Supplemental Digital Content is available in the text
Keywords: central nervous system, depression, enterovirus
Abstract
Enterovirus (EV) infection is common among children and adolescents. Few studies have investigated the relationship of depression after EV infection. This study explores an association between EV infection and subsequent depression in children and adolescents and assesses the risk of depression after EV infection with central nervous system involvement in a nationwide population-based retrospective cohort.
A random sample of 1,000,000 people was derived from Taiwan National Health Insurance Research Database and we identified enrollees less than 18 years with EV infection before 2005 and followed up until December 2009. A total 48,010 cases with EV infection and 48,010 healthy controls matched for sex, age, and residence were obtained. Association between EV infection and depression risk was assessed by Cox proportional hazards models to determine the hazard ratios (HRs) and confidence intervals (CIs). We further stratified EV infection into with central nervous system (CNS) involvement and without and compared with matched cohort.
Children and adolescents with EV infection had no elevated risk of depression compared with healthy controls (adjusted HR, aHR = 1.00, 95% CI: 0.83–1.21). However, CNS EV infection was associated with increased risk of depression (aHR = 1.62, 95% CI: 1.02–2.58) in the fully adjusted Cox regression model.
To the best of our knowledge, this is the first study investigating depression in children and adolescents with CNS EV infection. The results suggested that children and adolescents with CNS EV infection were a susceptible group for subsequent depressive disorders.
1. Introduction
Epidemiological studies have shown that major depression is comparatively rare among children, but more common among adolescents, with up to an 11% lifetime prevalence by the end of adolescence.[1] About 3% of prepubertal children and 6% of postpubertal children and adolescents were noted to suffer from depressive disorders.[2] Depressive disorder beginning early in life can lead to impairment in physical activities and has serious functional consequences.[3] Understanding the depressive disorder during this developmental stage may be critical for developing effective intervention strategies. Most children with depression will suffer from some noticeable changes in social and physical activities, a loss of interest in attending school, or poor academic performance.[4] Children may also have illegal substance abuse, alcohol use, or cigarettes smoking related to depressed mood, and these maladaptive behaviors further exacerbate depression.[5] The causes of depression in children and adolescents remain largely unknown. Depression may be due to any combination of poor physical health, environment stress, genetic vulnerability, and life events such as being abused, losing a parent or divorce of parents.[6] Early onset of major depression is an established risk factor for depression in adulthood.[7,8]
Major depressive disorder is associated with increased production of proinflammatory cytokines, such as interleukin-1 (IL-1), IL-6, and interferon gamma (IFN-γ).[9] Some chronic viral infections are also known to increase inflammatory cytokine like IL-1, IL-6, and tumor necrosis factor alpha (TNF-α).[10,11] The inflammatory model of depression provides a possible link between the infection and major depressive disorder. Research has shown that some mental disorders are related to chronic viral infection.[12–14] Increased macrophage migration inhibitory factor was found in patients with major depression.[15] Serum high-sensitivity C-reactive protein was an independent risk factor for major depressive disorder in women in 1 retrospective cohort study. Results supported an etiological role for inflammatory activity in the pathophysiology of depression.[16] One population-based study indicated that influenza infection was a risk factor for subsequent depressive disorders.[17] Dysfunction of the immune and inflammatory system has been found in a subgroup of treatment-resistant affective and schizophrenic spectrum disorder patients.[18] Noncentral nervous system (CNS) pathogenic microorganisms (i.e., in the gastrointestinal tract) may also activate the immune system and produce serotonin and gamma-aminobutyric acid, which act on the gut–brain axis and exert its effect on depression.[19]
The genus Enterovirus belongs to one of the families of Picornaviridae, and it is composed of icosahedral, nonenveloped, and single-stranded RNA viruses.[20] Enterovirus consists of several species that are defined by the molecular structure and serological characteristics. Enterovirus contains the following species: Enterovirus (EV)-A that includes EV-71 and some Coxsackievirus group A viruses, EV-B that includes Coxsackievirus group B viruses and echoviruses, EV-C that includes polioviruses 1 to 3, EV-D that includes EV-68 and 70, and the rhinoviruses.[21] EV are known to cause a spectrum of clinical manifestations in humans. Most EV infections are asymptomatic or mild, such as upper respiratory infection, herpangina, and hand-foot-and-mouth disease (HFMD). The more severe syndromes associated with EV were meningitis, encephalitis, myocarditis, and sepsis. Since its discovery in 1969, EV-71 has been identified as the cause of epidemic HFMD associated with severe neurological complications, including aseptic meningitis, brainstem encephalitis, acute flaccid paralysis, and neurogenic pulmonary edema in children under 5 years of age.[22] Human EV-71 has emerged as an important etiology of viral encephalitis throughout the Asia-Pacific region over the past 15 years. Increased epidemic activity and endemic circulation of EV-71 has also been observed since 1997, and this may be associated with the regular emergence of new genetic lineages.[23] During the EV-71 outbreak in Taiwan in 1998, there were more than 130,000 cases of HFMD with 405 severe cases and 78 deaths.[24] Despite that most of the HFMDS caused by EV-71 will not induce serious complications, high rates of neurological complications, such as meningoencephalitis (ME), pulmonary complications, and even fatal cases, were noted during the outbreaks of HFMD caused by EV-71 from 2000 to 2002.[25] Most survivors of brainstem encephalitis with cardiopulmonary failure have long-term neurologic sequelae, psychiatric disorders, and impaired cognition in young children.[26,27] Although EV infection is common among children and adolescents, very few studies have investigated the relationship of psychiatric sequelae after EV infection. We hypothesized that EV infection is related to depression in children and adolescents and that the severe form of EV infection further elevates the risk. We tested the hypothesis in the nationwide population-based retrospective cohort.
2. Materials and methods
2.1. Database
The data were derived from the Taiwan National Health Insurance Research Database (NHIRD). The National Health Insurance (NHI) established a single-payer insurance system in Taiwan since 1995. The disbursement of all national healthcare funds covering ambulatory, outpatient, dental services, and hospital inpatient care was centralized by this government insurer. The characteristics of NHI include 1 global budget, payroll-based premiums shared by employers, employees and the government, and the case payment system. Since NHI launched in 1995, the coverage rate rose from 92% in the beginning to 99.5% of medical claims in 2009 in Taiwan. The registration of all medical claims is demanded by the bureau of NHI. The data of registration include demographic data of patients, dates and numbers of clinical visits, medical prescriptions, physician specialties, and diagnostic codes of the Ninth Revision of the International Classification of Diseases, Clinical Modification (ICD-9-CM). The NHIRD gathers these data and provides a random sample of 1,000,000 people (about 5% of the national population of Taiwan) from the registry of NHI enrollees for research use. A systemic sampling method is applied and there is no statistically significant difference in age, sex, or health care utilization between the sample and total enrollees.[28]
2.2. Study subjects and design
This study used a population-based retrospective cohort from the Longitudinal Health Insurance Database. The cohort was composed of insured children under 18 years old before 2005 with EV infection and followed up until December 2009. We identified 48,340 children with incident diagnose of EV infection within the observation period. The EV infection was defined as having at least 2 outpatient diagnoses within 1 year or at least 1 inpatient diagnosis of EV infection. The diagnostic codes of EV infection included meningitis due to EV (ICD-9-CM codes 047, 047.0, and 047.1), other EV diseases of CNS (ICD-9-CM codes 048), specific diseases related to Coxsackievirus (ICD-9-CM codes 074, 074.0, 074.1, 074.2, 074.20, 074.21, 074.23, 074.3, and 074.8), Echovirus and Coxsackievirus infection in conditions classified elsewhere and of unspecified site (ICD-9-CM codes 079.1 and 079.2), and enteritis due to EV (ICD-9-CM codes 008.67).[29] Exclusion criteria were age 18 years old or older and diagnosis of depression before EV infection. We matched the study cohort with 1 control per case from the remaining sample without EV infection by randomly sampling and matching for sex, age, residence, and the index date of EV infection diagnosed. The process of obtaining the analyzed sample is shown in Fig. 1. EV with CNS involvement was defined by the diagnosis of encephalitis in viral diseases (ICD-9-CM codes 323.0), other encephalitis due to infection (ICD-9-CM codes 323.4), and unspecified cause of encephalitis (ICD-9-CM codes323.9), which were diagnosed within 1 month after diagnosis of EV infection.
Figure 1.
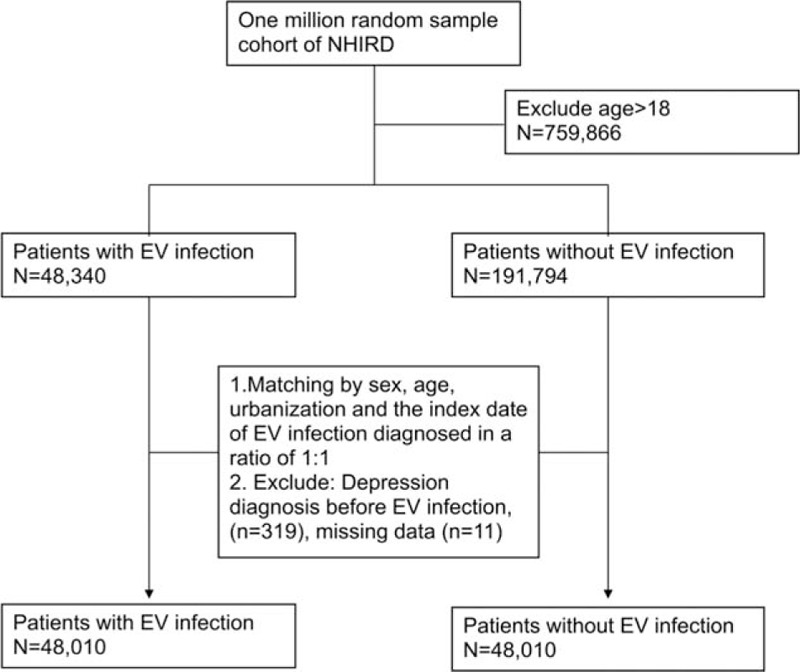
Flow chart of data collection for ICD codes of EV infection and matching cohort. EV = enterovirus, ICD = International classification of diseases.
2.3. Case identification of depression
Cases with the diagnosis of major depressive disorder, single episode (ICD-9-CM code 2962), major depressive disorder, recurrent episode (ICD-9-CM code 2963), neurotic depression (ICD-9-CM code 3004), or depressive disorder, not elsewhere classified (ICD-9-CM code 311) were identified as depression in this study. Those who had at least 2 outpatient diagnoses within 1 year or at least 1 inpatient diagnosis were included in the study analysis. The definition is the same as previous studies.[30]
2.4. Confounding factors
The pathogenesis of depression remains unclear.[31] Several risk factors for depression including genetic inheritance, low birth weight, psychosocial stress, allergic disease, and traumatic brain injury have been reported.[32,33] In this study, low birth weight (ICD-9-CM codes 760–764, 766–779, and V137) and allergic diseases, such as asthma (ICD-9-CM code 493), allergic rhinitis (ICD-9-CM code 477.9), allergic conjunctivitis (ICD-9-CM codes 372.05, 372.14), and atopic dermatitis (ICD-9-CM codes 691, 691.8) were identified as risk factors for depression and adjusted in Cox regression analysis. The ICD codes used in the study are shown in supplement Table 1.
2.5. Ethics
No identifying information from any of the subjects was available or accessed. Institutional Review Board approval was approved at Chang Gung Medical University Hospital.
2.6. Statistical analysis
Descriptive analyses were carried out for the distribution of demographic factors and the frequency of comorbidities in the groups of the children with and without EV infection. The log-rank test was used to investigate variation in the chance for depression and Cox proportional hazards models were applied to determine the hazard ratios (HRs) accompanying 95% CIs after adjusting for sex, age at entry, residence, asthma, allergic rhinitis, atopic dermatitis, conjunctivitis (including acute atopic conjunctivitis, and other chronic allergic conjunctivitis), disorders related to short gestation and unspecified low birth weight, and years of follow-up. Significant differences were defined as 2-tailed P value ≤ 0.05. The death date was retrieved from the national mortality database. Enrollees with a death date or loss of follow-up during the study period were excluded from analyses. All analyses in our study were performed using SAS statistical software (Version 9.4; SAS Institute, Cary, NC).
3. Results
3.1. Characteristics of the subjects
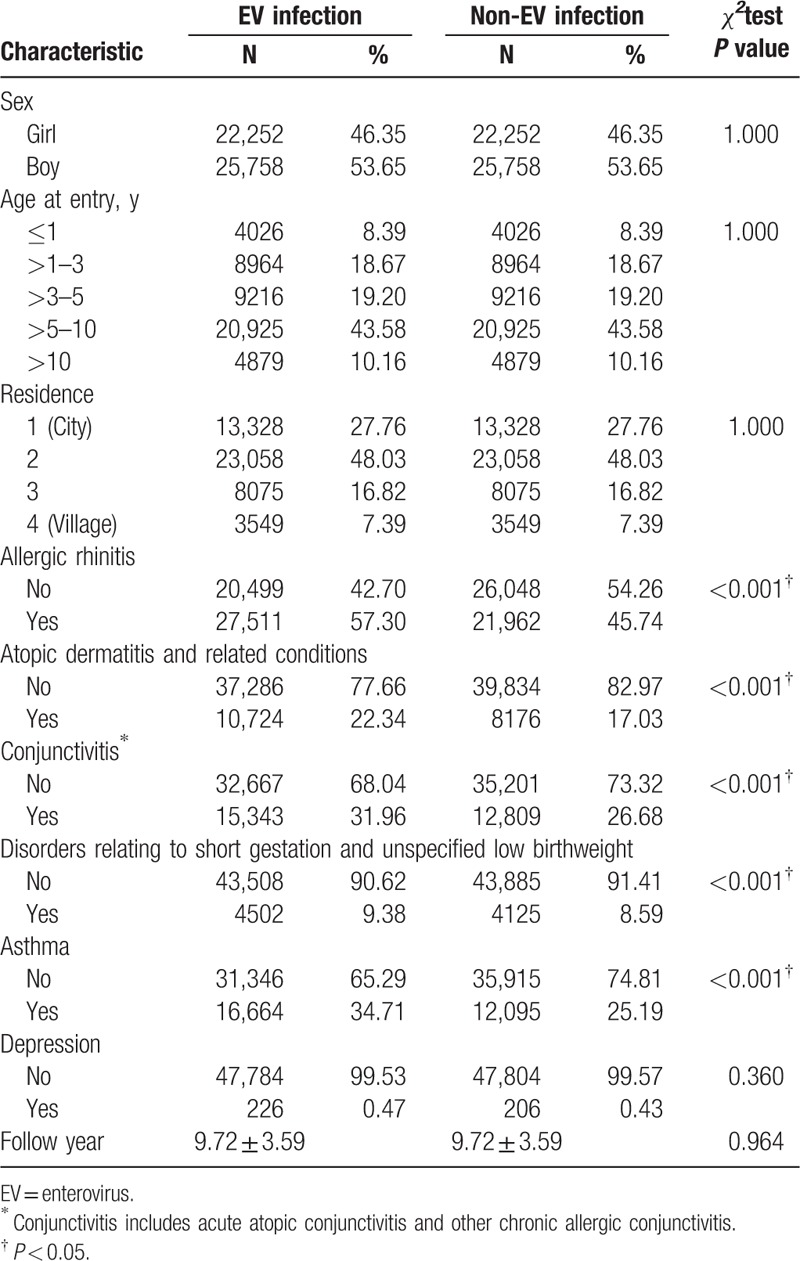
Two study groups were patients with EV infection (N = 48,010) and without EV infection (N = 48,010). EV infection with CNS involvement was identified in 1192 patients (supplement Table 2). The characteristics of cases with EV infection and the matched controls are shown in Table 1. There were significant differences between 2 groups in allergic rhinitis (P < 0.001), atopic dermatitis and related conditions (P < 0.001), conjunctivitis (P value < 0.001), asthma (P < 0.001), and disorders related to short gestation and unspecified low birth weight (P < 0.001). A total of 432 patients received the diagnosis of depression during the study period: 226 (0.47%) in EV infection cohort and 206 (0.43%) cases in non-EV infection cohort.
Table 1.
Characteristics of EV infection cases and their matched controls.
3.2. Association between EV infection and risk of depression
Analysis of the association between EV infection and risk of depression is shown in Tables 2 and 3. There was no difference in risk for depression between the EV infection and non-EV infection cohorts after adjusting for sex, age, residence, atopic diseases, and disorders relating to short gestation and unspecified low birthweight (adjusted HR, aHR = 0.999, 95% CI: 0.826–1.209).
Table 2.
Cox proportional hazard regression analysis for the adjusted HRs of depression for enterovirus infection, demographic characteristics, and comorbidity.
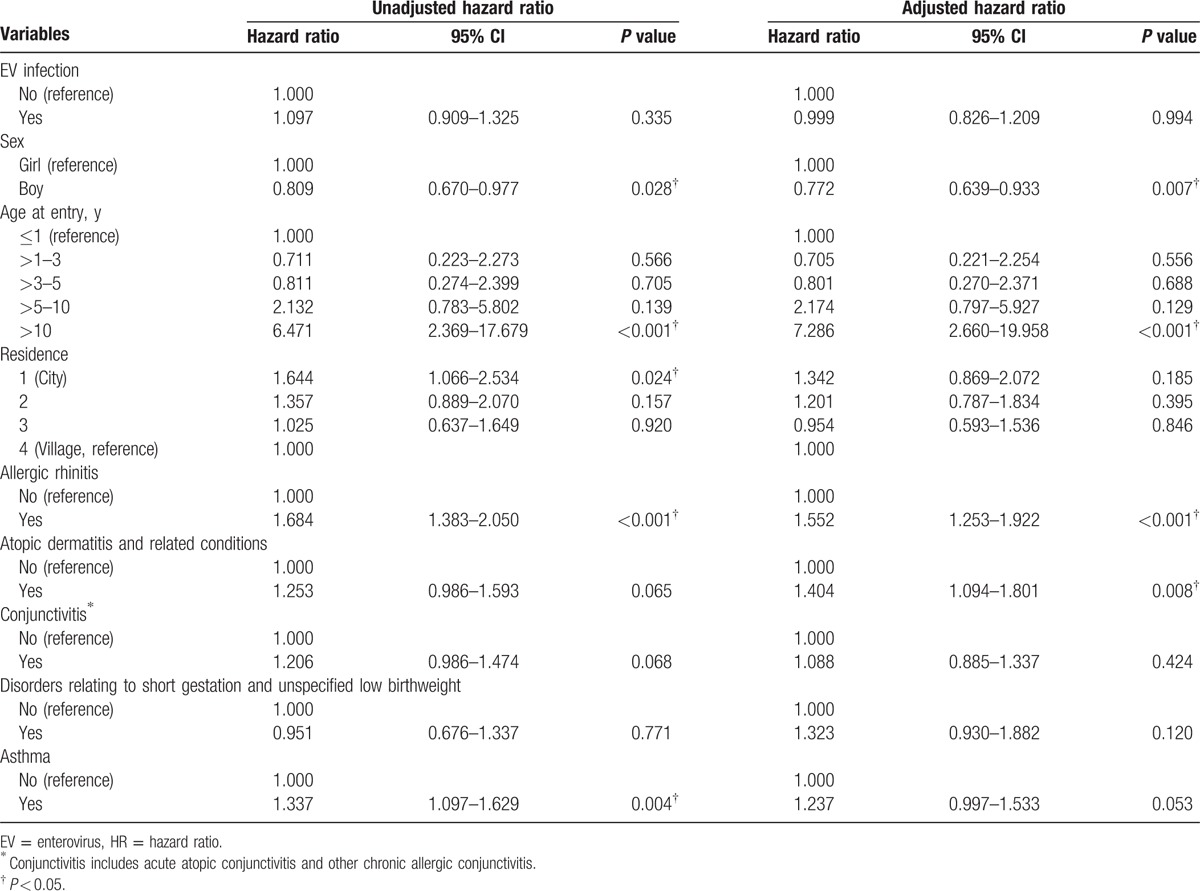
Table 3.
Cox proportional hazard regression analysis for the adjusted HRs of depression for CNS enterovirus infection, demographic characteristics, and comorbidity.
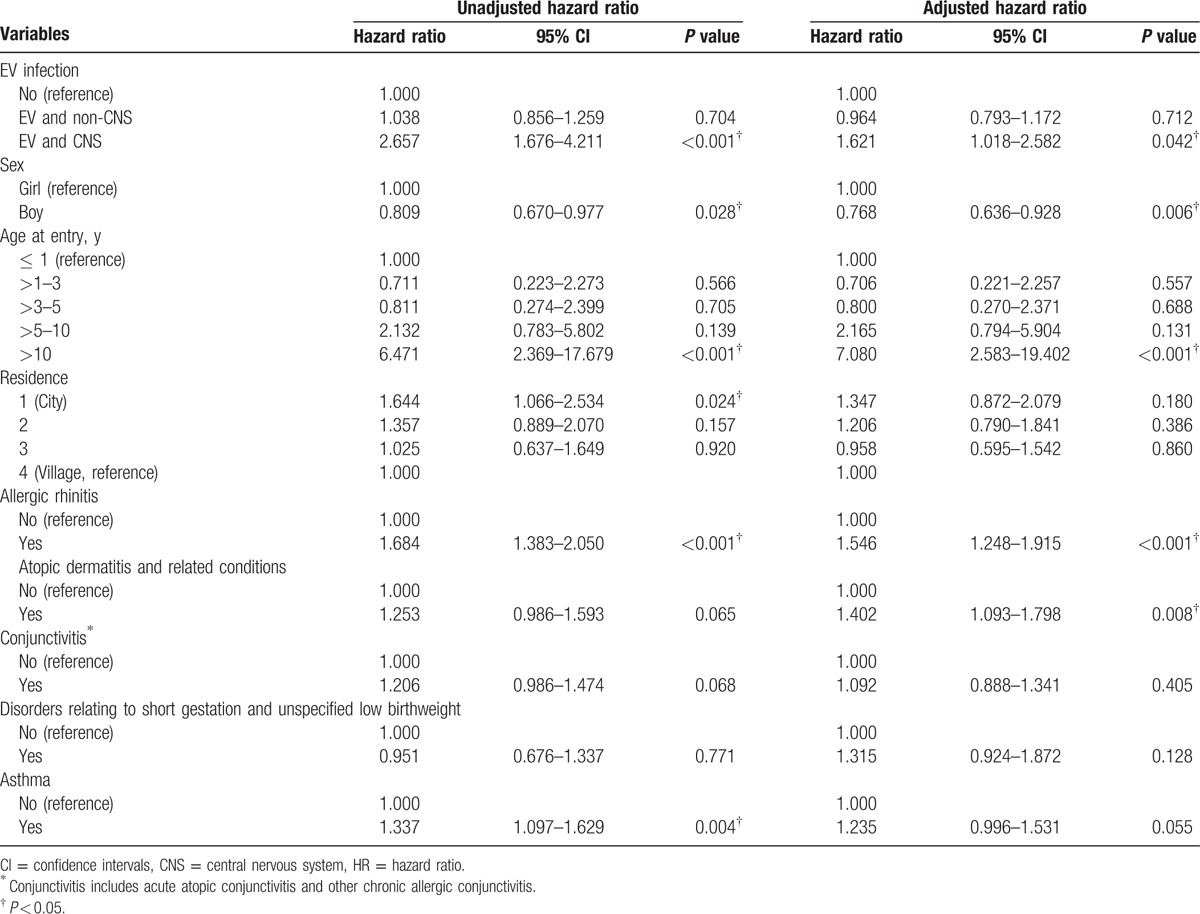
EV infection was positively correlated with depression in CNS involved cases (aHR = 1.621, 95% CI:1.018–2.582) in the fully adjusted Cox regression model for HRs after adjusting for sex, age, urbanization, allergic rhinitis, atopic dermatitis and related conditions, conjunctivitis, disorders relating to short gestation and unspecified low birth weight, and asthma. The incidences of depression were consistently higher for the EV infection than non-EV infection cohort with entry age more than 10 years old (adjusted HR = 7.080, 95% CI: 2.583–19.402), allergic rhinitis (aHR = 1.546, 95% CI: 1.248–1.915), atopic dermatitis and related conditions (aHR = 1.402, 95% CI: 1.093–1.798), and lower incidence of depression with boy (aHR = 0.768, 95% CI: 0.636–0.928).
4. Discussion
To the best of our knowledge, the present study is the first nationwide population-based cohort study to investigate the relationship between EV infection and depression in children under 18 years old. The results indicated that CNS EV infection increased by more than 60% the HR of depression compared with healthy control after adjusting for sex, age, residence, short gestational age, unspecified low birth weight, asthma, and atopic diseases, whereas EV infection per se did not increase the risk of subsequent depression.
Depression is a heterogeneous psychological disorder with multiple biological and psychosocial risk factors. Viral infection has been postulated as one of the risk factors of depression, and human immunodeficiency virus, hepatitis C virus, varicella-zoster virus, and human T-cell lymphotropic virus have been found to be related to depression.[34–37] There is only 1 small case series study (n = 39) in 1989 which considers the role of EV in depressive-like symptoms. Wilson et al[38] found that a combination of dysphoric symptoms, such as unexplained crying with clinging, anxious behavior, and preoccupation with death, was observed in children with Coxsackievirus B infection. The present study investigated depression related to more species of EV than the previous studies and in a much larger population. The results were in line with previous studies that CNS EV infection was positively associated with depression. Furthermore, we added more evidence of the role of EV infection in the development of subsequent depression with a longitudinal design and possible linkage of viral meningoencephalitis and depression.
The pathogenesis of depression has been hypothesized to be related to decreased monoamine neurotransmitter availability. Recently, the implication of immune system in depression has been extensively studied, and this inspired the cytokine hypothesis of depression. Cytokines related to depression included IL-6, IL-1β, TNF-α, and IFN-γ. The regulation of serotonin transporter function by pro-inflammatory cytokines provides a mechanistic link between the monoamine and cytokine theories of depression.[39]
A study of cytokine changes in the blood and cerebrospinal fluid (CSF) from 93 children infected with EV-71 showed elevated plasma levels of IL-1beta and IL-6 in cases with CNS involvement when compared with patients with no CNS involvement and normal controls. Higher CSF levels of IL-6, IL-8, and IL-1β were also found in acute phase in critical patients with CNS involvement.[40]
A similar study of 97 children with EV-71-related ME found higher levels of IFN-γ and IL-6 in ME than in febrile convulsion. The results might imply the severity CNS involvement correlated with more IL-6 overproduction in CNS inflammatory responses.[41] Another cross-sectional study enrolled 165 patients with CNS infection and found robust proinflammatory cytokine pattern, higher levels of IL-6, TNF, and IL-17, in CSF among 13 EV meningoencephalitis cases.[42] In conclusion, IL-1β, IL-6, and IFN-γ were associated with EV-71-induced neuropathology and depression might serve as a part of the neurological sequelae of CNS EV infection. This might provide an explanation for the differences between CNS and non-CNS EV infection on subsequent depression risks.
Atopy diseases, except asthma, were positively associated with depression risks in our results. Previous studies presented mixed results whether persons with asthma had elevated risk for depression.[43]
The present study is the first to utilize a comprehensive and large representative nationwide population-based cohort to investigate the association between EV infection and subsequent depression. The longitudinal analysis from the registration medical claim based data avoided recall bias. The use of a population-based cohort prevented selection bias. By excluding depression before EV-related diagnoses, we eliminated the reverse causal relationship between EV infection and depression. Nevertheless, there were several limitations of our study. First, we defined depression with a more broad range of diagnoses, including major depressive disorder, single and recurrent episode, neurotic depression and depressive disorder, not elsewhere classified (NOS). Neurotic depression and depression NOS comprised a heterogeneous patient group with overlapping minor mental disorders such as anxiety and adjustment disorder. There is also a strong association between hospitalization, depressive, and anxiety disorders.[44] Thus, depression could be the result of hospitalization, burden from severe physical illness, or disability that was not the direct effect of EV infection per se. In a subgroup analysis, we found patients with EV infection-related hospitalization had a nonsignificant risk for depression (HR 1.315, 95% CI: 0.997–1.735, not shown in table) compared with patients with nonhospitalized EV infection and healthy controls in the adjusted model. The Taiwan NHIRD lacked the data of extent of disability and disease burden and thus no further stratification of patient groups or analysis was performed. We matched the study cohort in large sample size with the control group for sex, age, and urbanization level of residence to avoid confounding. However, the family psychiatric history, parents’ marital status, child abuse, socioeconomic status, and other risk factors for depressive disorders were not included in NHIRD and potential bias was possible. Second, the diagnosis of depression was strictly based on ICD codes rather than a formal diagnostic process such as uniform structured clinical interview or questionnaires. There was no validation study specifically on diagnoses of depression in Taiwan. Central nervous system involvement of EV infection did not require cerebrospinal fluid study as a must to confirm the diagnosis in registration. NHIRD provided no concomitant data to check whether these diagnostic procedures had been performed, either. However, diagnoses in registration data were made by board-certified psychiatrist or physicians and the accuracy of disease diagnoses in national health insurance system had been reported between 60% and 90%.[45]
We found CNS EV infection was associated with increased risk of depression in children and adolescents in a nationwide population-based cohort study. Future biological research may provide the mechanism or pathophysiology of depression associated with EV infection. In clinical practice, we suggest that children with CNS EV infection should receive proper psychological assessment and monitoring for emerging depression with the rationale of these children being a susceptible group for depressive disorder.
Acknowledgments
The authors thank Center of Excellence for Chang Gung Research Datalink for the comments and assistance in data analysis.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, CNS = central nervous system, EV = enterovirus, HFMD = hand-foot-and-mouth disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Clinical Modification, IFN-γ = interferon gamma, IL = interleukin, ME = meningoencephalitis, NHI = National Health Insurance, TNF-α = tumor necrosis factor alpha.
M-HH contributed equally as the first author.
This study was supported by a grant from Chang Gung Memorial Hospital, Chia-yi Branch, and based on the National Health Insurance Research Database provided by the Central Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Avenevoli S, Swendsen J, He JP, et al. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015;54:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dolle K, Schulte-Korne G. The treatment of depressive disorders in children and adolescents. Deutsch Arztebl Int 2013;110:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nagar S, Sherer JT, Chen H, et al. Extent of functional impairment in children and adolescents with depression. Curr Med Res Opin 2010;26:2057–64. [DOI] [PubMed] [Google Scholar]
- [4].Rapee RM, Bogels SM, van der Sluis CM, et al. Annual research review: conceptualising functional impairment in children and adolescents. J Child Psychol Psychiatry 2012;53:454–68. [DOI] [PubMed] [Google Scholar]
- [5].Wilkinson AL, Halpern CT, Herring AH. Directions of the relationship between substance use and depressive symptoms from adolescence to young adulthood. Addict Behav 2016;60:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Niarchou M, Zammit S, Lewis G. The Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort as a resource for studying psychopathology in childhood and adolescence: a summary of findings for depression and psychosis. Soc Psychiatry Psychiatr Epidemiol 2015;50:1017–27. [DOI] [PubMed] [Google Scholar]
- [7].Kovacs M, Obrosky S, George C. The course of major depressive disorder from childhood to young adulthood: recovery and recurrence in a longitudinal observational study. J Affect Disord 2016;203:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Messerschmitt P. Depression in childhood. Ann Pediatr 1993;40:149–56. [PubMed] [Google Scholar]
- [9].Dahl J, Ormstad H, Aass HC, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014;45:77–86. [DOI] [PubMed] [Google Scholar]
- [10].Guo J, Ma Q, Zhou X, et al. Inactivation of p27kip1 promotes chemical hepatocarcinogenesis through enhancing inflammatory cytokine secretion and STAT3 signaling activation. J Cell Physiol 2013;228:1967–76. [DOI] [PubMed] [Google Scholar]
- [11].Guha D, Chatterjee R. Cytokine levels in HIV infected and uninfected Indian women: correlation with other STAs. Exp Mol Pathol 2009;86:65–8. [DOI] [PubMed] [Google Scholar]
- [12].Cunha EC, Behrensdorf MF, Bavaresco V, et al. Genotype 1 of hepatitis C virus increases the risk of major depression: a 12-week prospective study. Gen Hosp Psychiatry 2015;37:283–7. [DOI] [PubMed] [Google Scholar]
- [13].Del Guerra FB, Fonseca JL, Figueiredo VM, et al. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol 2013;19:314–27. [DOI] [PubMed] [Google Scholar]
- [14].Whitford TJ, Wood SJ, Yung A, et al. Structural abnormalities in the cuneus associated with Herpes Simplex Virus (type 1) infection in people at ultra high risk of developing psychosis. Schizophr Res 2012;135:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Musil R, Schwarz MJ, Riedel M, et al. Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression—no influence of celecoxib treatment. J Affect Disord 2011;134:217–25. [DOI] [PubMed] [Google Scholar]
- [16].Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry 2010;197:372–7. [DOI] [PubMed] [Google Scholar]
- [17].Bornand D, Toovey S, Jick SS, et al. The risk of new onset depression in association with influenza—a population-based observational study. Brain Behav Immun 2016;53:131–7. [DOI] [PubMed] [Google Scholar]
- [18].Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res 2010;44:321–30. [DOI] [PubMed] [Google Scholar]
- [19].Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci 2015;13:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tebruegge M, Curtis N. Enterovirus infections in neonates. Semin Fetal Neonatal Med 2009;14:222–7. [DOI] [PubMed] [Google Scholar]
- [21].Tapparel C, Siegrist F, Petty TJ, et al. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 2013;14:282–93. [DOI] [PubMed] [Google Scholar]
- [22].Hsiung GD, Wang JR. Enterovirus infections with special reference to enterovirus 71. J Microbiol Immunol Infect 2000;33:1–8. [PubMed] [Google Scholar]
- [23].Kok CC. Therapeutic and prevention strategies against human enterovirus 71 infection. World J Virol 2015;4:78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen KT, Chang HL, Wang ST, et al. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007;120:e244–52. [DOI] [PubMed] [Google Scholar]
- [25].Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002;109:e88. [DOI] [PubMed] [Google Scholar]
- [26].Chang LY. Enterovirus 71 in Taiwan. Pediatr Neonatol 2008;49:103–12. [DOI] [PubMed] [Google Scholar]
- [27].McMinn PC. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr Opin Virol 2012;2:199–205. [DOI] [PubMed] [Google Scholar]
- [28].Institutes NHR. Introduction to the National Health Insurance Research Database (NHIRD). Taiwan 2013. [Google Scholar]
- [29].Lin HC, Wang CH, Tsai FJ, et al. Enterovirus infection is associated with an increased risk of childhood type 1 diabetes in Taiwan: a nationwide population-based cohort study. Diabetologia 2015;58:79–86. [DOI] [PubMed] [Google Scholar]
- [30].Chuang CS, Yang TY, Muo CH, et al. Hyperlipidemia, statin use and the risk of developing depression: a nationwide retrospective cohort study. Gen Hosp Psychiatry 2014;36:497–501. [DOI] [PubMed] [Google Scholar]
- [31].Hasler G. Pathophysiology of depression: do we have any solid evidence. World Psychiatry 2010;9:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nkansah-Amankra S, Tettey G. Association between depressive symptoms in adolescence and birth outcomes in early adulthood using a population-based sample. Prev Med Rep 2015;2:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Saluja G, Iachan R, Scheidt PC, et al. Prevalence of and risk factors for depressive symptoms among young adolescents. Arch Pediatr Adolesc Med 2004;158:760–5. [DOI] [PubMed] [Google Scholar]
- [34].Coughlin SS. Anxiety and depression: linkages with viral diseases. Public Health Rev 2012;34:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol 2015;28:440–7. [PMC free article] [PubMed] [Google Scholar]
- [36].Chen MH, Wei HT, Su TP, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med 2014;76:285–91. [DOI] [PubMed] [Google Scholar]
- [37].Stumpf BP, Carneiro-Proietti AB, Proietti FA, et al. Higher rate of major depression among blood donor candidates infected with human t-cell lymphotropic virus type 1. Int J Psychiatry Med 2008;38:345–55. [DOI] [PubMed] [Google Scholar]
- [38].Wilson PM, Kusumakar V, McCartney RA, et al. Features of Coxsackie B virus (CBV) infection in children with prolonged physical and psychological morbidity. J Psychosom Res 1989;33:29–36. [DOI] [PubMed] [Google Scholar]
- [39].Haase J, Brown E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—a central role for the serotonin transporter? Pharmacol Ther 2015;147:1–1. [DOI] [PubMed] [Google Scholar]
- [40].Ye N, Gong X, Pang LL, et al. Cytokine responses and correlations thereof with clinical profiles in children with enterovirus 71 infections. BMC Infect Dis 2015;15:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li H, Li S, Zheng J, et al. Cerebrospinal fluid Th1/Th2 cytokine profiles in children with enterovirus 71-associated meningoencephalitis. Microbiol Immunol 2015;59:152–9. [DOI] [PubMed] [Google Scholar]
- [42].Bastos MS, Coelho-Dos-Reis JG, Zauli DA, et al. Divergent cerebrospinal fluid cytokine network induced by non-viral and different viral infections on the central nervous system. BMC Infect Dis 2015;15:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gao YH, Zhao HS, Zhang FR, et al. The relationship between depression and asthma: a meta-analysis of prospective studies. PLoS One 2015;10:e0132424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Willis TA, Gregory AM. Anxiety disorders and sleep in children and adolescents. Sleep Med Clin 2015;10:125–31. [DOI] [PubMed] [Google Scholar]
- [45].Yeoh C, Davies H. Clinical coding: completeness and accuracy when doctors take it on. BMJ 1993;306:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


