Abstract
Hematologic parameters of systemic inflammation are receiving attention as promising prognostic indicators in cancer patients. Here, we investigated the relation and compared the prognostic values of circulating blood cell-based parameters and tumor 18F-fluoro-2-deoxyglucose (FDG) uptake in patients with stage I nonsmall cell lung cancers (NSCLC).
Subjects were 1034 patients with newly diagnosed stage I NSCLC who underwent FDG positron emission tomography/computed tomography (PET/CT) followed by curative resection. Total white blood cell (WBC) count, absolute neutrophil, lymphocyte and platelet counts, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) were obtained. Tumor FDG uptake was measured as SUVmax.
WBC, neutrophil and lymphocyte counts, and NLR demonstrated weak but significant correlation to tumor SUVmax. Using the upper quartile as cutoff, patients with high tumor SUVmax had significantly higher WBC, neutrophil and lymphocyte counts, and greater NLR. There were 144 recurrences (13.9%) over a median follow-up of 29.5 months. On Cox proportional hazards regression analysis, WBC count, tumor SUVmax, age, gender, smoking, cell type, and tumor stage were significant univariate prognostic factors. On multivariate analysis, high tumor SUVmax (HR = 2.22; 95% CI, 1.52–3.25; P < 0.001), tumor stage 1B (HR = 2.11; 95% CI, 1.47–3.01; P < 0.001), and old age (HR = 1.03; 95% CI, 1.01–1.05; P = 0.002) were significant independent predictors of poor survival. Finally, high tumor SUVmax remained a significant predictor of prognosis in both low and WBC count groups.
Circulating blood counts showed significant correlation to tumor FDG uptake in early stage NSCLC. WBC count was a significant univariate variable, but tumor FDG uptake was a superior and independent predictor of outcome. Hence, tumor FDG uptake effectively stratified prognosis in patients with low as well as high WBC count.
Keywords: FDG, lung cancer, neutrophil-to-lymphocyte ratio, PET/CT, prognosis
1. Introduction
Lung cancer is the leading cause of cancer deaths in both men and women.[1] The most important prognostic factor for nonsmall cell lung cancers (NSCLC) that constitute approximately 85% of all lung cancers is tumor stage.[2] Patients with advanced stage NSCLC have the poorest prognosis, and only those with early-stage disease are candidates for complete resection. However, even following curative surgery, patients have a 5-year survival rate between 60% and 80%,[3] with recurrence as the major cause of mortality. As such, prognostic indicators that help predict risk of cancer relapse should allow more appropriate monitoring of patients with early-stage NSCLC following curative resection.
18F-fluoro-2-deoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) provides quantitative information on the metabolic activity of tumors, and is useful not only for staging and assessing treatment response but also for predicting patient prognosis. In NSCLC, metabolic parameters derived from FDG PET have been shown to provide prognostic information incremental to that obtained by conventional imaging.[4,5]
A more recent class of prognostic markers in cancer disease that is receiving intense attention is hematologic parameters of systemic inflammation based on circulating blood cell counts.[6] It is now recognized that cancer-associated inflammation plays an important role in the development and progression of most types of cancers. Furthermore, significant systemic inflammatory responses can exert negative influence on the nutritional, functional, and immunologic status of the host.[6] While the precise mechanism is currently under investigation, it is becoming increasingly apparent that the presence and severity of systemic inflammatory responses is closely associated with the outcome of cancer patients, and therefore has significant prognostic value. Cellular components of the host hematologic system that have been linked to cancer patient survival include circulating cells of inflammation, immunity, and hemostasis. Neutrophils and lymphocytes are known to play fundamental roles in cell-mediated destruction of cancer cells.[7] Mounting recent evidence supports that blood count of these cells can help predict patient outcome in many types of cancers. Furthermore, neutrophil-to-lymphocyte count ratio (NLR), an early marker of global inflammation, has been shown to possess significant prognostic value in various tumors including NSCLC.[8,9] A similar prognostic role has also been suggested for platelet-to-lymphocyte count ratio (PLR).[8,9]
Since tumor FDG uptake is significantly influenced by intratumoral inflammatory processes,[10] it is tempting to speculate a relationship between this and hematologic predictors of cancer prognosis, but this possibility has not been previously explored. Conversely, the prognostic information provided by these parameters may be sufficiently independent, and therefore add incremental value to each other. Given the routine testing of blood cell counts in virtually all patients, it would be of significant clinical relevance to determine the prognostic association between hematologic parameters and tumor FDG uptake in patients with cancer. We thus explored the relation between tumor FDG uptake and circulating blood cell-based parameters in patients with stage I NSCLC, and further investigated their prognostic associations following curative resection. The study was performed in patients with stage I disease since the presence of metastatic lesions may confound the relation between circulating blood cell counts and tumor FDG uptake.
2. Materials and methods
2.1. Study subjects
We retrospectively reviewed consecutive patients with newly diagnosed stage 1 NSCLC, who underwent FDG PET/CT for initial staging at our institute from January 2008 to December 2012. Inclusion criteria were performance of curative surgical resection and the presence of negative resection margins, which resulted in 1035 cases. Exclusion criteria were as follows: neoadjuvant or adjuvant therapy, clinical signs of or microbiologically proven preoperative infection, recent steroid therapy, and presence of coexisting hematologic or autoimmune disorders. Thirty-nine patients presenting infectious symptom with leukocytosis (total white blood cell count ≥10,000/μL) were also excluded. Thus, a total of 1034 subjects were finally included for analysis. The study protocol was approved by our Institutional Review Board, and the need to obtain written informed consents was waived.
Clinical profiles and survival information were obtained by reviewing medical records. Tumor histology and pathologic tumor size were determined from surgical pathology reports. Histology was classified according to the WHO guidelines, and tumor staging followed the TNM classification.
2.2. 18F-FDG PET/CT and uptake measurement
Patients fasted for at least 6 hours before PET/CT studies, and blood glucose level was <200 mg/dL at the time of FDG injection in all cases. At 60 minutes after injection of FDG (5.5 MBq/kg), PET and unenhanced CT images were acquired on a Discovery STe PET/CT scanner (GE Healthcare, Chicago, IL). CT images were acquired on a 16-slice helical CT with 30 to 170 mAs adjusted to the patient's body weight at 140 kVp and 3.75 mm section width. This was followed by an emission PET scan from the thigh to the head for 2.5 minutes per bed position in 3-dimensional mode. Attenuation-corrected PET images (3.9 × 3.9 × 3.3 mm) using CT data were reconstructed with an ordered subsets expectation maximization algorithm (20 subsets, 2 iterations).
To measure the magnitude of tumor FDG uptake, regions of interest were drawn along the margin of the primary tumor on a tomographic PET/CT slice with the highest tumor activity. Uptake levels were expressed as maximum standardized uptake value (SUVmax) normalized to patient body-weight.
2.3. Parameters from blood cell count and patient grouping
Hematologic parameters were obtained from peripheral venous blood samples taken within 30 days of the PET/CT prior to surgery. Blood cell counts were measured on a fully automated counting system and confirmed by laboratory technicians. Hematologic parameters of interest included total white blood cell (WBC) count, absolute neutrophil count, lymphocyte count, and platelet count. NLR and PLR were calculated by respective cell counts.
Cut-off values were selected by receiver operating characteristics analysis. After comparing mean values and several different percentile values, upper quartile values were selected as cutoff to categorize subjects into groups with high and low hematologic parameters and FDG uptake.
2.4. Statistical analysis
Linear regression analysis was used to determine the correlation between hematologic parameters and tumor SUVmax. Disease-free survival (DFS), defined as the interval between surgery and first cancer recurrence, was determined as primary clinical outcome. The prognostic significances of variables for DFS were assessed by univariate and multivariate analyses using Cox proportional hazards regression models. Survival curves were estimated using the Kaplan–Meier method, and differences between groups were carried out with log-rank tests. SPSS 20.0 (SPSS Inc, Chicago, IL) software and MedCalc version 13.1 (MedCalc Software, Mariakerke, Belgium) were used for statistical analyses. All tests were 2-sided, and P values <0.05 were considered statistically significant.
3. Results
3.1. Clinical characteristics of the entire cohort and groups according to tumor SUVmax
The clinical characteristics of our study subjects are summarized in Table 1. The entire study cohort had a mean age of 61.6 years (range, 16–89 years); there were 609 males (58.9%); 98.5% had Eastern Cooperative Oncology Group performance status 0, and 49.4% were current or former smokers. Histological subtype was adenocarcinomas in 793 (76.7%), squamous cell carcinomas in 180 (17.4%), and unspecified types in 61 patients (5.9%). The pathologic stage was IA in 727 and IB in 307 patients. The mean tumor SUVmax was 5.5 ± 4.9.
Table 1.
Demographic and clinical characteristics of study subjects.
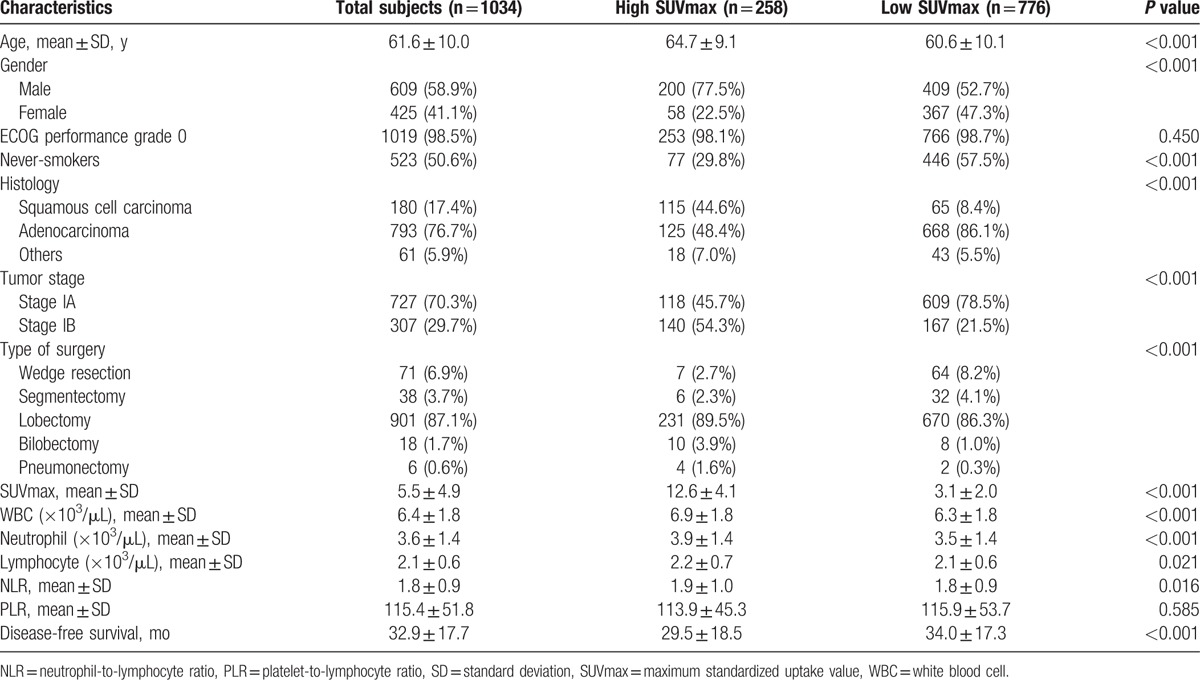
When a 75 percentile cut-off value of 7.83 was applied, 258 patients had high tumor SUVmax and 776 had low tumor SUVmax. Patients with high tumor SUVmax were slightly older and had greater proportions of males, ever-smokers, squamous carcinomas, and stage IB tumors (Table 1).
3.2. Blood cell counts and relation to tumor SUVmax
The results of blood cell counts are summarized in Table 1. Compared with the low SUVmax group, the high SUVmax group had significantly greater WBC, neutrophil and lymphocyte counts, and higher NLR. This prompted us to examine the relation between tumor SUVmax and blood cell parameters. As a result, linear regression analysis demonstrated weak but significant correlations of tumor SUVmax with total WBC count, neutrophil count, lymphocyte count, and NLR (Fig. 1).
Figure 1.
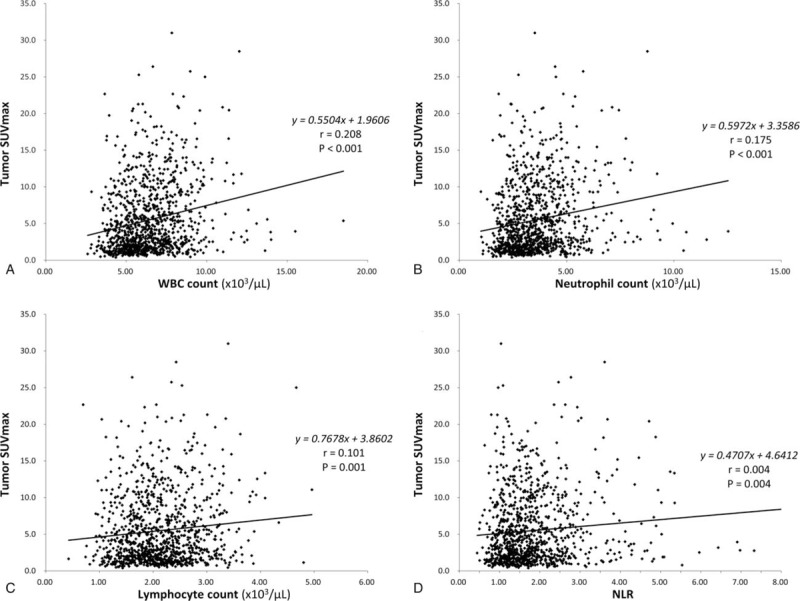
Correlations between tumor SUVmax and total WBC count (A), neutrophil count (B), lymphocyte count (C), and neutrophil-to-lymphocyte ratio (NLR; D). SUVmax = maximum standardized uptake value, WBC = white blood cell.
3.3. Patient outcome and univariate predictors of prognosis
The study subjects were followed up for a median duration of 29.5 months. While there were no deaths during this period, loco-regional or distant recurrence was documented in a total of 144 patients (13.9%). This led to an overall disease-free survival of 95% at 1 year and 90% at 2 years. DFS was significantly shorter for the high tumor SUVmax group (29.5 ± 18.5 months) compared with the low SUVmax group (34.0 ± 17.3 months; Table 1).
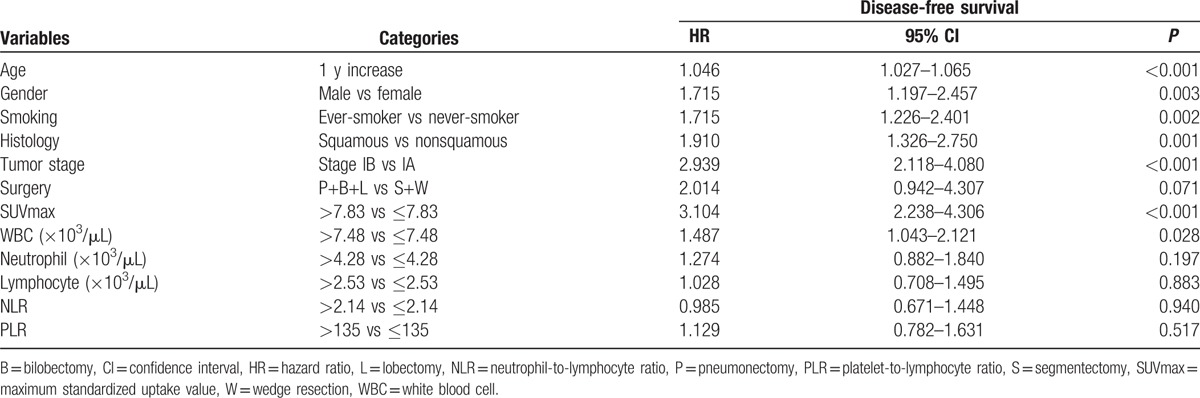
We next analyzed prognostic factors using univariate Cox-regression hazard models. The 75 percentile cut-off values for hematologic parameters were 7.48 × 103/μL for WBC count, 4.38 × 103/μL for neutrophil counts, 2.53 × 103/μL for lymphocyte count, 2.14 for NLR, and 135 for PLR. By definition, there were 258 patients with high value and 776 patients with low value for all parameters. Univariate analysis demonstrated that greater age, male gender, ever-smokers, squamous cancer cell type, tumor stage IB, high tumor SUVmax, and high total WBC count were significant predictors of poor prognosis. High tumor SUVmax had the greatest hazard ratio (HR) of 3.10 (95% CI, 2.24–4.31, P < 0.001), followed by tumor stage IB (HR = 2.94; 95% CI, 2.12–4.08; P = 0.001). Notably, neither NLR nor PLR was significantly associated with patient survival (Table 2).
Table 2.
Univariate Cox regression analysis of survival.
3.4. Multivariate predictors of prognosis and survival analysis
Multivariate Cox analysis using significant univariate variables revealed that older age (HR = 1.03; 95% CI, 1.01–1.05; P = 0.002), tumor stage 1B (HR = 2.11; 95% CI, 1.47–3.01; P < 0.001), and high tumor SUVmax (HR = 2.22; 95% CI, 1.52–3.25; P < 0.001) were significant independent predictors for poor prognosis (Table 3). Kaplan–Meier survival analysis showing significant survival benefits of low tumor SUVmax and low total WBC count are illustrated in Fig. 2.
Table 3.
Multivariate Cox regression analysis of survival.
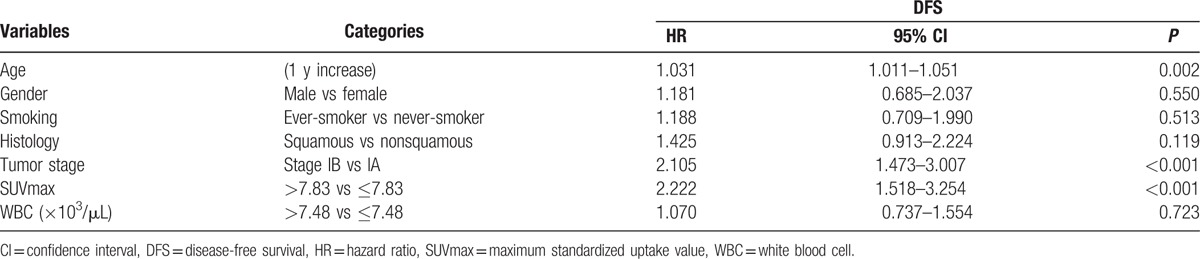
Figure 2.
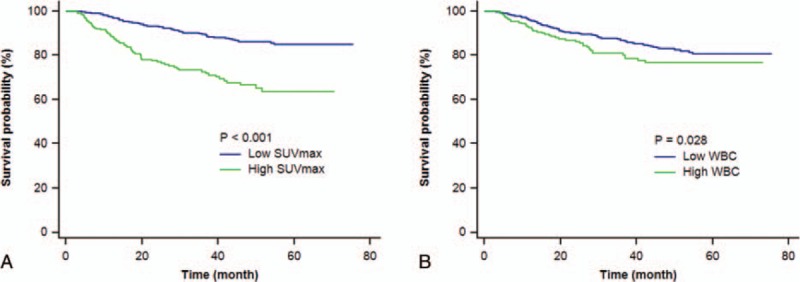
Kaplan–Meier curves of disease-free survival of study subjects (n = 1034) stratified by tumor SUVmax level (A) and total WBC count (B). Variables are those at the time of PET/CT staging. PET/CT = positron emission tomography/computed tomography, SUVmax = maximum standardized uptake value, WBC = white blood cell.
Finally, we performed Kaplan–Meier survival analysis in patients categorized according to tumor SUVmax and WBC count. As a result, total WBC count failed to significantly influence survival in both high and low tumor SUVmax groups (Fig. 3). In contrast, high SUVmax was significantly associated with worse survival in both high (HR, 3.33; 95% CI, 2.24–4.94; P < 0.001) and low WBC count groups (HR, 2.38; 95% CI, 1.31–4.33; P = 0.004; Fig. 3).
Figure 3.
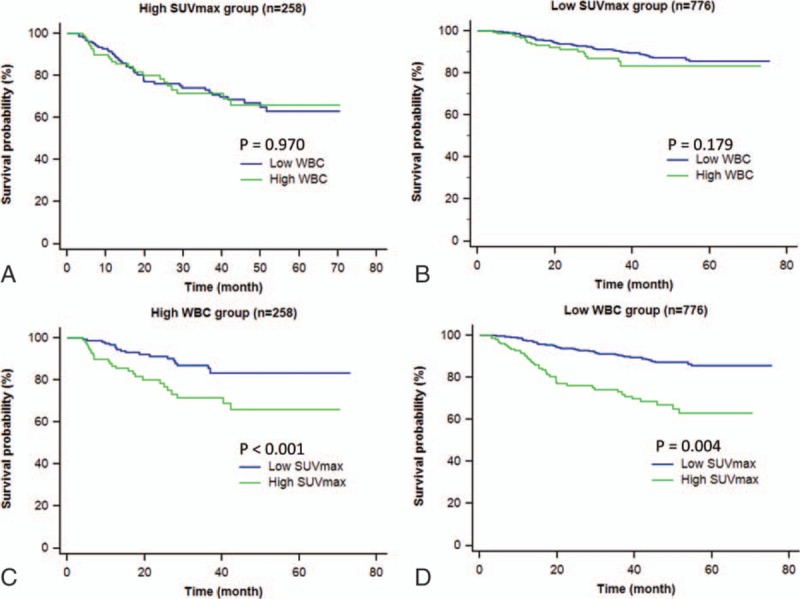
Kaplan–Meier curves of disease-free survival of study subjects categorized according to SUVmax and WBC levels. A, B, Survival curves of high SUVmax (n = 258; A) and low SUVmax groups (n = 776; B) stratified by WBC count. C, D, Survival curves of high WBC (n = 258; C) and low WBC groups (n = 776; D) stratified by SUVmax. SUVmax = maximum standardized uptake value, WBC = white blood cell.
4. Discussion
In this cohort of stage I NSCLC patients, circulating white blood cell, neutrophil and lymphocyte counts, as well as NLR were found to have significant correlations with tumor FDG uptake. Accordingly, patients with high tumor SUVmax had significantly greater counts of total white blood cells, neutrophils and lymphocytes, and higher NLR compared with those with low tumor SUVmax.
Although correlations have previously been reported between blood neutrophil count and FDG uptake in the bone marrow,[11] to our knowledge, this is the first demonstration that circulating blood cell counts can correlate to tumor FDG uptake. While the basis underlying this weak but significant association is not clear, a possible link may be found in intratumoral inflammatory processes that can contribute to tumor FDG activity.[10] Infiltrating macrophages, granulocytes, and lymphocytes have been shown to elevate tumor FDG accumulation, especially when the cells are activated.[12] Conversely, many tumors can secrete inflammatory cytokines that promote systemic inflammation processes. Indeed, one of the causes of elevated WBC count in cancer patients is production of granulocyte colony stimulating factor (G-CSF) by the tumor, and some NSCLCs can secrete G-CSF into the blood in quantities sufficient to raise peripheral WBC counts.[13] Furthermore, patients with G-CSF-producing tumors are reported to show abnormally high tumor FDG uptake with histopathologic findings of increased inflammatory cell infiltration.[14] However, these possibilities were not pursued in the present study, and further studies will be required to clarify this issue. Another possible explanation for our observed correlation is that high tumor FDG uptake and elevated inflammatory cell counts are indirectly associated through their link with tumor aggressiveness.
When Cox regression analysis of DFS was performed, total WBC count and tumor SUVmax, in addition to several clinical factors, were identified as significant univariate predictors of outcome. A WBC count threshold based on the 75 percentile value was able to stratify favorable from poor prognosis. Although the hazards ratio by this variable was relatively small, the clinical implication is potentially large because hematologic parameters are widely available from simple, inexpensive, and reproducible blood tests. Notably, however, neither neutrophil count nor NLR was able to show significant prognostic value in our study cohort. Moreover, total WBC count, which was the only significant univariate variable among hematologic parameters, did not show significant independent prognostic value when adjustment for other univariate variables was performed by multivariate analysis. This finding is divergent from the findings of several recent studies that report significant prognostic associations of 1 or more hematologic parameters in patients with NSCLC. Tomita et al[15] previously found total WBC and platelet counts to be significant univariate and multivariate predictors of outcome in 289 patients with NSCLC. In a more recent study, this group found NLR to be a significant univariate and multivariate indicator of prognosis in 84 patients with stage IV NSCLC.[16] Similar prognostic values for NLR were also observed by Kacan et al[17] in 299 NSCLC patients, Sarraf et al[18] in 177 NSCLC patients, and Pinato et al[8] in 220 NSCLC patients who underwent surgical resection, and Cedres et al[19] in 171 NSCLC patients with stage IV disease. Furthermore, positive associations were not restricted to advanced stage disease. Zhang et al[20] studied 400 NSCLC patients with pN0 disease and observed total WBC count, lymphocyte count, neutrophil count, NLR, and PLR as univariate predictors, and NLR as a multivariate predictor of survival following curative resection. In the report of Sarraf et al,[18] NLR was able to stratify prognosis in 83 of the study subjects who had stage I disease. Cannon et al[21] evaluated 149 patients with early-stage NSCLC and found NLR to be a significant univariate and multivariate predictor of prognosis.
Although the majority of previous studies in patients with lung cancer report positive associations with outcome, hematologic parameters have not always been shown to provide significant prognostic information. In the study by Zhang et al,[20] PLR did not have independent prognostic value, even though NLR did. Conversely, the study by Cannon et al[21] found an association between nonlocal failure and PLR but not NLR. More recently, Sim et al[22] evaluated 250 NSCLC patients with EGFR mutations and found that NLR was a significant prognostic factor for first-line progression-free survival in the chemotherapy group, but not in patients treated with tyrosine kinase inhibitors.
The reason our study with a large number of patients only managed to show total WBC count as a prognostic indicator may be explained by difference in subject characteristics. Even previous reports with early-stage NSCLC patients may have some differences in study population compared with our study. For instance, although subjects in the study by Cannon et al[21] had early-stage NSCLC, they were medically inoperable patients who underwent stereotactic radiotherapy instead of curative surgery. Also, the rate of recurrence or deaths in our study cohort (13.9%) was lower than that of the study by Zhang et al[20] (50.1%) or the study by Cannon et al[21] (24.1%), which cannot be explained by differences in follow-up duration (29.5 months vs 46 months and 17 months). Furthermore, only a small portion of our patients (3.8%) had WBC counts exceeding >10,000/μL. These facts suggest that our study population had more favorable prognostic characteristics compared with those of previous studies.
Unlike the weak prognostic association of hematologic parameters, tumor SUVmax was confirmed to be a powerful prognostic factor with the highest hazards ratio. A close relation between high tumor SUVmax and poor outcome in patients with NSCLC is well recognized, and this has also been observed with early-stage disease.[23–26] In our study, a tumor SUVmax of 7.83 effectively stratified patients with favorable from those with poor prognosis. This finding is similar to the report of Vansteenkiste et al,[25] who found a tumor SUVmax of over 7.0 to be associated with adverse outcome in NSCLC patients. High tumor SUVmax was a significant independent risk factor by multivariate analysis, along with old age and tumor stage 1B. Tumor SUVmax was also able to stratify favorable from poor outcome in both high and low WBC count groups. These results demonstrate that tumor FDG uptake remains a strong prognostic indicator in patients with resected stage I NSCLC after adjustment for hematologic parameters in addition to other clinical variables.
Limitations of our study include its retrospective design. Another potential limitation is the interval between PET/CT and blood tests, which reached 30 days in a few patients. However, the majority of our subjects had a much shorter interval, which averaged only 3.4 days. Only 3% of the subjects had an interval greater than 2 weeks, and during this interval, all were confirmed to be free of events that might affect blood counts such as infectious symptom, fever, trauma, blood loss, or medication. Also, the possibility that tumor SUVmax may have been underestimated by partial volume effect in some of the small tumors cannot be excluded. Finally, since our subjects were limited to stage I NSCLC, the prognostic impacts of tumor SUVmax and hematologic markers may be different in patients with more advanced disease. Therefore, further studies with patients with a broader range of NSCLC stage are required.
In patients with stage I NSCLC, circulating blood counts showed weak but significant correlation to tumor FDG uptake. Although total WBC count was a significant univariate risk factor, the prognostic value of tumor FDG uptake was substantially superior. Tumor SUVmax was an independent predictor of outcome after adjustment for other variables including WBC count, and effectively stratified prognosis in patients with low as well as high WBC count.
Footnotes
Abbreviations: DFS = disease-free survival, FDG = 18F-fluoro-2-deoxyglucose, HR = hazard ratio, NLR = neutrophil-to-lymphocyte count ratio, NSCLC = nonsmall cell lung cancers, PET/CT = positron emission tomography/computed tomography, PLR = platelet-to-lymphocyte ratio, SUVmax = maximum standardized uptake value, WBC = white blood cell.
EJ and SHH contributed equally to this work.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (# 2016R1C1B2013411).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- [2].Myrdal G, Lambe M, Gustafsson G, et al. Survival in primary lung cancer potentially cured by operation: influence of tumor stage and clinical characteristics. Ann Thorac Surg 2003;75:356–63. [DOI] [PubMed] [Google Scholar]
- [3].Sakurai H, Asamura H, Goya T, et al. Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol 2010;5:1594–601. [DOI] [PubMed] [Google Scholar]
- [4].Stroobants SG, D’Hoore I, Dooms C, et al. Additional value of whole-body fluorodeoxyglucose positron emission tomography in the detection of distant metastases of non-small-cell lung cancer. Clin Lung Cancer 2003;4:242–7. [DOI] [PubMed] [Google Scholar]
- [5].Viney RC, Boyer MJ, King MT, et al. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 2004;22:2357–62. [DOI] [PubMed] [Google Scholar]
- [6].Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [7].Kobayashi T, Teruya M, Kishiki T, et al. Inflammation-based prognostic score and number of lymph node metastases are independent prognostic factors in esophageal squamous cell carcinoma. Dig Surg 2010;27:232–7. [DOI] [PubMed] [Google Scholar]
- [8].Pinato DJ, Shiner RJ, Seckl MJ, et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer 2014;110:1930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yao Y, Yuan D, Liu H, et al. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother 2013;62:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim M, Achmad A, Higuchi T, et al. Effects of intratumoral inflammatory process on 18F-FDG uptake: pathologic and comparative study with 18F-fluoro-alpha-methyltyrosine PET/CT in oral squamous cell carcinoma. J Nucl Med 2015;56:16–21. [DOI] [PubMed] [Google Scholar]
- [11].Murata Y, Kubota K, Yukihiro M, et al. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: measurements by PET/CT. Nucl Med Biol 2006;33:999–1004. [DOI] [PubMed] [Google Scholar]
- [12].Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972–80. [PubMed] [Google Scholar]
- [13].Kasuga I, Makino S, Kiyokawa H, et al. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer 2001;92:2399–405. [DOI] [PubMed] [Google Scholar]
- [14].Morooka M, Kubota K, Murata Y, et al. (18)F-FDG-PET/CT findings of granulocyte colony stimulating factor (G-CSF)-producing lung tumors. Ann Nucl Med 2008;22:635–9. [DOI] [PubMed] [Google Scholar]
- [15].Tomita M, Shimizu T, Hara M, et al. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res 2009;29:2687–90. [PubMed] [Google Scholar]
- [16].Tomita M, Shimizu T, Ayabe T, et al. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res 2011;31:2995–8. [PubMed] [Google Scholar]
- [17].Kacan T, Babacan NA, Seker M, et al. Could the neutrophil to lymphocyte ratio be a poor prognostic factor for non small cell lung cancers? Asian Pac J Cancer Prev 2014;15:2089–94. [DOI] [PubMed] [Google Scholar]
- [18].Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425–8. [DOI] [PubMed] [Google Scholar]
- [19].Cedres S, Torrejon D, Martinez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol 2012;14:864–9. [DOI] [PubMed] [Google Scholar]
- [20].Zhang T, Jiang Y, Qu X, et al. Evaluation of preoperative hematologic markers as prognostic factors and establishment of novel risk stratification in resected pN0 non-small-cell lung cancer. PLoS One 2014;9:e111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol 2015;10:280–5. [DOI] [PubMed] [Google Scholar]
- [22].Sim SH, Beom SH, Ahn YO, et al. Pretreatment neutrophil-lymphocyte ratio is not a significant prognostic factor in epidermal growth factor receptor-mutant non-small cell lung cancer patients treated with tyrosine kinase inhibitors. Thorac Cancer 2016;7:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yoo Ie R, Chung SK, Park HL, et al. Prognostic value of SUVmax and metabolic tumor volume on 18F-FDG PET/CT in early stage non-small cell lung cancer patients without LN metastasis. Biomed Mater Eng 2014;24:3091–103. [DOI] [PubMed] [Google Scholar]
- [24].Goodgame B, Pillot GA, Yang Z, et al. Prognostic value of preoperative positron emission tomography in resected stage I non-small cell lung cancer. J Thorac Oncol 2008;3:130–4. [DOI] [PubMed] [Google Scholar]
- [25].Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol 1999;17:3201–6. [DOI] [PubMed] [Google Scholar]
- [26].Sakai T, Tsushima T, Kimura D, et al. A clinical study of the prognostic factors for postoperative early recurrence in patients who underwent complete resection for pulmonary adenocarcinoma. Ann Thorac Cardiovasc Surg 2011;17:539–43. [DOI] [PubMed] [Google Scholar]


