Supplemental Digital Content is available in the text
Keywords: chronic kidney disease, cystatin-C, donor nephrectomy, living-donor kidney transplantation
Abstract
Donor nephrectomy in living-donor kidney transplantation may result in hyperfiltration injury in remnant kidney; however, its clinical implication in partial recovery of kidney function (PRKF) in remnant kidney and chronic kidney disease (CKD) progression remains unclear. Thus, we investigated the effect of PRKF on CKD development in the residual kidney and the utility of cystatin-C (Cys-C) in evaluating renal function in living-donor kidney transplantation donors.
The electronic medical records and laboratory results of 1648 kidney transplant (KT) donors and 13,834 healthy nondonors between January 2006 and November 2014 were reviewed. The predictors of PRKF and CKD diagnosed by Kidney Disease: Improving Global Outcomes (KDIGO) criteria were evaluated by multivariate analysis. CKD risk was compared between KT donors and healthy nondonors using Cox proportional hazard regression analysis following propensity score matching (PSM).
The incidence of PRKF for KT donors was 49.3% (813). CKD incidence was 24.8% (408) in KT donors and 2.0% (277) in healthy nondonors. The predictors of PRKF were, male sex (odds ratio [OR], 17.32; 95% confidence interval [CI] 9.16–32.77), age (OR, 1.02; 95% CI, 1.00–1.04; P < 0.001), Cys-C concentration (OR, 1.02; 95% CI, 1.00–1.04; P = 0.02), and preoperative albumin level (OR, 0.49; 95% CI, 0.27–0.89; P = 0.02). The predictors of CKD were age (hazards ratio [HR], 1.04; 95% CI, 1.02–1.05; P < 0.001), Cys-C concentration (HR, 1.024; 95% CI, 1.012–1.037; P < 0.001), and PRKF (HR, 1.41; 95% CI, 1.04–1.92; P = 0.03). After PSM, the risk of progression to CKD was higher in KT donors than in healthy nondonors (HR, 58.4; 95% CI, 34.2–99.8; P < 0.001).
Donor nephrectomy is associated with PRKF and progression to CKD. Cys-C is a useful early marker for detecting PRKF and CKD.
1. Introduction
Kidney transplantation (KT) is considered as the best strategy to treat end-stage renal disease (ESRD).[1] However, in addition, because of a paucity of deceased donors, living-donor kidney transplantation is also widely performed. However, this is problematic because unilateral nephrectomy in living donors is associated with an abrupt loss of renal tissue, an accompanying compensatory increase in single-nephron glomerular filtration rate (GFR), and long-term structural damage to the remnant kidney.[2] Recent studies have found that chronic kidney disease (CKD) risk is increased in living KT donors,[3–5] which has led to greater interest in assessing the risk of CKD in living-donor kidney transplantation.
Cystatin-C (Cys-C), an endogenous cysteine proteinase inhibitor produced by nucleated cells, is freely filtered by the glomerulus and is subsequently reabsorbed and catabolized by the healthy tubular epithelium.[6] Thus, as its production stays constant independent of gender, age, or muscle mass; given its exclusive sensitivity to GFR changes,[7] changes in Cys-C concentration allow for the early detection of deterioration in renal function. Indeed, recent studies have shown that estimated GFR (eGFR) based on Cys-C is more accurate than eGFR using serum creatinine (sCr).[8–11]
The Acute Dialysis Quality Initiative Group (ADQI) defined recovery of kidney function after insult into 3 levels; complete if the patient's level returns to within 50% of baseline sCr, partial when the patient is off renal replacement therapy but one does not attain 50% of baseline sCr, and no recovery was defined as persistent need for renal replacement.[12] Renal functional reserve, which is the capacity of the kidney to compensate or increase its function in states of demand or disease, became virtually zero after donor nephrectomy.[13] Although renal recovery slowly progresses as time goes by, the impact of partial recovery of kidney function (PRKF) on the progression to CKD in living KT donors is not known.
In this study, we investigated the impact of partial recovery of the remnant kidney function (PRKF) in terms of development of CKD in living KT donors compared with the healthy general population. In addition, we evaluated the role of Cys-C concentration in predicting AKI incidence and progression to CKD in living KT donors.
2. Methods
2.1. Patient population
2.1.1. KT donors
We reviewed the electronic medical records and laboratory results of all patients who underwent donor nephrectomy between January 2006 and November 2014 at Asan Medical Center. A total of 1669 patients were identified for this study; among these patients, 15 cadaveric donors and 6 living donors with insufficient laboratory data were excluded. Thus, a total of 1648 donors were included in the final analysis. The KT donor cohort was divided into 2 groups on the basis of CKD development during the follow-up-period (normal vs CKD) (Fig. 1); CKD were defined using the Kidney Disease Improving Global Outcomes classification.[14] PRKF was defined as the ratio of the last sCr during hospital stay to baseline sCr ≥ 1.5. At our center, all donors underwent a series of studies to exclude those with potential risks associated to kidney donation, including sCr, eGFR, urinary examination, renal sonography, and effective renal plasma flow. Potential donors with any infection, proteinuria, malignancy, or end-stage kidney disease were excluded. Our study protocol was approved by the institutional review board of Asan Medical Center (2015-0383).
Figure 1.
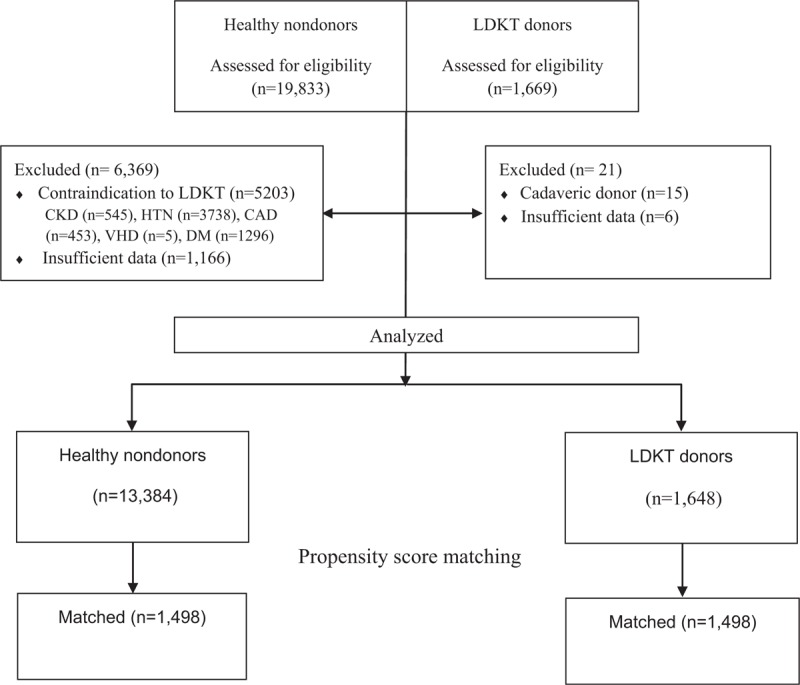
Study flow chart. CAD = coronary artery disease, CKD = chronic kidney disease, Cys-C = cystatin-C, DM = diabetes mellitus, HTN = hypertension, LDKT = living donor kidney transplantation, VHD = valvular heart disease.
2.1.2. Clinical data
Demographic, laboratory, and intraoperative data on all patients were obtained from the electronic medical records system of our institution (Asan Medical Center Information System Electrical Medical Records). Demographic data included age, sex, body mass index (BMI), and comorbidities (ie, hypertension [HTN], diabetes mellitus [DM], pulmonary tuberculosis, hepatitis, and cardiovascular disease). HTN was defined as the use of any antihypertensive medication at admission, whereas DM was defined as the use of any hypoglycemic agents. Hepatitis was defined as the presence of a serological marker, such as the e and s antigens of the hepatitis B virus or the IgG antibody of the hepatitis C virus. Cardiovascular disease was defined as the presence of at least one of ischemic heart disease, arrhythmia, valvular heart disease, and vascular occlusive disease.
Laboratory data included hemoglobin, total protein, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, sodium, blood urea nitrogen (BUN), sCr, and eGFR. Of these, the latter was estimated from the preoperative sCr concentration using the Modification of Diet in Renal Disease (MDRD) study equation for adult patients, adjusted for each 1.73 m2 of body surface area.[15] Intraoperative data included the amount of fluid administered, intraoperative use of furosemide or mannitol, intraoperative urine output, anesthesia duration, lowest intraoperative mean blood pressure, serum Cys-C concentration, and eGFR estimated by Cys-C concentration. In addition, Cys-C concentrations and eGFR values estimated by Cys-C concentration were obtained after nephrectomy; thus, for a total of 1648 donors included in the final analysis, Cys-C concentration was available for 739.
2.1.3. Outcome variables
KT donors were evaluated for postoperative PRKF and progression to CKD according to a review of electronic medical records.
2.1.4. Healthy nondonors
The healthy nondonor population was drawn from patients who visited Asan Medical Center health promotion clinic for annual check-ups for at least 5 years between January 2006 and December 2014. Data from a total of 19,833 patients were collected, and 5203 patients who were identified with contraindications for kidney transplantation, including CKD (n = 545) and comorbidities affecting renal function such as cardiovascular disease (HTN [n = 3837], coronary artery disease [n = 453], arrhythmia/valvular heart disease/peripheral arterial disease [n = 5]), and endocrine diseases (DM [n = 1296]) were excluded. In addition, 1166 healthy nondonors were excluded because of insufficient demographic or laboratory data. CKD was defined using the Kidney Disease Improving Global Outcomes classification according to a review of laboratory data from the electronic medical records.
2.1.5. Clinical data
In this cohort, medical information was obtained from patient self-reports, physical examination, and laboratory test results in our computerized database (Asan Biomedical Research Environment). Demographic data included age, body weight, height, BMI, and systolic and diastolic blood pressure at annual health check examinations, whereas sCr and eGFR were retrospectively reviewed from the computerized database.
2.1.6. Outcome
Healthy nondonors were evaluated for the development of CKD.
2.2. Statistical analysis
Continuous variables were reported as means ± SD, or as median values with interquartile range (IQR), as appropriate. For KT donors, patient age, BMI, laboratory data, amount of fluids administered, diuretic dose, urine output, lowest mean blood pressure, and anesthetic time were compared with Student t tests or the Mann–Whitney U test. Categorical variables were described as frequencies and percentages and analyzed with Fisher exact test, or chi-squared tests. Multiple logistic regression analysis was used to identify independent predictors of PRKF in KT donors, and all variables with P < 0.1 on univariate analysis were included in the multivariate analysis. Model discrimination was assessed using C statistics, and calibration was evaluated on the basis of the Hosmer–Lemeshow test. Multivariate Cox proportional hazard regression analyses were used to assess the prognostic value of Cys-C concentration in CKD development; the proportional-hazards assumption for each variable was checked using Schoenfeld residuals and the double-log method.
To investigate CKD risk in KT donors and healthy nondonors, KT donors were matched with healthy nondonors using the propensity score matching (PSM) method to adjust demographic differences between KT donors and healthy nondonors. We calculated the propensity score for each patient using age, sex, BMI, hemoglobin, albumin, sCr, and follow-up period, and subsequently used these propensity scores to match 1648 patients in the KT donor group with healthy nondonors at a ratio of 1:1 using the greedy matching algorithm. During this process, KT donors with DM, HTN, and CKD were excluded from the PSM. After PSM, the balance in baseline covariates were assessed using standardized mean differences and McNemar test, as appropriate. For matched groups, donor nephrectomy as a risk factor for CKD development was assessed using multivariate Cox proportional hazard regression analysis. All P values of <0.05 were considered statistically significant; data manipulation and statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) and R software version 2.10.1.
3. Results
A total of 1648 KT donors and 13,834 healthy nondonors were included in the final analysis (Fig. 1). Open nephrectomy was performed in 92 (5.6%) KT donors, and hand assisted laparoscopic surgery technique was performed in 1556 (94.4%) KT donors. The proportion of right sided nephrectomy was 42.1%. The duration of hospital stay was 8.1 ± 2.7 days.
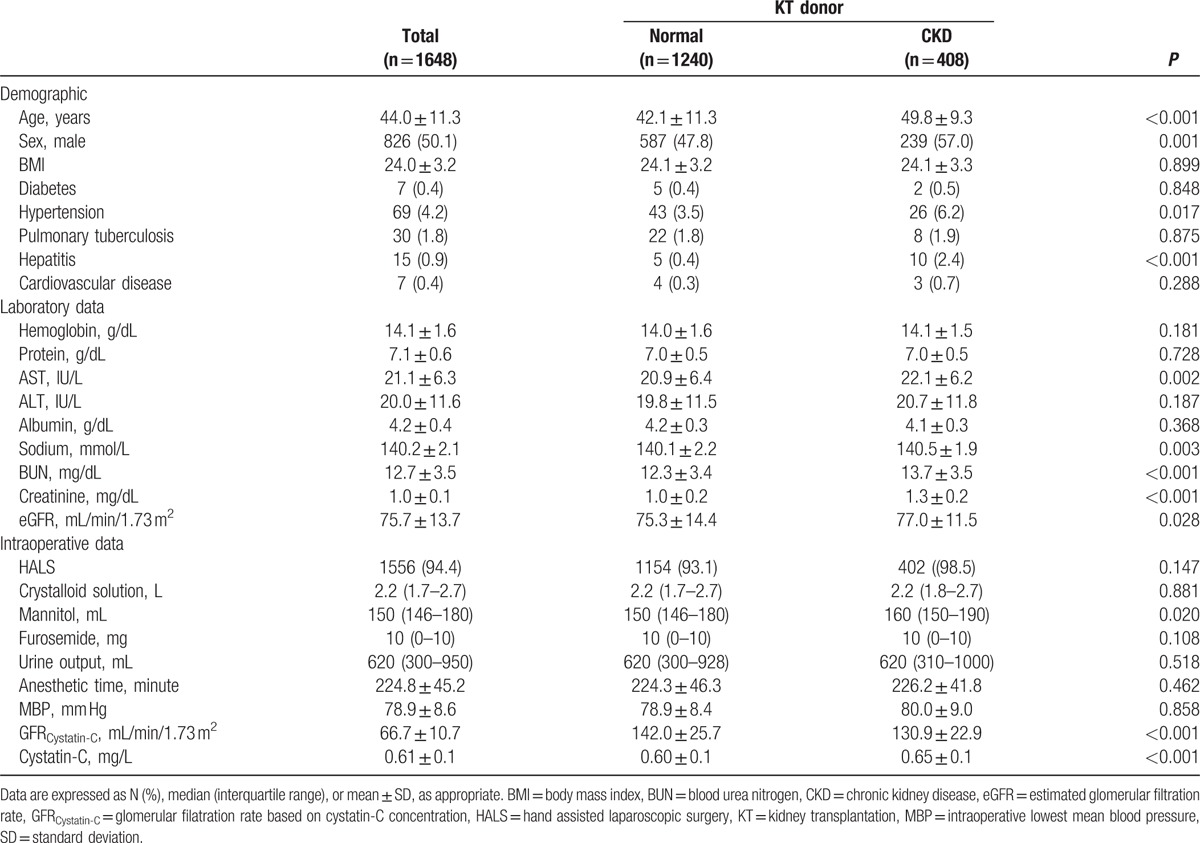
The median follow-up for KT donors and healthy nondonors were 1.1 (IQR, 0.5–2.1 years) and 6.1 (IQR, 4.0–7.8 years) years, respectively. Demographic, preoperative, and intraoperative characteristics of KT donors are summarized in Table 1. After donor nephrectomy, 408 KT donors (24.8%) were diagnosed with CKD; patients with CKD were older and male, and hypertension and hepatitis incidences were higher in KT donors developing CKD. In addition, these patients had higher concentrations of preoperative sodium, BUN, sCr, Cys-C, and lower eGFR estimated on the basis of sCr or serum Cys-C. The incidence of postoperative PRKF in KT donors was 49.3% (n = 813). The last sCr was obtained on postoperative day (POD) 4 (n = 1146, 69.5%), POD 5 (n = 364, 22.1%), and POD 6 and 7 (n = 138, 8.4%), respectively.
Table 1.
Demographic, preoperative, and intraoperative characteristics of kidney transplant donors.
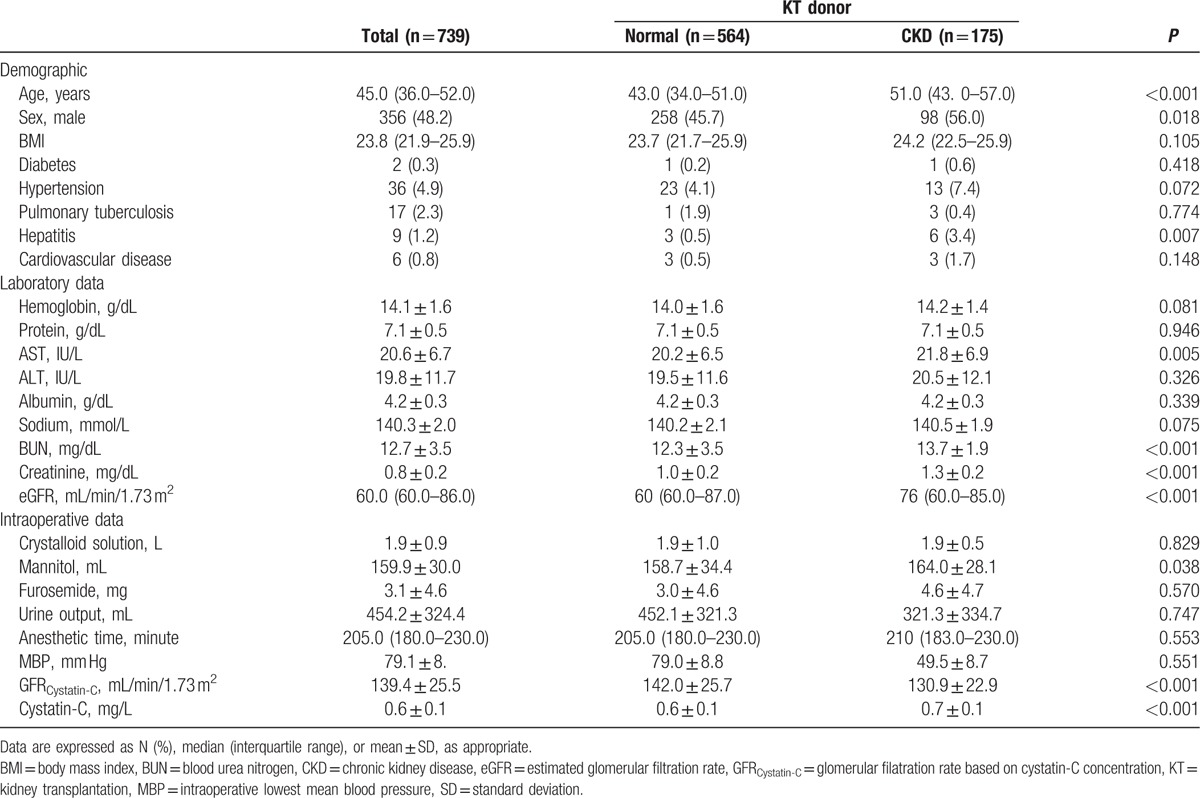
Demographic, preoperative, and intraoperative characteristics of KT donors who performed Cys-C concentration measurement are summarized in Table 2. Among 739 donors, the incidence of CKD was 23.7% (n = 175). The demographic, preoperative, and intraoperative characteristics were similar between whole study population (n = 1648) and donors with Cys-C concentration (n = 739).
Table 2.
Demographic, preoperative, and intraoperative characteristics of kidney transplant donors with cystainc C values.
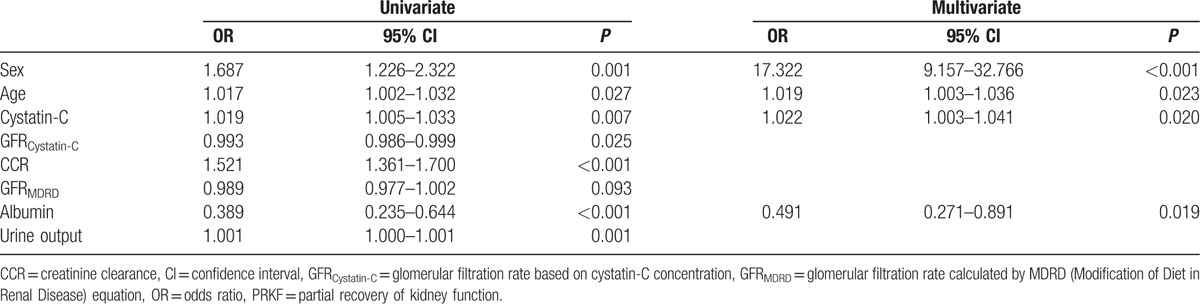
The predictors of PRKF determined by multivariate logistic regression are shown in Table 3; male sex (odds ratio [OR], 17.322; 95% confidence interval [CI], 9.157–32.766; P < 0.001), age (OR, 1.019; 95% CI, 1.003–1.036; P = 0.023), Cys-C concentration (OR, 1.022; 95% CI, 1.003–1.041; P = 0.020), and preoperative albumin level (OR, 0.491; 95% CI, 0.271–0.891; P = 0.019) were associated with PRKF.
Table 3.
Logistic regression analysis to identify predictors of PRKF.
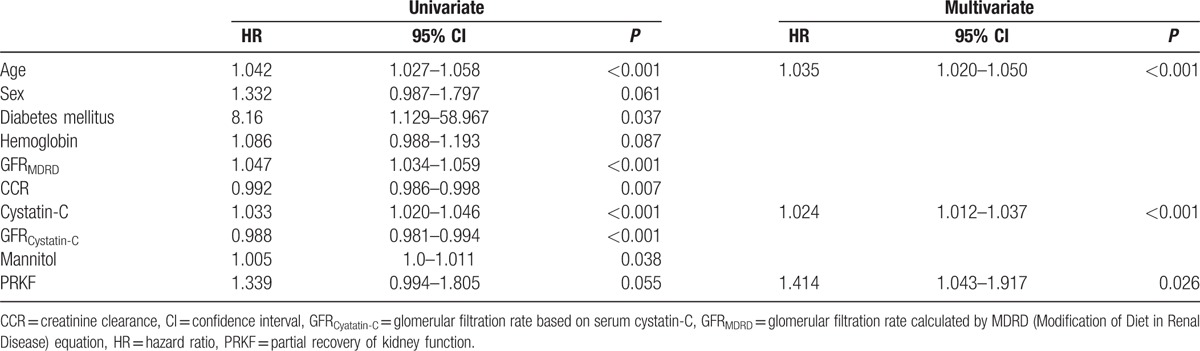
CKD incidence in KT donors was 24.8% (n = 408). Cox proportional hazard regression analysis showed that age (HR, 1.035; 95% CI, 1.020–1.050; P < 0.001), high intraoperative Cys-C concentrations (HR, 1.024; 95% CI, 1.012–1.037; P < 0.001), and PRKF (HR, 1.414; 95% CI, 1.043–1.917; P = 0.026) were associated with CKD (Table 4).
Table 4.
Cox proportional hazard regression analysis to identify predictors of chronic kidney disease in transplant donors.
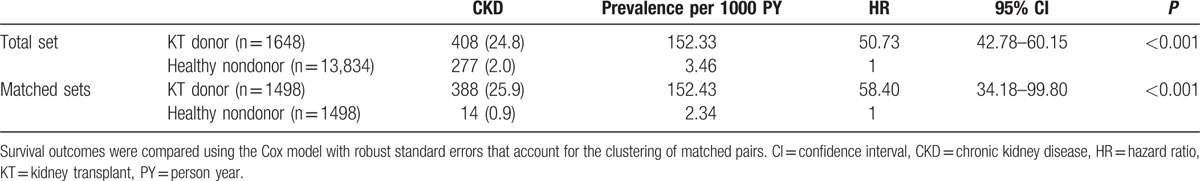
CKD incidence in healthy nondonors was 2.0% (n = 277) (Table 5), whereas the hazard ratio (HR) of kidney donation for CKD was 50.73 (95% CI, 42.78–60.15; P < 0.001). Healthy nondonors (n = 1498) were matched at a 1:1 ratio with KT donors (n = 1498); however, after PSM, CKD risk was still higher in KT donors (HR, 58.40; 95% CI, 34.18–99.80; P < 0.001) than in healthy nondonors. The balance between healthy nondonors and KT donors is summarized in Table 1S.
Table 5.
Risk comparison of progression to chronic kidney disease between live donors and healthy nondonors.
4. Discussion
In this study, we have demonstrated that PRKF is associated with progression to CKD after donor nephrectomy. In addition, Cys-C concentration is a useful early marker to detect PRKF and CKD. The CKD incidence and risk are significantly higher in KT donors than in healthy nondonors. Our analysis indicates that the independent variables related to PRKF were male sex, age at donation, intraoperative Cys-C concentration, and the preoperative albumin level, whereas the predictors of CKD were age at donation, intraoperative Cys-C concentration, and PRKF.
Live donor nephrectomy is associated with a sudden loss of approximately 50% renal tissue. However, the remaining kidney compensates within a relatively short time with a 20% to 40% functional increase via adaptive hyperfiltration.[16] A previous study using an animal model has demonstrated that this adaptive hyperfiltration leads to a severe reduction in functional renal mass and contributes to the progressive destruction of remaining glomeruli.[17] More recently, it has been suggested that kidney injury might develop right after donor nephrectomy.[18,19] Rossi et al[19] reported that the concentrations of uremic toxins, indoxyl sulfate, and p-cresyl sulfate, in particular, were significantly increased in patients undergoing donor nephrectomy. Both indoxyl sulfate and p-cresyl sulfate induce the nuclear factor-κB pathway, resulting in both oxidative stress and proinflammatory cytokine stimulation,[20–22] and are associated with CKD progression,[23,24] cardiovascular morbidity,[25,26] and mortality.[27,28] Indeed, one recent study found that a urinary biomarker, α-1-microglobulin, was increased in donors undergoing nephrectomy, suggesting that kidney injury was induced by either altered blood flow, or hypertrophy and dedifferentiation of tubular cells in the remaining kidney.[18] Therefore, acute deterioration of kidney function may occur as a result of hyperfiltration and renal toxic insult following donor nephrectomy, which develops abruptly and continued in progression to CKD in remnant kidney of donors. However, the findings of studies investigating long-term complications following donor nephrectomy remain contradictory.[29,30] According to a longitudinal study of live KT donors, a slow and steady increase in GFR was observed over a 10-year period.[31] Also, ESRD risk did not increase compared to the general population in a study with a mean follow-up period of 12.9 ± 9.2 years.[32]
As KT donors are always very healthy individuals with no comorbidities affecting renal function, comparison of these donors versus the general population with various comorbidities leads to an underestimation of potential kidney dysfunction risk following donor nephrectomy. Previous studies have reported that increased blood pressure, albuminuria, and decreased renal function, all of which may occur in KT donors following nephrectomy, were associated with increased all-cause and cardiovascular mortality rates.[33,34] Therefore, concerns related to increased cardiovascular morbidity and mortality because of kidney donation remain.[35] Several recent studies have reported that ESRD risk, as well as the all-cause mortality rate, was increased in KT donors compared with healthy nondonors,[3,29] and incidences of proteinuria, hypertension, and ESRD were increased in KT donors.[36,37] Thus, longer follow-up periods are needed to assess the impact of nephrectomy in donors.
In our study, CKD risk was very high in the KT donor group. Although the follow-up period was shorter in KT donors than in healthy nondonor controls, the HR of CKD was 43 times higher in KT donors than in healthy nondonors, and CKD risk was associated with postoperative PRKF. Therefore, because delayed renal recovery might result from hyperfiltration and toxic renal injury following nephrectomy in donors and may abruptly progress to CKD in the remaining kidney, it is critical to identify the predictors of PRKF to prevent its development.
The current criteria used for CKD diagnosis are based on eGFR.[38] Of the available models that are used to measure GFR, the MDRD equation is the most widely used, which evaluates the function of the remaining kidney in living donors. However, the MDRD equation was developed using data from patients with established CKD who had underlying renal pathologies. In contrast, living kidney donors do not have significant pathological lesions in the remnant kidney. A study using eGFR by the MDRD equation found that the prevalence of CKD was high in KT donors; however, measured GFR was higher than eGFR by MDRD in this group.[39] In addition, GFR in healthy individuals might not be accurately estimated using the MDRD equations,[40] raising concerns regarding its relevance in the assessment of kidney function in living donors. Cys-C concentration is increasingly being used to evaluate renal function as an alternative to eGFR calculated on the basis of sCr[10]; Cys-C is a low molecular weight protein secreted by most cells in the body and is independent of gender, age, and muscle mass,[7] in contrast to sCr. Thus,Cys-C is a useful marker for the early detection of abnormal renal function.[41] Moreover, recent studies have suggested that eGFR based on Cys-C is superior to eGFR based on sCr in predicting poor outcomes related to renal function.[42] Moreover, postoperative Cys-C based estimation of the GFR was reported to be helpful to predict the recovery of kidney donors due to its high specificity.[43] Although its concentrations can also be affected by various disease states,[6] Cys-C, either alone or in combination with sCr, is useful in predicting ESRD risk and death.[8] In accordance with this earlier study, our analysis demonstrates that Cys-C is a predictor of PRKF and progression to CKD in KT donors. Indeed, considering the limited ability of acute changes in sCr in predicting the development of adverse renal outcomes,[41] Cys-C should be considered as a surrogate marker of eGFR during the follow-up for KT donors.
The main limitation of our study is its retrospective design, although we used statistical methods such as PSM analysis to control bias. However, there is a possibility that additional confounding parameters that could not be entirely excluded might influence our findings. For example, the follow-up period of KT donors was relatively short, based on the average time period included in the protocols at our hospital during the study period. Considering the deterioration of function of remnant kidney over long-term period, longer follow-up period is required. Also, it is still not evident whether progression of kidney donors to CKD possesses as same risk of significant medical complication as that of general CKD population.[44,45]
In conclusion, PRKF in KT donors was associated with progression to CKD. Moreover, CKD risk is higher in KT donors than in healthy nondonors. Intraoperative Cys-C is a useful marker for detecting PRKF risk and progression to CKD in KT donors after nephrectomy.
Supplementary Material
Footnotes
Abbreviations: CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, PRKF = partial recovery of kidney function, sCr = serum creatinine.
Authorship: JYB, JGS: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, study supervision. SOK: acquisition of data and statistical analysis. SGK: acquisition of data, analysis, and interpretation of data. GSH: study design and supervision.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int 2009;75:1088–98. [DOI] [PubMed] [Google Scholar]
- [2].Vergho D, Burger M, Schrammel M, et al. Matched-pair analysis of renal function in the immediate postoperative period: a comparison of living kidney donors versus patients nephrectomized for renal cell cancer. World J Urol 2015;33:725–31. [DOI] [PubMed] [Google Scholar]
- [3].Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int 2014;86:162–7. [DOI] [PubMed] [Google Scholar]
- [4].Kido R, Shibagaki Y, Iwadoh K, et al. How do living kidney donors develop end-stage renal disease? Am J Transplant 2009;9:2514–9. [DOI] [PubMed] [Google Scholar]
- [5].Muzaale AD, Massie AB, Kucirka LM, et al. Outcomes of live kidney donors who develop end-stage renal disease. Transplantation 2016;100:1306–0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem 2015;68:57–69. [DOI] [PubMed] [Google Scholar]
- [7].Filler G, Bokenkamp A, Hofmann W, et al. Cystatin C as a marker of GFR – history, indications, and future research. Clin Biochem 2005;38:1–8. [DOI] [PubMed] [Google Scholar]
- [8].Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013;369:932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63:820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem 2002;48:699–707. [PubMed] [Google Scholar]
- [11].Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002;40:221–6. [DOI] [PubMed] [Google Scholar]
- [12].Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 2014;127:94–100. [DOI] [PubMed] [Google Scholar]
- [14].Group KDIGOKCw. KDIGO 2012 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–50. [DOI] [PubMed] [Google Scholar]
- [15].Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- [16].Saxena AB, Myers BD, Derby G, et al. Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am J Physiol Renal Physiol 2006;291:F629–34. [DOI] [PubMed] [Google Scholar]
- [17].Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol 2001;12:1315–25. [DOI] [PubMed] [Google Scholar]
- [18].Hoogendijk-van den Akker JM, Warle MC, van Zuilen AD, et al. Urinary biomarkers after donor nephrectomy. Transpl Int 2015;28:544–52. [DOI] [PubMed] [Google Scholar]
- [19].Rossi M, Campbell KL, Johnson DW, et al. Uremic toxin development in living kidney donors: a longitudinal study. Transplantation 2014;97:548–54. [DOI] [PubMed] [Google Scholar]
- [20].Lekawanvijit S, Adrahtas A, Kelly DJ, et al. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 2010;31:1771–9. [DOI] [PubMed] [Google Scholar]
- [21].Motojima M, Hosokawa A, Yamato H, et al. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int 2003;63:1671–80. [DOI] [PubMed] [Google Scholar]
- [22].Watanabe H, Miyamoto Y, Otagiri M, et al. Update on the pharmacokinetics and redox properties of protein-bound uremic toxins. J Pharm Sci 2011;100:3682–95. [DOI] [PubMed] [Google Scholar]
- [23].Lin CJ, Liu HL, Pan CF, et al. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res 2012;43:451–6. [DOI] [PubMed] [Google Scholar]
- [24].Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 2011;26:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009;4:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meijers BK, Bammens B, De Moor B, et al. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008;73:1174–80. [DOI] [PubMed] [Google Scholar]
- [27].Bammens B, Evenepoel P, Keuleers H, et al. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 2006;69:1081–7. [DOI] [PubMed] [Google Scholar]
- [28].Liabeuf S, Barreto DV, Barreto FC, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010;25:1183–91. [DOI] [PubMed] [Google Scholar]
- [29].Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA 2014;311:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fehrman-Ekholm I, Duner F, Brink B, et al. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation 2001;72:444–9. [DOI] [PubMed] [Google Scholar]
- [31].Saran R, Marshall SM, Madsen R, et al. Long-term follow-up of kidney donors: a longitudinal study. Nephrol Dial Transplant 1997;12:1615–21. [DOI] [PubMed] [Google Scholar]
- [32].Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med 2009;360:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- [34].Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Garg AX, Prasad GV, Thiessen-Philbrook HR, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation 2008;86:399–406. [DOI] [PubMed] [Google Scholar]
- [36].Hakim RM, Goldszer RC, Brenner BM. Hypertension and proteinuria: long-term sequelae of uninephrectomy in humans. Kidney Int 1984;25:930–6. [DOI] [PubMed] [Google Scholar]
- [37].Rogers NM, Lawton PD, Jose MD. Indigenous Australians and living kidney donation. N Engl J Med 2009;361:1513–6. [DOI] [PubMed] [Google Scholar]
- [38].Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1–50. [Google Scholar]
- [39].Ibrahim HN, Rogers T, Tello A, et al. The performance of three serum creatinine-based formulas in estimating GFR in former kidney donors. Am J Transplant 2006;6:1479–85. [DOI] [PubMed] [Google Scholar]
- [40].Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 2004;43:112–9. [DOI] [PubMed] [Google Scholar]
- [41].Herget-Rosenthal S, Pietruck F, Volbracht L, et al. Serum cystatin C – a superior marker of rapidly reduced glomerular filtration after uninephrectomy in kidney donors compared to creatinine. Clin Nephrol 2005;64:41–6. [DOI] [PubMed] [Google Scholar]
- [42].Park M, Hsu CY, Li Y, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012;23:1725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Han HH, Choi KH, Yang SC, et al. Clinical assessment of follow-up cystatin C-based eGFR in live kidney donors. Korean J Urol 2012;53:721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tan JC, Ho B, Busque S, et al. Imprecision of creatinine-based GFR estimates in uninephric kidney donors. Clin J Am Soc Nephrol 2010;5:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barri Y, Parker T, 3rd, Kaplan B, et al. Primum non Nocere: is chronic kidney disease staging appropriate in living kidney transplant donors? Am J Transplant 2009;9:657–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


