Abstract
To assess the safety and efficacy of tranexamic acid (TXA) for decreasing perioperative blood loss in cervical laminectomy with lateral mass screw fixation and bone grafting (CLF), in which all surgical procedures are identical.
From November 2014 to April 2016, we performed a retrospective comparative analysis of 119 patients with multilevel cervical spondylotic myelopathy who had undergone a CLF from C3 to C6 in our center. All surgeries were performed on the patients using a consistent, standard procedure. Patients were divided into control (46) and TXA (73) groups according to whether or not they had received TXA treatment before and during surgery. Demographic profiles of patients such as gender, age, body weight, height, and body mass index were collated and differences between the 2 groups compared. Preoperative and postoperative hematological data in addition to intraoperative and postoperative blood loss were compared between the 2 groups. Additionally, any complications of TXA were also evaluated to assess safety.
There was no statistically significant difference in demographic traits between the 2 groups. Intraoperative blood loss in the TXA group (179.66 ± 81.45 mL) was significantly lower than that of the control group (269.13 ± 94.68 mL, P < 0.001), as was postoperative blood loss (108.08 ± 44.31 and 132.83 ± 49.39 mL, respectively; P = 0.005). Total blood loss in the TXA group (287.74 ± 115.40 mL) was also significantly lower than that of the control group (401.96 ± 127.88, P < 0.01). No major intraoperative complications occurred in any of the cases.
TXA significantly reduced perioperative blood loss in CLF with no major side effects.
Keywords: blood loss, cervical laminectomy with lateral mass screw fixation and bone grafting, multilevel cervical spondylotic myelopathy, tranexamic acid
1. Introduction
Multilevel cervical spondylotic myelopathy (MCSM) is a serious disease which can lead to spinal cord dysfunction and a substantial decrease in quality of life. In addition, MCSM requiring multilevel cervical spine surgery can be accompanied by extensive blood loss. As spinal surgery has become increasingly complex, so control of perioperative bleeding has become an important clinical issue for spine surgeons.[1–3] Excessive blood loss can lead to a range of comorbidities such as anemia, hypotension, hematoma formation, and inadequate oxygenation of organs, thereby affecting patient outcome. Excessive blood loss often requires allogeneic blood transfusion, the risks of which are numerous and among which immunological reactions and transmission of viruses are considered the most serious.[4–6] In addition, hematoma formation within a few millimeters of the spinal canal can cause considerable neurological damage. Although occurrences of postoperative spinal hematoma formation requiring emergency surgery are rare, it is nevertheless important to control perioperative bleeding to decrease its incidence.[7–9]
Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine which operates through competitive inhibition of the activation of plasminogen to plasmin by binding specific sites on both plasminogen and plasmin, thereby retarding fibrinolysis, the degradation of blood clots.[3,10,11] It has been widely used in various medical fields such as cardiac surgery, gynecology, dentistry, urological surgery, and liver transplantation to reduce the perioperative blood loss, as it is relatively inexpensive and has not been cited in the literature as causing any significant untoward side effects. In addition, TXA has been reported to reduce blood loss and transfusion requirements during orthopedic surgery, most commonly in knee and hip joint replacement.[10,12–14] Although the benefits of TXA in spinal surgery have been reported, its use in cervical spinal surgery is somewhat limited. Indeed, there are no studies of TXA in cervical laminectomy with lateral mass screw fixation and bone grafting (CLF) so far.
CLF has been widely used for many years for treating MCSM caused by multilevel cervical spinal cord compression. Although some authors suggest that laminoplasty is superior to laminectomy with lateral mass screw fixation and bone grafting regarding preserved range of motion,[15] indications for the use of CLF are broader than that of laminoplasty and recent studies showed good outcomes for CLF.[16–18] Thus, the aim of this study is to evaluate the efficacy and safety of TXA in controlling blood loss during CLF for the treatment of MCSM.
2. Material and methods
This study was approved by the biomedical research ethical committee of Honghui Hospital. A retrospective comparative analysis was performed in patients with MCSM undergoing CLF of vertebrae C3 to C6. Patients with cirrhosis of the liver, serious cardiac disease, chronic renal failure, cancer, allergy to TXA, a history of thromboembolic disease (deep vein thrombosis, ischemic heart disease, pulmonary embolism, transient ischemic attack, strokes, or subarachnoid hemorrhage), bleeding disorders, hypercoagulation status, disseminated intravascular coagulation, pregnancy, combined anterior and posterior spinal fusions, patients receiving antiplatelet and/or anticoagulant therapy at the time of the study, and treatments of vertebrae outside of C3 to C6 were excluded from the study. Between November 2014 and April 2016, 119 CLFs fitting the inclusion criteria were performed in our center. Each surgeon (Hao, Liu, He, Wu, Wang, Zheng, and Zhao) in this study had more than 20 years of experience in spinal surgery. The surgical procedure was performed consistently in each case. The 73 patients who received TXA were categorized as the TXA group. The control group consisted of 46 patients who underwent CLF for the treatment of MCSM in our institution without being given TXA. In both groups, we analyzed patient demographic trait (age at surgery, gender, body weight, height, and body mass index) and duration of surgery which was defined as the time from the initial incision to the completion of wound closure. The quantity of intraoperative and postoperative blood loss and the preoperative and day 1 postoperative hematological data for each patient were obtained in the 2 groups. Total blood loss was calculated as the sum of intraoperative and postoperative blood loss (the quantity during the 1st 16 hours). No patient required a preoperative blood transfusion.
2.1. Surgical procedure
All patients in the 2 groups underwent the same CLF intraoperative technique. Patients were placed in a prone position on the operating table under general anesthesia. The spinous processes, lamina, and lateral mass facet complexes were exposed using a standard posterior midline opening after longitudinally dividing the nuchal fascia in line with a midline skin incision. Laminectomy was performed using a high speed matchstick burr to drill troughs in the bone at the lateral edge of the lamina on each side, prior to complete removal of the entire lamina and associated ligamentum flavum of the target vertebrae (C3–C6). The excised lamina and spinous processes were cleaned of soft tissue and cut into pieces for use as autograft material. Lateral mass screws were placed bilaterally on vertebrae C3 to C6 then fixed with rods using the Margerl technique.[19] Pieces of autograft material were carefully placed posterolaterally. The incision was rinsed and hemostasis achieved. Eventually, the wound was closed using a layer-to-layer suture.
2.2. Drug dose
All patients in the TXA group were given a dose of 15 mg/kg of TXA (Transamin; Daiichi Pharmaceutical, Tokyo, Japan) before a skin incision was made,[6] followed immediately with a maintenance dose of 100 mg/hour, and continued until wound closure.[7,20–22] No patient in the control group received intraoperative administration of TXA or any other antifibrinolytic drug.
2.3. Statistical analysis
Continuous variable data were presented as mean and standard deviation while categorical variable data were presented as a number and its specific value. Statistical differences between the 2 experimental groups were compared using a chi-square test or Fisher exact test for categorical variables and Student t test for continuous variables. A value of P < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., United States of America).
3. Results
There was no statistical difference in demographic values (patients’ age, gender, body weight, height, and body mass index) between the 2 groups (Table 1). No statistically significant difference in duration of surgery was observed between the 2 groups, with a mean time of 155.72 ± 15.59 and 153.52 ± 11.91 minutes in the control and TXA groups, respectively (P = 0.387). There was significantly less intraoperative blood loss in the TXA group compared to the control (Fig. 1), at 179.66 ± 81.45 and 269.13 ± 94.68 mL, respectively (P < 0.001). The TXA group had significantly less postoperative blood loss during the 1st 16 hours compared to the control group (108.08 ± 44.31 vs 132.83 ± 49.39 mL, P = 0.005). Total blood loss in the control group (401.96 ± 127.88 mL) was significantly higher than that in the TXA group (287.74 ± 115.40 mL, P < 0.001). Changes in blood hemoglobin (Hgb) content and hematocrit (Hct) were not statistically significantly different between the 2 groups: preoperative Hgb: 138.28 ± 9.12 g/L (control) and 140.42 ± 10.05 g/L (TXA); postoperative Hgb: 122.35 ± 10.74 g/L (control) and 125.21 ± 11.29 g/L (TXA); preoperative Hct: 41.78 ± 2.62% (control) and 42.07 ± 2.08% (TXA); and postoperative Hct: 36.50 ± 3.22% (control) and 37.32 ± 2.41% (TXA). Although the Hgb and Hct values in the TXA group were all higher than those of the control group, the differences were not statistically significant (Fig. 2). No patient required an allogeneic blood transfusion during or after the surgery in either group and no serious intra- or postoperative complications, for example, dural tear, infection, epidural hematoma formation, deep-vein thrombosis, pulmonary embolism, allergic reaction, renal failure, or cardiopulmonary complications were observed in either group. Furthermore, no minor side effects associated with the use of TXA such as nausea, vomiting, headache, or diarrhea occurred in either of the groups.
Table 1.
Demographic parameters of patients in each group.
Figure 1.
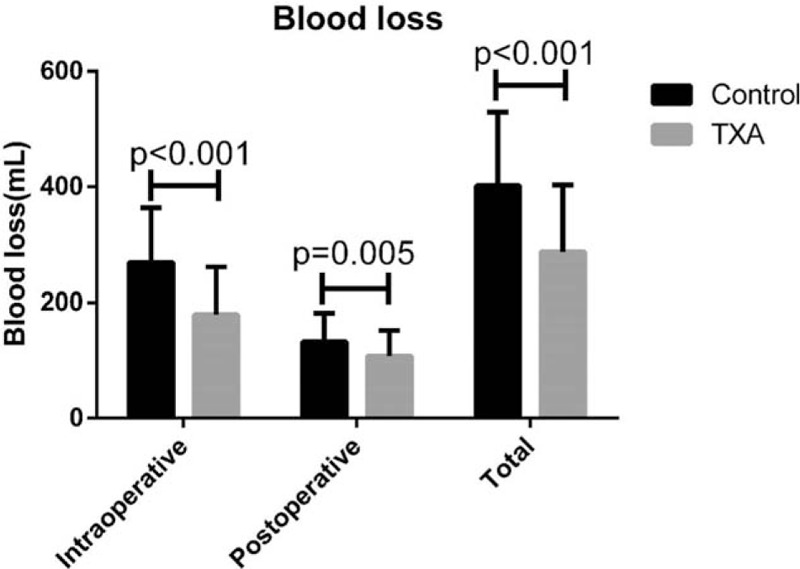
The TXA (tranexamic acid) group had significantly less intraoperative, postoperative, and total blood loss compared to the control group.
Figure 2.
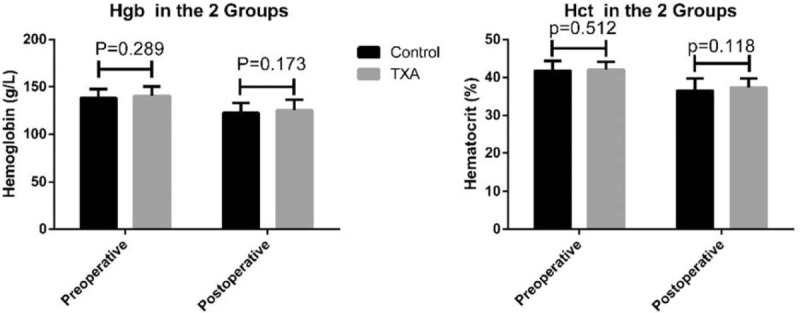
There were no significantly different in blood Hgb Hct between the control and TXA groups. Hgb = hemoglobin, Hct = content and hematocrit, TXA = tranexamic acid.
4. Discussion
Numerous studies have supported the use of TXA in orthopedic surgery.[10,12–14,23,24] Although it has been evaluated in many studies, the safety and efficacy of TXA for blood loss reduction in spinal surgery has not been clearly demonstrated, especially for CLF.[25,26]
CLF for the treatment of MCSM has proved to be a successful technique for restoring normal cervical lordotic alignment, recovering neurological function, and decreasing the morbidity of C5 palsy and axial pain.[27] The principal disadvantage of this approach is a significant decrease in motion due to the fixation of the target vertebrae. Laminoplasty allows a better range of neck motion although it becomes more restricted in flexion and extension over time. For patients with MCSM combined with simple instability or correctable kyphosis, CLF is a more suitable method than laminoplasty, even if the consequence is neck stiffness.
Fibrinolysis increases transiently when patients undergo surgery, and it has been shown that it contributes to perioperative blood loss during spinal surgery.[28] Fibrinolytic activation is the result of an enzymatic cascade process. TXA inhibits fibrinolysis by blocking the lysine-binding sites of plasminogen, plasmin, and tissue plasminogen activator.[6] Since fibrinolysis is activated immediately during and after surgery, TXA should be administered before surgery begins.[29] The majority of studies utilize intravenous TXA using a wide range of recommended doses. The half-life of TXA is approximately 80 minutes in patients with normal renal function.[6] Pharmacokinetic evidence suggests a loading dose of 10 to 15 mg/kg followed by a maintenance dose of 1 mg/kg/hour or repeated dosing.[6] Li et al[26] conducted a meta-analysis of 6 randomized controlled trials and believed that higher TXA dosage (≥15 mg/kg) reduced perioperative blood loss and blood transfusion in surgery. Yang et al[30] conducted a meta-analysis of 9 randomized controlled trials which had a similar sample to Li's study.[26] The 2 meta-analyses arrived at a similar conclusion although the quantity of blood loss and incidence of blood transfusion were different. Elwatidy et al[1] used a high single dose (30 mg/kg) of TXA and found that it reduced total blood loss and incidence of blood transfusion. Raksakietisak et al[25] reported that 2 doses of TXA (15 mg/kg) reduced perioperative blood loss and incidence of blood transfusion among low-risk adult patients undergoing elective complex thoracolumbar spine surgery. A single bolus dose of TXA may also effective.[7,20] In this study, we evaluated the effects of TXA with a dose of 15 mg/kg followed by a maintenance dose of 100 mg/hour. Our results are in line with the results of the studies cited above. The TXA treatment group lost significantly less blood than that of the control group, including during the intra- and postoperative periods. Total blood loss in the TXA group was 28% less than that of the control group. Although the Hgb levels and Hct in the TXA group were higher than that of the control group, the differences were not significant, possibly because the sample size was too small and the perioperative blood loss in CLF relatively minor.
A theoretical anxiety associated with the use of TXA is its potential for inducing thromboembolic complications. However, many studies have shown that the administration of TXA does not increase this risk.[22,31,32] In our study, there were no clinical symptoms or signs of thromboembolic events, such as deep-vein thrombosis or symptomatic pulmonary embolism. In addition, no adverse effects related to TXA occurred in our study. Thus, the use of TXA in patients undergoing CLF should be regarded as safe.
A weakness of this study is the small sample size and retrospective data collection. Additionally, we are unsure whether the complexity of the surgery has an effect on the benefit of TXA. Another limitation is that the control group did not receive a placebo. A prospective randomized controlled study would better determine the safety and efficacy of TXA during laminectomy with lateral mass screw fixation and bone grafting. We are planning such a prospective randomized controlled trial.
5. Conclusions
In this study, blood loss (both intra- and postoperative) in the TXA group was significantly lower than that in the control group, and no major intraoperative complications occurred. Our results indicate that the use of intravenous TXA is both safe and effective in reducing blood loss in CLF. Prophylactic TXA may provide the benefit of limiting excessive blood loss in posterior approach cervical spinal surgery. A future prospective randomized controlled trial will provide superior evidence of the efficacy and safety of TXA.
Acknowledgements
The authors thank National Natural Science Foundation of China (No. 81601898) for the support.
Footnotes
Abbreviations: CLF = cervical laminectomy with lateral mass screw fixation and bone grafting, Hct = content and hematocrit, Hgb = hemoglobin, MCSM = multilevel cervical spondylotic myelopathy, TXA = tranexamic acid.
C-CY and W-JG contributed equally to this work.
Funding/support: This study was supported by the National Natural Science Foundation of China (No. 81601898).
The authors have no conflicts of interest to disclose.
References
- [1].Elwatidy S, Jamjoom Z, Elgamal E, et al. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine 2008;33:2577–80. [DOI] [PubMed] [Google Scholar]
- [2].Hu SS. Blood loss in adult spinal surgery. Eur Spine J 2004;13(Suppl 1):S3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xie J, Lenke LG, Li T, et al. Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J 2015;15:647–54. [DOI] [PubMed] [Google Scholar]
- [4].Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br 2008;90:1128–36. [DOI] [PubMed] [Google Scholar]
- [5].Zollo RA, Eaton MP, Karcz M, et al. Blood transfusion in the perioperative period. Best Pract Res Clin Anaesthesiol 2012;26:475–84. [DOI] [PubMed] [Google Scholar]
- [6].Soviero F, Geraci A, Termine S, et al. Bleeding in orthopaedic surgery: the role of blood transfusion and erythropoietin alpha. Acta Biomed 2010;81:125–9. [PubMed] [Google Scholar]
- [7].Tsutsumimoto T, Shimogata M, Ohta H, et al. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine 2011;36:1913–8. [DOI] [PubMed] [Google Scholar]
- [8].Luo XB, Zhou X, Wang Q, et al. The classification of recurrent spinal epidural hematoma: a review of the literature and a comparison with the cases. Eur Spine J 2016;25(Suppl 1):224–9. [DOI] [PubMed] [Google Scholar]
- [9].Minato T, Miyagi M, Saito W, et al. Spinal epidural hematoma after thoracolumbar posterior fusion surgery without decompression for thoracic vertebral fracture. Case Rep Orthop 2016;2016: 6295817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang Y, Zhang L, Ma X, et al. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Der Orthopade 2016;45:616–21. [DOI] [PubMed] [Google Scholar]
- [11].Cheriyan T, Maier SP, 2nd, Bianco K, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J 2015;15:752–61. [DOI] [PubMed] [Google Scholar]
- [12].Reina N, Fennema P, Hourlier H. The impact of mild peri-operative hypothermia on the effectiveness of tranexamic acid in total hip arthroplasty. Int Orthop 2017;41:55–60. [DOI] [PubMed] [Google Scholar]
- [13].Yue C, Pei F, Yang P, et al. Effect of topical tranexamic acid in reducing bleeding and transfusions in TKA. Orthopedics 2015;38:315–24. [DOI] [PubMed] [Google Scholar]
- [14].Yang Y, Lv YM, Ding PJ, et al. The reduction in blood loss with intra-articular injection of tranexamic acid in unilateral total knee arthroplasty without operative drains: a randomized controlled trial. Eur J Orthop Surg Traumatol 2015;25:135–9. [DOI] [PubMed] [Google Scholar]
- [15].Seng C, Tow BP, Siddiqui MA, et al. Surgically treated cervical myelopathy: a functional outcome comparison study between multilevel anterior cervical decompression fusion with instrumentation and posterior laminoplasty. Spine J 2013;13:723–31. [DOI] [PubMed] [Google Scholar]
- [16].Lee CH, Lee J, Kang JD, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: a meta-analysis of clinical and radiological outcomes. J Neurosurg Spine 2015;22:589–95. [DOI] [PubMed] [Google Scholar]
- [17].Yuan W, Zhu Y, Liu X, et al. Laminoplasty versus skip laminectomy for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Arch Orthop Trauma Surg 2014;134:1–7. [DOI] [PubMed] [Google Scholar]
- [18].Lao L, Zhong G, Li X, et al. Laminoplasty versus laminectomy for multi-level cervical spondylotic myelopathy: a systematic review of the literature. J Orthop Surg Res 2013;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Silveri CP, Vaccaro AR. Posterior atlantoaxial fixation: the Magerl screw technique. Orthopedics 1998;21:455–9. [DOI] [PubMed] [Google Scholar]
- [20].Wang Q, Liu J, Fan R, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J 2013;22:2035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop 2005;76:314–9. [PubMed] [Google Scholar]
- [22].Yagi M, Hasegawa J, Nagoshi N, et al. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine 2012;37:E1336–42. [DOI] [PubMed] [Google Scholar]
- [23].Xie J, Ma J, Kang P, et al. Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases. Thromb Res 2015;136:234–8. [DOI] [PubMed] [Google Scholar]
- [24].Wu Q, Zhang HA, Liu SL, et al. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol 2015;25:525–41. [DOI] [PubMed] [Google Scholar]
- [25].Raksakietisak M, Sathitkarnmanee B, Srisaen P, et al. Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: a prospective randomized study. Spine 2015;40:E1257–63. [DOI] [PubMed] [Google Scholar]
- [26].Li ZJ, Fu X, Xing D, et al. Is tranexamic acid effective and safe in spinal surgery? A meta-analysis of randomized controlled trials. Eur Spine J 2013;22:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yeh KT, Lee RP, Chen IH, et al. Laminoplasty with adjunct anterior short segment fusion for multilevel cervical myelopathy associated with local kyphosis. J Chin Med Assoc 2015;78:364–9. [DOI] [PubMed] [Google Scholar]
- [28].Neilipovitz DT. Tranexamic acid for major spinal surgery. Eur Spine J 2004;13(Suppl 1):S62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tanaka N, Sakahashi H, Sato E, et al. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 2001;83:702–5. [DOI] [PubMed] [Google Scholar]
- [30].Yang B, Li H, Wang D, et al. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PloS One 2013;8:e55436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gill JB, Chin Y, Levin A, et al. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am 2008;90:2399–407. [DOI] [PubMed] [Google Scholar]
- [32].Winter SF, Santaguida C, Wong J, et al. Systemic and topical use of tranexamic acid in spinal surgery: a systematic review. Global Spine J 2016;6:284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


