Supplemental Digital Content is available in the text
Keywords: gastritis, IL-1β, polymorphism, risk assessment, single nucleotide
Abstract
Background:
Helicobacter pylori (H. pylori) infection of the human stomach regularly leads to chronic gastric inflammation. The cytokine gene interleukin (IL)-1β has been implicated in influencing the pathology of inflammation induced by H. pylori infection. Currently, several studies have been carried out to investigate the association of IL-1β-511 (rs16944) and IL-1β-31 (rs1143627) polymorphisms with gastritis risk; however, the results are inconsistent and inconclusive. To assess the effect of IL-1β polymorphisms on gastritis susceptibility, we conducted a meta-analysis.
Methods:
Up to March 15, 2016, 2205 cases and 2289 controls were collected from 12 published case–control studies. Summarized odds ratios and corresponding 95% confidence intervals (CIs) for IL-1β-511 and IL-1β-31 polymorphisms and gastritis risk were estimated using fixed- or random-effects models when appropriate. Heterogeneity was assessed by chi-squared-based Q-statistic test, and the sources of heterogeneity were explored by subgroup analyses and logistic meta-regression analyses. Publication bias was evaluated by Begg funnel plot and Egger test. Sensitivity analyses were also performed.
Results:
The results provided evidences that the single nucleotide polymorphisms (SNPs) in IL-1β-31 might be associated with the gastritis risk, especially in the Caucasian population, while SNPs in the IL-1β-511 might not be.
Conclusion:
Our studies may be helpful in supplementing the disease monitoring of gastritis in the future, and additional studies to determine the exact molecular mechanisms might inspire interventions to protect the susceptible subgroups.
1. Introduction
Helicobacter pylori (H. pylori) infection of the human stomach regularly leads to chronic gastric inflammation. This infection first induces chronic superficial (nonatrophic) gastritis, which can progress through chronic atrophic gastritis, and finally toward gastric carcinoma. However, many H. pylori colonized individuals never develop these pathologies, implying that besides bacterial factors, genetic characteristics of the host as well as the environmental factors may be involved in the gastric pathological course.[1,2]
Epidemiological studies have indicated that the inflammation induced by H pylori infection was regulated by several interleukins (ILs), including proinflammatory cytokine IL-1β. IL-1β acts as an inhibitor of gastric acid secretion and plays important roles in initiating and amplifying the inflammatory responses to H pylori infection, yet finally allowing expansion of H pylori colonization from the gastric antrum to the corpus, leading to further progression of severe atrophic gastritis.[3]
Two allelic variants IL-1β-511 (rs16944) and IL-1β-31 (rs1143627) locate in the promoter region of the IL-1β gene, and they have been proved to affect the expression level of IL-1β.[4] Currently, several studies have been carried out to investigate the association between IL-1β promoter polymorphisms and gastritis risk; however, the results are inconsistent and inconclusive.[5–8]
Until recently, there has been no meta-analysis assessing the association between IL-1β promoter polymorphisms and gastritis risk. Therefore, we carried out a meta-analysis on all published case–control studies to estimate the overall gastritis risk of IL-1β-511 and IL-1β-31 polymorphisms and to investigate heterogeneity between the individual studies as well as the existence of potential publication bias. The association between IL-1β promoter polymorphisms and gastritis risk in H pylori infected patients was also evaluated.
2. Materials and methods
This study was carried out in accordance with the checklist proposed by Systematic Review and Meta-Analysis (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
2.1. Selection of published studies
We searched the MEDLINE and Embase databases (the last search on March 15, 2016) using Pubmed and Ovid search engines for all articles on the association between IL-1β gene polymorphisms and gastritis risk. The following terms were used: “IL-1β, or IL-1β or IL-1 beta” and “gastritis” and “polymorphism or variant or variation.” Additional eligible studies were identified by hand searching of references of retrieved articles. For inclusion, a study had to meet the following criteria: it had to be a case–control or case–cohort study; the study evaluated the association between IL-1β-511 and/or IL-1β-31 polymorphisms and gastritis risk; original data for odds ratios (ORs) calculation was reported; and records were published in English. The major exclusion criteria were as follows: not a case–control study;[4,9,10] no original data available for ORs.[11,12] The publication that was a deviation from Hardy–Weinberg equilibrium (HWE) was excluded.[13–15] The publication year of eligible studies ranged from 2002 to 2014. No contacts with authors were carried out.
A total of 69 relevant articles were retrieved from MEDLINE database. After title and abstract screening, 49 publications which did not investigate the association between gastritis risk and the polymorphisms of interest were excluded; and then, the remaining 20 publications were carefully reviewed according to the criteria described in “Section 2.” Another 6 publications were further removed, among which, 1 publication was not a case–control study,[9] 4 had no original data for ORs,[7,12,16,17] and 1 did not evaluate the association between IL-1β-511 and/or IL-1β-31 polymorphisms and gastritis risk.[11] After the evaluation of deviation from HWE, 2 studies were removed because of deviation from HWE in controls[13,15] (Fig. S1). Finally, 12 case–control studies were included in this meta-analysis, including 2205 cases and 2289 controls.[8,18–28] Among them, 8 articles studied the association between the IL-1β-31 polymorphism and gastritis risk, and 12 articles studied the association between the IL-1β-511 polymorphism and gastritis risk. Ethical approval and informed patient consent was not required as this study was a literature review and had no direct patient contact or influences on patient care.
2.2. Data extraction
Two investigators (XS and ZL) independently extracted the following data: the first author's name, year of publication, country of the first author, patient ethnicity, source of control groups, numbers of cases and controls, genotyping methods, matching variables, minor allele frequency in controls, and the number of H pylori infected persons. A 3rd investigator (SL) checked the data further and solved the inconsistency, if any, through discussion together. Different ethnicity descents were categorized as Asian, Caucasian, and mixed (Mexico) populations.
2.3. Quality score assessment
The quality of included studies were assessed using the Newcastle–Ottawa Scale.[29] A “☆” rating system was used to judge the quality of the included studies. The score of each study was ranked by the total number of “☆” given. WY and GD assessed the quality of the studies independently, and the inconsistencies were resolved in a consensus meeting with all authors. Studies with a score ≥5 were considered to be of high quality.
2.4. Statistical analysis
Deviation from HWE in the control group was examined by χ2 test. P < 0.05 was considered to be statistically significant. The strength of relationship association between IL-1β polymorphisms and gastritis risk was assessed using the pooled OR with 95% confidence intervals (CIs). The significance of the pooled OR was determined using the Z test, with P < 0.05 considered statistically significant. We evaluated the risk using the allele model, the homozygous model, the heterozygous model, the dominant model, and the recessive model. The statistical heterogeneity within studies was detected with the Chi-squared based Q test and I2 metric (0%–25%, no heterogeneity; 25%–50%, moderate heterogeneity; 50%–75%, large heterogeneity; and 75%–100% extreme heterogeneity). When P > 0.05 or I2 < 50%, the fixed-effects model was used (the DerSimonian and Laird method). Otherwise, the random-effects model was selected (the Mantel–Haenszel method). If any apparent heterogeneity existed, logistic meta-regression would be used to explore the sources of heterogeneity: ethnicities, genotyping methods (if one method contains only one study, it was merged into the “other” group), source of control (hospital-based studies and population-based studies), and sample size (<200 and ≥200 subjects). Subgroup analysis based on ethnicity, source of controls, genotype method was performed. Funnel plots and Egger linear regression were used to evaluate publication bias. Sensitivity analyses were performed to assess the stability of the results by excluding one study at a time. All analyses were done using STATA software, version 10.0 (STATA Corp., College Station, TX). All graphs were obtained also by STATA software. All the P values were 2-sided.
3. Results
3.1. Characteristics of studies
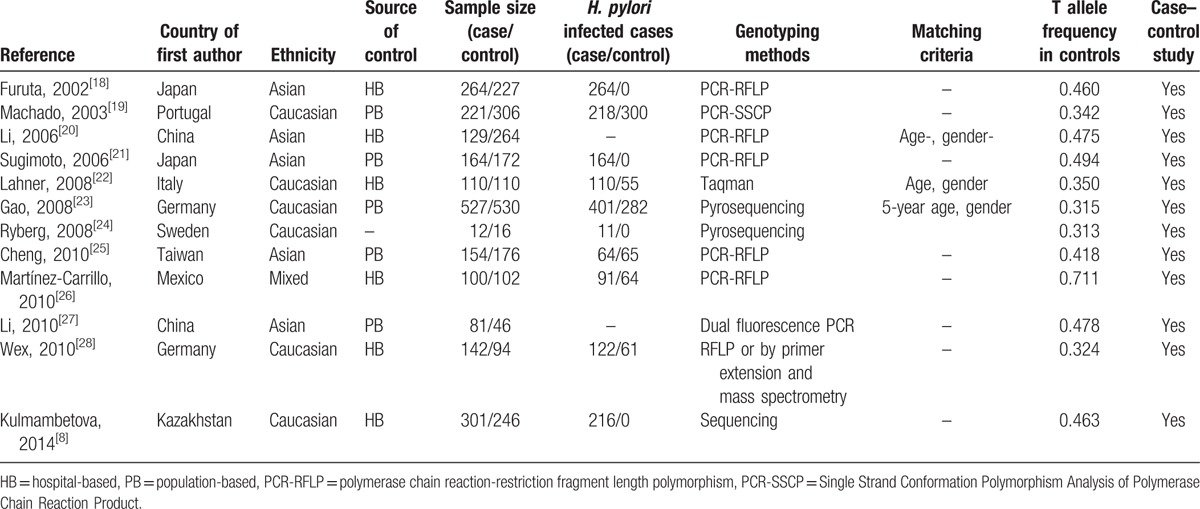
We identified 12 articles using the above search terms, including 2205 cases and 2289 controls, concerning IL-1β-511 and/or IL-1β-31 polymorphisms of IL-1β gene and gastritis risk. The study characteristics were summarized in Tables 1 and 2. There were 6 studies of Caucasian descendents, 5 studies of Asian descendents, and 1 mixed population of Mexican descendents. Several genotyping methods were used, including Taq-Man, polymerase chain reaction (PCR)-restriction fragment length polymorphism, pyrosequencing, Taqman, dual fluorescence PCR, Single Strand Conformation Polymorphism Analysis of PCR Products, and primer extension and mass spectrometry. Nevertheless, only 25% (3/12) of these studies included described genotyping quality control measures, such as blindness to the case–control status, a different genotyping assay to confirm the data, and random repetition of a portion of samples.
Table 1.
Characteristics of literatures on IL-1β-31 polymorphism included in the meta-analysis.
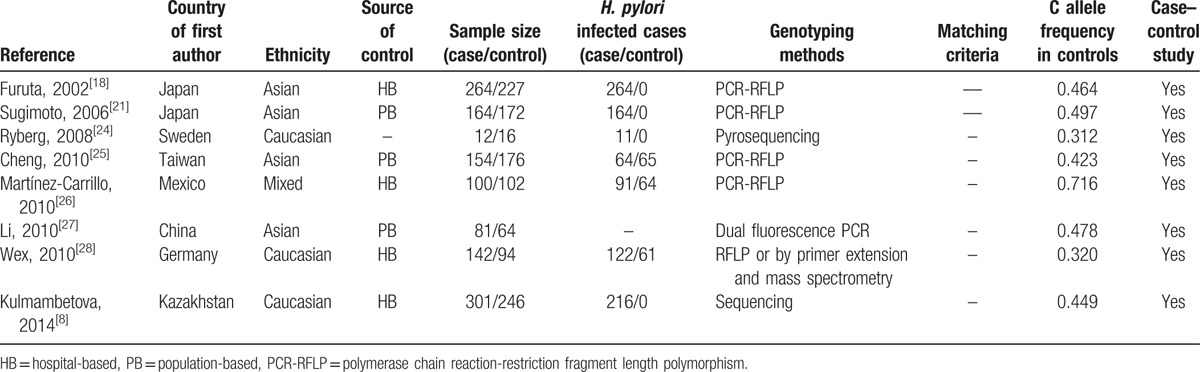
Table 2.
Characteristics of literatures on IL-1β-511 polymorphism included in the meta-analysis.
The study quality was assessed using the Newcastle–Ottawa Scale. Most studies scored 5, with all studies being of high quality in terms of selection and exposure. However, on comparability, only 3 studies included cases comparable with the controls (Supplementary Table S1).
3.2. Quantitative synthesis
The mean frequencies of minor allele in controls varied between different populations. The mean frequency of IL-1β-31C allele was 0.47 for Asian, 0.36 for Caucasian, and 0.72 for Mexican. The mean frequency of IL-1β-511T allele was 0.47 for Asian, 0.35 for Caucasian, and 0.71 for Mexican (Tables 1 and 2).
The evaluations of the association of IL-1β-31 polymorphism with gastritis risk are shown in Table 3. There was no evidence of any association between IL-1β-31 polymorphism and gastritis risk in overall analysis under any genetic comparisons (Table 3). However, when stratified by ethnicity, significantly elevated risks were observed in the Caucasian population in 3 genetic comparisons (C vs T: OR = 1.24, 95% CI 1.01–1.51; CC vs TT: OR = 1.60, 95% CI 1.05–2.44; recessive model: OR = 1.44, 95% CI 1.00–2.06), while no associations were found in any comparison models in the Asian population. Interestingly, decreased risk was observed in the mixed population (C vs T: OR = 0.53, 95% CI 0.35–0.80; CC vs TT: OR = 0.28, 95% CI 0.11–0.73; recessive model: OR = 0.43, 95% CI 0.24–0.77) (Table 3 and Fig. 1). Hence, the limitation of this study must be considered, as there is only one study with a mixed population, which only provided 100 cases and 102 controls. No associations were found in any comparison when stratified by source of control or genotype method.
Table 3.
Stratified analyses of the IL-1β-31 polymorphism on gastritis risk.
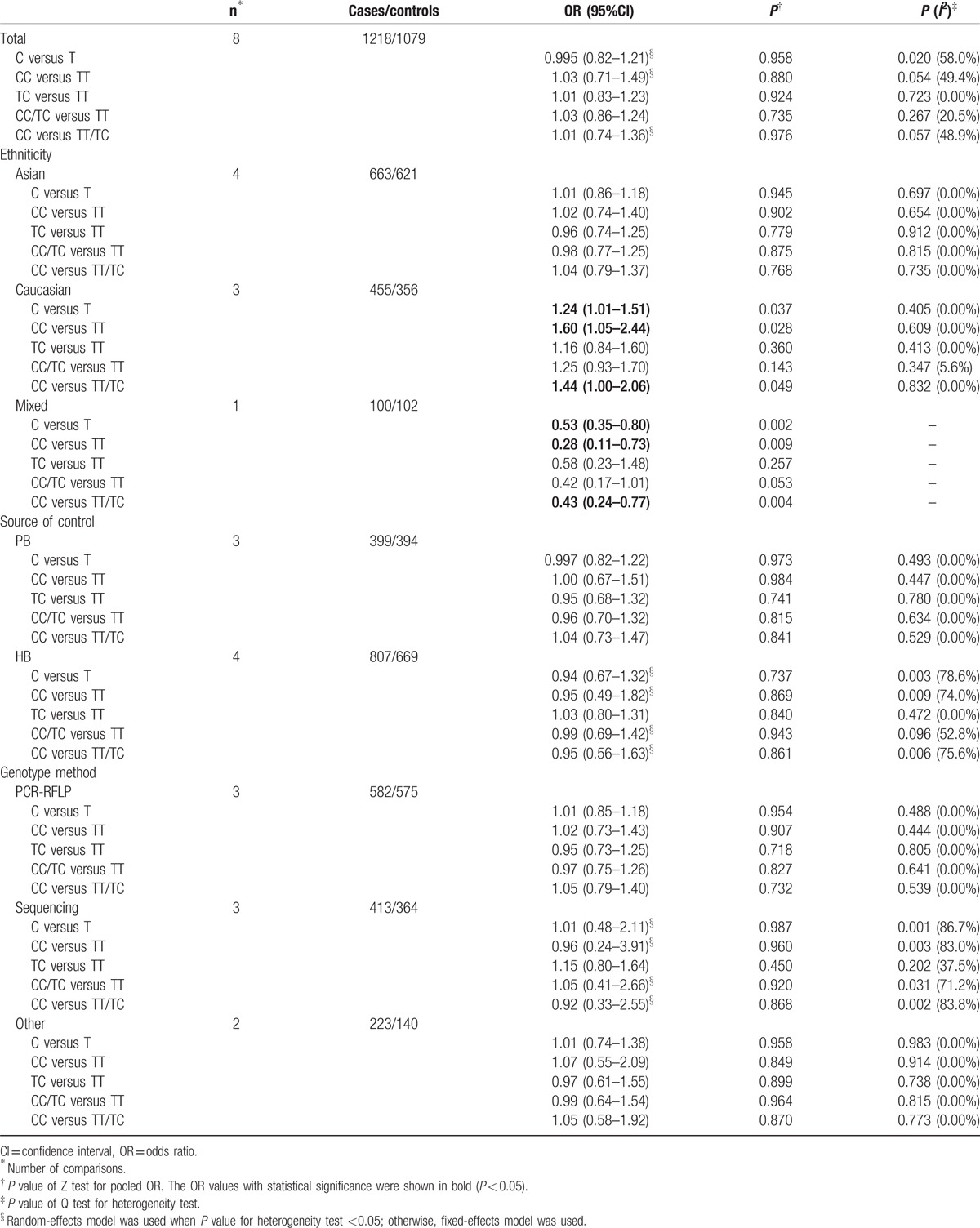
Figure 1.
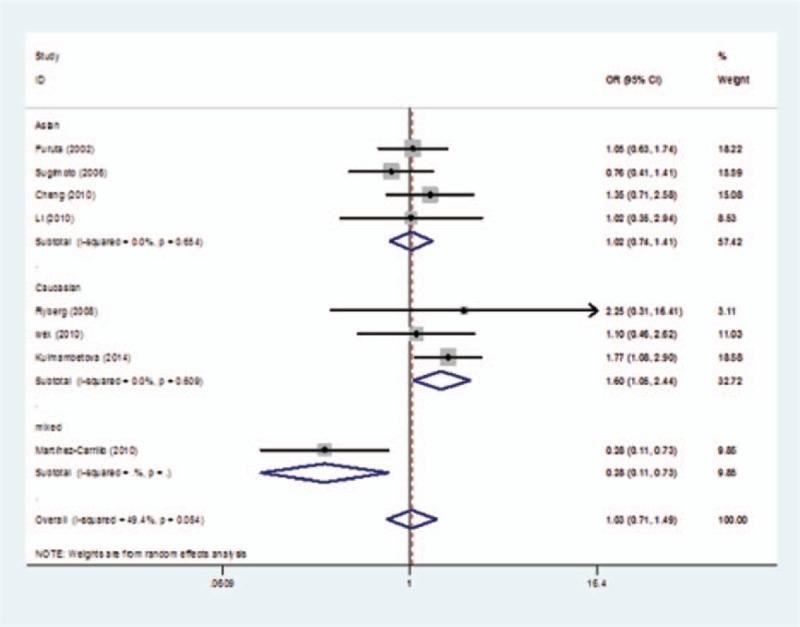
Forest plot of gastritis risk associated with the CC genotypes compared with the TT genotype in interleukin (IL)-1β-31 polymorphism in subgroup analysis of “ethnicity.”
No significant association was found between IL-1β-511 polymorphism and gastritis risk in overall analyses or any subgroup analyses (Supplementary Table S2).
The data of patients infected with H pylori were also extracted. The evaluations of the association of IL-1β-31 or IL-1β-511 polymorphisms with gastritis risk were carried out between H pylori infected patients and control groups[8,18,20,21,24,25] or H pylori noninfected controls.[8,18,20,21,24,25] No significant association was found in any comparison model of either polymorphism site.
3.3. Evaluation of heterogeneity
There was heterogeneity among studies in overall comparisons and also subgroup analyses in IL-1β-31 polymorphism. To explore sources of heterogeneity, we evaluated the following variables by meta-regression: ethnicities, source of control, study quality, genotyping methods, and sample size.
For the IL-1β-31 polymorphism, there was significant heterogeneity in overall comparisons of allele model (P = 0.02), homozygous model (P = 0.054), and dominant model (P = 0.057). Interestingly, when stratified by “ethnicity,” the heterogeneity disappeared in all comparison models. However, meta-regression analyses revealed that sample size could explain 14.02% (allele model), 14.44% (dominant model), and 15.11% (recessive model) of the τ2, and ethnicity could explain 9.02% (recessive model) of the τ2. None of the other possible variables could explain the heterogeneity between studies by meta-regression analysis.
3.4. Sensitivity analysis
To assess the stability of the results, sensitivity analyses were performed to assess the influence of each individual study on the pooled OR. The omission of any study made no significant difference in each comparison in the polymorphisms of IL-1β-31 and IL-1β-511, indicating that the results of this meta-analysis were statistically reliable (Fig. 2).
Figure 2.
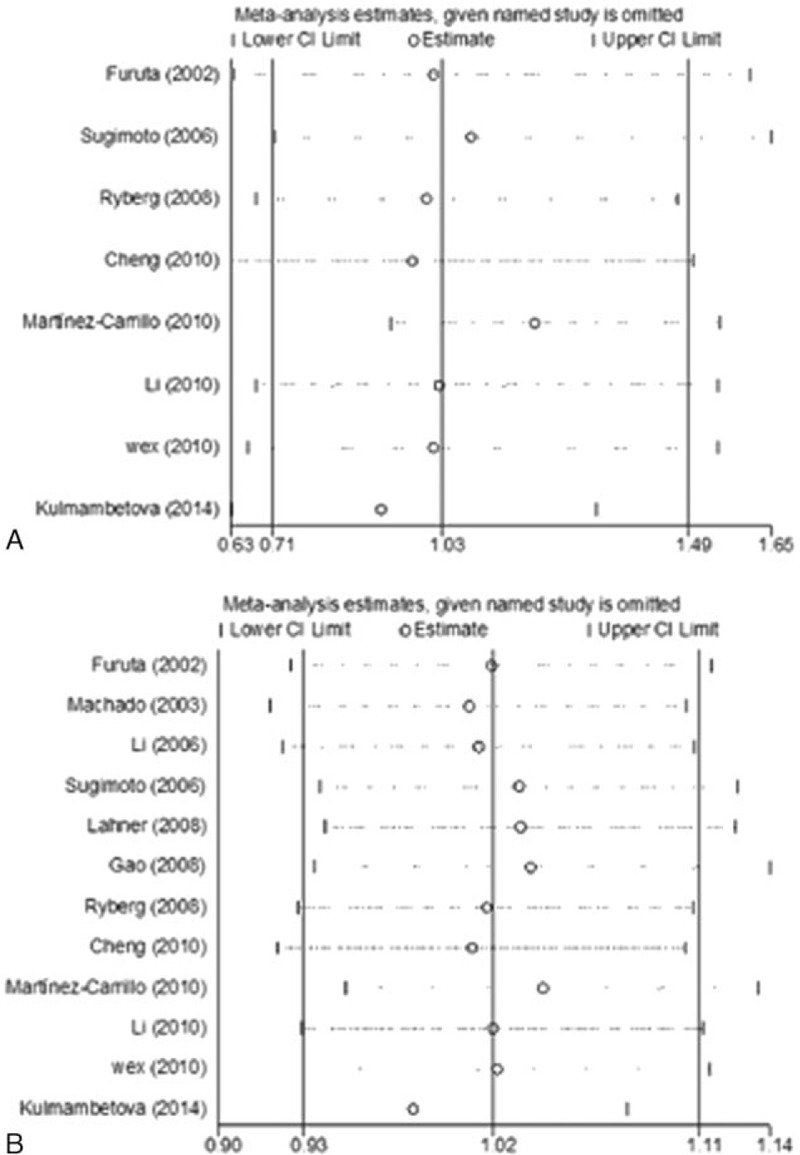
(A) Sensitivity analysis of the homozygous model in interleukin (IL)-1β-31 polymorphism. (B) Sensitivity analysis of the allele model in IL-1β-511 polymorphism.
3.5. Publication bias
There was no publication bias in polymorphisms of IL-1β-31 or IL-1β-511 in the current study. The shape of either the Begg funnel plots or the Egger's plots did not reveal any evidence of obvious asymmetry in all comparison models (Fig. 3, Fig. 4).
Figure 3.
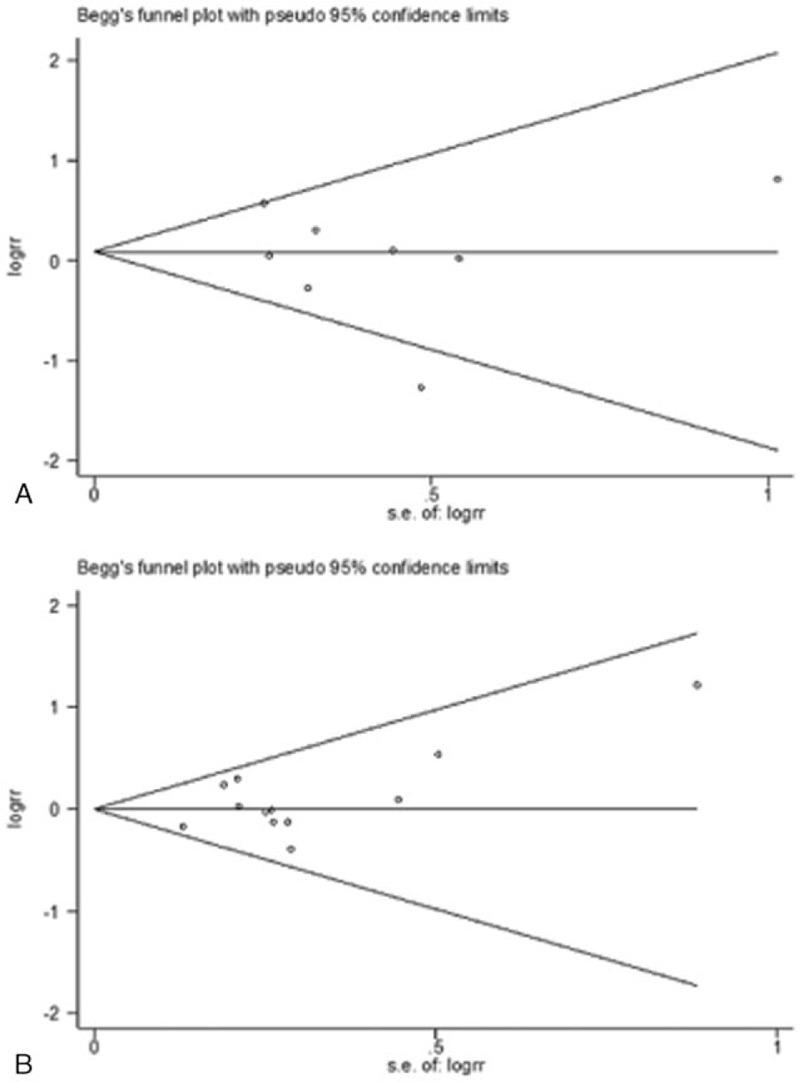
(A) Begg funnel plot for publication bias test under homozygous model in interleukin (IL)-1β-31 polymorphism. (B) Begg funnel plot for publication bias test under heterozygous model in IL-1β-511 polymorphism. Each point represents a separate study for the indicated association.
Figure 4.
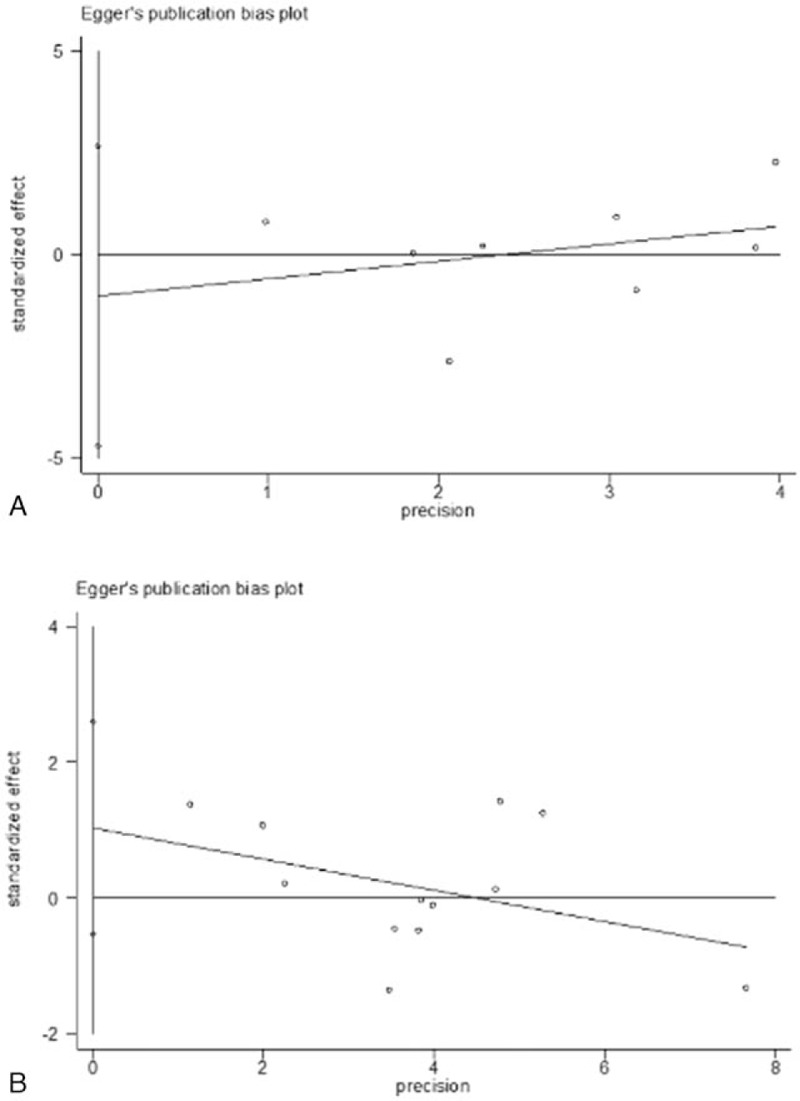
(A) Egger funnel plot for publication bias test under homozygous model in interleukin (IL)-1β-31 polymorphism. (B) Egger funnel plot for publication bias test under heterozygous model in IL-1β-511 polymorphism. Each point represents a separate study for the indicated association.
4. Discussion
In this study, we performed a systematic review of association between IL-1β promoter polymorphisms and gastritis risk based on 12 case–control studies. This review is also the 1st attempt to explore the individual association between the polymorphisms of IL-1β-31 or IL-1β-511 and gastritis risk. The results provided evidences that the IL-1β-31 polymorphisms might be associated with the gastritis risk, especially the Caucasian population, while the IL-1β-511 polymorphisms might not be.
IL-1β is involved in the host's immune response to many antigenic challenges, including H pylori infection.[30] Upon H pylori infection, a local increase of IL-1β was induced. IL-1β may work together with other inflammatory cytokines to recruit and activate neutrophils in the gastric mucosa, resulting in mucosal inflammation.[31]IL-1β could also inhibit the gastric acid secretion by modulating functions of several gastric epithelial cells.[32] As a consequence, the chronic superficial gastritis induced by H pylori infection could gradually develop into chronic atrophic gastritis and gastric carcinoma finally. However, this progression occurs in only some patients, implying that genetic characteristics and environmental factors may be involved in the process.[17] The association of IL-1β polymorphisms and gastric cancer has been implied by several studies,[4,33] whereas the association of IL-1β promoter polymorphisms and gastritis risk is still obscure.
Single nucleotide polymorphisms at positions -31 and -511 of the IL-1β gene were associated with increased expression of IL-1β. IL-1β-31 got a TATA-box polymorphism that directly affects DNA-protein interactions.[4] The IL-1β-511 polymorphism was in nearly complete linkage disequilibrium with IL-1β-31. EI-Omar et al[4] proved IL-1β-31T was associated with increased IL-1β expression in Scottish and Polish populations. Hwang et al[34] showed the IL-1β-511 TT genotype was associated with increased IL-1β expression in the Japanese population. In the context of H pylori infected gastritis, the IL-1β gene is the prime candidate for studies of genetic factors involved in gastritis pathology. Many studies have been carried out to investigate the association between IL-1β promoter polymorphisms and gastritis risk; however, the results remained inconclusive. The H pylori infected patients carrying IL-1β-511T/-31C were found to have an increased risk of atrophic gastritis in the German population.[3] However, IL-1β-511 CT/CC genotypes have been found to be associated with the chronic gastritis in the Brazilian population,[15] as well as the Mexican population.[26] In the Japanese population, Furuta et al[18] failed to find any significant difference in IL-1β-511 polymorphisms between gastritis patients and controls. Achyut et al[13] also failed to observe any significant association of IL-1β-511 polymorphisms with gastritis in the Indian population. As far as IL-1β-31 polymorphism was concerned, the study results remained contradictory. The T allele of IL-1β-31 polymorphism was found to be associated with vulnerability to persistent H pylori infection in Hamajima study,[35] while IL-1β-31 CC may be associated with risk of development of relatively severe gastritis in South China.[36] The distribution of IL-1β-31 polymorphism genotype differed significantly between H pylori infected patients and the noninfected group, with frequency of TT genotype lower in the former group.[6]
In our meta-analysis, IL-1β-31 C allele or CC genotype could increase the gastritis risk in the Caucasian population, while it could decrease the gastritis risk in the Mexican population. However, no association was found between IL-1β-31 polymorphisms and gastritis risk in the background of H pylori infection. The contradictory results between the Caucasian population and the Mexican population may be due to the population constitutions and genotype distributions. In our study, the frequencies of IL-1β-31 C allele and IL-1β-511 T allele in the Mexican population varied largely from the frequencies in the other 2 populations. The Mexican population is a mixed population, and there is genomic diversity in different regions of Mexico. In our analysis, there had only been 1 study of a Mexican population, with it being the population of Guerrero constituting of dominant African ancestors and minor European ancestors. However, other studies based on the Mexican population proved the IL-1β-31 CC genotype was associated with gastric cancer.[37,38] More studies of Mexican or African populations should be taken into the meta-analysis to further confirm our results.
Different population constitutions may also account for this inconsistency between the Asian and Caucasian populations. It is hypothesized that the lack of association between IL-1β polymorphisms and gastritis severity in the Indian population may be due to the comparatively lower level of cytokines secreted in the mucosa than the Caucasian population.[9] As the IL-1β polymorphisms lack association with gastritis risk in the Asian population entirely, the cytokines expression level may also influence the epidemiology study results of the whole Asian population.
However, in our study, the IL-1β-511 polymorphism has no association with gastritis risk in overall comparison or subgroup analysis, which is not consistent with the linkage disequilibrium between IL-1β-511 and IL-1β-31 polymorphisms. One possible reason is that IL-1β-31 locates in TATA box which could directly influence the expression of IL-1β, while IL-1β-511 does not. Our results of IL-1β-31 polymorphism were consistent with previous study,[3,8,9] and there seems to be no publication bias in both polymorphisms. However, the limited studies included in our study may also influence the analysis results. In the future, more studies should be included for reconfirmation.
Cam[39] showed a higher prevalence of familial history of gastric cancer in H pylori infected children, demonstrating the effect of genetic factors in the gastric pathological course of H pylori infected patients. However, neither of the polymorphism sites was proved to be able to affect the gastritis risk of H pylori infected patients in our study. The induction of gastritis by H pylori infection is a chronic process, during which multiple factors exert effects, including IL-1β, IL-8, tumor necrosis factor-alpha, etc., as well as environmental factors. The IL-1β protein may play a role in the development of gastritis, and the IL-1β-31 polymorphism may affect gastritis risk as a genetic factor. However, the polymorphisms in IL-1β promoter may not contribute to the gastritis risk of H pylori infected patients, according to our study. We hypothesized that the influence of IL-1β on gastritis development in H pylori infected patients may not depend on the IL-1β promoter activity, implying clues for gastric pathological research. In addition, other IL-1β single nucleotide polymorphisms may affect gastritis risk in H pylori infected patients, such as +3954C>T polymorphism indicated by Hnatyszyn et al.[11] In any case, our results were inconsistent with Santos’ study of the Brazilian population;[15] thus, more studies need to be carried out to confirm our results.
For a sound meta-analysis, 1 major concern is publication bias. No publication bias for the 2 polymorphisms was found by Begg funnel plot or Egger test. The shapes of the funnel plot were symmetrical in all the comparison models. Heterogeneity between studies is another major concern. In our study, there was no heterogeneity in IL-1β-511 polymorphism by Q test. However, there is heterogeneity between studies in the allele model, homozygous model, and recessive model of IL-1β-31 polymorphism. The heterogeneity disappeared in subgroup analysis by “ethnicity.” The “sample size” and “ethnicity” could only explain part of the τ2 by logistic meta-regression analyses. There may be other reasons accounting for the heterogeneity in IL-1β-31 polymorphism. Nevertheless, sensitivity analysis proved that our meta-analysis results were statistically reliable.
Different ethnic groups, especially a mixed population such as the Mexican and the Brazilian, have their own migration history, genetic drift, allele-frequency-affecting mating status, lifestyle, and disease susceptibility. Therefore, in epidemiology investigation, ethnic differences may induce discrepancies in these studies. In our study, the ethnicity could induce heterogeneity and affect the meta-analysis results. In the future, the ethnicity should be paid more attention to when designing the study method and explaining study results in epidemiology investigation of IL-1β polymorphisms.
In conclusion, the current meta-analysis provides data to support the existence of association between IL-1β-31 polymorphism and gastritis risk in the Caucasian population. It is necessary to conduct more studies with larger sample sizes and various ethnic populations to provide further evidence of the results. In addition, further studies evaluating the gene–environment interaction effects may provide a more comprehensive explanation for the association between IL-1β promoter polymorphisms and gastritis risk.
Supplementary Material
Acknowledgments
The authors thank Ms Grace Tan for the help on English language editing.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy–Weinberg equilibrium, IL = interleukin, OR = odds ratio, PCR = polymerase chain reaction.
Funding/support: The study was funded by the PhD initiation funds from Xuzhou Medical University (code A53591505, A53591504), the University Science Research Project of Jiangsu Province (code 16KJB340002).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Basiri Z, Safaralizadeh R, Bonyadi MJ, et al. Helicobacter pylori vacA d1 genotype predicts risk of gastric adenocarcinoma and peptic ulcers in northwestern Iran. Asian Pac J Cancer Prev 2014;15:1575–9. [DOI] [PubMed] [Google Scholar]
- [2].Vannarath S, Vilaichone RK, Rasachak B, et al. Virulence genes of Helicobacter pylori in gastritis, peptic ulcer and gastric cancer in Laos. Asian Pac J Cancer Prev 2014;15:9027–31. [DOI] [PubMed] [Google Scholar]
- [3].Rad R, Dossumbekova A, Neu B, et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut 2004;53:1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000;404:398–402. [DOI] [PubMed] [Google Scholar]
- [5].Zorzetto V, Tollardo M, Plebani M, et al. Interleukin 1beta and tumor necrosis factor-alpha polymorphisms in autoimmune gastritis. Eur J Gastroenterol Hepatol 2011;23:196. [DOI] [PubMed] [Google Scholar]
- [6].Caleman Neto A, Rasmussen LT, de Labio RW, et al. Gene polymorphism of interleukin 1 and 8 in chronic gastritis patients infected with Helicobacter pylori. J Venom Anim Toxins Incl Trop Dis 2014;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murphy G, Thornton J, McManus R, et al. Association of gastric disease with polymorphisms in the inflammatory-related genes IL-1B, IL-1RN, IL-10, TNF and TLR4. Eur J Gastroenterol Hepatol 2009;21:630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kulmambetova GN, Imanbekova MK, Logvinenko AA, et al. Association of cytokine gene polymorphisms with gastritis in a kazakh population. Asian Pac J Cancer Prev 2014;15:7763–8. [PubMed] [Google Scholar]
- [9].Moorchung N, Srivastava AN, Gupta NK, et al. Cytokine gene polymorphisms and the pathology of chronic gastritis. Singapore Med J 2007;48:447–54. [PubMed] [Google Scholar]
- [10].Achyut BR, Moorchung N, Srivastava AN, et al. Risk of lymphoid follicle development in patients with chronic antral gastritis: role of endoscopic features, histopathological parameters, CagA status and interleukin-1 gene polymorphisms. Inflamm Res 2008;57:51–6. [DOI] [PubMed] [Google Scholar]
- [11].Hnatyszyn A, Wielgus K, Kaczmarek-Rys M, et al. Interleukin-1 gene polymorphisms in chronic gastritis patients infected with Helicobacter pylori as risk factors of gastric cancer development. Arch Immunol Ther Exp (Warsz) 2013;61:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamada S, Matsuhisa T, Makonkawkeyoon L, et al. Helicobacter pylori infection in combination with the serum pepsinogen I/II ratio and interleukin-1beta-511 polymorphisms are independent risk factors for gastric cancer in Thais. J Gastroenterol 2006;41:1169–77. [DOI] [PubMed] [Google Scholar]
- [13].Achyut BR, Moorchung N, Mittal B. Genetic association of interleukin-1 haplotypes with gastritis and precancerous lesions in North Indians. Clin Exp Med 2008;8:23–9. [DOI] [PubMed] [Google Scholar]
- [14].Kupcinskas L, Wex T, Kupcinskas J, et al. Interleukin-1B and interleukin-1 receptor antagonist gene polymorphisms are not associated with premalignant gastric conditions: a combined haplotype analysis. Eur J Gastroenterol Hepatol 2010;22:1189–95. [DOI] [PubMed] [Google Scholar]
- [15].Santos JC, Ladeira MS, Pedrazzoli J, Jr, et al. Relationship of IL-1 and TNF-alpha polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Braz J Med Biol Res 2012;45:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zabaleta J, Camargo MC, Piazuelo MB, et al. Association of interleukin-1beta gene polymorphisms with precancerous gastric lesions in African Americans and Caucasians. Am J Gastroenterol 2006;101:163–71. [DOI] [PubMed] [Google Scholar]
- [17].Forte GI, Cala C, Scola L, et al. Role of environmental and genetic factor interaction in age-related disease development: the gastric cancer paradigm. Rejuvenation Res 2008;11:509–12. [DOI] [PubMed] [Google Scholar]
- [18].Furuta T, El-Omar EM, Xiao F, et al. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 2002;123:92–105. [DOI] [PubMed] [Google Scholar]
- [19].Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology 2003;125:364–71. [DOI] [PubMed] [Google Scholar]
- [20].Li C, Xia HH, Xie W, et al. Association between interleukin-1 gene polymorphisms and Helicobacter pylori infection in gastric carcinogenesis in a Chinese population. J Gastroenterol Hepatol 2007;22:234–9. [DOI] [PubMed] [Google Scholar]
- [21].Sugimoto M, Furuta T, Shirai N, et al. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J Gastroenterol Hepatol 2007;22:51–9. [DOI] [PubMed] [Google Scholar]
- [22].Lahner E, Corleto VD, D’Ambra G, et al. Is interleukin-1 genotyping useful for the clinical management of patients with atrophic body gastritis? Aliment Pharmacol Ther 2008;27:355–65. [DOI] [PubMed] [Google Scholar]
- [23].Gao L, Weck MN, Nieters A, et al. Association between a pro-inflammatory genetic profile and the risk of chronic atrophic gastritis among older adults from Germany. Eur J Cancer 2009;45:428–34. [DOI] [PubMed] [Google Scholar]
- [24].Ryberg A, Borch K, Sun YQ, et al. Concurrent genotyping of Helicobacter pylori virulence genes and human cytokine SNP sites using whole genome amplified DNA derived from minute amounts of gastric biopsy specimen DNA. BMC Microbiol 2008;8:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng HH, Chang CS, Wang HJ, et al. Interleukin-1beta and -10 polymorphisms influence erosive reflux esophagitis and gastritis in Taiwanese patients. J Gastroenterol Hepatol 2010;25:1443–51. [DOI] [PubMed] [Google Scholar]
- [26].Martinez-Carrillo DN, Garza-Gonzalez E, Betancourt-Linares R, et al. Association of IL1B -511C/-31T haplotype and Helicobacter pylori vacA genotypes with gastric ulcer and chronic gastritis. BMC Gastroenterol 2010;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li ZW, Wu Y, Sun Y, et al. Inflammatory cytokine gene polymorphisms increase the risk of atrophic gastritis and intestinal metaplasia. World J Gastroenterol 2010;16:1788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wex T, Leodolter A, Bornschein J, et al. Interleukin 1 beta (IL1B) gene polymorphisms are not associated with gastric carcinogenesis in Germany. Anticancer Res 2010;30:505–11. [PubMed] [Google Scholar]
- [29].Wells GA SB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed December 15, 2014]. [Google Scholar]
- [30].Huang FY, Chan AO, Lo RC, et al. Characterization of interleukin-1beta in Helicobacter pylori-induced gastric inflammation and DNA methylation in interleukin-1 receptor type 1 knockout (IL-1R1(-/-)) mice. Eur J Cancer 2013;49:2760–70. [DOI] [PubMed] [Google Scholar]
- [31].El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut 2001;48:743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wallace JL, Cucala M, Mugridge K, et al. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol 1991;261(4 Pt 1):G559–64. [DOI] [PubMed] [Google Scholar]
- [33].Park MJ, Hyun MH, Yang JP, et al. Effects of the interleukin-1beta-511C/T gene polymorphism on the risk of gastric cancer in the context of the relationship between race and H. pylori infection: a meta-analysis of 20,000 subjects. Mol Biol Rep 2014. [DOI] [PubMed] [Google Scholar]
- [34].Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology 2002;123:1793–803. [DOI] [PubMed] [Google Scholar]
- [35].Hamajima N, Matsuo K, Saito T, et al. Interleukin 1 polymorphisms, lifestyle factors, and Helicobacter pylori infection. Jpn J Cancer Res 2001;92:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li J, Wang F, Zhou Q, et al. IL-1 polymorphisms in children with peptic symptoms in South China. Helicobacter 2011;16:246–51. [DOI] [PubMed] [Google Scholar]
- [37].Sicinschi LA, Lopez-Carrillo L, Camargo MC, et al. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer 2006;118:649–57. [DOI] [PubMed] [Google Scholar]
- [38].Garza-Gonzalez E, Bosques-Padilla FJ, El-Omar E, et al. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer 2005;114:237–41. [DOI] [PubMed] [Google Scholar]
- [39].Cam S. Risk of gastric cancer in children with Helicobacter pylori infection. Asian Pac J Cancer Prev 2014;15:9905–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


