Abstract
Even though more than a century later, after the first case of gastrectomy has been successfully performed, the best surgical treatment for distal gastric cancer still remains controversial. Thus, the present study was designed to compare the survival impact of distal (DG) or total gastrectomy (TG) for distal gastric cancer. A total of 1262 distal gastric cancer patients were enrolled in current study including 1157 patients who underwent DG and 157 patients who underwent TG. The postoperative complications and 5-year overall survival were compared between the 2 groups. TG group presented a longer surgical time, a higher volume of intraoperative bleeding, and a larger number of excised lymph nodes (all P < 0.05) compared with the DG group. The postoperative complications were comparable (all P >0.05). The 5-year overall survival rate of DG group was significantly higher than that of TG group (67.6% vs 44.3%, P < 0.001). However, multivariate analysis showed that type of resection was not an independent prognostic factor for distal gastric cancer (P > 0.05). The factor-stratified multivariate analysis showed that only in the subgroup of Tumor-node-metastasis staging system (TNM) stage III (P = 0.049), TG was the independent prognostic factor for poor survival. In conclusion, DG was as feasible as TG; however, TG did not increase the survival rate. DG brought better long-term survival than TG in patients with TNM stage III tumor. We recommended that DG should be the optimal surgical procedure for distal gastric cancer under the premise of negative resection margin.
Keywords: complication, distal gastrectomy, distal gastric cancer, prognosis, total gastrectomy
1. Introduction
Although a significantly decreasing incidence trend of gastric cancer has been observed worldwide, gastric cancer is still the second most common carcinoma in China.[1] Surgical resection including proximal, distal (DG), or total gastrectomy (TG) with extended lymphadenectomy is the only curative treatment for gastric cancer by now. Even more than a hundred years later, since the first case of subtotal gastrectomy and total gastrectomy had been successfully performed in 1881 and 1897,[2,3] respectively, the best surgical procedure for distal gastric cancer still remains controversial.
The type of resection for gastric cancer is assessed and determined by the tumor size and location as well as the distance of proximal resection edge.[4] Complete resection with at least a 4 cm proximal margin length for gastric cancer is recommended by the 2016 edition of NCCN guidelines.[5] However, McNeer et al[6] proposed that TG should be performed even an R0 margin can be obtained by DG. There is no consensus on the selection of operations for distal gastric cancer under the premise of sufficient proximal margin length, since study based on a comparison of survival superiority between DG and TG was lacking. The preference of surgical resection for distal gastric cancer is much more dependent on surgeons’ experience and varies between different regions.[7–9]
Therefore, the current study aimed to compare the survival impact between DG and TG for distal gastric cancer in order to achieve the optimal treatment strategy.
2. Patients and methods
From september 2008 to March 2015, a total of 1262 distal gastric cancer who received radical gastrectomy in Xijing Hospital, Fourth Military Medical University, were retrospectively enrolled in the present study. The inclusion criteria were listed as follows: (1) with a lower third gastric cancer; (2) without neoadjuvant chemotherapy; (3) without distal metastasis; (4) with radical gastrectomy; (5) with negative proximal margin; (6) with complete follow-up records. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from all patients before surgery.
All of the patients received DG or TG according to the recommendation of Japanese Gastric Cancer Treatment guidelines.[10] All the surgeries were performed by experienced surgeons in our center. The Tumor-node-metastasis staging system (TNM) stages were defined on the basis of 7th edition of AJCC cancer staging manual.[11]
Clinicopathological data including age, gender, tumor size, histologic type, tumor depth, lymph node metastasis, and TNM stage were recorded and analyzed. The perioperative outcomes including surgical time, intraoperative bleeding, number of excised lymph nodes, pulmonary infection, wound dehiscence, wound infection, anastomotic leakage, chylous fistula, intraperitoneal hemorrhage, postoperative 30-day mortality, and hospital stay were also analyzed.
Data were processed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL). Numerical variables were expressed as mean ± SD. Discrete variables were analyzed using the Chi-square test or Fisher's exact test. Risk factors for survival were identified by univariate analysis and Cox's proportional hazards regression model was employed for multivariate analysis. Overall survival was analyzed by the Kaplan–Meier method and differences between curves were compared using the log-rank test. P values were considered to be statistically significant at the 5% level.
3. Results
3.1. General features between DG and TG groups
The clinicopathological features were summarized in Table 1. There were 923 males and 339 females. The median age was 56 years (range 21–86 years). Among the enrolled patients, 1157 (91.7%) patients received DG and 105 (8.3%) patients received TG. The distribution of tumor size, histologic type, tumor depth, lymph node metastasis, and TNM stage were significantly different between the DG and TG groups (all P < 0.005).
Table 1.
Clinicopathological features of distal gastric cancer patients between DG and TG group.
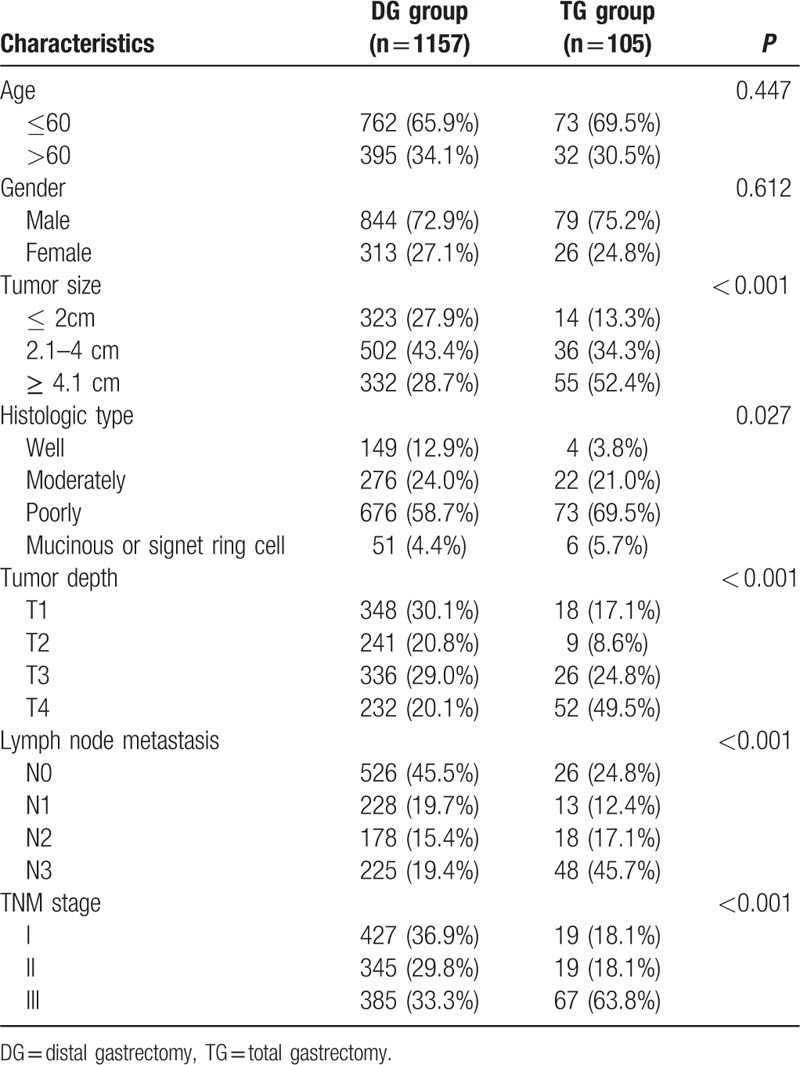
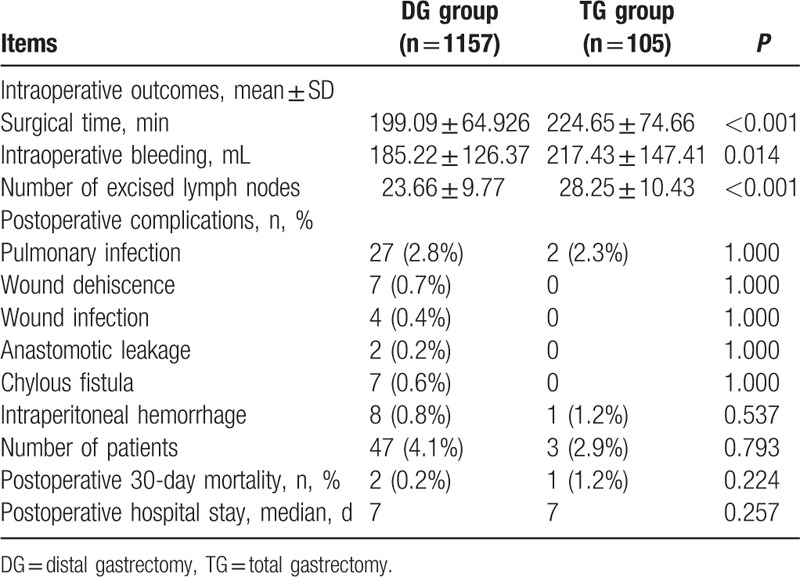
As showed in Table 2, the TG group presented a longer surgical time (224.65 min vs 199.09 min, P < 0.001), a higher volume of intraoperative bleeding (217.43 mL vs 185.22 mL, P = 0.014) and a larger number of excised lymph nodes (28.25 vs 23.66, P < 0.001) in comparison with the DG group. The postoperative complications including surgical time, intraoperative bleeding, number of excised lymph nodes, pulmonary infection, wound dehiscence, wound infection, anastomotic leakage, chylous fistula, intraperitoneal hemorrhage, postoperative 30-day mortality were comparable between the 2 groups (all P > 0.05). The postoperative hospital stay had no statistical difference either (median, 7 d vs 7 d, P = 0.257).
Table 2.
Perioperative outcomes of distal gastric cancer patients between DG and TG groups.
3.2. Overall survival analysis
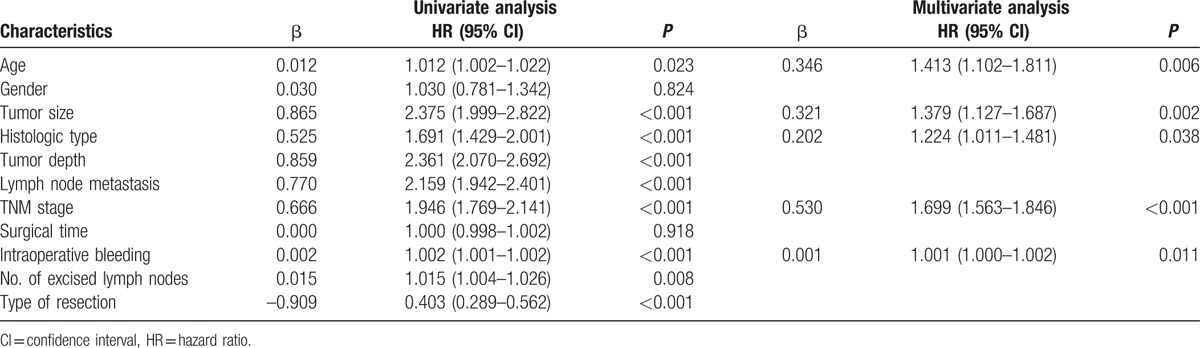
Survival was analyzed in 1262 distal gastric cancer patients with the range of follow-up from 0.17 to 76 months (mean, 29 months; median, 25.83 months). A 65.8% 5-year overall survival rate for the entire cohort was found in the current study. The 5-year overall survival rate of DG group was significantly higher than that of TG group (67.6% vs 44.3%, P < 0.001, Fig. 1). The presence of age, tumor size, histologic type, tumor depth, lymph node metastasis, TNM stage, intraoperative bleeding, number of excised lymph nodes, and type of resection were associated with prognosis according to the univariate analysis (all P < 0.05, Table 3). However, multivariate analysis showed that type of resection was not an independent prognostic factor for distal gastric cancer (P > 0.05, Table 3).
Figure 1.
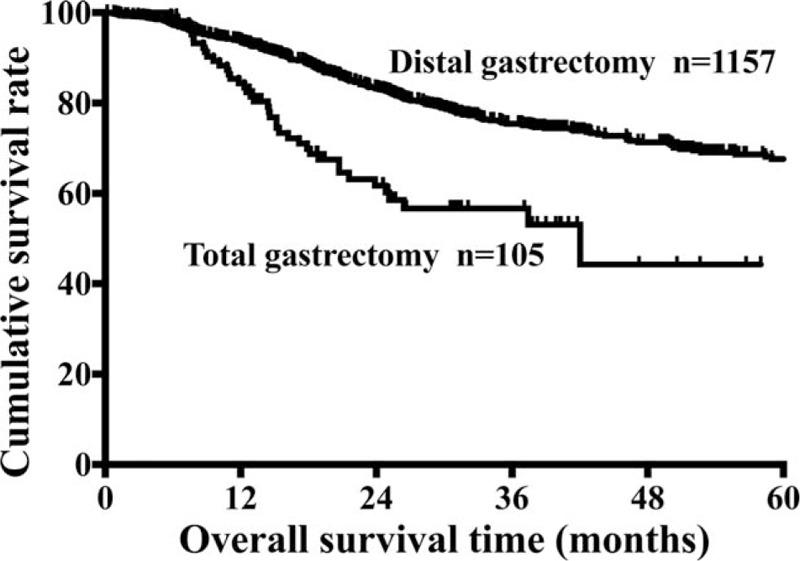
Comparison of 5-year survival rates of distal gastric cancer patients between DG and TG groups. DG = distal gastrectomy, TG = total gastrectomy.
Table 3.
Univariate and multivariate analyses of prognostic factors for patients with distal gastric cancer.
3.3. Survival analysis according to subgroups
In order to further compare the survival of DG and TG groups, we analyzed the 5-year overall survival rates of patients according to the subgroups of all the clinicopathological factors listed in Table 1, using the Kaplan–Meier method (Table 4). The results showed that TG was associated with poor survival in subgroups of age (≤60, > 60), gender (male, female), tumor size (2.1–4 cm), histologic type (differentiated, undifferentiated), tumor depth (T4), lymph node metastasis (positive), and TNM stage (stage III) (all P < 0.05). The survival rates had no significant differences between the 2 groups in the rest of the subgroups (all P > 0.05).
Table 4.
Kaplan–Meier survival analysis of patients’ clinicopathological factors.
We conducted univariate and multivariate analyses for each subgroup. In consistent with the Kaplan–Meier method, the same results were also found by univariate analysis (data not show). The multivariate analysis showed that only in the subgroup of TNM stage III, TG was the independent prognostic factor indicating poor survival (all P = 0.049, Table 5). The survival curves of the 2 subgroups were showed in Fig. 2.
Table 5.
Univariate and multivariate analyses of prognostic factors for patients with stage III distal gastric cancer.
Figure 2.
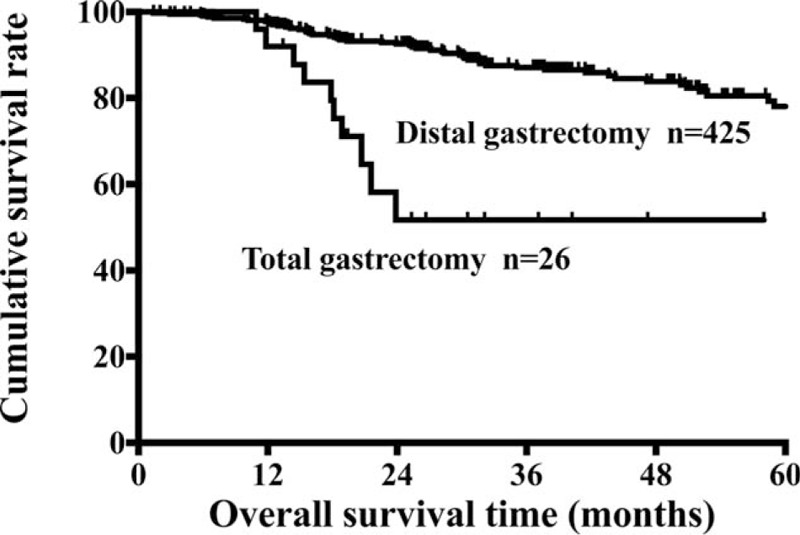
Survival curves of patients with TNM stage III distal gastric cancer between DG and TG groups. DG = distal gastrectomy, TG = total gastrectomy, TNM = tumor-node-metastasis staging system.
4. Discussion
The current study focused on the survival impact of DG and TG for distal gastric cancer. We found that the 5-year overall survival rate after DG for distal gastric cancer patients was higher than that of TG, but the resection type was not an independent prognostic factor for the cohort. Only in TNM stage III, TG brought a worse prognosis for distal gastric cancer than DG according to multivariate analysis.
Although a variety of novel molecular targets have been found and the targeted therapies have shown encouraging results in gastric cancer patients,[12–16] curative resection is considered to be the ideal primary choice that not only brings favorable long-term survival but also causes a low morbidity rate.[17,18] However, consideration regarding the extent of surgical resection depends on multiple factors.[19,20] Till now, there was no consensus about the surgical procedure for distal gastric cancer. A previous extensive survey of 62 centers in Europe including 16,594 patients showed that 44% surgeons would chose TG for antrum tumor of stomach.[21] The national Cancer Data Base report of United States comprising 6400 patients showed that approximately 12.3% distal gastric cancer patients received TG.[9] In our cohort, only 8.3% patients received TG which was obviously lower than the proportion reported previously.
Actually, TG could cause several complications such as weight loss, diarrhea, anorexia, and metabolic changes.[22] Meanwhile, there is also some superiority of TG compared with DG; for instance, avoiding tumor local recurrence and reducing the occurrence risk of remnant gastric cancer.[23] However, a previous randomized clinical trial demonstrated that the postoperative complications were comparable between DG and TG.[24] At the current time, the comparison of perioperative morbidity and mortality between the 2 groups were still under debate.[22,25,26] In the present study, DG showed significant superiority to TG during the surgical procedure. The postoperative complications and hospital stay were comparable between the 2 groups. From the point of view of safety, DG instead of TG was feasible. Previous studies demonstrated that extended lymph node dissection had not shown any benefit for gastric cancer so far.[27–29] In the current study, the number of excised lymph nodes was not an independent prognostic factor either.
Long-term survival is the most important criteria when choosing the extent of resection. A French prospective controlled study including 201 patients with gastric antrum cancer indicated that TG did not increase the survival rate compared with DG.[30] In consistent with the conclusion above, another randomized clinical trial including 618 patients with tumor of the distal stomach from 28 institutions, demonstrated that there is no superiority in extending resection, which showed familiar 5-year survival rate between DG and TG groups.[31] The similar results were also found in the other studies.[32–34] In our study, DG brought a significantly better overall survival than TG for distal gastric cancer patients. But, the multivariate analysis showed that type of resection was not an independent prognostic factor for the entire cohort. The poor survival after TG may be due to the higher stage of tumor in the TG group.
Under this case, further clinicopathological factor-stratified survival analysis was necessary. Multivariate analysis indicated that TG was an independent risk factor for poor prognosis in subgroup of TNM stage III. Thus, patients with distal gastric cancer who received TG should be treated more carefully and followed up closely, when assessed as TNM stage III degree postoperatively by the pathologists.
There are several limitations in our present study. First, it was a retrospective study of a single center's experience. Multicenter studies are needed to verify the survival impact of these 2 types of gastrectomy. Second, the postoperative quality of life of patients who underwent either DG or TG was not analyzed. Third, the numbers of patients in the 2 groups were unbalanced.
5. Conclusions
Distal gastrectomy was as feasible as total gastrectomy for distal gastric cancer regarding the intraoperative procedure. Type of resection was not an independent prognostic factor for distal gastric cancer in the cohort. Distal gastrectomy was significantly superior to total gastrectomy in subgroup of TNM stage III. We recommended distal gastrectomy as the optimal surgical procedure for distal gastric cancer under the premise of negative resection margin.
Footnotes
Abbreviations: CI = confidence interval, DG = distal gastrectomy, HR = hazard ratio, TG = total gastrectomy.
ZL, FF, and MG contributed equally to this work.
Funding: This study was supported in part by grants from the National Natural Scientific Foundation of China [NO. 31100643, 31570907, 81300301, 81572306, 81502403, XJZT12Z03].
The authors have no conflicts of interest to disclose.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Billroth T. Offenes schreiben an Herrn Dr. L. Wittelshofer. Wien Med Wochenschr 1881;31:161–5. [Google Scholar]
- [3].Schlatter K. A unique case of complete removal of the stomach-successful esophago-enterostomy recovery. Med Rec 1897;52:909–14. [Google Scholar]
- [4].Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005;241:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ajani JA, D’Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:1286–312. [DOI] [PubMed] [Google Scholar]
- [6].McNeer G, Bowden L, Booner RJ, et al. Elective total gastrectomy for cancer of the stomach: end results. Ann Surg 1974;180:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- [8].Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993;218:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hundahl SA, Menck HR, Mansour EG, et al. The National Cancer Data Base report on gastric carcinoma. Cancer 1997;80:2333–41. [DOI] [PubMed] [Google Scholar]
- [10].Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–23. [DOI] [PubMed] [Google Scholar]
- [11].Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077–9. [DOI] [PubMed] [Google Scholar]
- [12].Saito R, Abe H, Kunita A, et al. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in Epstein–Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol 2016;doi: 10.1038/modpathol.2016.202. [DOI] [PubMed] [Google Scholar]
- [13].Amedei A, Munari F, Bella CD, et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 2014;9:303–9. [DOI] [PubMed] [Google Scholar]
- [14].Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014;15:1007–18. [DOI] [PubMed] [Google Scholar]
- [15].Satoh T, Lee KH, Rha SY, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2015;18:824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717–26. [DOI] [PubMed] [Google Scholar]
- [17].Stein HJ, Sendler A, Siewert JR. Site-dependent resection techniques for gastric cancer. Surg Oncol Clin N Am 2002;11:405–14. [DOI] [PubMed] [Google Scholar]
- [18].Clark CJ, Thirlby RC, Picozzi V, Jr, et al. Current problems in surgery: gastric cancer. Curr Probl Surg 2006;43:566–670. [DOI] [PubMed] [Google Scholar]
- [19].Roukos DH. Current advances and changes in treatment strategy may improve survival and quality of life in patients with potentially curable gastric cancer. Ann Surg Oncol 1999;6:46–56. [DOI] [PubMed] [Google Scholar]
- [20].Takiguchi S, Yamamoto K, Hirao M, et al. A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Gastric Cancer 2012;15:198–205. [DOI] [PubMed] [Google Scholar]
- [21].Heberer G, Teichmann RK, Kramling HJ, et al. Results of gastric resection for carcinoma of the stomach: the European experience. World J Surg 1988;12:374–81. [DOI] [PubMed] [Google Scholar]
- [22].Le A, Berger D, Lau M, et al. Secular trends in the use, quality, and outcomes of gastrectomy for noncardia gastric cancer in the United States. Ann Surg Oncol 2007;14:2519–27. [DOI] [PubMed] [Google Scholar]
- [23].Meyer HJ, Jahne J, Wilke H, et al. Surgical treatment of gastric cancer: retrospective survey of 1,704 operated cases with special reference to total gastrectomy as the operation of choice. Semin Surg Oncol 1991;7:356–64. [DOI] [PubMed] [Google Scholar]
- [24].Bozzetti F, Marubini E, Bonfanti G, et al. Total versus subtotal gastrectomy: surgical morbidity and mortality rates in a multicenter Italian randomized trial. The Italian Gastrointestinal Tumor Study Group. Ann Surg 1997;226:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Meyer C, Rohr S, Vix J, et al. Outcome of surgical treatment of cancer of the stomach. Report of 330 cases. Chir Ital 1997;49:27–33. [PubMed] [Google Scholar]
- [26].Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol 2006;101:2485–92. [DOI] [PubMed] [Google Scholar]
- [27].Bozzetti F. Rationale for extended lymphadenectomy in gastrectomy for carcinoma. J Am Coll Surg 1995;180:505–8. [PubMed] [Google Scholar]
- [28].Robertson CS, Chung SC, Woods SD, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg 1994;220:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069–77. [DOI] [PubMed] [Google Scholar]
- [30].Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg 1989;209:162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bozzetti F, Marubini E, Bonfanti G, et al. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 1999;230:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gockel I, Pietzka S, Gonner U, et al. Subtotal or total gastrectomy for gastric cancer: impact of the surgical procedure on morbidity and prognosis—analysis of a 10-year experience. Langenbecks Arch Surg 2005;390:148–55. [DOI] [PubMed] [Google Scholar]
- [33].Pugliese R, Maggioni D, Sansonna F, et al. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc 2010;24:2594–602. [DOI] [PubMed] [Google Scholar]
- [34].Morgagni P, Marfisi C, Gardini A, et al. Subtotal gastrectomy as treatment for distal multifocal early gastric cancer. J Gastrointest Surg 2009;13:2239–44. [DOI] [PubMed] [Google Scholar]


