Abstract
Contribution of decompressive laparotomy within the framework of the complex therapeutic algorithm of abdominal compartment syndrome (ACS) is cited with an extremely heterogeneous percentage in terms of survival. The purpose of this study was to present new data regarding contribution of each therapeutic step toward decreasing the mortality of this syndrome.
This is a longitudinal prospective study including 134 patients with risk factors for ACS. The intra-abdominal pressure was measured every hour indirectly based on transvesical approach and the appearance of organ dysfunction. Specific therapy for ACS was based on the 2013 World Society of Abdominal Compartment Syndrome guidelines, which include laparotomy decompression. Management of the temporarily open abdomen included an assisted vacuum wound therapy.
Of 134 patients, 66 developed ACS. The average intra-abdominal pressure significantly decreased after therapy and decompression surgery. The overall rate of mortality was 27.3% with statistical significance in necrotizing infected pancreatitis. Surgical decompression performed within the first 24 hours after the onset of ACS had a protective role against mortality (odds ratio <1). The average time after which laparotomy decompression was performed was 16.23 hours. The complications occurred during TAC were 2 wound suppurations and 1 intestinal obstruction. Wound suppurations evolved favorably by using vacuum wound-assisted therapy associated with the general treatment, whereas for occlusion, resurgery was performed after which adhesions dissolved. The final closure of the abdomen was performed at a mean of 11.7 days (min. = 9, max. = 14). The closure type was primary suture of the musculoaponeurotic edges in 4 cases, and the use of dual mesh in the other 11 cases.
The highest mortality rate in the study group was registered in patients with necrotizing pancreatitis and the lowest in trauma group. Surgical decompression within the framework of the complex algorithm treatment of ACS contributed to the reduction of mortality by 8.7%. It is extremely important that the elapsed time since the initiation of the ACS until the surgical decompression is minimal (under 24 hours).
Keywords: abdominal compartment syndrome, decompressive laparotomy, intra-abdominal pressure, open abdomen
1. Introduction
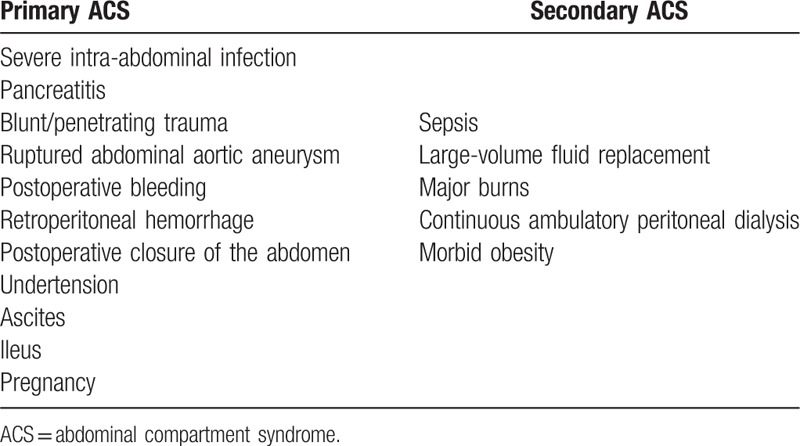
Although abdominal compartment syndrome (ACS) has long been known, the true basis and definitions of this concept were revealed in 2004 at Noosa, Australia, when The World Society of the Abdominal Compartment Syndrome (WSACS) was founded.[1] ACS is now a well-known entity defined by increased intra-abdominal pressure (IAP) that is >20 mmHg (with or without arterial perfusion pressure [APP] ≤60 mmHg) and is associated with organ dysfunction. Primary ACS is triggered by the condition located in the abdominal-pelvic region, and secondary ACS develops owing to certain diseases outside the abdominal-pelvic region (Table 1). Recurrent ACS develops following previous surgical or medical treatment of primary or secondary ACS.[2] Additionally, IAP is sometimes associated with lower degree organ failure in critical patients. Therefore, in 1996, Burch et al[3] concluded that even first stage of intra-abdominal hypertension (IAH:10–15 mmHg) involves organ failure leading to same clinical course as ACS.
Table 1.
The key parameter in detecting the syndrome is the increased IAP. There are several methods for measuring IAP,[7,8] both directly and indirectly invasive. However, the transvesical IAP measurement has become the criterion standard owing to its simplicity, efficacy, and absence of side effects.[9–12] According to the Guidelines of 2013—Definitions and Recommendations, IAP measurement is recommended whenever there is a risk of IAP development with possible development of ACS. The data from the literature are quite contradictory in terms of mortality rates after the occurrence of ACS. If not treated, >90% of the cases lead to death, and after treatment, the mortality is between 25% and 75%.[13]
The purpose of this article was to present new data regarding terms of mortality rate and importance of decompressive laparotomy (DL), relying on interdisciplinary collaboration and the high addressability of the patients who are at risk of developing ACS. We mention that the Intensive Care department is the largest in Transylvania, with 75 beds and a regional center of medical and surgical emergencies, covering 5.8 million of Romania's population.
2. Materials
The study took place between January 2016 and September 2016 and included a total of 134 patients. The informed consent was obtained from each patient, and in case of comatose patients, it was signed by relatives according to the Romanian State legislation and the agreement of the Medical Research Ethics Committee of the University of Medicine and Pharmacy in Tîrgu-Mureş, a document registered under no. 118/01.22.2016.
The study inclusion criteria were as follows:
-
-
Patients with abdominal surgery for acute peritonitis caused by abdominal organ perforation or postoperative fistulas following digestive anastomoses, infected necrotic acute pancreatitis, traumas to the intra-abdominal organs as a result of polytrauma, and intestinal occlusion caused by adhesion processes of the abdominal scarring.
-
-
Patients suffering from acute pancreatitis clinically confirmed by laboratory data and imaging, but without surgical indication.
The exclusion criteria were patients with secondary ACS, isolated IAH.
The data collected in the study were: biographical data (sex, age), primary disease, presence of surgery and its type, comorbidities, IAP after the primary surgery, after medical treatment of ACS, and after decompression laparotomy, organ dysfunctions, response to conservative or surgical treatment, mortality rate, and how it is influenced by surgical decompression.
3. Methods
The measurement of IAP was determined indirectly, transvesically, by using a dedicated kit, Abviser ABV 331. Monitoring consisted of value recording every hour and was started as soon as the study was initiated. It was ended when the measurement of IAP repeated over 24 hours got <15 mmHg or in case of the patient's death.
3.1. Description of the IAP measuring technique
The autovalve of the kit is mounted between the Foley catheter and the collecting bag; the pressure transducer is fixed at the level of the midaxillary line on the iliac crest; the tubing is connected to a bag of saline solution; after emptying the air in the kit components, the monitor is calibrated by bringing the reference value to 0; the measurement of the abdominal pressure is started by aspiration of 25 mL saline solution into the kit's syringe and injecting it into the bladder. The value displayed on the monitor represents the IAP in millimeters of mercury (Fig. 1). After about 2 to 3 minutes, the saline solution injected is discharged by the autovalve into the collecting bag, and to perform a new reading, the syringe is refilled with 25 mL saline solution and the previous steps are repeated. Each new measurement adds 25 mL to the calculation of urine output every 24 hours.
Figure 1.
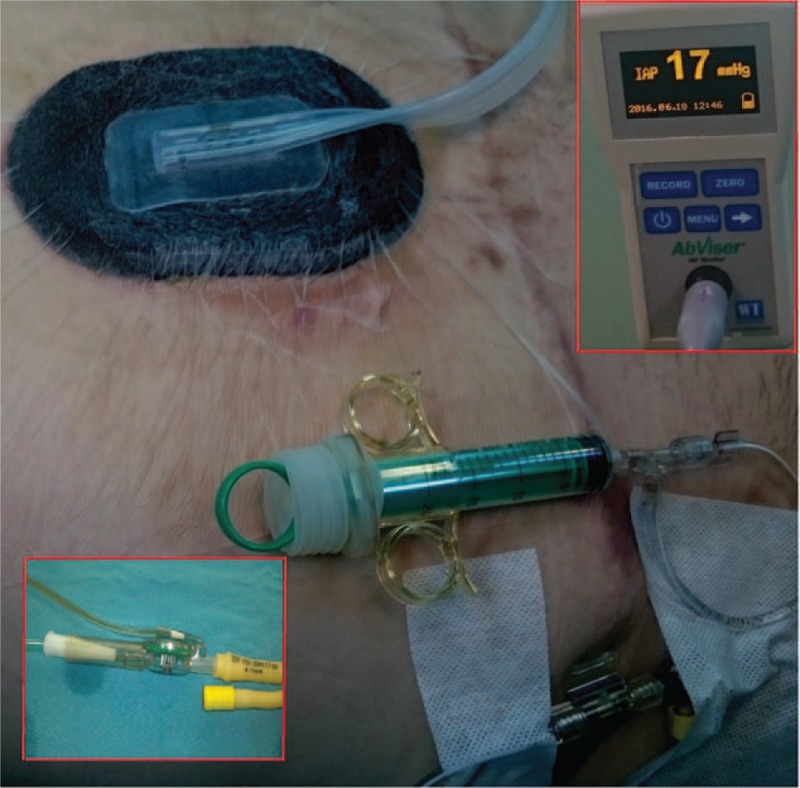
Intra-abdominal pressure measurement using the Abviser ABV 331 kit.
Three average IAP values were defined (at least 3 determinations) as follows: IAP1 = average of the pressures recorded during the ACS diagnosing, before initiation of the specific treatment; IAP2 = average of the pressures recorded after initiation of the ACS specific medical treatment; IAP3 = average of the pressures recorded after the DL.
If the IAP exceeded 20 mmHg and patients have developed a de novo organ failure or a new one added to the existing one, the diagnosis of ACS was clear and we proceeded to implement the medical therapy specific to this syndrome, according to the WSACS Guidelines of 2013.
3.2. Therapy protocol
Stage I comprise of nasogastric tube, adequate sedation of the patient, avoidance of the excess parenteral fluid intake, setting the fluid balance at zero or negative.
Stage II comprise of reducing enteral nutrition, evacuation enemas, parenteral administration of crystalloid or colloid hypertonic solutions to extract tissue edema, Trendelenburg position, administration of diuretics if the hemodynamic status allows it.
Stage III comprise of ceasing enteral feeding or discontinuing administration, colon decompression by inserting a rectosigmoid suction catheter with intermittent aspiration, curare administration to the patient, intubation and mechanical ventilation, evacuation puncture guided by ultrasound or computed tomography, hemodialysis.
Stage IV comprise of abdominal decompression by median laparotomy with specific management of open abdomen by the assisted vacuum wound therapy method using the Vivanotec abdominal kit system.
DL was performed in the following situations: the IAP increased despite the medical treatment (at least 3 measurements) and high levels of IAP remained (up to 20 mmHg) during the medical treatment for more than 24 hours.
We note that each stage of the ACS treatment was associated with the specific treatment of the primary disease.
3.3. Open abdomen management
DL was performed by a midline incision above and below the umbilicus, enough to release the loops and epiploon from the peritoneal cavity. From the beginning, the subsequent TAC management was dictated by the use of a negative pressure continuous suction system having dual roles: aspiration of collections and reducing intra-abdominal tissue edema, and avoiding musculo-aponeurotic retraction of the wound edges and the induction of granulation tissue. To this end, the Vivano system (Hartmann TM) was used from which the Vivano®Med Abdominal Kit was chosen. The entire system assembly consisted of the VivanoTec suction unit and consumables. The single use abdominal kit contains: mesh to protect the viscera with the diameter of 65 cm fitted with superior pockets to facilitate insertion, 2 polyurethane foams of 38 × 25 × 1.6 cm, 1 suction port, and hydrofilm strips for sealing.
Immediately after laparotomy, the intestines and epiploon were protected with mesh from the kit described above, by inserting it into the upper inter-hepato-phrenic spaces, laterally into the flaps toward the right and left parietocolic grooves, and lower into the posterior plane behind the urinary bladder. On top of the mesh, polyurethane foam was mounted in 2 or 3 layers, by adjusting their dimensions to the incision made. After installing the sealing strips over the entire wound without creating tension, the suction port connected to the collection tank was secured. The VivanoTech device was originally set to a gentle pressure of −105 mmHg. The dressings were changed every 2 to 3 days using the abdominal kit if the patient's condition was critical, maintaining ACS. After the third replacement of the abdominal kit, the pressure was lowered to −135 mmHg and continued in the same manner until the final closure. Subsequently, for the favorable developments, the reconstruction of the abdominal wall was performed by primary fascial closure or using a substitute dual mesh (polyester + dimethyl siloxane—Cousin BiotechTM), sutured on the musculoapneurotic edges with Prolene 3.0 continuous threads secured with 10 separate suture points.
3.4. Statistical analysis
All statistical calculations were performed using Graph Pad Software (San Diego, CA). Continuous variables were tested for normal distribution with the Kolmogorov–Smirnov test. We characterized variables as mean and standard deviation (SD) or as median and range for variables with normal and abnormal distribution, respectively. We chose adequate statistical tests according to data distribution. Differences between the mean ages for each sex were determined by the Student t test. Intra-abdominal pressure, mortality, and decompression laparotomy results were analyzed using analysis of variance (ANOVA) test (associated with the Bonferroni multiple comparison test). In a Box-and-Whisker plot, the central box represents values from the lower to upper quartile (25–75 percentile). The middle line represents the median. A line extends from the minimum to the maximum value. All the tests were interpreted relative to the significance threshold P = 0.05 and statistical significance was considered below the significance threshold value.
4. Results
Of the total of 134 patients included in the study with the risk of developing ASC, 76 of them developed IAH >20 mmHg, and 66 of them developed ACS (Fig. 2).
Figure 2.
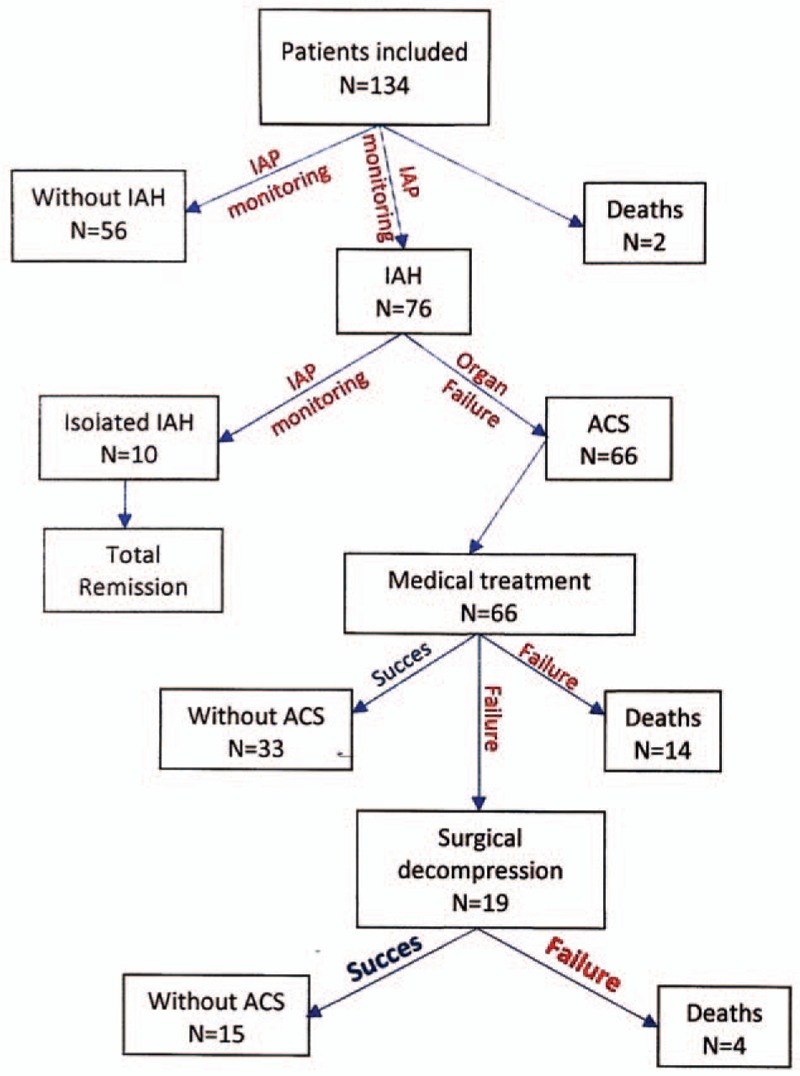
Study design.
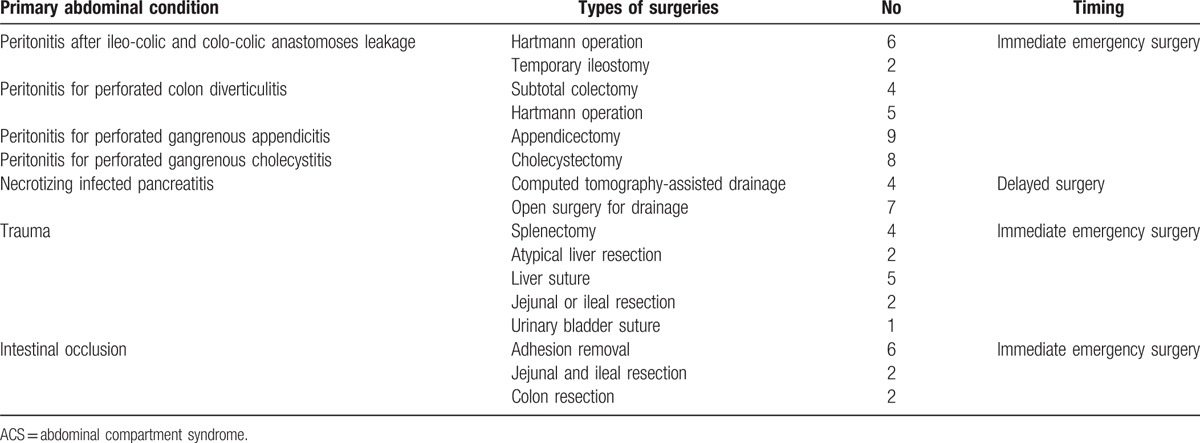
The primary abdominal diseases who leaded to ACS were: peritonitis, necrotizing infected pancreatitis, noninfected pancreatitis (without surgery requiring), intestinal occlusion, and trauma. Each primary abdominal disease, which led to ACS, excepting noninfected acute pancreatitis, followed initial specific surgical treatment. Acute peritonitis, trauma, and intestinal occlusion benefited from immediate emergency surgery (in the first 24 hours). Infected necrotizing pancreatitis represented the unfavorable evolution of acute pancreatitis despite aggressive medical treatment and underwent delayed surgical treatment (after 3–8 days from initial diagnosis) (Table 2). In all cases, ACS installed after 3.62 days and decompressive laparotomies were performed in the first 16.23 hours.
Table 2.
Primary abdominal diseases which led to ACS, and their surgrical approach.
The average age of the patients who developed ACS was 68.85 years (min–max = 43–82, St. deviation = 10.22), with a higher incidence in males (62.1%). Of the IAH-associated dysfunctions, kidney failure was present in 93.9%, liver failure in 80.3%, and cardiocirculatory failure in 66.7% of patients. IAP analysis of the early-stage ACS, after medical therapy and after surgical decompression, showed significant difference between the groups (ANOVA with Bonferroni multiple comparability test) (Table 3, Fig. 3).
Table 3.
Statistical comparison between the decrease in intra-abdominal pressure before and after the 2 stages of the treatment.

Figure 3.
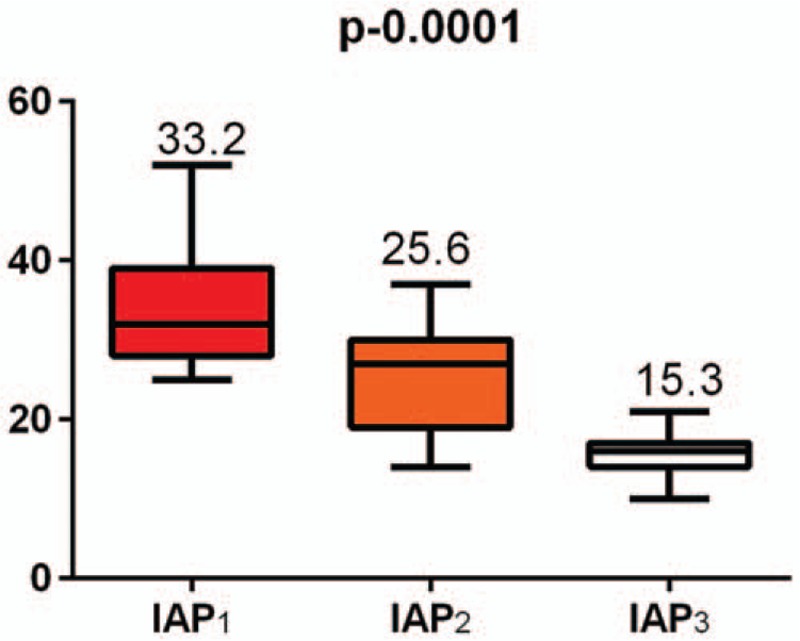
Intra-abdominal hypertensionvariation before and after the therapeutic stages.
The overall mortality in 66 patients with ACS was 27.3%. By statistically analyzing the conservative treatment and the surgical treatment, we found that DL was protective against mortality (odds ratio [OR]<1). However, it was not statistically significant (P > 0.05) (Table 4).
Table 4.
Statistical comparison between the 2 main stages of treatment, decompression laparotomy, and mortality rates.

The primary abdominal conditions were statistically compared regarding the mortality rate using the χ2 test. The acute peritonitis group consisted of 34 patients and mortality was 44.4% (P = 0.58, OR = 0.67, 95% confidence interval [CI]: 0.22–2.01). Necrotizing infected pancreatitis included 10 patients, mortality was 38.9% (P = 0.003, OR = 9.54, 95% CI: 2.10–42.9), and the bowel occlusion group comprised 9 patients with 16.7% mortality rate (P = 0.69, OR = 1.50, 95% CI: 0.31–6.31). In the groups of 4 cases of noninfected acute pancreatitis (P = 0.33, OR = 1.40, 95% CI: 0.20–1.62) and 9 cases of trauma (P = 0.04, OR = 0.46, 95% CI: 0.21–0.74), mortality was 0%. Therefore, in the study group, necrotizing acute pancreatitis was statistically significant correlated with a high mortality rate, and trauma was statistically significant correlated with low mortality rate.
The causes of death were septic shock (in the third and fifth day after surgery) in 1 case (generalized peritonitis after perforated colon diverticulitis), respiratory failure in 2 cases, following bronchopneumonia in 2 patients with peritonitis associating chronic obstructive pulmonary disease (COPD) (on the 7th and 15th postoperative day), and severe bleeding in 1 case after necrotizing infected pancreatitis (after 6 weeks).
4.1. Postoperative complications
The complications occurred during TAC were 2 wound suppurations in patients who had undergone surgeries for generalized peritonitis after colorectal anastomosis fistula, and 1 intestinal obstruction owing to adhesions in a patient with frozen abdomen. Wound suppurations evolved favorably by using vacuum wound-assisted therapy associated with the general treatment, whereas for occlusion, resurgery was performed after which adhesions dissolved. The final closure of the abdomen was performed at a mean of 11.7 days (min. = 9, max. 14). The closure type was primary suture of the musculoaponeurotic edges in 4 cases, and the use of dual mesh in the other 11 cases.
5. Discussion
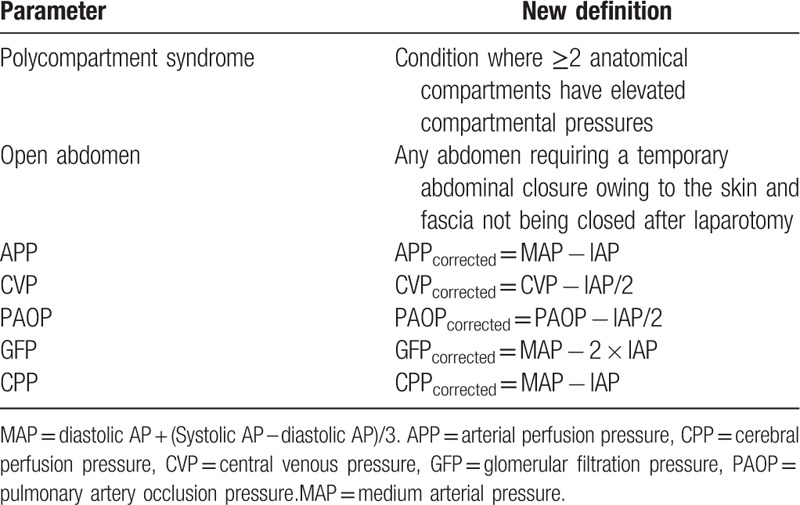
After founding WSACS, the grounds for the definitions and recommendations regarding the epidemiology, diagnostic algorithm, and treatment of ACS were laid down.[2] They remained largely valid; some new definitions were also added (Table 5).
Table 5.
Pressure parameters correction formula in case of intraabdominal pressure (MAP = diastolic AP + [systolic AP – diastolic AP]/3).[2,14–16]
Even the ACS was first described after abdominal traumatic injuries, WSACS identified 3 main risk factors for primary ACS developing: peritonitis, acute pancreatitis, and trauma.[14] In different studies were added also intestinal obstruction, abdominal aortic aneurism, abdominal tumors, ascitis, complication of pregnancy, and operated incisional hernias with high tension in abdominal wall.[4,6]
Pathophysiological mechanisms leading to increased abdominal pressure, regardless of the cause, include tissue edema, bowel and mesenteric edema, retroperitoneal space edema, and the accumulation of free abdominal fluid as a result of extracapillary extravasations. Intra-abdominally, the increased pressure leads to capillary compression exceeding the critical tissue perfusion threshold that translates as intestinal ischemia.[17] Cardiac output decreases with an increase in the peripheral and abdominal hypoperfusion.[18] In the lungs, it induces hypoxia and hypercapnia by decreasing the compliance of the thoracic wall and diaphragm, increasing pleural pressure and accumulation of intrapleural fluid.[19] Renally, the arterial hypoperfusion and venous compression lead to progressive alterations of the glomerular filtration rate.[18] Muftuoglu et al[20] reported that in case of ACS caused by primary abdominal sepsis, the liver is rapidly and severely affected, and is considered the most severe hepatic tissue injury. The ischemia and infection from the portal blood flow activate Kupffer cells, which release inflammatory mediators affecting the activity of hepatocytes and sinusoidal cells. Thus, hepatocellular retention of bile, biliary acids, and exogenous substances develops.[21–23] In our study, liver failure was present in 80.3% patients with no statistical correlations with the mortality rate. Betro and Kaplan have described 3 types of patients that are most likely to develop ACS. These types include those with massive transfusion during surgeries, medical patients that require large volumes of fluid resuscitation for severe sepsis, and surgical patients that require large volume resuscitation for an intra-abdominal underlying disease.[24] Peritonitis, necrotizing acute pancreatitis, abdominal traumas, and aortic abdominal aneurisms are the main causes of primary ACS.[17,25]
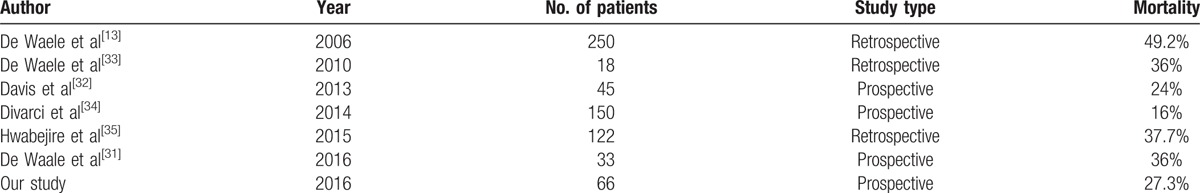
ACS therapy must be implemented immediately. The treatment algorithm is well established by the WSACS Guidelines of 2013.[14] No therapeutic approach can improve the progression of the syndrome by itself. A multiple approach must be applied with most important points being sedation, setting the fluid balance to 0 =or negatively, nasogastric and rectal probe, and neuromuscular blockade.[14] Surgical decompression reduces IAP, but it is not equally correlated with the mortality rate.[26] In our study, the IAH significantly decreased after each therapeutically step. However, the same trend was not observed in the survival rate. The overall mortality depends on the underlying condition. If occurs after an already severe pathologic background, the mortality rate increases, but it is reduced after trauma, which suddenly occurs on healthy people. When we compared the groups of patients, we noticed the highest mortality rate from the study group after necrotizing pancreatitis and the lowest after trauma (P < 0.05) After Vidal et al,[5] the respiratory and circulatory failure are the end-points of pathophysiologycal mechanism, being considered the main direct causes of death. The influence of surgical decompression on the survival rate during the complex treatment of ACS remains a topic with extremely heterogeneous data in the literature (Table 6). A very important aspect is represented by the time after which the DL was performed. There are studies that revealed a mortality rate close to 100% when DL was performed 48 hours following ACS diagnosis.[13,26] In our study, the medium time between diagnosis and DL was 16.23 hours (min 6 hours, max 24 hours). However, improved survival parameters after surgical decompression are owing to the positive effects on the underlying disease, especially in acute pancreatitis and septic abdomen.[27–30] In our study, DL was not significantly correlated with the mortality rate (P > 0.05) but was protective (OR <1), reducing mortality by 8.7%.
Table 6.
The abdominal compartment syndrome mortality rates quoted by different authors in the literature and our study.[13,31–35]
The use of a wound vacuum system is one of the new recommendations in the WSACS Guidelines of 2013.[14] The continuous aspiration of extravasated fluids decreases the IAP, removes the unwanted secretions, significantly reduces systemic effects of their toxicity, and significantly decreases the main open abdominal complications—lateral retraction of the wound edges.[36–38]
After the procedure was performed by Brock et al and Barker et al in 1995,[39,40] the development of vacuum therapy-dedicated kits started. TAC approach by vacuum-assisted wound therapy technique has also been the WSACS recommendation since the 2013 consensus.[14] In our case, the chosen solution was VivanoTech (HartmannTM). Initially appearing as individual methods, the use of meshes to prevent lateral retraction was subsequently combined with the vacuum therapy techniques: Polydioxanone (PDS) mesh + vacuum therapy,[41] polypropylene + vacuum therapy,[42] or ABRA system—the combination of transfascial elastomeric fibers tensed with buttons placed on the skin, associated with vacuum therapy.[43] All these latter techniques have significantly increased the percentage of late fascial closure up to 100% according to some authors.[44] Regarding the final closure of the abdominal wall, it should be done without tension. Depending on the TAC technique used, primary fascial closure varies. In our study, it could be carried out in 4 of the 19 cases. The closure without tension can be achieved by the use of meshes.[45] If visceral protection with large omentum can be performed, or the granulation tissue is well enough developed after vacuum therapy, polypropylene meshes can be used.[46] A safe alternative are the dual-meshes made from polypropylene, polyester, or expanded polytetrafluoroethylene (e-PTFE), which can be sutured to the aponeurotic edges and applied safely over the viscera.[47] The modern meshes of cross-link and non-cross-link types, manufactured in the laboratory, are very expensive for the time being. As a result, they should only be used in the reserved cases.[48] In our study, we had very good results with fewer complications using silicone polyester dual-meshes. Complications caused by VAC technique use include hemorrhage as a result of large vessels (portal vein, splenic vein) present in the open abdominal wound of necrotizing pancreatitis.[29,37] Xiao et al[49] reported kidney failure and numbers of laparotomies as main predictive factors for bleeding occurrence in necrotizing acute pancreatitis. In our study, one of the deaths was caused by hemorrhagic shock through the splenic vein fistula. The patient underwent 4 laparotomies in 6 weeks. The general postoperative complications are usually the consequence of primary disease, and wound complications derive from open abdomen management. If the general ones sometimes leaded to death, the local ones were successfully treated using the vacuum wound therapy management with no related mortality. Regarding the advantage of VAC technique use, Cirocchi et al,[50] analyzed all related studies, published until July 2015. In a systematic review and meta-analysis performed on 1225 patients from 8 articles of the most important research area, they compared the negative pressure technique with other 4 non-negative pressure techniques (Bogota bag, Mesh-foil laparostomy, Midline zip laparostomy and others). After statistical analysis was made, they observed that there are no significant differences in between VAC technique and all others, regarding the fascial closure, postoperative 30-day overall morbidity, postoperative enteroatmospheric fistulae rate, in the postoperative bleeding rate, and postoperative abdominal abscess rate. Instead, statistical significance was found in the postoperative mortality rate (28.5% vs. 41.4%) and in the length of stay in the intensive care unit.
6. Conclusions
Despite the new therapeutic protocols, ACS still has high mortality. Primary ACS most often occurs after events contaminating the peritoneal cavity. The highest overall mortality rate was after necrotizing pancreatitis. The specific medical therapy significantly reduced the mortality rate compared with no treatment of the syndrome. Decompression laparotomy was protective against mortality reducing it by 8.7%, and should be used as soon as possible in case of medical resuscitation failure. The prolonged duration (over 24 hours) between the occurrence of ACS and surgical decompression negatively influenced the prognosis. Recovery following decompression may depend on the severity of the primary disease. General postoperative complications are because of underlying disease and in some cases leaded to death, but local complications were easily reduced using the vacuum wound therapy system with no mortality correlated.
Footnotes
Abbreviations: ACS = abdominal compartment syndrome, CI = confidence interval, DL = decompressive laparotomy, IAH = intra-abdominal hypertension, IAP = intra-abdominal pressure, OA = open abdomen, OR = odds ratio, St. deviation = standard deviation, TAC = temporally abdominal closure, WSACS = World Society of Abdominal Compartment Syndrome.
This project is financed through internal research grants provided by the University of Medicine and Pharmacy of Targu Mures, Romania (No. 17800/10/22.12.2015).
The authors report no conflicts of interest.
References
- [1].Midbrain MLNG, De Laet I, Cheatham M. Consensus conference definitions and recommendations on intra-abdominal hypertension (IAH) and the abdominal compartment syndrome (ACS)-the long road to the final publications, how did we get there? Acta Clin Belg 2007;62(suppl 1):44–59. [DOI] [PubMed] [Google Scholar]
- [2].Midbrain MLNG, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-Abdominal hypertension and Abdominal Compartment Syndrome. Part I. Definitions. Intensive Care Med 2006;32:1722–32. [DOI] [PubMed] [Google Scholar]
- [3].Burch JM1, Moore EE, Moore FA, et al. The abdominal compartment syndrome. Surg Clin North Am 1996;76:833–42. [DOI] [PubMed] [Google Scholar]
- [4].Muckart DJJ, Ivatury RR, Leppaniemi A. Ivatury RR, Cheatham ML, Malbrain M, Sugrue M, et al. Definitions. Abdominal Compartment Syndrome. Georgetown, TX: Landis Bioscience; 2006;4: 8–18. [Google Scholar]
- [5].Vidal MG, Weisser JR, Gonzalez F, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med 2008;36:1823–31. [DOI] [PubMed] [Google Scholar]
- [6].Muresan M, Muresan S, Bara T, et al. The intraabdominal pressure: a real indicator of the tension free principle during anterior wall repair procedure after incisional hernias. Ann Ital Chir 2015;86:421–6. [PubMed] [Google Scholar]
- [7].Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med 2004;30:357–71. [DOI] [PubMed] [Google Scholar]
- [8].Malbrain M, Jones F. Ivatury R, Cheatham M, Malbrain M, et al. Intra-abdominal pressure measurement techniques. Landes Bioscience, Abdominal Compartment Syndrome. Texas:2006. [Google Scholar]
- [9].Chopra SS, Wolf S, Rohde V, et al. Pressure measurement techniques for abdominal hypertension: conclusions from an experimental model. Crit Care Res Pract 2015;278139; doi: 10.1155/2015/278139. Epub 2015 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Malbrain MLNG. You don’t have any excuse, just start measuring abdominal pressure and act upon it!. Minerva Anestesiol 2008;74:1–2. [PubMed] [Google Scholar]
- [11].De Laet I, Hoste E, De Waele JJ. Transvesical intra-abdominal pressure measurement using minimal instillation volumes: how low can we go? Intensive Care Med 2008;34:746–50. [DOI] [PubMed] [Google Scholar]
- [12].Desie N, Willems A, De Laet I, et al. Intra-abdominal pressure measurement using the Foley manometer does not increase the risk for urinary tract infection in critically ill patients. Ann Intensive Care 2012;2(suppl 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Waele JJ, Hoste EA, Malbrain ML. Decompressive laparotomy for abdominal compartment syndrome-a critical analysis. Critical Care 2006;10:R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kirkpatrick AW, Roberts DJ, De Wale J. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Joseph DK, Dutton RP, Aarabi B, et al. Decompressive laparotomy to treat intractable intracranial hypertension after traumatic brain injury. J Trauma 2004;57:687–93. [DOI] [PubMed] [Google Scholar]
- [16].Kirkpatrick AW, Colistro R, Laupland KB, et al. Renal arterial resistive index response to intraabdominal hypertension in a porcine model. Crit Care Med 2007;35:207–13. [DOI] [PubMed] [Google Scholar]
- [17].Luckianow GM, Ellis M, Governale D, et al. Abdominal compartment syndrome: risk factors, diagnosis, and current therapy. Crit Care Res Pract 2012;908169. doi:10.1155/2012/908169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mullens W, Abrahams Z, Francis GS, et al. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Card Fail 2008;14:508–14. [DOI] [PubMed] [Google Scholar]
- [19].Malbrain ML. Respiratory effects of increased intra-abdominal pressure. Réanimation 2007;16:49–60. [Google Scholar]
- [20].Muftuoglu MA, Aktekin A, Ozdemir NC, et al. Liver injury in sepsis and abdominal compartment syndrome in rats. Surg Today 2006;36:519–24. [DOI] [PubMed] [Google Scholar]
- [21].Nesseler N, Launey Y, Aninat C, et al. Clinical review: the liver in sepsis. Crit Care 2012;16:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Z, Han M, Chen T, et al. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int 2010;30:782–94. [DOI] [PubMed] [Google Scholar]
- [23].Guo K, Ren J, Wang G, et al. Early liver dysfunction in patients with intra-abdominal infections. Medicine 2015;94:e1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Betro G, Kaplan LJ. Abdominal compartment syndrome. In: Kellum JA, Ronco C, Bellomo R. Critical Care Nephrology. 2nd ed. Elsevier; 2008; 36: 212–215. [Google Scholar]
- [25].Mehta M, Mehta M, Darling RC, et al. Factors associated with abdominal compartment syndrome complicating endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2005;42:1047–51. [DOI] [PubMed] [Google Scholar]
- [26].Rollins MD, Deamorim-Filho J, Scaife ER, et al. Decompressive laparotomy for abdominal compartment syndrome in children on ECMO: effect on support and survival. J Pediatr Surg 2013;48:1509–13. [DOI] [PubMed] [Google Scholar]
- [27].Ejike JC, Mathur M. Abdominal decompression in children. Crit Care Res Pract 2012;180797; doi: 10.1155/2012/180797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sun Z, Huang H, Zhou H. Indwelling catheter and conservative measures in the treatment of abdominal compartment syndrome in fulminant acute pancreatitis. World J Gastroenterol 2006;12:5068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen H, Li F, Sun JB, et al. Abdominal compartment syndrome in patients with severe acute pancreatitis in early stage. World J Gastroent 2008;14:3541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Waele JJ. Abdominal compartment syndrome in severe acute pancreatitis-when to decompress? Eur J Trauma Emerg Surg 2008;34:11–6. [DOI] [PubMed] [Google Scholar]
- [31].De Waele JJ, Kimball E, Malbrain M, et al. Decompressive laparotomy for abdominal compartment syndrome. Br J Surg 2016;103:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davis PJB, Eltawil KM, Abu-Wasel B, et al. Effect of obesity and decompressive laparotomy on mortality in acute pancreatitis requiring intensive care unit admission. World J Surg 2013;37:318–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Waele JJ, Desender L, De Laet I, et al. Abdominal decompression for abdominal compartment syndrome in critically ill patients: a retrospective study. Acta Clin Belg 2010;65:399–403. [DOI] [PubMed] [Google Scholar]
- [34].Divarci E, Karapinar B, Yalaz M, et al. Incidence and prognosis of intraabdominal hypertension and abdominal compartment syndrome in children. J Ped Surg 2014;51:50–7. [DOI] [PubMed] [Google Scholar]
- [35].Hwabejire JO, Nembhard CE, Oyetunji AT, et al. Abdominal compartment syndrome in traumatic hemorrhagic shock: is there a fluid resuscitation inflection point associated with increased risk? Am J Surg 2015;211:733–8. [DOI] [PubMed] [Google Scholar]
- [36].Bjorck M, Bruhin A, Cheatham M, et al. Classification-important step to improve management of patients with an open abdomen. World J Surg 2009;33:1154–7. [DOI] [PubMed] [Google Scholar]
- [37].Navsaria P, Nicol A, Hudson D, et al. Negative pressure wound therapy management of the “open abdomen” following trauma: a prospective study and systematic review. World J Emerg Surg 2013;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ventsislav M, Mutafchiyski GI, Popivanov K, et al. Open abdomen and VAC® in severe diffuse peritonitis. R Army Med Corps 2016;162:30–4. [DOI] [PubMed] [Google Scholar]
- [39].Brock WB, Barker DE, Burns RP. Temporary closure of open abdominal wounds: the vacuum pack. Am Surg 1995;61:30–5. [PubMed] [Google Scholar]
- [40].Barker DE, Green JM, Maxwell RA, et al. Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am CollSurg 2007;204:784–92. [DOI] [PubMed] [Google Scholar]
- [41].Burlew CC, Moore EE, Biffl WL, et al. One hundred percent fascial approximation can be achieved in the postinjury open abdomen with a sequential closure protocol. J Trauma Acute Care Surg 2012;72:235–41. [DOI] [PubMed] [Google Scholar]
- [42].Petersson U, Acosta S, Björck M. Vacuum-assisted wound closure and mesh-mediated fascial traction–a novel technique for late closure of the open abdomen. World J Surg 2007;31:2133–7. [DOI] [PubMed] [Google Scholar]
- [43].Haddock C, Konkin DE, Blair NP. Management of the open abdomen with the abdominal reapproximation anchor dynamic fascial closure system. Am J Surg 2013;205:528–33. [DOI] [PubMed] [Google Scholar]
- [44].Cothren CC, Moore EE, Johnson JL, et al. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg 2006;192:238–42. [DOI] [PubMed] [Google Scholar]
- [45].Bjarnason T, Montgomery A, Ekberg O, et al. One-year follow-up after open abdomen therapy with vacuum-assisted wound closure and mesh-mediated fascial traction. World J Surg 2013;37:2031–8. [DOI] [PubMed] [Google Scholar]
- [46].Fansler RF, Taheri P, Cullinane C, et al. Polypropylene mesh closure of the complicated abdominal wound. Am J Surg 1995;170:15–8. [DOI] [PubMed] [Google Scholar]
- [47].Chuo CB, Thomas SS. Absorbable mesh and topical negative pressure therapy for closure of abdominal dehiscence with exposed bowel. J Plastic Rec Aesth Surg 2008;61:1378–81. [DOI] [PubMed] [Google Scholar]
- [48].Slater NJ, van der Kolk M, Hendriks T, et al. Biologic grafts for ventral hernia repair: a systematic review. Am J Surg 2013;205:220–30. [DOI] [PubMed] [Google Scholar]
- [49].Xiao S, Jing S, Jingzhu Z, et al. Risk factors and outcome for massive intra-abdominal bleeding among patients with infected necrotizing pancreatitis. Medicine 2015;94:e1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cirocchi R, Birindelli A, Biffl WL, et al. What is the effectiveness of the negative pressure wound therapy (NPWT) in patients treated with open abdomen technique? A systematic review and meta-analysis. J Trauma Acute Care Surg 2016;81:575–84. [DOI] [PubMed] [Google Scholar]


