Abstract
Adenoid cystic carcinoma (ACC) is characterized by slow growth, frequent local recurrences, and high incidence of distant metastasis (DM). The aim of this study was to evaluate predictive factors for local-regional (LR) recurrence, DM, and survival in ACC.
A retrospective review of the medical records for patients with salivary glands ACC from 1990 to 2015 was performed. The clinical parameters were assessed to identify correlations with the development of LR recurrence, DM, and survival of these patients.
Among 228 patients who underwent surgery as definitive treatment, 210 (92.1%) were followed up in the study. DM was detected in 64 (30.5%) patients, LR recurrence was detected in 58 (27.6%) patients. The estimated 5, 10, and 15-year overall survival rates were 84.7%, 70.8%, and 34.0%, respectively. Multivariate analysis revealed that the presence of lymphovascular invasion and a high T classification were very strong adverse factors, which independently influenced LR recurrence, DM, and survival of ACC patients. Positive/close margin and N+ status were independent risk factors for DM and LR recurrence, respectively. Survival of ACC patents was also affected by tumor location.
Presence of lymphovascular invasion and a high T classification were very strong adverse factors and independent predictors for ACC patients’ prognosis, which influenced LR control, DM control, and survival.
Keywords: adenoid cystic carcinoma, lymphovascular invasion, postoperative radiotherapy, salivary gland
1. Introduction
Adenoid cystic carcinoma (ACC) is a rare tumor accounting for approximately less than 1% of all malignant tumors of the head and neck.[1,2] Yet, it is the most common malignant tumor in the minor salivary glands and 2nd most frequent malignancy in the major salivary glands.[3,4] ACC is characterized by an indolent but persistent course, with high rates of both local-regional (LR) recurrence and distant metastasis (DM)[5,6] that can develop even many years after initial treatment of primary tumor. DM is reported in up to 52% of ACC patients[7] and can occur with/without LR recurrence.[8] Although most patients with ACC are alive at 5 years, a majority of patients die from their disease 5 to 20 years after diagnosis.[6,8] The long-term outcomes continue to be guarded, with an estimated 10-year overall survival (OS) of <70%.[8–10] Therefore, understanding more about patient and tumor characteristics in regards to prognosis is critical.
Due to the low prevalence of ACC, most reports are based on very small patient populations or single-institution retrospective series.[5,11–13] The few population-based studies that are available were established in the United States, Europe, and Denmark from the Surveillance, Epidemiology and End Results, EUROCARE-3, and Danish Head and Neck Cancer Group databases[8–10,14] which all demonstrated the importance of staging on the survival of ACC.[8–10,14] Margin status,[10] age at diagnosis,[8,10] nodal status,[8] T classification,[8] gender,[9] and primary site[8,9] from these studies were mentioned as independent factors to survival. Unfortunately, only 1 of the 4 population-based studies identified risk factors concerning LR recurrence,[10] which included staging, margin status, and vascular invasion as significant variables. However, risk factors related to DM were not reported in these trials.
The aim of the present study was to report a cohort of sizable patients with salivary glands ACC of the head and neck who were previously untreated in South China and to investigate predictors for LR recurrence, DM, and survival.
2. Patients and methods
2.1. Study design
We retrospectively reviewed all the records for patients with salivary glands ACC of the head and neck who were treated at Sun Yat-sen University (Guanghua Hospital of Stomatology, Sun Yat-en University Cancer Center, Sun Yat-sen Memorial Hospital, and The First Affiliated Hospital), from 1990 to 2015. From the pooled data, 228 patients who underwent definitive surgical treatment were eligible for the study. Demographic data, clinical characteristics, and follow-up information were recorded. TNM classification and clinical staging were documented according to the American Joint Committee on Cancer staging criteria.[15] Surgical margin status was recorded from the pathological reports in the medical records. According to NCCN clinical practice Guidelines in Oncology, Head and Neck Cancers (Version I, 2015),[16] a clear margin is defined as the distance from the invasive tumor front that is 5 mm or more from the resected margin; a close margin is defined as the distance from the invasive tumor front to the resected margin that is less than 5 mm; a positive margin is defined as carcinoma in situ or as invasive carcinoma at the margin of resection. Institutional Review of Board approval was obtained for this study.
A head and neck pathologist reviewed all the pathological slides. Histologic information including the tumor subtype (cribriform, tubular, or solid), perineural/nerve invasion, lymphovascular invasion, and nodal status were recorded. Margin status was collected from medical records. When >30% of tumor was presented as a solid component, it was classified as a solid pattern.
2.2. Treatment
All patients underwent surgery as definitive treatment. The indications for postoperative radiotherapy (RT) included late-stage tumors (stage III–IV), close/positive margins, perineural/nerve invasion, and lymphovascular invasion. Of the 228 patients, 104 patients (45.6%) received postoperative RT. The average radiation dose delivered to the primary site was 59.3 Gy (range 18–75 Gy). In brief, the prophylactic irradiation dose was 46 to 50 Gy, with a 60 to 66 Gy boost to high-risk areas. For patients with adverse factors such as positive margins, the dose of radiation was over 66 Gy. Two patients withdrew from postoperative RT due to acute toxicity at 18 and 54 Gy. Due to poor general health or refusal, 14/95 patients with positive/close margins and 17/81 patients with stage III–IV disease did not receive postoperative RT. Nineteen patients with nodal disease, local recurrence, or DM received chemotherapy, each with different regimens.
2.3. Statistical analysis
Estimated survival rates and curves describing survival were generated using the Kaplan–Meier method. Statistical significance of the differences between certain curves was determined using the log-rank test. Correlations between clinicopathological factors and the outcomes were analyzed using a Cox proportional hazards regression model. A 2-tailed P value of <0.05 was considered significant. Statistical analyses were performed with SPSS (version 22.0; IBM Corporation, Somers, NY) software application.
3. Results
3.1. Demographic data and clinicopathological characteristics
The cohort consisted of 99 males and 129 females with a median age of 48 years at diagnosis (range, 13–78 years). The peak incidence was during the 5th decade of life, accounting for 28.9% (66/228) of all tumors.
Almost all the patients (218/228, 95.6%) presented with a gradually growing mass. Other incidental symptoms included facial paralysis (3.5%), sensory/motor neural deficit (3.9%), rapid tumor growth (6.1%), ulceration with/without bleeding (3.5%), tooth mobility (3.9%), and nasal obstruction (3.1%). The course of disease ranged from 2 months to 11 years.
Of the 228 tumors, 120 (52.6%) originated from the minor salivary glands, and 108 (47.4%) occurred in the major salivary glands. The palate (67/228) was the most frequently affected site, followed by the parotid gland (53/228) and the submandibular gland (44/228) (Table 1).
Table 1.
Tumor clinicophathological features.
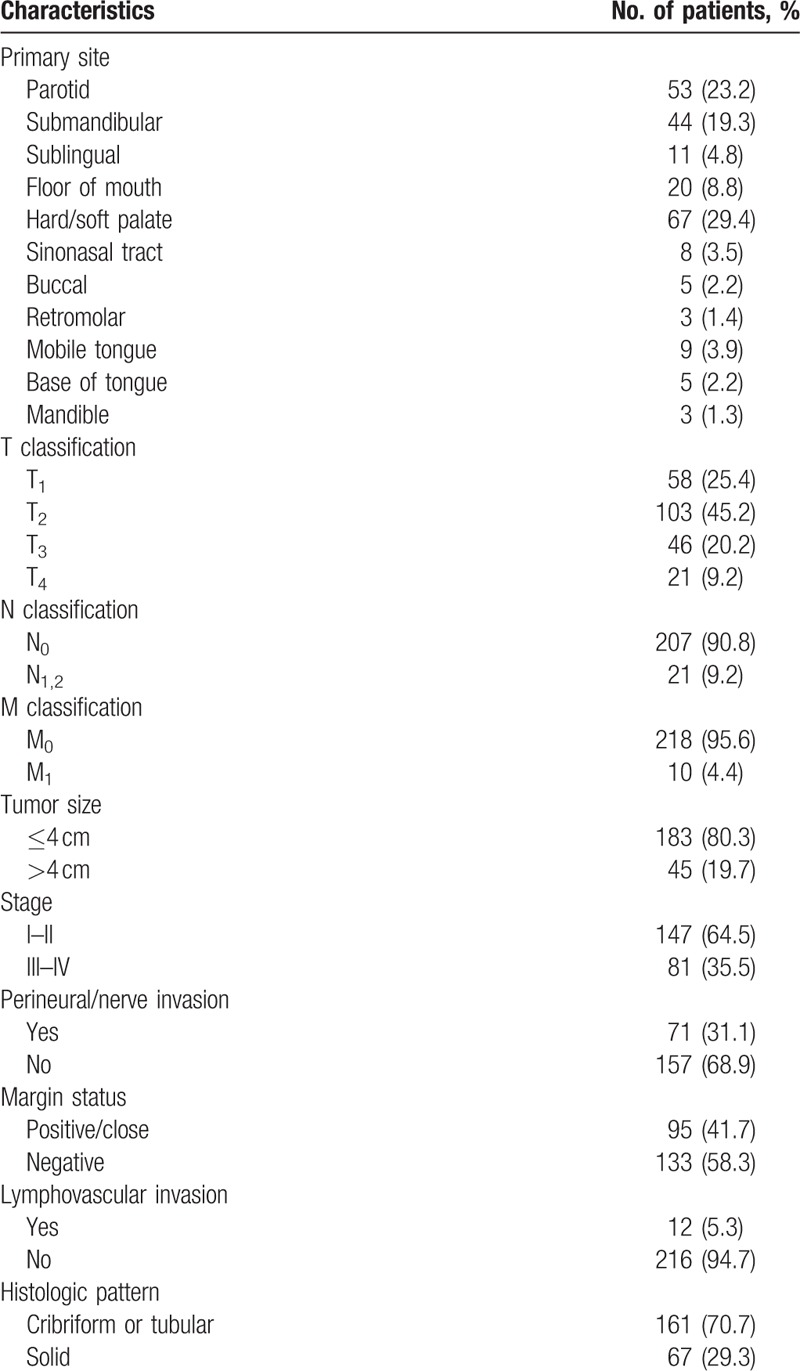
At the time of diagnosis, 57 patients (25.0%) presented with stage I disease, 90 patients (39.4%) with stage II disease, 46 patients (20.2%) with stage III disease, and 35 patients (15.4%) with stage IV disease. Twenty-one patients (9.2%) were detected with pN+ classification and 10 patients (4.4%) with M1 disease all metastasizing to the lung at initial treatment. Histologically, 161 tumors (70.7%) were identified as cribriform or tubular growth pattern, and 67 (29.3%) solid pattern. Other clinical and histologic features were presented in Table 1.
3.2. Follow-up information
Regular follow-up visit was required every 3 months/6 months within/beyond 5 years after treatment. A thorough head and neck clinical examination was mandatory. Chest radiograph was recommended every 6 to 12 months. MRI, CT, and/or PET-CT were indicated if there was any suspicion of LR recurrence or DM.
Follow-up information was available for 210/228 (92.1%) of the patients. Follow-up duration for living patients ranged from 12 to 288 months (mean 74.6 months, and median 66.0 months). At the end of the follow-up period, 70 patients had died: 17 patients died from LR recurrence, 33 of DM, 17 had both LR recurrence and DM, and 3 of other causes. In total, LR recurrence contributed to 34/67 (50.7%) of the disease-related death while DM accounted for 50/67 (74.6%). A total of 140 (66.7%) patients remained alive, including 32 patients with residual disease. Fifty-one patients (26.7%) developed LR recurrence (Table 2), where recurrence ranged from 2 to 176 months (median of 24 months) after the initial treatment. Fifty-four patients developed DM from 3 to 212 months (median 37 months) after treatment of the primary disease. Including 10 patients who already had lung metastasis at diagnosis, total DM rate is 30.5%. Patients with DM had a median survival time of 37.0 months. A total of 74% of the LR recurrence and 68.5% of the DM were detected within 5 years after treatment.
Table 2.
Follow-up results with local-regional recurrence and distant metastasis information.

3.3. Survival analysis
The estimated median OS and median disease-free survival (DFS) for all patients was 133 and 86 months, respectively. The estimated 5-, 10-, and 15-year OS rates were 84.7%, 70.8%, and 34.0%, respectively. Five-, 10-, and 15-year DFS rates were estimated at 59.7%, 38.0%, and 20.9%, respectively (Fig. 1).
Figure 1.
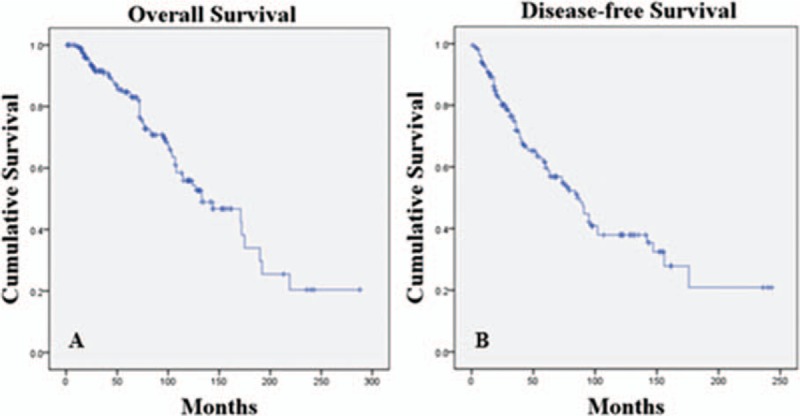
Kaplan–Meier survival curves of (A) overall survival and (B) disease-free survival of ACC patients.
Patients who developed LR-recurrence had a significantly lower median OS (104 months) than patients who did not have LR-recurrence (172 months; P = 0.014). The estimated 5- and 10- year OS in patients with no LR-recurrence were 86.1% and 72.2%, respectively, while patients with LR-recurrence were 81.2% and 32.5%, respectively (Fig. 2A). Similarly, the estimated 5- and 10-year OS rates were significantly lower in patients with DM (69.7% and 29.0%, respectively) than those who did not have DM (93.8% and 79.4%, respectively; P < 0.001) (Fig. 2B).
Figure 2.
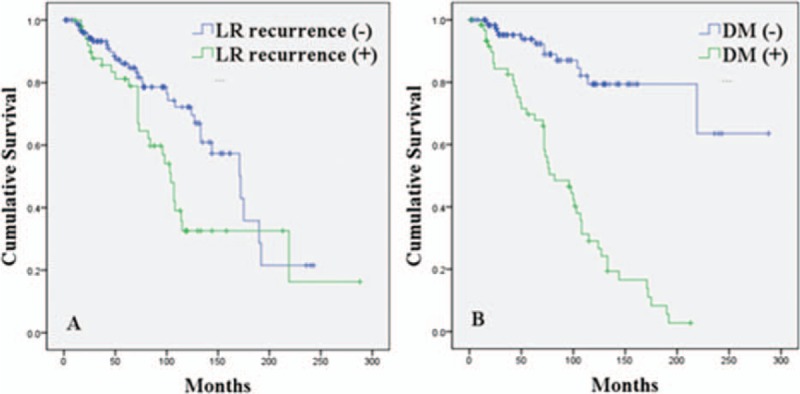
Cumulative survival curves of ACC patients. (A) Overall survival was significantly reduced in patients with LR recurrence (green line) compared with patients without LR recurrence (blue line). P = 0.014. (B) Overall survival was significantly reduced in patients with DM. P < 0.001. ACC = adenoid cystic carcinoma, LR = local-regional, DM = distant metastasis.
3.4. Risk factors for local-regional recurrence, distant metastasis, and survival
Risk factors were analyzed using a Cox proportional hazards regression model. Variables achieving significance in the univariate analysis were subsequently incorporated into multivariate analysis using Stepwise method. For OS, age >50 years (P = 0.010), T3–4 (P = 0.001), N+ (P < 0.001), M1 (P < 0.001), presence of lymphovascular invasion (P < 0.001), perineural/nerve invasion (P = 0.038), and positive/close margin status (P < 0.001) were significant predictors for decreased survival. Survival was significantly worse with tumor sites located in the floor of month (FM)/sublingual area (SL)/tongue complex, sinonasal tract, and hard palate compared to those in parotid, submandibular gland (SG), and the “other” group (mandible, buccal, soft palate, base of tongue, and retromolar region) (P < 0.001). However, gender (P = 0.089), histologic patterns (P = 0.396), and postoperative RT (P = 0.269) did not appear to influence OS (Fig. 3). Furthermore, T3–4, N+, age >50 years, presence of lymphovascular invasion, positive/close margin, primary tumor location in FM/SL/tongue area, sinonasal tract, and hard palate were identified as independent factors adversely affecting the OS from the multivariate analysis (Table 3).
Figure 3.
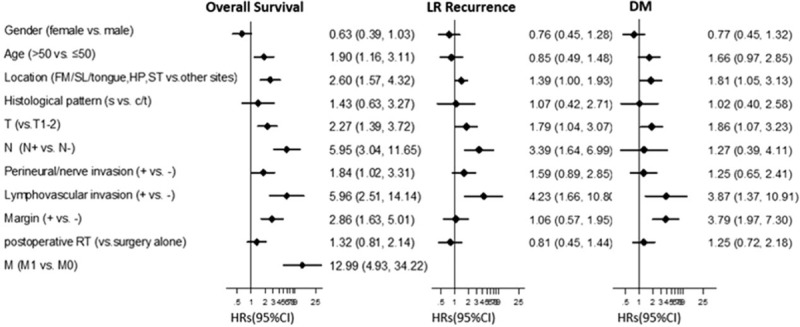
Summary of univariate analysis for LR recurrence, DM, and overall survival. HR and 95% CI of HR were showed in Forest plot. Factors of significance for overall survival were: age (P = 0.010), T classification (P = 0.001), N classification (P < 0.001), M classification (P < 0.001), location (P < 0.001), margin status (P < 0.001), perinerual/nerve invasion (P = 0.038), and lymphovascular invasion (P < 0.001). For LR-recurrence they were: T classification (P = 0.034), N classification (P = 0.001), location (P = 0.047), and lymphovascular invasion (P = 0.003). Significant factors for DM development were: T classification (P = 0.028), margin status (P < 0.001), location (P = 0.033), and lymphovascular invasion (P = 0.011). s, solid; c, cribriform; t, tubular. CI = confidence interval, DM = distant metastasis, FM = floor of mouth, HP = hard palate, HR = hazard ratio, LR = local-regional, RT = radiotherapy, SL = sublingual, ST = sinonasal tract.
Table 3.
Summary of multivariate analysis for overall survival, local-regional control, and distant metastasis control.
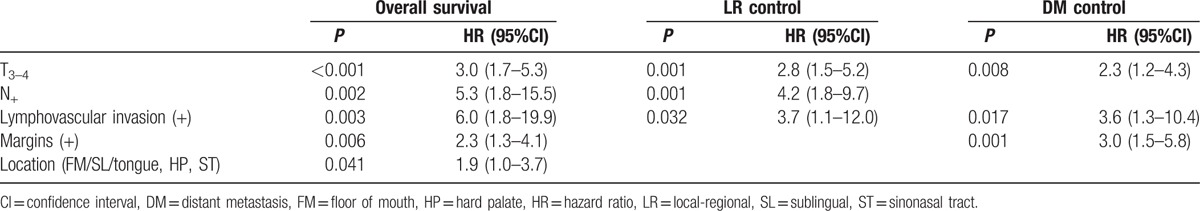
T3–4 (P = 0.034), N+ (P = 0.001), tumor location in the FM/SL/tongue complex, sinonasal tract, and hard palate (P = 0.047), and lymphovascular invasion (P = 0.003) were significantly associated with a higher risk of LR recurrence (Fig. 3). Multivariate analysis of these significant factors revealed that T3–4 classification, positive nodal status, and presence of lymphovascular invasion were independent factors correlated with LR recurrence (Table 3).
Meanwhile, as shown in Fig. 3 and Table 3, significant parameters toward the development of DM included T3–4 classification (P = 0.028), positive/close margin (P < 0.001), lymphovascular invasion (P = 0.011), and primary tumor site located in FM/SL/tongue complex, sinonasal tract, and hard palate (P = 0.033). T3–4 classification, the presence of lymphovascular invasion, and positive/close margin were each independent risk factors for DM.
4. Discussion
ACC is characterized by an indolent but persistent course by local infiltrative growth and a high incidence of DM. Our findings indicated that T3–4, presence of lymphovascular invasion, and positive/close margins were independent risk factors for DM. Meanwhile, LR recurrence was affected independently by T3–4, presence of lymphovascular invasion, and N+ status. Besides the factors mentioned above, multivariate analysis also revealed lower survival rates among patients with tumors located in FM/SL/tongue, hard palate, and sinonasal tract compared to tumors originating from with the parotid, submandibular gland, buccal, base of tongue, and soft palate.
The present study highlighted lymphovascular invasion as a strong independent variable associated with survival, LR recurrence, and DM. Oplatek et al[17] and Min et al[18] both reported similar observations that lymphovascular invasion presented in the head and neck ACC was independently associated with LR failure. Thompson et al[19] suggested patients with sinonasal tract and nasopharyngeal ACC demonstrating lymphovascular invasion had an increased incidence of recurrence and lower survival probability. Therefore, pathological diagnoses of ACC should place emphasis on detecting lymphovascular invasion. Once a patient is found to have lymphovascular involvement, close monitoring and periodical chest radiographs for detecting DMs should be strictly implemented. In our own practice, chest radiograph was recommended every 6 to 12 months. Multidisciplinary treatment should also be considered in these patients.
The findings of the present study confirmed T3–4 classification rather than disease staging[9–11,14] was predictor for lower survival. Moreover, multivariate analysis also identified T3–4 as another independent risk factor for both LR recurrence and DM. We recorded a rate of 9.2% on initial presentation with an additional 7.6% developing positive lymph nodes subsequently. The rate of lymph node involvement over the course of the disease was 12.3%. These rates were comparable to other studies.[6,8,18] In concurrence with other reports, our study also determined that positive nodal status was an independent predictor for worse prognosis, adversely affecting the LR control and survival.[17,18,20] Whether nodal status has an influence on DM development is controversial. Previously, Bhayani et al[21] found positive lymph nodes on neck dissection and lymph nodes with extracapsular spread had statistically significant correlations with the development of DM in early-stage ACC. However, in our cohort, we failed to confirm that nodal status had an influence on DM development, which was consistent with Zhang report.[13] Due to the low incidence of nodal metastasis, so far there is no consensus on the relationship between nodal status and DM development, and further investigation is warranted.
Although many authors reported histopathological subtype was a major predictor for LR recurrence,[13] DM,[21] and survival,[11,13,22,23] we did not find such a correlation in our study. Tumor subtypes (solid/cribiform/tubular pattern) achieved nonsignificant results in the univariate analysis and the Cox multivariate analysis, suggesting that subtypes have no impact on LR recurrence, DM development, or survival. Our results are consistent with data from Spiro et al[6,12] as they also could not confirm the impact of solid subtype on prognosis. Unfortunately, the limited information provided from the 3 population-based studies from the EUROCARE-3[14] and Surveillance, Epidemiology and End Results[8,9] databases neither cannot provide a conclusive correlation whether tumor subtype influences survival. Therefore, until there is more clinical evidence in the future, whether a different pathological subtype affects ACC prognosis currently is still controversial.
The present study revealed that positive/close margin was independent predictor for DM and OS, which is consistent with the findings from other studies.[8,9] Interestingly, margin status did not seem to impact LR recurrence in our findings. Unexpectedly our results have suggested that perineural/nerve invasion has no significant influence on recurrence, metastasis, or survival. Amit et al[24] have reported intraneural rather than perineurral invasion as a factor trending for poor prognosis while Garden et al[25] found that perineural invasion was an adverse prognostic factor only when a major (named) nerve invasion was involved. This inconsistency raises the necessity for further research on the effects of different patterns of nerve invasion of ACC.
Our results indicated that tumors originating from the FM/SL/tongue area, sinonasal tract, and hard palate were adverse predictors for survival. This may be due to the following reasons: first, the complex anatomy of these areas increases the difficulty of surgery planning and treatment implementation, and surgery approaches to the advanced tumors in these locations are complicated; second, tumors originating from these sites usually present an indolent and asymptomatic course, which leads to the delayed patients’ help seeking process; third, the frequent movement of the mobile tongue may increase the risk of nodal involvement; and last, advanced-stage hard palate and sinus ACC may involve skull base and cranial nerves. However, we found no significant differences of the recurrence, DM, and survival outcome between major and minor salivary glands.
Age appears to impact survival, as several authors reported worse survival in older patients with ACC[13,21] which is consistent to our univariate analysis results where age at diagnosis had a significant influence on survival and DM. Since ACC is characterized by an indolent but persistent clinical course, older patients are more likely to present with late-stage tumors that are more prone for recurrence and DM.[14] Also, older patients are more likely to suffer from other existing comorbidities which may limit their treatment response and tolerance for an aggressive multidisciplinary approach compared to younger patients. However, in the multivariate analysis, age was not an independent factor for ACC prognosis.
Long-term outcome of ACC patient did not improve over time.[14] Treatment modalities are limited and have not reached a universal consensus. However, current standards indicate surgery as first choice for treatment with the aim to obtain free margins. RT is usually indicated for local disease control following surgery, and used alone for patients with nonresectable tumors or those who cannot tolerate surgery. Although the role of RT in ACC treatment is still controversial, postoperative RT is reported to be widely implemented in up to 75% to 100% of ACC cases.[10,13,21] Surprizingly, in our analysis, the inclusion of postoperative RT did not appear to impact LR recurrence, DM, or OS (Fig. 3). However, our results must be interpreted carefully since there are multiple covariables that need to be considered before such a conclusion can be made including irradiation technique, dosage, and patient compliance. The role of chemotherapy is still currently a subject of debate as there is no definitive protocol established for ACC treatment. Currently limited evidences showed that mitoxantrone or vinorelbine is both reasonable 1st-line single-agent options, while cisplatin and anthracycline are recommended for combination chemotherapy.[1] Identifying novel targets and individualized chemotherapies directed at the key genes involved in the tumorigenesis of ACC offers a potential treatment option for chemotherapy to help improve ACC survival. Given the low incidence of ACC, performing prospective randomized controlled clinical trials would be very difficult. A lack of high-level clinical evidence will continue to be an obstacle for treatment planning and decision-making.
There were a number of limitations in our present study. Our study does not offer the highest level of clinical evidence since it is a retrospective study design. Furthermore, considering the study covered a time period of 25 years, heterogeneity of treatments including the extent of surgery, postoperative RT, and chemotherapy should be taken into account. Given these short-comings, large-scale prospective studies are warranted before reaching final conclusions.
5. Conclusions
The presence of lymphovascular invasion and a high T classification were very strong adverse factors and independent predictors for ACC patients’ prognosis including LR control, DM control, and survival. Positive nodal status also adversely impacted the LR control and survival. Positive/close margin was another independent predictor for DM and survival. Furthermore, prognosis was also modified by location of the primary site. ACC patients with such risk factors should be more carefully managed during treatment and follow-up.
Acknowledgments
The authors thank Ya-ming Zou for the statistical analysis and statistical conclusions (MS, Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, Guangdong, China).
Footnotes
Abbreviations: ACC = adenoid cystic carcinoma, DM = distant metastasis, LR = local-regional, OS = overall survival, RT = radiotherapy.
Funding/support: The project was supported by grants from National Natural Science Foundation of China (No. 81371160), Natural Science Foundation of Guangdong Province (No.2014A030310076), and Seed Funding Programme for Basic Research, HKU (No. 201501159006).
The authors have no conflicts of interest to disclose.
References
- [1].Laurie SA, Ho AL, Fury MG, et al. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol 2011;12:815–24. [DOI] [PubMed] [Google Scholar]
- [2].Wang YL, Zhu YX, Chen TZ, et al. Clinicopathologic study of 1176 salivary gland tumors in a Chinese population: experience of one cancer center 1997–2007. Acta Otolaryngol 2012;132:879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck – an update. Oral Oncol 2015;51:652–61. [DOI] [PubMed] [Google Scholar]
- [4].Brown J. Booth PW, Schendel SA, Hausamen J-E. Prognostic factors in oral, oropharyngeal and salivary gland cancer. Maxillofacial Surgery. Vol. 1. Edinburgh, London, New York: Churchill Livingstone; 1999. 291–308. [Google Scholar]
- [5].Sung MW, Kim KH, Kim JW, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 2003;129:1193–7. [DOI] [PubMed] [Google Scholar]
- [6].Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg 1997;174:495–8. [DOI] [PubMed] [Google Scholar]
- [7].Matsuba HM, Simpson JR, Mauney M, et al. Adenoid cystic salivary gland carcinoma: a clinicopathologic correlation. Head Neck Surg 1986;8:200–4. [DOI] [PubMed] [Google Scholar]
- [8].Lloyd S, Yu JB, Wilson LD, et al. Determinants and patterns of survival in adenoid cystic carcinoma of the head and neck, including an analysis of adjuvant radiation therapy. Am J Clin Oncol 2011;34:76–81. [DOI] [PubMed] [Google Scholar]
- [9].Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973–2007 Surveillance, Epidemiology, and End Results data. Cancer 2012;118:4444–51. [DOI] [PubMed] [Google Scholar]
- [10].Bjorndal K, Krogdahl A, Therkildsen MH, et al. Salivary adenoid cystic carcinoma in Denmark 1990-2005: outcome and independent prognostic factors including the benefit of radiotherapy. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2015;51:1138–42. [DOI] [PubMed] [Google Scholar]
- [11].Nascimento AG, Amaral AL, Prado LA, et al. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer 1986;57:312–9. [DOI] [PubMed] [Google Scholar]
- [12].Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg 1974;128:512–20. [DOI] [PubMed] [Google Scholar]
- [13].Zhang CY, Xia RH, Han J, et al. Adenoid cystic carcinoma of the head and neck: clinicopathologic analysis of 218 cases in a Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:368–75. [DOI] [PubMed] [Google Scholar]
- [14].Ciccolallo L, Licitra L, Cantu G, et al. Survival from salivary glands adenoid cystic carcinoma in European populations. Oral Oncol 2009;45:669–74. [DOI] [PubMed] [Google Scholar]
- [15].Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Handbook. New York: Springer; 2010. [Google Scholar]
- [16].Pfister DG, Spencer S, Brizel DM, et al. Head and Neck Cancers, Version 1.2015. J Natl Compr Canc Netw 2015;13:847–55. 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oplatek A, Ozer E, Agrawal A, et al. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope 2010;120:65–70. [DOI] [PubMed] [Google Scholar]
- [18].Min R, Siyi L, Wenjun Y, et al. Salivary gland adenoid cystic carcinoma with cervical lymph node metastasis: a preliminary study of 62 cases. Int J Oral Maxillofac Surg 2012;41:952–7. [DOI] [PubMed] [Google Scholar]
- [19].Thompson LD, Penner C, Ho NJ, et al. Sinonasal tract and nasopharyngeal adenoid cystic carcinoma: a clinicopathologic and immunophenotypic study of 86 cases. Head Neck Pathol 2014;8:88–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shen C, Xu T, Huang C, et al. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol 2012;48:445–9. [DOI] [PubMed] [Google Scholar]
- [21].Bhayani MK, Yener M, El-Naggar A, et al. Prognosis and risk factors for early-stage adenoid cystic carcinoma of the major salivary glands. Cancer 2012;118:2872–8. [DOI] [PubMed] [Google Scholar]
- [22].Eby LS, Johnson DS, Baker HW. Adenoid cystic carcinoma of the head and neck. Cancer 1972;29:1160–8. [DOI] [PubMed] [Google Scholar]
- [23].Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer 2001;96:149–58. [DOI] [PubMed] [Google Scholar]
- [24].Amit M, Binenbaum Y, Trejo-Leider L, et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 2015;37:1038–45. [DOI] [PubMed] [Google Scholar]
- [25].Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys 1995;32:619–26. [DOI] [PubMed] [Google Scholar]


