Abstract
Myofascial pain syndrome (MPS) of the trapezius muscle (TM) is a frequently occurring musculoskeletal disorder. However, the treatment of MPS of the TM remains a challenge. We investigated the effects of ultrasound (US)-guided pulsed radiofrequency (PRF) stimulation on the interfascial area of the TM. In addition, we compared its effect with that of interfascial block (IFB) with 10 mL of 0.6% lidocaine on the interfascial area of the TM. Thirty-six patients with MPS of the TM were included and randomly assigned into 2 groups. Eighteen patients underwent PRF stimulation on the interfascial area of the TM (PRF group) and 18 patients underwent IFB with lidocaine on the same area (IFB group). Pain intensity was evaluated using a numerical rating scale (NRS) at pretreatment, 2, 4, and 8 weeks after treatment. At pretreatment and 8 weeks after treatment, quality of life was assessed using the Short Form-36 Health Survey (SF-36), which includes the physical component score (PCS) and the mental component score (MCS). One patient in the PRF group was lost to follow-up. Patients in both groups showed a significant decrease in NRS scores at 2, 4, and 8 weeks after treatments and a significant increase in PCS and MCS of the SF-36 at 8 weeks after treatments. Two weeks after each treatment, the decrements of NRS scores were not significantly different between the 2 groups. However, 4 and 8 weeks after the procedures, we found that the NRS score was significantly lower in the PRF group than in the IFB group. At 8 weeks after the treatments, PCS and MCS of the SF-36 in the PRF group were significantly higher than those in the IFB group. For the management of MPS of the TM, US-guided interfascial PRF had a better long-term effect on reducing the pain and the quality of life compared to US-guided IFB. Therefore, we think US-guided PRF stimulation on the interfascial area of the TM can be a beneficial alternative to manage the pain following MPS of the TM.
Keywords: interfascial block, lidocaine, myofascia, myofascial pain syndrome, pulsed radiofrequency, trapezius muscle
1. Introduction
Myofascial pain syndrome (MPS), characterized by chronic pain in multiple myofascial trigger points and fascial constrictions, is one of the most common causes of musculoskeletal pain in clinical practice; it occurs in 30% of patients in pain clinics.[1] MPS is often unresponsive to pharmacological or nonpharmacological treatments, which reduces quality of life and sometimes causes psychological problems such as anxiety or depression.[2] The trapezius muscle (TM) is one of the most frequently affected muscles; thus, clinicians have tried to manage pain following MPS in the TM.[3] In the past several decades, several pain relief methods, including stretching exercises, trigger point injection, acupuncture, and intramuscular electric stimulation, have been used.[4–7] However, although clinicians used these methods to manage MPS in the TM, many patients continued to complain of persistent pain.
Recently, Domingo et al[4] performed interfascial block (IFB) with 8 to 10 mL of 0.125% bupivacaine in patients with MPS in the TM and demonstrated the possible therapeutic effects of their procedure. They also found abundant piercing nerve branches related to myofascial pain in the interfascial area and suggested that blocking these branches could reduce the pain. On the basis of this finding, clinicians are now using IFB to relieve MPS in various muscles. However, the clinical effects of this procedure, such as the degree of pain relief, duration of pain relief, and quality of life, were not clearly demonstrated. In addition, in our clinical practice, the effect of the IFB was not sustained for a long period after the injection.
Pulsed radiofrequency (PRF), a new therapeutic option that is increasingly being employed to treat chronic pain, has been reported to be safe and effective in alleviating many types of pain.[8] It works by delivering an electric field and heat bursts to targeted nerves or tissues without damaging these structures. Continuous radiofrequency (CRF) thermo-coagulation exposes target nerves or tissues to continuous electrical stimulation and ablates the structures by increasing the temperature around the RF needle tip. In contrast to CRF, PRF applies a brief electrical stimulation followed by a long resting phase. Thus, PRF does not produce sufficient heat to cause structural damage. The precise mechanism of action of PRF remains unclear, but it has been proposed that the electrical fields produced by PRF can alter pain signals.[9] So far, several studies have reported the positive effect of PRF on managing MPS.[10–13] As for the treatment of MPS in the TM, only 1 case study reported that using PRF on the TM was able to control the pain effectively.[12]
In the current study, we evaluated the effect of ultrasound (US)-guided PRF stimulation on the interfascial area of the TM. Moreover, we compared the effect of PRF to that of IFB with 10 mL of 0.6% lidocaine on the interfascial area of the TM.
2. Methods
2.1. Patients
This study was a prospective, randomized, and controlled clinical trial. Patients were recruited from the rehabilitation department of a university hospital from January 2015 to July 2016. Thirty-six patients (19 men, 17 women; mean age 51.1 ± 7.5 years, range 39–64 years) were recruited according to the following inclusion criteria[14–16]: (1) aged 20 to 70 years, (2) complaint of myofascial pain in the TM that is not confined to 1 dermatome or myotome during physical examination, and the presence of taut bands in the muscle with 1 or more identifiable trigger points along the muscle, (3) symptoms that persisted for at least 3 months, (4) normal results on the neurological examination, including deep tendon reflexes, manual muscle testing, and sensory exam, (5) pain that is rated at least 3 on a numerical rating scale (NRS, 0 = no pain, 10 = the worst pain). The exclusion criteria were as follows: (1) the presence of other diagnoses such as herniated cervical disc, cervical spinal stenosis, or nerve entrapment syndromes, which were excluded using cervical spine magnetic resonance imaging, computed tomography, electromyography, and nerve conduction study, (2) pregnancy, (3) the presence of coagulopathy, or the use of anticoagulants, (4) TM pain caused by malignant, or autoimmune disease, (5) the presence of chronic medical conditions that might preclude participation in the study, such as systemic inflammatory disorders or neurological abnormalities, (6) a history of surgery on the TM. Informed consent was obtained from all participants. This study was approved by the Institutional Review Board of a university hospital. Based on our previous study,[17] we calculated a sample size. In that study, the difference of the NRS reduction after each treatment (PRF stimulation and lidocaine block) was 1.00 ± 0.97 (mean ± standard deviation). When we adopted Type I error of 0.05, power of 80%, and 2-sided test, 15 subjects per group were found to be necessary for our study. Considering 20% as the dropout rate, we needed to recruit 18 subjects.
Thirty-six patients with MPS in the TM were randomly assigned to 2 groups: 18 patients received PRF stimulation on the interfascial area of the TM (PRF group) and 18 patients received IFB with lidocaine on the interfascial area of the TM (IFB group). Randomization was performed through a simple randomization method via a random table. All patients were advised not to use any oral medication during the course of treatment. Treatment was carried out only once for each patient.
2.2. Procedures
To increase the accuracy and the safety of the procedures (PRF and IFI), US (LOGIQ P6, General electric company) guidance was used. In the PRF group, the PRF treatment was performed in the sitting position. The trigger point was established using physical signs (hypersensitive bundle or nodule of muscle fibers that were harder than normal upon palpation).[18] Then, the skin over the tender area was marked. The skin was sterilized using chlorhexidine gluconate, and a sterile surgical towel was placed on the TM area. A linear array transducer probe was used to scan the marked area in the sagittal and axial planes and identify the TM and its fascia. After all the preparation steps above, under US guidance, a 22-gauge 10 cm cannula with a 10-mm active tip (Cosman RF Cannula, CC10522, Cosman medical) was inserted into the interfascial area of the TM (i.e., interfascial area between trapezius and levator scapulae muscles or between trapezius and rhomboid muscles, or between trapezius and supraspinatus muscles) (Fig. 1). The cannula was positioned on the interfascial area just beneath the most painful point in the TM. Before starting the PRF stimulation, 5 mL of normal saline solution was infused through the cannula to decrease resistance and increase the electrical field (Fig. 1). After an electrode was connected to the PRF needle, the interfascial area was stimulated by the PRF (Cosman G4 radiofrequency generator, COSMAN MEDICAL). PRF treatment was administered at 5 Hz and a 5-ms pulsed width for 360 seconds at 55 V under the condition that the electrode tip temperature did not exceed 42°C. In the IFB group, all the preparation steps were the same as the PRF group. Because steroid injection often causes adverse effects, such as muscle/fat atrophy and suppression of the pituitary-adrenal axis,[19,20] we used only local anesthetic for IFB.[17,21–23] Under US guidance, we injected 3 mL of 2% lidocaine mixed with 7 mL of normal saline solution (i.e., 10 mL of 0.6% lidocaine) into the interfascial area of the TM with a 25-gauge 1.5-inch needle.
Figure 1.
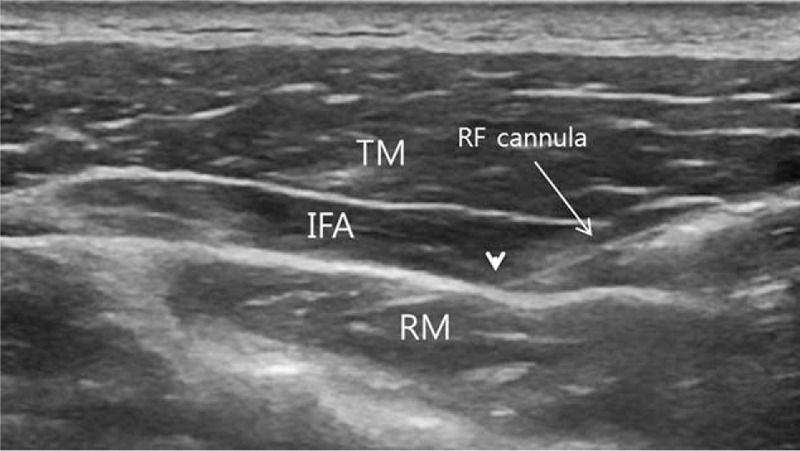
(A) Radiofrequency (RF) cannula (arrow) was inserted into the interfascial space between the trapezius muscle (TM) and the rhomboid muscle (RM) under ultrasound guidance, and 5 mL of normal saline solution was infused into the interfascial area (IFA) through the cannula to decrease resistance and increase the electrical field. IFA = interfascial area, RF = radiofrequency, RM = rhomboid muscle, TM = trapezius muscle.
These PRF and IFB procedures were performed once for each patient by the same physician who has 20 years of training and experience. The physician who performed the procedures was not involved in measuring outcomes.
2.3. Outcomes measures
The assessments at pretreatment and follow-up periods were performed by 1 investigator; this investigator was blinded to the grouping of the patients and did not participate in any treatments. As the primary outcome measure, pain intensities were assessed using an NRS with values between 0 and 10, with the end-points set as “no pain” and “the most intense pain imaginable.”[24] The NRS scores were measured before treatment, and 2, 4, and 8 weeks after treatment. Successful treatment was defined as more than 50% reduction in the NRS score at 8 weeks compared to the pretreatment NRS score. Changes in NRS scores were also calculated by the difference between the NRS scores pretreatment and 8 weeks after treatment in order to validate the degree of change in pain reduction (change in NRS [%] = [pretreatment score – score at 8 weeks after treatment]/pretreatment score × 100).
The secondary outcome measurement was performed using the Short Form 36 Health Survey (SF-36), which is a well-known generic measure of health-related quality of life. The SF-36 evaluates the impact of a disease on the patient and has been proven reliable, valid, and responsive in musculoskeletal disorders.[25] In the SF-36, 2 separate subscales, physical component score (PCS) and mental component score (MCS), are included. The PCS and MCS, reflecting overall physical and mental health status, respectively, are derived from the 8 original scales of the SF-36. The PCS and MCS of SF-36 were measured before treatment and 8 weeks after treatment. Adverse effects were evaluated at each visit in order to detect pain flare-ups and newly developed deficits after the procedures.
2.4. Statistical analysis
Data were analyzed using the Statistical Package for Social Science (SPSS, v. 22.0, IBM Corporation, Armonk, NY). Demographic data were compared between the 2 groups using the Mann–Whitney U test. The changes in NRS score, SF-36 PCS, and SF-36 MCS in each group were evaluated using repeated measure 1-factor analysis. To compare clinical changes over time between groups, repeated measure 2-factor analysis was used. Multiple comparison results were obtained following an adjustment using the Bonferroni correction. The level of statistical significance was set at P < 0.05.
3. Results
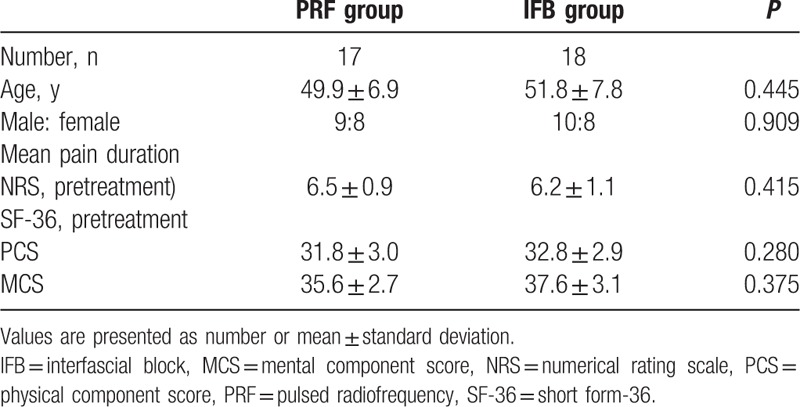
One patient in the PRF group was lost to follow-up. However, adverse events were not observed in both groups throughout the study. No significant intergroup differences were observed for demographic data (P > 0.05) (Table 1).
Table 1.
Demographic characteristics of patients in the PRF and IFB groups.
The average NRS of the pain of MPS in the TM declined from 6.2 ± 1.1 pretreatment to 2.9 ± 0.7 at 2 weeks, 2.5 ± 0.8 at 4 weeks, and 3.2 ± 0.9 at 8 weeks after interfascial PRF (Fig. 2). After IFB, the average NRS declined from 6.5 ± 0.9 pretreatment to 3.6 ± 0.8 at 2 weeks, 4.2 ± 1.2 at 4 weeks, and 5.3 ± 1.0 at 8 weeks. The average SF-36 PCS increased from 32.8 ± 3.0 to 40.7 ± 4.6 at 8 weeks after interfascial PRF; the IFB group SF-36 PCS increased from 31.9 ± 3.0 to 34.3 ± 3.1 at 8 weeks after treatment. As for the SF-36 MCS, the average score increased from 35.6 ± 2.7 to 44.6 ± 5.1 for the PRF group, and from 37.6 ± 3.1 to 39.1 ± 3.5 for the IFB group.
Figure 2.
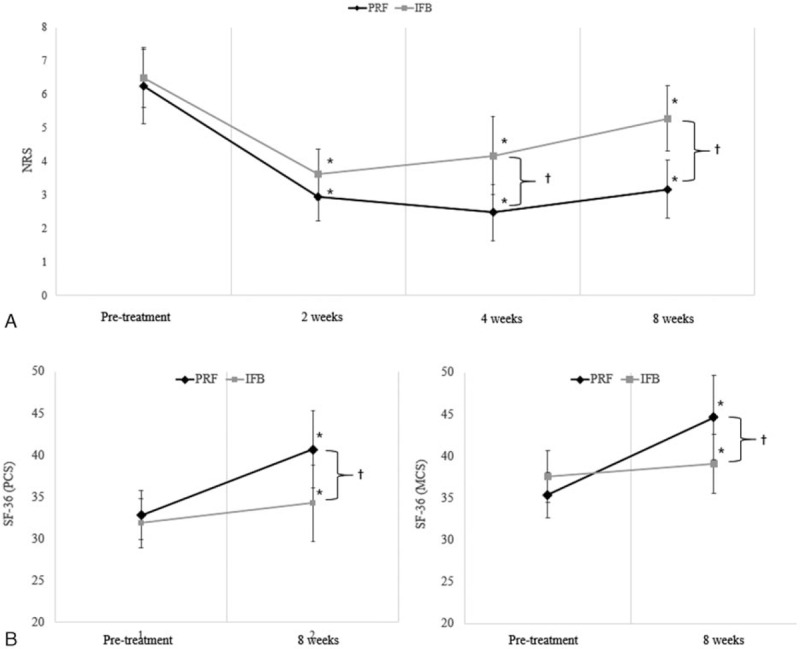
(A) Change in the numerical rating scale (NRS) and (B) change in the physical component score (PCS) and mental component score (MCS) of short form 36 health survey (SF-36) in pulsed radiofrequency (PRF) and interfascial block (IFB) groups. Both groups showed a significant decrease in NRS scores at 2, 4, and 8 weeks after treatments and a significant increase in SF-36 PCS and MCS at 8 weeks after treatments. Two weeks after each treatment, the decrements of NRS scores were not significantly different between the 2 groups. However, 4 and 8 weeks after the procedures, the NRS score was significantly lower in the PRF group. At 8 weeks after the treatments, SF-36 PCS and MCS in the PRF group were significantly higher than those in the IFB group. ∗P < 0.05: intragroup comparison between post-treatment 2, 4, 8 weeks, and pretreatment (repeated measure 1 factor analysis), †P < 0.05: intergroup comparison in each time point (repeated measure 2 factor analysis). IFB = interfascial block, MCS = mental component score, NRS = numerical rating scale, PCS = physical component score, PRF = pulsed radiofrequency, SF-36 = short form 36 health survey.
NRS scores in each group were significantly changed over time (P = 0.000). Two, 4, and 8 weeks after each procedure, NRS scores were significantly lower compared to the scores before the procedure in both groups (P = 0.000) (Fig. 2). In addition, the changes in NRS scores over time were significantly different between groups (P = 0.000). Two weeks after each procedure, the decrements of NRS scores were not significantly different between the 2 groups (P = 0.145). However, 4 and 8 weeks after the procedures, we found that the NRS score was significantly lower in the PRF group than in the IFB group (P = 0.000) (Fig. 2). In addition, 12 (70.6%) out of the 17 patients reported successful pain relief (pain relief of ≥ 50%) at 8 weeks after the PRF, whereas no patient reported successful pain relief at 8 weeks after the IFB.
SF-36 PCS and MCS in each group were significantly higher at 8 weeks after the procedures (P = 0.000). However, the changes in SF-36 PCS and MCS over time were significantly different between the groups (P = 0.000). At 8 weeks after the procedures, SF-36 PCS and MCS in the PRF group were higher than those in the IFB group (PCS, P = 0.001; MCS, P = 0.000).
4. Discussion
In this study, we evaluated the clinical effect of US-guided PRF and IFB in patients with MPS in the TM, and compared the effects of both procedures. Our results showed that the severity of pain, which was measured using the NRS score, was significantly reduced after each US-guided PRF and IFB with lidocaine. At 2 weeks after the procedures, the degrees of pain reduction were not significantly different between the 2 groups. However, at 4 and 8 weeks after the procedures, the patients who had PRF showed a significantly higher reduction in pain compared to the IFB group. Furthermore, about 70% of the patients reported successful pain relief at 8 weeks following US-guided PRF on the interfascial area of the TM. In contrast, 8 weeks after IFB, no patient reported successful pain relief. In summary, the short-term pain-relieving effect was similar between PRF and IFB, but the pain relief from the PRF was maintained longer than that from the IFB. In addition, although SF-36 PCS and MCS at 8 weeks post-treatment were increased in both groups, the patients in the PRF group showed higher scores compared to the IFB group. Higher SF-36 PCS and MCS are indicative of higher physical and mental quality of life, respectively. Therefore, our results indicate that PRF treatment can provide better physical and mental quality of life in patients with MPS in the TM than IFB treatment. Our results were correlated with those of previous study, which showed that intraoperative pain control with dexmedetomidine significantly reduced pain and fatigue and promoted the recovery after the operation.[26] Therefore, we recommend PRF for managing chronic pain following MPS in the TM and improving the quality of life of patients.
Most free nerve endings of muscles are known to be nociceptive, which are connected to the central nervous system by thin myelinated (A-delta) and unmyelinated (C) afferent fibers. The nociceptive free nerve endings and the afferent nerve fibers predominantly contain substance P (SP) and calcitonin gene-related peptide (CGRP), which play important roles in the development of pain. The free nerve endings containing SP and CGRP are abundantly located around muscle fascia.[27] The occurrence of MPS of the TM is attributed to the excitement of the free nerve endings inside the muscle fascia. Recently, studies have reported that IFB relieved myofascial pain by reducing the excitability of these nerve endings.[4,28,29]
The mechanisms of how PRF reduces the pain remain unclear. However, some studies have investigated the therapeutic mechanisms of PRF.[13,30–33] In 2009, Erdine et al[30] studied the structural effects of PRF on sensory nociceptive axons using electron microscopy. They found the ultrastructural lesion of the axon following exposure to PRF. This lesion was selectively greater on the smaller principal sensory nociceptors, C-fibers, and A-delta fiber, and lesser on the larger nonpain-related sensory fiber, the A-beta fiber. In the same year, Hagiwara et al[34] reported that the electromagnetic field of the PRF enhances the noradrenergic and serotonergic descending pain inhibitory pathways and the inhibition of excitatory C-fibers. In addition, in 2002, Moffett et al[31] studied the therapeutic mechanism of PRF at the molecular level. They found that the PRF energy fields could increase the levels of endogenous opioid precursor mRNA and the corresponding opioid peptide. Based on these previous studies, we think that the interfascial PRF reduced the number of nociceptive free nerve endings and afferent nerves or modulated the pain signals from pain-generating nerves. In addition, increased opioid materials after PRF appear to be related to pain reduction in the PRF group. The electrical field induced by the PRF electrode placed in soft tissue is rapidly diluted at increasing distances from the electrode.[35] Thus, we think that PRF stimulation is limited to broad areas in the interfascial area. As a countermeasure, we infused 5 mL of normal saline solution into the interfascial area of the TM. The conducting solutions (e.g., saline or local anesthetics) decrease resistance and increase the electrical field.[36] Accordingly, the current induced by PRF seems to have affected broad areas of fascia and the interfascial area of the TM. An injection of lidocaine blocks the transmission of pain signals and reduces ectopic discharges in nociceptive C-fibers.[37] It also has anti-inflammatory effects.[37] However, the duration of the pain relief provided by the IFB was less than that of the PRF. At 8 weeks after IFB with lidocaine, no patient reported successful pain relief. This result is in agreement with previous studies, in which PRF has better and longer effects compared to local anesthesia when used on radiculopathy, neuropathy, joint arthritis, or complex regional pain syndrome.[17,38–40]
Several studies have evaluated the feasibility or the effect of IFB.[4,33,41–43] In 2011, Domingo et al[4] performed IFB with 8 to 10 mL of 0.125% bupivacaine between the TM and the levator scapulae or between the TM and the rhomboid major in 25 patients with MPS in the TM. After IFB, NRS scores were reduced from 6.4 at pretreatment to 1 at 10 minutes after IFB. As for the application of PRF on MPS, to the best of our knowledge, 5 studies have been conducted. In 2008, Bevacqua and Fattouh[10] demonstrated the effect of PRF on painful trigger points in 10 patients with MPS, but they did not describe any data related to the outcome of PRF treatment. In 2009, Tamimi et al[11] conducted PRF in 6 patients with MPS in the abdominal, lower back, and neck muscles. They reported that PRF was followed by injection of triamcinolone 20 mg in some cases. All the patients experienced more than 50% reduction of pain at 4 weeks after PRF, and the effect of PRF persisted from 6 months to 1 year in 5 patients. In 2012, Park et al[12] inserted cannula into the TM and performed PRF stimulation on a patient with MPS in the TM. Two months after PRF, the NRS score was reduced from 8 to 2–3. In the same year, Niraj[13] conducted PRF stimulation by inserting cannulas and injecting 3 mL of 0.5% levobupivacaine into trigger points in 12 patients with MPS in the cervicothoracic or abdominal wall muscles. Eight (66%) out of 12 patients reported more than 50% pain relief at 6 months after the procedure. In 2016, Park et al[17] evaluated the effect of US-guided PRF stimulation on the interfascial area of the GCM in 20 patients. The NRS score was reduced from 5 to 2.4, and the effect of PRF was sustained for at least 4 weeks. In the studies of Tamimi et al and Niraj, they injected a corticosteroid into a trigger point prior to PRF stimulation; thus, we think that these 2 previous studies did not strictly control for the evaluation of PRF effect on MPS. Moreover, in Park et al's study, trigger points in the TM were directly stimulated. Therefore, our study is the first to demonstrate the therapeutic effects of US-guided PRF on the interfascial area to treat MPS in the TM. In addition, we also compared the effects of PRF and IFB, and showed that PRF is superior in pain relief ability and duration. However, some limitations of this study should be considered. First, a small number of subjects were recruited. Second, we evaluated the effects of PRF and IFB in only 8 weeks. Third, we could not clearly explain the mechanism of action of PRF in reducing pain induced by MPS. Fourth, although recruitment of a placebo group would have been unethical, our study might be criticized for the lack of a placebo group. Therefore, further studies are required to compensate for these limitations.
In conclusion, we found that US-guided PRF stimulation and IFB with lidocaine on the interfascial area of the TM significantly relieved MPS at 2, 4, and 8 weeks after the procedures. There was less pain at 4 and 8 weeks in the PRF group than in the IFB group. The rate of successful pain relief at 8 weeks after PRF was found to be approximately 70%, while that after IFB was 0%. Physical and mental quality of life at 8 weeks after treatment was better in the PRF group. Therefore, we think US-guided PRF stimulation in the interfascial area can be a beneficial treatment option for patients who are suffering from MPS in the TM.
Footnotes
Abbreviations: CGRP = calcitonin gene-related peptide, CRF = continuous radiofrequency, IFB = interfascial block, MCS = mental component score, MPS = myofascial pain syndrome, NRS = numerical rating scale, PCS = physical component score, PRF = pulsed radiofrequency, SF-36 = Short Form 36 Health Survey, SP = substance P, TM = trapezius muscle, US = ultrasound.
Funding: This work was supported by the 2016 Yeungnam University Research Grant
The authors have no conflicts of interest to disclose.
References
- [1].Skootsky SA, Jaeger B, Oye R. Prevalence of myofascial pain in general internal medicine practice. West J Med 1989;151:157–60. [PMC free article] [PubMed] [Google Scholar]
- [2].Robert D, Gerwin Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep 2001;5:412–20. [DOI] [PubMed] [Google Scholar]
- [3].Kamanli A, Kaya A, Ardicoglu O. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int 2005;25:604–11. [DOI] [PubMed] [Google Scholar]
- [4].Domingo T, Blasi J, Casals M, et al. Is interfascial injection with ultrasound guided puncture useful in treatment of myofascial pain of the trapezius muscle? Clin J Pain 2011;27:297–303. [DOI] [PubMed] [Google Scholar]
- [5].Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil 1994;73:256–63. [DOI] [PubMed] [Google Scholar]
- [6].Chu J, Yuen KF, Wang BH, et al. Electrical twitch-obtaining intramuscular stimulation in lower back pain: a pilot study. Am J Phys Med Rehabil 2004;83:104–11. [DOI] [PubMed] [Google Scholar]
- [7].Lee JC, Lin DT, Hong CZ. The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger point. J Musculoskeletal Pain 1997;5:81–90. [Google Scholar]
- [8].Van Zundert J, de Louw AJ, Joosten EA. Pulsed and continuous radiofrequency current adjacent to the cervical dorsal root ganglion of the rat induces late cellular activity in the dorsal horn. Anesthesiology 2005;102:125–31. [DOI] [PubMed] [Google Scholar]
- [9].Abejon D, Reig E. Is pulsed radiofrequency a neuromodulation technique? Neuromodulation 2003;6:1–3. [DOI] [PubMed] [Google Scholar]
- [10].Bevacqua B, Fattouh M. Pulsed radiofrequency for treatment of painful trigger points. Pain Pract 2008;8:149–50. [DOI] [PubMed] [Google Scholar]
- [11].Tamimi MA, McCeney MH, Krutsch J. A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med 2009;10:1140–3. [DOI] [PubMed] [Google Scholar]
- [12].Park CH, Lee YW, Kim YC, et al. Treatment experience of pulsed radiofrequency under ultrasound guided to the trapezius muscle at myofascial pain syndrome—a case report. Korean J Pain 2012;25:52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Niraj G. Ultrasound-guided pulsed radiofrequency treatment of myofascial pain syndrome: a case series. Br J Anaesth 2012;109:645–6. [DOI] [PubMed] [Google Scholar]
- [14].Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil 2008;89:157–9. [DOI] [PubMed] [Google Scholar]
- [15].Gemmell H, Miller P. Immediate effect of ischaemic compression and trigger point pressure release on neck pain and upper trapezius trigger points: a randomised controlled trial. Clin Chiropractic 2008;11:30–6. [Google Scholar]
- [16].Simons DG, Travell JG, Simons LS. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. 1st, 2nd ed. Williams & Wilkins: Baltimore; 1999. [Google Scholar]
- [17].Park SM, Cho YW, Ahn SH, et al. Comparison of the effects of ultrasound-guided interfascial pulsed radiofrequency and ultrasound-Guided interfascial injection on myofascial pain syndrome of the gastrocnemius. Ann Rehabil Med 2016;40:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jaeger B. Myofascial trigger point pain. Alpha Omegan 2013;106:14–22. [PubMed] [Google Scholar]
- [19].Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician 2002;5:182–99. [PubMed] [Google Scholar]
- [20].Park SK, Choi YS, Kim HJ. Hypopigmentation and subcutaneous fat, muscle atrophy after local corticosteroid injection. Korean J Anesthesiol 2013;65:S59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cardone DA, Tallia AF. Joint and soft tissue injection. Am Fam Physician 2002;66:283–8. [PubMed] [Google Scholar]
- [22].Lavelle W, Lavelle ED, Lavelle L. Intra-articular injections. Med Clin North Am 2007;91:241–50. [DOI] [PubMed] [Google Scholar]
- [23].Stephens MB, Beutler AI, O’Connor FG. Musculoskeletal injections: a review of the evidence. Am Fam Physician 2008;78:971–6. [PubMed] [Google Scholar]
- [24].Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of change in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [25].Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: QualityMetric Inc.; 2001. [Google Scholar]
- [26].Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci Rep 2016;23:21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Benjamin M. The fascia of the limbs and back—a review. J Anat 2009;214:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pasqualucci A, Varrassi G, Braschi A, et al. Epidural local anesthetic plus corticosteroid for the treatment of cervical brachial radicular pain: Single injection versus continuous infusion. Clin J Pain 2007;23:551–7. [DOI] [PubMed] [Google Scholar]
- [29].Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain 2000;87:7–17. [DOI] [PubMed] [Google Scholar]
- [30].Erdine S, Bilir A, Cosman ER, et al. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract 2009;9:407–17. [DOI] [PubMed] [Google Scholar]
- [31].Moffett J, Fray LM, Kubat NJ. Activation of endogenous opioid gene expression in human keratinocytes and fibroblasts by pulsed radiofrequency energy fields. J Pain Res 2012;5:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vallejo R, Tilley DM, Williams J, et al. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician 2013;16:601–13. [PubMed] [Google Scholar]
- [33].Forero M, Neira VM, Heikkila AJ, et al. Continuous lumbar transversus abdominis plane block may spread to supraumbilical dermatomes. Can J Anaesth 2011;58:948–51. [DOI] [PubMed] [Google Scholar]
- [34].Hagiwara S, Iwasaka H, Takeshima N, et al. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain 2009;13:249–52. [DOI] [PubMed] [Google Scholar]
- [35].Sluijter ME, Teixeira A, Serra V, et al. Intra-articular application of pulsed radiofrequency for arthrogenic pain—report of six cases. Pain Pract 2008;8:57–61. [DOI] [PubMed] [Google Scholar]
- [36].Misirlioglu TO, Akgun K, Palamar D, et al. Piriformis syndrome: comparison of the effectiveness of local anesthetic and corticosteroid injections: a double-blinded, randomized controlled study. Pain Physician 2015;18:163–71. [PubMed] [Google Scholar]
- [37].Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand 2006;50:265–82. [DOI] [PubMed] [Google Scholar]
- [38].Gofeld M, Restrepo-Garces CE, Theodore BR, et al. Pulsed radiofrequency of suprascapular nerve for chronic shoulder pain: a randomised double-blind active placebo-controlled study. Pain Pract 2013;13:96–103. [DOI] [PubMed] [Google Scholar]
- [39].Djuric V. Pulsed radiofrequency treatment of complex regional pain syndrome: a case series. Pain Res Manag 2014;19:186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shanthanna H, Chan P, McChesney J, et al. Pulsed radiofrequency treatment of the lumbar dorsal root ganglion in patients with chronic lumbar radicular pain: a randomized, placebo-controlled pilot study. J Pain Res 2014;7:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Manassero A, Bossolasco M, Ugues S, et al. Ultrasound-guided obturator nerve block: interfascial injection versus a neurostimulation-assisted technique. Reg Anesth Pain Med 2012;37:67–71. [DOI] [PubMed] [Google Scholar]
- [42].Ueshima H, Oku K, Otake H. Ultrasound-guided thoracolumbar interfascial plane block: a cadaveric study of the spread of injectate. J Clin Anesth 2016;34:259–60. [DOI] [PubMed] [Google Scholar]
- [43].López-Matamala B, Fajardo M, Estébanez-Montiel B, et al. A new thoracic interfascial plane block as anesthesia for difficult weaning due to ribcage pain in critically ill patients. Med Intensiva 2014;38:463–5. [DOI] [PubMed] [Google Scholar]


