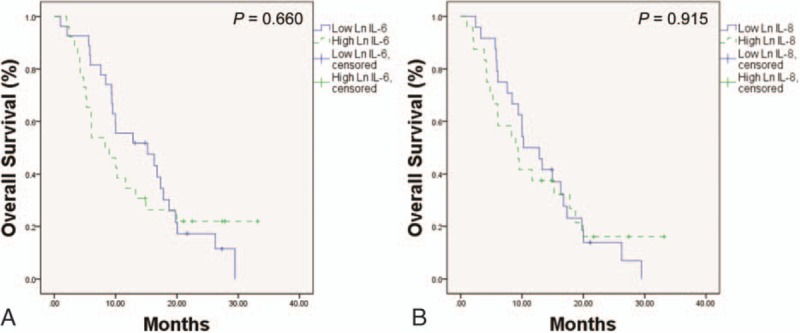
(A) Kaplan–Meier analysis of overall survival according to the median serum values of Ln IL-6. (B) Kaplan–Meier analysis of overall survival according to the median serum values of Ln IL-8. Ln = natural logarithm, IL = interleukin.
4. Discussion
The role of chronic pancreatic inflammation in tumor progression has been demonstrated to be major.[2,19] It has been known that IL-6 or IL-8, proinflammatory cytokines, are involved with tumor progression in PDAC patients.[3–6] This study defined the tumor progression pattern according to the hepatic metastatic burden of PDAC at the last follow-up. We attempted to investigate whether serum IL-6 and IL-8 could predict tumor progression pattern and overall survival. The serum level of IL-6 of the progressed group was significantly greater than the limited group, and higher IL-6 was the only independent risk factor for progression to extensive hepatic metastasis. Therefore, it would be suggested that more aggressive treatment from the beginning might be considered in PDAC patients with high IL-6.
IL-6 is a proinflammatory cytokine that is synthesized by many cell types, including macrophages, fibroblasts, endothelial cells, and myeloid cells, and it promotes synthesis of CRP from hepatocyte.[20,21] This study revealed that IL-6 is correlated with inflammatory markers such as CRP and NLR, and that it was an independent risk factor for progression to extensive hepatic metastasis.
IL-8 is a proinflammatory cytokine and a member of the CXC chemokine family.[22] Several studies showed that IL-8 plays a crucial role in tumor progression including cancer invasion and angiogenesis.[5,6,15] However, this study did not show that IL-8 is correlated with inflammatory markers, and that it was shown to be able to predict the tumor progression pattern.
Although IL-6 was an independent risk factor for progression to extensive hepatic metastasis, higher IL-6 and IL-8 were not associated with shorter survival in this study. The authors cannot explain exactly why high IL-6 indicating extensive hepatic metastasis in the future, does not project onto lower survival. Small sample size might be influence the outcome. On the contrary to this, this study showed that a higher CA19-9, larger primary tumor size, or advanced initial disease status was associated with shorter survival, but not risk factors for progression to extensive hepatic metastasis.
Previous autopsy studies showed that most PDAC patients die with a broad range of metastatic burden, from limited to extensive.[11,23] Furthermore, 10 to 20% of PDAC patients die of locally advanced disease, without metastatic disease.[11,24] This study revealed that mortality due to disease progression account for almost all causes of death in the progressed group patients. However, in limited group patients, localized infection and tumor bleeding in relation to the primary tumor, in addition to disease progression, accounted for 25% of overall death. Findings of this study were consistent with a previous autopsy study.
The present study has limitations. First, it is retrospective design. Second, the tumor progression pattern was assessed by the abdomen computed tomography not autopsy, and by using measurable lesion of the hepatic metastatic burden except for nonmeasurable lesion such as peritoneal seeding, lymphatic pulmonary metastasis, and bone destructive metastasis. To the best of our knowledge, we investigate of the relationship between proinflammatory cytokines and tumor progression pattern for the first time. In conclusion, higher serum IL-6 was an independent risk factor for progression to extensive hepatic metastasis of PDAC, even though it did not correlate with survival. Therefore, more aggressive chemotherapy from the beginning should be considered in PDAC patients with high IL-6.
Acknowledgments
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses. The authors are indebted to J. Patrick Barron, Professor Emeritus, Tokyo Medical University and Adjunct Professor, Seoul National University Bundang Hospital for his pro bono editing of this manuscript.
Supplementary Material
Footnotes
Abbreviations: CA19-9 = carbohydrate antigen 19-9, CI = confidence interval, CRP = C-reactive protein, ECOG = Eastern Cooperative Oncology Group, IL = interleukin, Ln = natural logarithm, NLR = neutrophil-lymphocyte ratio, PDAC = pancreatic ductal adenocarcinoma.
Funding: This study was supported by grant no. 06-2010-067 and 02-2010-017 from SNUBH Research fund.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:2964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol 2009;7:S48–54. [DOI] [PubMed] [Google Scholar]
- [3].Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011;19:456–69. [DOI] [PubMed] [Google Scholar]
- [4].Fukuda A, Wang SC, Morris JPt, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011;19:441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ikeda O, Egami H, Ishiko T, et al. Signal of proteinase-activated receptor-2 contributes to highly malignant potential of human pancreatic cancer by up-regulation of interleukin-8 release. Int J Oncol 2006;28:939–46. [PubMed] [Google Scholar]
- [6].Kuwada Y, Sasaki T, Morinaka K, et al. Potential involvement of IL-8 and its receptors in the invasiveness of pancreatic cancer cells. Int J Oncol 2003;22:765–71. [PubMed] [Google Scholar]
- [7].Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. [DOI] [PubMed] [Google Scholar]
- [8].Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- [9].Morizane C, Okusaka T, Morita S, et al. Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas 2011;40:415–21. [DOI] [PubMed] [Google Scholar]
- [10].Forssell H, Wester M, Akesson K, et al. A proposed model for prediction of survival based on a follow-up study in unresectable pancreatic cancer. BMJ Open 2013;3:e004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Talar-Wojnarowska R, Gasiorowska A, Smolarz B, et al. Clinical significance of interleukin-6 (IL-6) gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig Dis Sci 2009;54:683–9. [DOI] [PubMed] [Google Scholar]
- [13].Ebrahimi B, Tucker SL, Li D, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer 2004;101:2727–36. [DOI] [PubMed] [Google Scholar]
- [14].Mitsunaga S, Ikeda M, Shimizu S, et al. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 2013;108:2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dima SO, Tanase C, Albulescu R, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas 2012;41:1001–7. [DOI] [PubMed] [Google Scholar]
- [16].Denley SM, Jamieson NB, McCall P, et al. Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:887–98. [DOI] [PubMed] [Google Scholar]
- [17].Tas F, Karabulut S, Ciftci R, et al. Serum levels of LDH, CEA, and CA19-9 have prognostic roles on survival in patients with metastatic pancreatic cancer receiving gemcitabine-based chemotherapy. Cancer Chemother Pharmacol 2014;73:1163–71. [DOI] [PubMed] [Google Scholar]
- [18].Xue P, Kanai M, Mori Y, et al. Comparative outcomes between initially unresectable and recurrent cases of advanced pancreatic cancer following palliative chemotherapy. Pancreas 2014;43:411–6. [DOI] [PubMed] [Google Scholar]
- [19].Masui T, Hosotani R, Doi R, et al. Expression of IL-6 receptor in pancreatic cancer: involvement in VEGF induction. Anticancer Res 2002;22:4093–100. [PubMed] [Google Scholar]
- [20].Lesina M, Wormann SM, Neuhofer P, et al. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol 2014;26:80–7. [DOI] [PubMed] [Google Scholar]
- [21].Young DP, Kushner I, Samols D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J Immunol 2008;181:2420–7. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y, Shi M, Yu GZ, et al. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol 2012;18:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nakahashi C, Oda T, Kinoshita T, et al. The impact of liver metastasis on mortality in patients initially diagnosed with locally advanced or resectable pancreatic cancer. Int J Gastrointest Cancer 2003;33:155–64. [DOI] [PubMed] [Google Scholar]
- [24].Kamisawa T, Isawa T, Koike M, et al. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 1995;11:345–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


