Abstract
Rationale:
There were a few case reports concerning epidermoid tumor coexisted with multiple cerebral aneurysms. Here, we present one case of coexistence of intracranial epidermoid tumor and multiple cerebral aneurysms and performed a literature review.
Patient concerns:
A 42 years old male patient was admitted to our institution with complaints of headache and dizziness.
Interventions:
The radiological examinations showed a hypointense lesion in the right parasellar and petrous apex region and an ipsilateral saccular aneurysm originated from the M2–M3 junction of the right middle cerebral artery (MCA) and a saccular aneurysm of the clinoid segment of right internal carotid artery (ICA).
Interventions:
The patients underwent a right frontotemporal approach for removal of the epidermoid tumor and clipping of the MCA aneurysm in one stage. The aneurysm located at the clinoid segment of ICA was invisible and untreated during operation.
Outcomes:
No postoperative complications were found in the patient. The patient's follow up after 5 years of surgical treatment was uneventful, and the untreated aneurysm remains stable.
Lessons:
The coexistence of intracranial epidermoid tumor and cerebral aneurysm is a rare event. The secondly inflammation in cerebral arterial wall may be responsible for the aneurysm formation. Surgical treatment of the intracranial epidermoid tumor and cerebral aneurysm repair may be an optimal scheme in one stage.
Keywords: clinical manifestations, intracranial epidermoid, multiple cerebral aneurysms, surgical treatment
1. Introduction
Cerebral aneurysms are seldom associated with primary intracranial tumor, which were reported in a wide range of tumor types, including meningioma,[1] glioma,[2,3] pituitary adenoma,[4] arachnoid cyst,[5] craniopharyngioma,[6] lymphoma,[7,8] and dermoid tumor.[9] It is estimated that the frequency of the association of primary brain tumor with intracranial aneurysm is approximately 1%.[10–13] The most common tumor coexisted with cerebral aneurysm was meningioma.[1] And there were a few case reports concerning epidermoid tumor, which is relatively a rare and congenital cystic lesion, coexisted with multiple cerebral aneurysms.[14–16]
We present 1 case of coexistence of epidermoid tumor and multiple cerebral aneurysms admitted to our institution, and a right frontotemporal approach and was chosen as surgical approach for removal of the epidermoid tumor and the middle cerebral artery (MCA) aneurysm repair in one stage. The aneurysm located at the clinoid segment of internal carotid artery (ICA), however, was invisible and untreated during operation, and its morphology in 5-year follow up remained stable.
2. Case report
The study protocol was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University. A 42 years old male patient was admitted to our institution with complaints of headache and dizziness for 1 month. Physical and neurological examinations showed no anomalies, there was no history of smoking, hypertension, diabetes, endocrine dysfunction, seizure, or physical disability at the time of admission. There was no abnormal in blood test and no endocrine dysfunction in laboratory investigations. Brain computed tomography (CT) and magnetic resonance imaging (MRI) examinations showed a hypointense lesion in the right parasellar and petrous apex region (Fig. 1A–F), and the lesion was extended to lateral wall of the sphenoid sinus. Three-dimensional digital subtraction angiography (3D-DSA) examination was performed to clarify the relationship between the tumor and ICA, and the images demonstrated an ipsilateral saccular aneurysm originated from the M2–M3 junction of MCA and a saccular aneurysm of the clinoid segment of right ICA (Fig. 1G–I).
Figure 1.
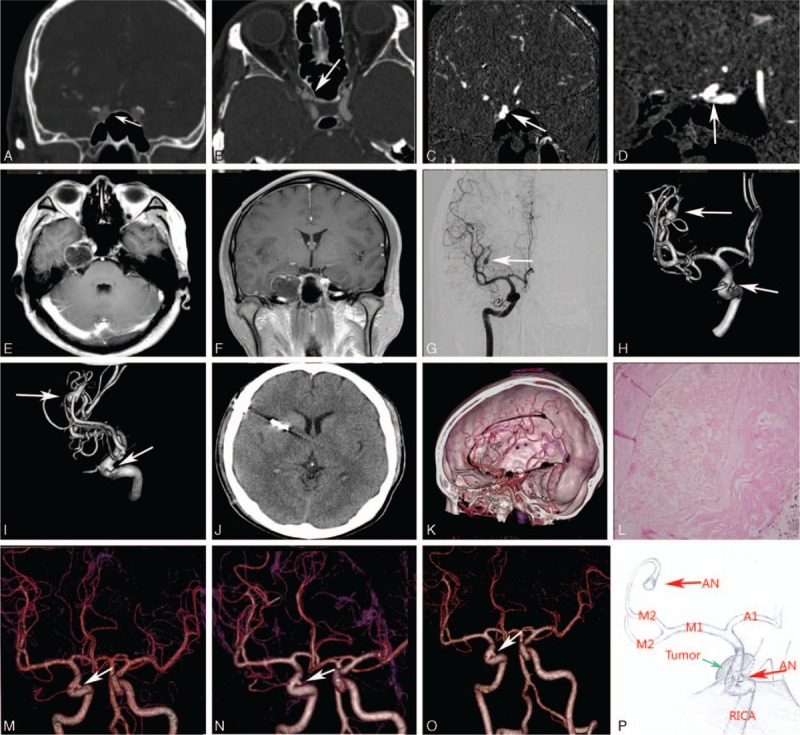
Brain CT and MRI examinations showed a hypointense lesion in the right parasellar and petrous apex region (A–F). DSA and 3D-DSA images demonstrated an right saccular aneurysm (arrow) originated from the M2–M3 junction of the right MCA and a saccular aneurysm of the clinoid segment of right internal carotid artery (ICA) (G–I). Postoperative CT and 3D-CTA images (J). The diagnosis of epidermoid tumor was confirmed by pathologic examination (L). 3D-CTA images showed the morphology of the untreated aneurysm of the clinoid segment of right ICA remained stable 3 times in 5-years follow-up (M–O). Illustration demonstrating the relative position between the epidermoid tumor and the untreated aneurysm (P).
Two days after admission, the patients underwent a right frontotemporal approach for removal of the epidermoid tumor and clipping of the cerebral aneurysm in one stage. The patient underwent thorough surgical planning that the aneurysm of M2–M3 junction of MCA should be firstly clipped to prevent intraoperative premature rupture of aneurysms before the epidermoid tumor was dissected. After sylvian fissure dissection and exposure of the aneurysm and its parent artery in the M2–M3 junction of MCA, strong adhesion of the unruptured aneurysm's dome to neighboring arachnoid membrane was revealed. Ultimately, the aneurysm was clipped successfully. After dissection of the arachnoid membrane of the right carotid cistern, a thin capsule of tumor which located in the right parasellar and petrous apex region was seen, and the capsule was strongly adherent to the surface of the right ICA. After the tumor capsule was incised, it was found that there was fluffy, white, waxy material in the tumor, which was avascular and lipid like in structure. Finally the tumor was removed with gentle dissection from the right parasellar and petrous apex region successfully. The aneurysm located at the clinoid segment of ICA, however, was invisible during operation, and it remained untreated.
No intracranial hemorrhage was detected on postoperative brain CT scan. No postoperative complications were found in the patient. The diagnosis of epidermoid tumor was confirmed by pathologic examination, and microscope shows a few lymphocytes infiltration into the capsule (Fig. 1L). The patients were discharged on the seventh day postoperatively. Brain MRI examination showed no recurrent tumor 3 months and 1 year after surgery. The patient's follow up after 5 years of surgical treatment was uneventful, and the morphology of the untreated aneurysm located at the clinoid segment of ICA remained stable (Fig. 1M–O).
3. Discussion
Coexistence of primary intracranial tumor and cerebral aneurysm is a rare finding, which was first reported by Pia et al.[12] Along with the development of neuroimaging technology and microsurgical techniques for central nervous system, which is widely used and facilitate the detection of cerebral aneurysms, the incidence of cerebral aneurysm associated with intracranial tumor is approximately 1%.[10–13]
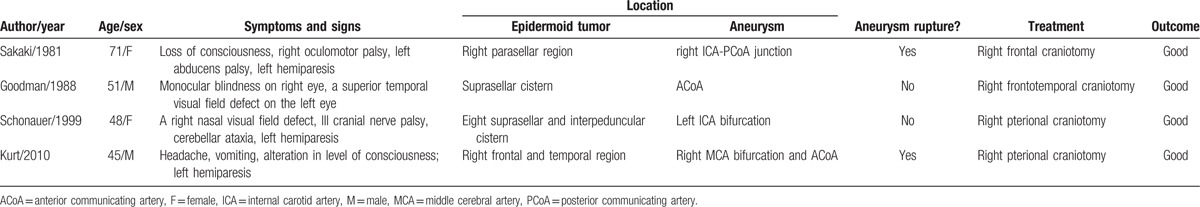
Intracranial epidermoid tumor, also known as cholesteatomas and pearly tumor, is congenital benign lesion and account for approximately 0.1% of all intracranial space occupying lesions. The coexistence of intracranial epidermoid tumor and unruptured/ruptured cerebral aneurysm is a rare event, only 4 cases aged 45 to 71 years have been reported in English literature since 1981 (Table 1).[14–17] the clinical manifestations can be divided into the following 4 categories according to the literature: asymptomatic; intracranial mass effect (e.g., headache, visual field defect, hemidysesthesia, and/or hypopituitarism); subarachnoid hemorrhage (SAH) (e.g., headache, nausea, vomiting, and/or loss of consciousness); intracranial mass effect and SAH. Two patients in the reported literature were admitted to the hospital because of SAH, and all 4 patients suffering from intracranial space-occupying lesions were presented. Three epidermoid tumors were located in the parasellar or suprasellar cistern, and 1 located in the right frontal and temporal region. There were no endocrine dysfunction in any of the four patients, and the aneurysms associated with epidermoid tumor were located in the anterior circulation. It is worth noting that clinical doctor will pay more attention to the epidermoid cyst when the unruptured aneurysm is asymptomatic, and the coexistence of cerebral aneurysm and intracranial tumor will be ignored. The missed diagnosis, therefore, is most likely to occur in the absence of cerebrovascular examination because of the low incidence of coexistence of the 2 lesions, and people will recognize that it is an accidental phenomenon, cerebrovascular examination is “excessive” and will increase medical expenses.
Table 1.
Summary of the literature data of patients with epidermoid tumor and cerebral aneurysm.
So far the question whether the formation of cerebral aneurysm is associated with intracranial tumor has not been satisfactorily answered. Several hypotheses have been proposed to explain the possible pathogenesis: Abundant vascular supplies of intracranial tumor results in an increase of directional blood flow, then the hemodynamic stress on a small segment of cerebral arterial wall have a role in the initiation process of aneurysm formation.[18–21] Growth hormone (GH) or insulin-like growth factor 1 (IGF-1) affects the cerebral vascular wall and plays a role in the formation of aneurysm in patients with pituitary adenomas.[22,23] The direct invasion and erosion of the vascular wall by tumor cells led to the formation of aneurysm.[3,24,25] However, epidermoid tumor is avascular, and there does not exist hemodynamic stress and hormonal effects contributing to the intracranial aneurysm formation. And the epidermoid tumor cell seldom directly invaded adjacent vessels. Thus, the above hypotheses cannot help explain mechanism of the aneurysm formation in epidermoid tumor.
Pathology showed that epidermoid tumor has a focal fibrous scars,[26] which may be strong adhesion to the surrounding neurovascular structures.[27] And the strong adhesion will lead to incomplete removal of the epidermoid tumor.[28] The reason for the above is that there exists an inflammation process in the epidermoid capsule, which has been confirmed by Bennett's findings.[29] All of those patients in the reported literature was found that the adjacent blood vessels were surrounded by the epidermoid tumor, and the aneurysms were close or clung to the capsule of the epidermoid tumor which firmly adhered to the vessel during operation (Fig. 2).[14–17] We, therefore, speculated that the close anatomical correlation between inflammatory capsule and cerebral artery were contributed to the development of the cerebral aneurysm in this case, the coexistence of intracranial epidermoid tumor and multiple cerebral aneurysm was not as an “accidental” phenomenon.[9] Furthermore, A direct evidence for inflammation was that a few lymphocytes infiltration into the capsule found by pathologic examination. However, the formation of the MCA aneurysm in this case was far away from the capsule of the tumor cannot be fully explained from the above viewpoint. An indirect evidence of aseptic inflammation in this case was the strong adhesion of the unruptured aneurysm's dome to neighboring arachnoid membrane was revealed. They, thus, also indirectly supports the hypothesis that the inflammatory response could lead to the formation of aneurysm,[30] which need to be further confirmed by direct evidence from pathology and immunology.
Figure 2.
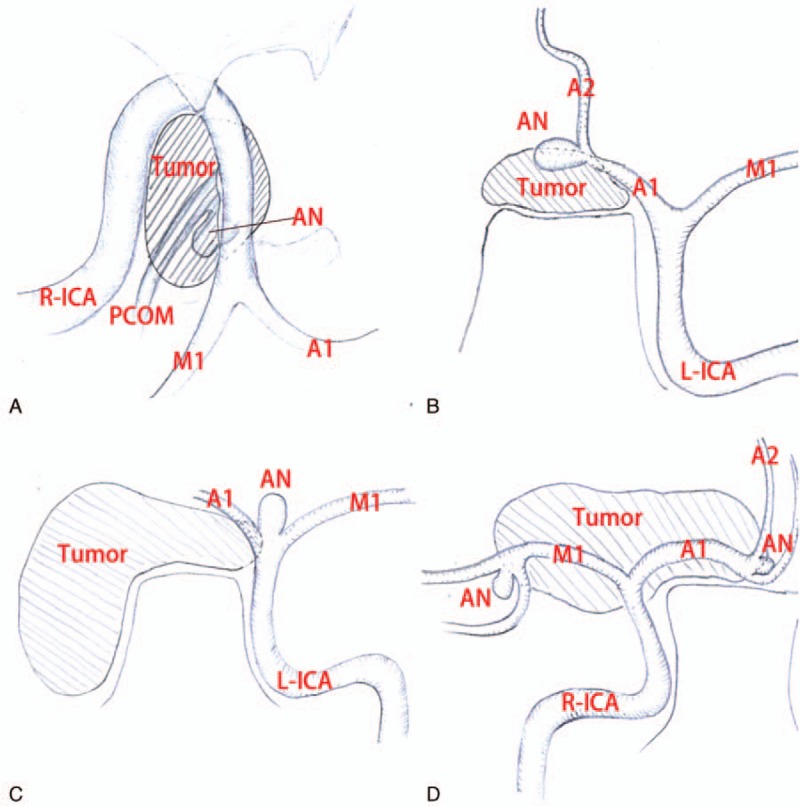
(A) Illustration demonstrating the relative position between the epidermoid tumor and the aneurysm in CT image from the paper “Sakaki S, Matsuo Y, Kuwabara H, et al. Rupture of an aneurysm into a parasellar epidermoid cyst: case report. J Neurosurg. 1981;55:629–632.” (B) Illustration demonstrating the relative position between the epidermoid tumor and the aneurysm in CT image from the paper “Goodman ML, Nelson PB. Association of an epidermoid tumor with an aneurysm of the anterior communicating artery. Neurosurgery. 1988;23:392–395.” (C) Illustration demonstrating the relative position between the epidermoid tumor and the aneurysm in MRI image from the paper “Schonauer C, Parlato C, Moraci A, et al. Association of an epidermoid tumour with a contralateral aneurysm of intracranial carotid bifurcation. Acta Neurochir (Wien). 1999;141:325–326.” (D) Illustration demonstrating the relative position between the epidermoid tumor and the aneurysm in MRI image from the paper “Kurt G, Cemil B, Çelik B., et al. Association of an epidermoid tumour with ipsilateral aneurysms of middle cerebral artery bifurcation and anterior communicating artery. J Neurol Sci. 2010;27:482–486.”
The intracranial epidermoid cysts in the 4 cases of the literature and our case occur above the tentorium, whose common locations are the parasellar, suprasellar, or sellar region. And we, therefore, recommend that cerebrovascular examinations should be carried out to rule out the possibility of the coexistence of cerebral aneurysm when epidermoid cysts occur above the tentorium. Surgical management in one stage for epidermoid cysts and aneurysm clipping may be the preferred treatment when epidermoid cysts located on ipsilateral side to the coexisting aneurysm. If epidermoid cysts locate on contralateral side, there is still the possibility that they be treated in one stage.[31,32] or the coexisting aneurysm should be treated before removal of epidermoid cysts.
4. Conclusions
The coexistence of intracranial epidermoid tumor and cerebral aneurysm is a rare event. Clinical manifestations of their coexistence were cause by the space occupying of tumor or/and the rupture of the aneurysm.[14–16] The secondly inflammation in cerebral arterial wall may be responsible for the cerebral aneurysm formation. Surgical treatment of the intracranial epidermoid tumor and cerebral aneurysm repair may be an optimal scheme in one stage.
Footnotes
Abbreviations: 3D-DSA = three-dimensional digital subtraction angiography, CT = computed tomography, GH = growth hormone, ICA = internal carotid artery, IGF-1 = insulin-like growth factor 1, MCA = middle cerebral artery, MRI = magnetic resonance imaging, SAH = subarachnoid hemorrhage.
Funding: This study was sponsored by the key clinical specialty discipline construction program of Fujian, P.R. China, major project of Fujian Provincial Department of Science and Technology (No. 2014YZ0003 and No. 2014YZ01 to D-ZK).
P-SY and Z-YL contributed equally to this article.
The authors have no conflicts of interest to disclose.
References
- [1].Kotapka MJ, Kalia KK, Martinez AJ, et al. Infiltration of the carotid artery by cavernous sinus meningioma. J Neurosurg 1994;81:252–5. [DOI] [PubMed] [Google Scholar]
- [2].Zuchner S, Kawohl W, Sellhaus B, et al. A case of gliosarcoma appearing as ischaemic stroke. J Neurol Neurosurg Psychiatry 2003;74:364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Paulis D, Nicosia G, Taddei G, et al. Intracranial aneurysms and optic glioma–an unusual combination: a case report. J Med Case Rep 2016;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Q, Lv H, Chen G, et al. Endoscopic endonasal removal of pituitary adenomas with paraclival internal carotid artery invasion. ORL J Otorhinolaryngol Relat Spec 2010;72:28–37. [DOI] [PubMed] [Google Scholar]
- [5].Chhabra VS, Zhang J, Olson JJ. Association between an arachnoid cyst and intracranial aneurysms misdiagnosed as a cystic tumor with a mural nodule. Case report and review of the literature. Neurosurg Focus 2007;22:E3. [DOI] [PubMed] [Google Scholar]
- [6].Takeuchi S, Wada K, Sakakibara F, et al. Anterior cerebral artery dissecting aneurysm associated with untreated craniopharyngioma. Br J Neurosurg 2013;27:102–4. [DOI] [PubMed] [Google Scholar]
- [7].Roitberg BZ, Cochran EJ, Thornton J, et al. Giant anterior communicating artery aneurysm infiltrated with a primary cerebral lymphoma: case report. Neurosurgery 2000;47:458–62. [DOI] [PubMed] [Google Scholar]
- [8].Suslu HT, Bozbuga M. Primary brain tumors associated with cerebral aneurysm: report of three cases. Turk Neurosurg 2011;21:216–21. [DOI] [PubMed] [Google Scholar]
- [9].Ahmad I, Tominaga T, Ogawa A, et al. Ruptured suprasellar dermoid associated with middle cerebral artery aneurysm: case report. Surg Neurol 1992;38:341–6. [DOI] [PubMed] [Google Scholar]
- [10].Brzezinski J, Kotwica Z, Polis Z. Brain tumors associated with intracranial vascular anomalies. Zentralbl Neurochir 1989;50:201–2. [PubMed] [Google Scholar]
- [11].Handa J, Matsuda I, Handa H. Association of brain tumor and intracranial aneurysms. Surg Neurol 1976;6:25–9. [PubMed] [Google Scholar]
- [12].Pia HW, Obrador S, Martin JG. Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien) 1972;27:189–204. [DOI] [PubMed] [Google Scholar]
- [13].Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821–8. [DOI] [PubMed] [Google Scholar]
- [14].Sakaki S, Matsuo Y, Kuwabara H, et al. Rupture of an aneurysm into a parasellar epidermoid cyst: case report. J Neurosurg 1981;55:629–32. [DOI] [PubMed] [Google Scholar]
- [15].Goodman ML, Nelson PB. Association of an epidermoid tumor with an aneurysm of the anterior communicating artery. Neurosurgery 1988;23:392–5. [DOI] [PubMed] [Google Scholar]
- [16].Schonauer C, Parlato C, Moraci A, et al. Association of an epidermoid tumour with a contralateral aneurysm of intracranial carotid bifurcation. Acta Neurochir (Wien) 1999;141:325–6. [DOI] [PubMed] [Google Scholar]
- [17].Kurt G, Cemil B, Çelik B, et al. Association of an epidermoid tumour with ipsilateral aneurysms of middle cerebral artery bifurcation and anterior communicating artery. J Neurol Sci 2010;27:482–6. [Google Scholar]
- [18].Kulcsar Z, Ugron A, Marosfoi M, et al. Hemodynamics of cerebral aneurysm initiation: the role of wall shear stress and spatial wall shear stress gradient. AJNR Am J Neuroradiol 2011;32:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim YH, Lee YJ, Han JH, et al. Association of intracranial aneurysms and meningiomas: a case-control study. J Neurosurg 2015;123:357–61. [DOI] [PubMed] [Google Scholar]
- [20].Ali R, Pabaney A, Robin A, et al. Glioblastoma and intracranial aneurysms and intracranial aneurysms: case report and review of literature. Surg Neurol Int 2015;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hashiguchi A, Morioka M, Ichimura H, et al. Glioblastoma with an intratumoral feeding-artery aneurysm. Clin Neurol Neurosurg 2007;109:302–4. [DOI] [PubMed] [Google Scholar]
- [22].Oshino S, Nishino A, Suzuki T, et al. Prevalence of cerebral aneurysm in patients with acromegaly. Pituitary 2013;16:195–201. [DOI] [PubMed] [Google Scholar]
- [23].Kulseng B, Myhre HO. Is insulin growth factor-1 (IGF-1) playing a role for aneurysm formation in patients with pituitary gland tumor? Int Angiol 2006;25:433–5. [PubMed] [Google Scholar]
- [24].Helmer FA. Oncotic aneurysm. Case report. J Neurosurg 1976;45:98–100. [DOI] [PubMed] [Google Scholar]
- [25].Zairi F, De Saint Denis T, Thines L, et al. Ruptured cerebral oncotic aneurysm from choriocarcinoma: report of two cases and review of the literature. Acta Neurochir (Wien) 2011;153:353–7. [DOI] [PubMed] [Google Scholar]
- [26].Ohara T, Maki S, Furuya T, et al. Elderly onset intramedullary epidermoid cyst in the conus medullaris: a case report. J Med Case Rep 2015;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim KH, Cho JH. Ruptured intracranial dermoid cyst associated with rupture of cerebral aneurysm. J Korean Neurosurg Soc 2011;50:453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lynch JC, Aversa A, Pereira C, et al. Surgical strategy for intracranial dermoid and epidermoid tumors: an experience with 33 patients. Surg Neurol Int 2014;5:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bennett WA. Intracranial epidermoids (cholesteatomas). Am J Orthod Oral Surg 1946;32:588–94. [DOI] [PubMed] [Google Scholar]
- [30].Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 2012;32:1659–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Andrade-Barazarte H, Kivelev J, Goehre F, et al. Contralateral approach to bilateral middle cerebral artery aneurysms: comparative study, angiographic analysis, and surgical results. Neurosurgery 2015;77:916–26. discussion 926. [DOI] [PubMed] [Google Scholar]
- [32].Caplan JM, Sankey E, Gullotti D, et al. Contralateral approach for clipping of bilateral anterior circulation aneurysms. Neurosurg Focus 2015;39(Video suppl 1):V9. [DOI] [PubMed] [Google Scholar]


