Abstract
Objective:
To describe a successful prophylactic protocol for managing an athlete with hemophilia playing at a high level of contact sports.
Background:
Published data show that team physicians are not comfortable either treating athletes with bleeding disorders or allowing them to participate in contact sports. Much of the literature historically has recommended against allowing athletes with bleeding disorders to play sports at all and certainly against playing contact sports. Hemophilia treatment can now include prophylactic injections of recombinant factor VIII to prevent bleeding episodes. Modern treatments hold the promise of allowing athletes with hemophilia to participate in contact sports.
Differential Diagnosis:
Mild, moderate, or severe hemophilia; von Willebrand disease; other factor deficiencies.
Treatment:
A treatment protocol was developed that included prophylactic factor VIII injections on a regular basis and when the athlete was injured.
Uniqueness:
This is the first published case report of an athlete with known hemophilia being successfully treated and participating in National Collegiate Athletic Association collegiate basketball for 2 full seasons.
Conclusions:
Sports medicine teams can successfully manage an athlete with hemophilia playing a contact sport.
Key Words: factor VIII, prophylactic hemophilia protocol, bleeding disorders
Hemophilia is a medical condition in which the ability of the blood to clot is severely reduced, causing bleeding episodes. This condition is typically caused by a hereditary lack of a coagulation factor, most often factor VIII. In recent years, young people with hemophilia have become more involved in physical activities as modern medications have provided better treatment of bleeding disorders and helped to prevent episodes of bleeding and their complications. These treatments have allowed people with hemophilia to assume nearly normal lives.1,2 Sports physicians and athletic trainers must be able to accommodate athletes with hemophilia in organized physical activities.
Recommendations both for and against participation in various types of athletic activities are present in the literature. The majority of authors3–6 have recommended against most types of athletic participation that carry the risk of contact and therefore a bleeding episode. With better, more consistent treatment options available, the hemophilia community has sought to increase the activity level of patients and encourage participation in organized sports.1,2
However, research indicates that most team physicians do not know enough about hemophilia or its management to feel comfortable managing athletes with bleeding disorders or clearing them to play.7 Additionally, little information in the literature approves of organized contact sports or documents the successful participation of those with hemophilia at this level.2
We present a case report of the successful protocol for treatment of a National Collegiate Athletic Association (NCAA) Division I collegiate basketball player with moderate hemophilia through 2 full competitive seasons. To our knowledge, this is the first documented successful prophylactic protocol used in a collegiate-level contact sport to manage an athlete with known hemophilia. We believe it is important to introduce this protocol into the literature so that future athletes with hemophilia might be cleared to play sports. This protocol represents the optimal standard of care for the sports medicine team.
CASE REPORT
In 2009, a 20-year-old male presented to our Division I NCAA university as a junior college transfer. During his preparticipation evaluation, no mention was made that he either had hemophilia or had had any previous problems related to a bleeding disorder, even though our preparticipation evaluation addresses bleeding disorders. He was evaluated routinely and cleared to play basketball.
About a month into preseason practice, the athlete twisted his ankle. The athletic trainer for the team heard from another player postinjury that the athlete went to his dormitory room and injected recombinant factor VIII. The sports medicine team subsequently learned that he had been taught self-treatment at an early age and was proficient in self-administering the intravenous medication.
When directly asked, he admitted that he had hemophilia. He was immediately withdrawn from further athletic participation pending a review of this additional medical history. The team medical staff, athletic trainers, coaches, and administrators then began a complete review of the medical risk for an athlete with hemophilia playing Division I basketball, which is clearly a contact-level sport. All NCAA rules and regulations, legal precedents, the Americans with Disabilities Act (ADA), and current and past literature involving athletes with hemophilia were carefully studied.
We could find no legal or medical precedent that would allow us to clear him to play basketball. At this point, we sought further guidance from the local hemophilia center that had been treating him since his initial diagnosis. Our question was whether “reasonable accommodations” might allow him to safely play basketball. We considered this consultation to be a formality because the published literature regarding hemophilia in sports at that time did not recommend participation in contact-level sports.3–6
To our surprise, his physicians at the local hemophilia center believed that a protocol could be developed that would allow the athlete to play basketball safely. After a discussion with all parties involved, the decision was to pursue the development of a prophylactic treatment protocol that would allow him to return to play.
The athletic trainers and medical staff developed and followed the protocol (Table) for our athlete over the next 2 years of participation on a NCAA Division I basketball team. He missed no games because of injury or complications of his bleeding disorder. He did miss 1 away game because no hemophilia treatment center was available within 75 miles of the game. According to the protocol, this disqualified him from playing in that game.
Table.
Prophylactic Protocol for Managing a Contact-Sport Athlete With Hemophilia
| 1. The athlete cannot have a history of spontaneous bleeds and playing cannot cause bleeding into the knees or ankles. |
| 2. No intramuscular injections of any kind are given. |
| 3. The athlete's management must be discussed with the school's insurance company to ensure coverage of any sport-related injury. Typically, coverage excludes direct hemophilia-related medications or treatment. |
| 4. The athlete or guardian (if the athlete is a minor) must sign a preexisting-condition medical-release agreement that contains standard wording for release of liability, acknowledgment of the awareness of risk by the athlete, and awareness of the possibility that the insurance company may not cover some expenses. |
| 5. Clinicians should know the untreated factor VIII level and factor VIII inhibitor status of the athlete. These data are used by the athlete's hematologist to determine the prophylaxis dose and track the response to treatment. |
| 6. With the help of the athlete's hematologist, the clinician should chart the recombinant antihemophilic factor (eg, Kogenate, RECOMBINATE [Baxalta US Inc, Bannockburn, IL], ADVATE [Baxalta US Inc], Bioclate [Cerner Multum, Inc, Denver, CO], ELOCTATE [Biogen, Cambridge, MA]) prophylaxis schedule. A typical schedule is 25–30 mg/kg 3 times/wk (eg, Monday, Wednesday, Friday) with an additional 40 mg/kg before games: the physician usually selects a single dose from among the available dosages, so that mixing is not necessary. |
| 7. The prophylactic dose is given 1–2 hours before a game. If a dose is scheduled for a practice day, the medication is given 1–2 hours before the practice. |
| 8. All injections are given in presence of the athletic trainer (or team physician if available) to ensure compliance. |
| 9. For travel to away games, the athlete should bring 6 doses of factor VIII because hospitals usually do not have recombinant factor VIII readily available. Light and heat are detrimental to the medication, so it should be kept at a maximum of room temperature and preferably in a cooler (but not frozen). The athletic trainer stows the medication in his or her carry-on luggage to avoid loss. |
| 10. The athlete cannot play at away games unless a hemophilia treatment center is within 75 miles. |
| 11. Injuries are treated with factor VIII administration. The RICE protocol (rest, ice, compression, elevation) is also important, although it does not take the place of factor VIII. |
| Dosages should be confirmed with the athlete's hematologist in advance: |
| Serious injury: 40 mg/kg as soon as possible and daily until bleeding is resolved (generally 3 days). |
| Joint hemarthrosis: 40 mg/kg twice per day for 3–4 days. |
| Head injury: 40 mg/kg as soon as possible and computed tomography imaging of the head. |
The athlete's hematologist determined that his factor VIII levels were 3% to 5% (ie, moderate hemophilia) and his factor inhibitors test was negative. A single unit dose of 3000 units of Kogenate FS recombinant antihemoglobin factor (Bayer AG, Leverkusen, Germany) was selected. This dose was given Monday, Wednesday, and Friday, with an additional 4000 units given 1 to 2 hours before games. The athletic trainer communicated with the hematologist to create a calendar with dosages specified for the entire season.
Our athlete regularly used cold whirlpool baths postexercise. His ankles were prophylactically taped and he wore padded compression shirts and shorts to help prevent injury.
While following this protocol, the athlete successfully played 2 full years of Division I collegiate basketball. During season 1, he had an episode of hip pain. He recalled no injury, and no extra treatment was given. In the off-season after that first season, the athlete sustained an abdominal strain with a weight workout. No extra treatment was necessary. During season 2, he sprained his wrist. No joint hemarthrosis developed, and he required no treatment beyond that specified in the protocol. In the second season, the athlete incurred a significant ankle sprain and a traumatic olecranon bursal bleed. Both injuries were treated with factor VIII per the protocol and resolved uneventfully.
Finally, it should be noted that this athlete was the star of the team, earning several team and NCAA conference-level honors. He also played professional-level basketball for 2 years after college.
DISCUSSION
Background
There was a time when hemophilia was characterized by patients suffering uncontrollable muscle and joint bleeding.8 These joint hemarthroses were very painful and led to early joint degeneration and contractures, most often involving the knee and the ankle, although other joints could be involved. Factor VIII was available but difficult to produce, and pooled human plasma was needed to provide enough factor VIII for treatment. Supplies were limited and, unfortunately, many patients developed hepatitis and HIV because of the large number of donors supplying the pooled plasma. The treatment for the disease was nearly as bad as the disease itself.
Improved techniques were developed in the 1980s: donors were screened and factor VIII was processed with virus-inactivation measures. These techniques significantly decreased the disease transmission rate.9 In 1992, recombinant factor VIII was created in human albumin in the laboratory, thus minimizing disease transmission and increasing availability. In 1997, it became possible to make recombinant factor VIII without albumin, thereby eliminating any risk of disease transmission. This breakthrough both increased the supply and lowered the number of complications. Increased availability of recombinant factor VIII in turn stimulated interest among young patients with hemophilia who wanted to participate in more physical activities.10 Factor VIII treatment has evolved from being a reactive treatment to, in some cases, being used prophylactically.11 However, acceptance of the involvement of athletes with hemophilia in contact-level sports has not been as forthcoming.12–14
Physiology
Hemophilia is a genetic disorder that affects the ability of blood to clot. The classic form of the disease, hemophilia A, prevents the appropriate production of factor VIII. This is an X chromosome–linked recessive gene defect occurring in 1 in 10 000 people. It should be noted that 30% of those with hemophilia have no family history of the disease; in these patients, spontaneous gene mutations are responsible.15
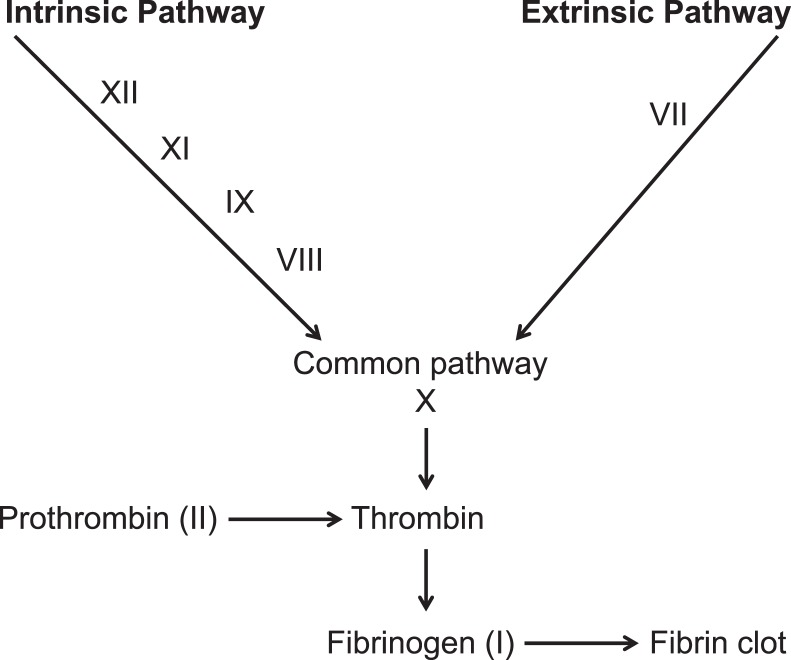
The clotting cascade involves 2 pathways. In the extrinsic pathway, injury activates factors in the soft tissue to form a clot. The intrinsic pathway responds to abnormalities in the blood vessel itself and is initiated by exposed endothelial cell surfaces. All components of the intrinsic pathway are found in the plasma, and factor VIII is an important component of this pathway. In the clotting cascade, the 2 pathways converge to form a clot (Figure).
Figure.
The coagulation cascade.
Bleeding disorders are also classified by severity. This classification is usually based on the percentage of the factor that is present in the blood. If factor VIII is 5% to 50% of normal, the disease is mild; 1% to 5%, moderate; or less than 1%, severe.15 Symptoms of hemophilia are typically related to the severity of the missing clotting factor. As a result, those with mild hemophilia often have no symptoms. However, patients with severe hemophilia can experience spontaneous bleeding into joints and muscles with no inciting trauma. Bleeding episodes of any kind in patients with hemophilia require prompt treatment with factor VIII. A bleed is defined as any episode of bleeding requiring additional clotting factor concentrate to be administered.15 Complications of a bleed can include acute compartment syndrome and an increased chance of myositis ossificans as well as the obvious problem of blood loss.16,17 Factor VIII inhibitors (antibodies) can develop with frequent administration of recombinant factor VIII. These levels are tracked by the athlete's hematologist and dosages adjusted accordingly. If inhibitor levels become too high, a period of time without prophylactic factor VIII administration may be necessary to bring the levels down. New medications have been designed to bind to these inhibitors so that the recombinant factor VIII remains effective.
Sports
So why should we even consider sport participation by patients with hemophilia? No good prophylactic protocol had been published previously to address their care. Obviously, for the sports medicine team, it would be easier to simply prohibit an athlete with a significant bleeding disorder from competing for fear of injury and complications. Additionally, parents and other care providers might prefer that their children with hemophilia not enter an unprotected environment, such as sports, to avoid injury and problems resulting from the bleeding disorder.
Yet not being physically active creates its own problems. Children with hemophilia are less physically fit and have an increased risk of obesity.18,19 Twenty percent of hemophiliac children are overweight.20 Obese patients with hemophilia are also at increased risk of hemophilia-associated arthropathy.21 Most of the recommendations in the sports literature today indicate that these children should be less physically active because of the perception that participation in physical activities will cause bleeding episodes.5–7 However, improved strength and coordination enhances insulin sensitivity, socialization, and self-esteem in these patients.22,23 Regular exercise builds muscle strength, increases joint motion, reduces pain, and decreases the frequency of joint hemorrhage. Activity also increases the level of factor VIII produced by the endothelium.18
In 2012, Manco-Johnson24 demonstrated a need for these patients to feel like part of a peer group. For many males, social encounters center on sports activities. Thus, participation in sports activities helps to keep them from feeling different because of their bleeding disorder and reduces the sense of isolation. The author showed that sports may also motivate the patient to learn to self-infuse prophylactic recombinant factor VIII. More body awareness also leads to earlier recognition of bleeds, so that treatment can be instituted quickly.
Historically, one of the earliest published accounts of an athlete playing sports with a bleeding disorder was of a collegiate hockey player in 1980.25 He had to stop playing because of complications from bleeding episodes. In 2002, a case report9 described a 21-year-old collegiate soccer player who developed a knee hemarthrosis during competition without any documented history of injuries or any knowledge of a bleeding disorder. He was treated with factor VIII and returned to play in 6 weeks. The authors delineated an emergency plan to treat players with hemophilia-related bleeding disorders. This plan included a referral to a hematologist for clearance and for injury, desmopressin acetate effectiveness testing, transport of factor VIII to all games, and knowledge of the locations of nearby hemophilia treatment centers for away games. Desmopressin acetate is available as a nasal spray and has been shown to release factor VIII from the endothelium in some patients with hemophilia,15 but it has now largely been replaced by recombinant factor VIII. The authors' plan was more of a reactive strategy to a bleeding episode, not a prophylactic plan.
Other than case reports, few discussions in the literature relate to organized sports participation and hemophilia. Three older Scandinavian studies26,27 documented somewhat successful athletic participation. However, these studies involved several Nordic-type sports, not the more common sports played in North America. No researchers have fully described the extent to which girls and boys with bleeding disorders participate in sports.
In 1990, McLain and Heldrich5 published some of the first sports guidelines for athletes with hemophilia. They addressed which sports were appropriate for athletes with bleeding disorders and which were not. The sports were divided into noncontact, contact, and collision activities; contact and collision sports were not thought to be appropriate. These same sport recommendations were republished in the Journal of Athletic Training9 in 2002, along with a discussion of the recently passed ADA. In 2005, Anderson and Forsyth3 categorized sports somewhat differently: level 1 sports were those in which significant collisions were not expected, level 2 sports involved possible significant collisions, and level 3 sports were characterized by significant, inevitable collisions. The National Hemophilia Foundation supported the Anderson and Forsyth3 classification.
In 2003, Fiala et al7 published the results of a survey of NCAA Division I athletic trainers and physicians to investigate if patients with hemophilia were participating in NCAA Division I sports. Among the returned surveys, only 1 reported an athlete who was actively participating in a contact sport. When they followed up on the unreturned surveys, the authors found that many team physicians did not know enough to answer the questions that were asked. The authors concluded that (1) certain sports were associated with a greater risk of musculoskeletal injury and should be avoided and (2) the effectiveness of desmopressin acetate was the most important variable in whether team physicians allowed patients to play. It was clear that most team physicians did not how to handle medical situations involving players with hemophilia and that they were unaware of the locations of hemophilia centers and that modern treatments with recombinant factor VII were available.
Our review of the literature confirmed that we were not alone in our lack of knowledge about the preparticipation clearance or potential treatment of our athlete with hemophilia. We also recognized the importance of carefully following the law and NCAA regulations to be fair in our evaluation of the athlete and to protect the university.28,29
The ADA is a federal statute that is frequently referred to and quoted but is not well understood by athletes, team physicians, and collegiate sports administrators.30,31 This effort started with the Rehabilitation Act of 1973,32 which says no otherwise “qualified person” because of a disability may be denied the participation in, be denied the benefits of, or be subjected to discrimination under any program or activity receiving federal financial assistance. This law was supplemented by the more familiar ADA in 1990, which prohibited any public entities from denying “otherwise qualified persons” with disabilities the right to participate.30 The ADA noted that a person with a disability is anyone who has a physical or mental impairment that substantially limits 1 or more of the major life activities. The person must be able to meet the essential eligibility requirements of a program with or without “reasonable accommodations” in spite of the restrictions imposed by the disability. Courts and colleges continue to struggle to identify these reasonable accommodations and procedures that will allow for nondiscriminatory participation. According to this statute, an athlete who otherwise meets all criteria for participation, including the skill level and strength and conditioning required to make the team, must be allowed to play.33
After a series of Supreme Court decisions narrowed the definition of disability in case law, President Bush signed the ADA Amendments Act of 2008,31 which clarified that people with bleeding disorders are also protected under the ADA as well.31,34 Team physicians must make participation decisions considering the type of sport and possible harm to an athlete's health. Our athlete's hematologist believed that a protocol including prophylactic administration of factor VIII could provide reasonable accommodations for his participation.
Recent studies show the effectiveness of this prophylactic treatment in children with hemophilia. In 2007, Manco-Johnson et al35 found that the risk of bleeding dropped by 82% in 65 children given prophylactic recombinant factor VIII. Gringeri et al,36 in 2011, observed that the risk of bleeding decreased by 48% in 43 children given prophylactic recombinant factor VIII. In 2009, Ross et al37 compared 299 high-impact seasons in 27 children with 46 low-impact seasons in 10 children, 92% of whom were receiving regular recombinant factor VIII prophylaxis. No difference in bleeding episodes was evident between the high-impact and low-impact seasons. However, bleeding events related to sports in children with severe hemophilia accounted for only a small percentage of total bleeding episodes, and the authors felt that athletic participation was not a prognostic factor for joint outcomes. Tiktinsky et al,38 in 2009, studied 44 children with hemophilia who were participating in sports and not using prophylaxis. Again, bleeding events related to sports specifically in children with severe hemophilia represented a small percentage of total bleeding episodes. These data from children with bleeding disorders receiving prophylactic treatment went against the prevailing thought about sport participation.
An important study by Broderick et al39 in 2012 noted that for 104 Australian children with moderate or severe hemophilia, the risk of bleeding events requiring acute factor VIII administration increased in level 2 and 3 sports. They concluded that factor VIII level below a critical threshold was a better determinant of bleeding risk than actual exposure to vigorous activity. They suggested dividing a weekly dose of recombinant factor VIII by the number of days of sports activities and giving each dose just before participation. We included this recommendation in our protocol.
Our protocol is limited mainly by a lack of supportive data. Its successful application to other athletes and whether it will work in collision-sport athletes are unknown. Also, our athlete had moderate hemophilia; the protocol's success in patients with more severe cases of hemophilia is uncertain.
Future authors investigating physical activities in patients with hemophilia should address the long-term effects of sports on the weight-bearing joints. The effects of subclinical microbleeding are completely unknown. Guidelines for the participation of athletes with hemophilia in collision sports are clearly needed. These guidelines should include the education of these athletes and their parents as well as involvement in conditioning and strengthening programs. In development are longer-acting factor VIII products that may allow improved prophylactic treatment of bleeding disorders in these athletes.40,41
CONCLUSIONS
Athletes with hemophilia can participate in contact sports if an appropriate prophylaxis protocol is followed. Preparticipation clearance and communication with the hematologist are necessary. Correct dosing and timing of recombinant factor VIII are critical and should be monitored throughout the athlete's season.
From a given level of trauma, the athlete with hemophilia is probably no more likely to bleed and will not bleed faster than any other athlete. Bleeding is simply prolonged until the serum factor VIII level is therapeutic. A bloodstream factor VIII level that is below a critical threshold is a better determinant of bleeding risk than actual exposure to vigorous activity.
REFERENCES
- 1. Fromme A, Dreeskamp K, Pollmann H, Thorwestern L, Mooren FC, Volker K. . Participation in sports and physical activity of haemophilia patients. Haemophilia. 2007; 13 3: 323– 327. [DOI] [PubMed] [Google Scholar]
- 2. Gomis M, Querol F, Gallach JE, Gonzalez LM, Aznar JA. . Exercise and sport in the treatment of haemophilic patients: a systematic review. Haemophilia. 2009; 15 1: 43– 54. [DOI] [PubMed] [Google Scholar]
- 3. Anderson A, Forsyth A. . Playing It Safe; Bleeding Disorders, Sports and Exercise. New York, NY: National Hemophilia Foundation; 2005. [Google Scholar]
- 4. Mann D. . Kids with hemophilia should be active, but avoid risky sports: study. Health Day Reporter Web site. http://consumer.healthday.com/circulatory-system-information-7/blood-disorder-news-68/kids-with-hemophilia-should-be-active-but-avoid-risky-sports-study-669483.html. Published October 9, 2012. Accessed September 19, 2014. [Google Scholar]
- 5. McLain LG, Heldrich FT. . Hemophilia and sports. Phys Sportsmed. 1990; 18 11: 73– 80. [DOI] [PubMed] [Google Scholar]
- 6. Owens S, Baglin T. . Recurrent haematomas of the thigh: a case of van Willebrand's disease presenting to a sports clinic. Br J Sports Med. 2000; 34 2: 122– 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiala KA, Hoffmann SJ, Ritenour DM. . A survey of team physicians on the participation status of hemophilic athletes in National Collegiate Athletic Association Division I athletics. J Athl Train. 2003; 38 3: 245– 251. [PMC free article] [PubMed] [Google Scholar]
- 8. Beyer R, Ingerslev J, Sorensen B. . Muscle bleeds in professional athletes: diagnosis, classification, treatment and potential impact in patients with haemophilia. Haemophilia. 2010; 16 6: 858– 865. [DOI] [PubMed] [Google Scholar]
- 9. Fiala KA, Hoffmann SJ, Ritenour DM. . Traumatic hemarthrosis of the knee secondary to hemophilia A in a collegiate soccer player: a case report. J Athl Train. 2002; 37 3: 315– 319. [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes C, Lillicrap D, Pasmino-Canizares J, et al. . Pharmacokinetics of recombinant factor VIII (Kogenate-FS) in children and causes of inter-patient pharmacokinetic variability. Haemophilia. 2006; 12 suppl 4: 40– 49. [Google Scholar]
- 11. Carlsson M, Berntorp E, Bjorkman S, Lindvall K. . Pharmacokinetic dosing in prophylactic treatment of hemophilia A. Eur J Haematol. 1993; 51 4: 247– 252. [DOI] [PubMed] [Google Scholar]
- 12. Buzzard BM. . Sports and hemophilia: antagonist or protagonist. Clin Orthop Relat Res. 1996; 328: 25– 30. [PubMed] [Google Scholar]
- 13. Committee on Sports Medicine and Fitness. American Academy of Pediatrics: medical conditions affecting sports participation. Pediatrics. 2001; 107 5: 1205– 1209. [DOI] [PubMed] [Google Scholar]
- 14. Mulder K, Cassis F, Seuser DR, Naryan P, Dalzell R, Poulsen W. . Risks and benefits of sports and fitness activities for people with haemophilia. Haemophilia. 2004; 10 suppl 4: 161– 163. [DOI] [PubMed] [Google Scholar]
- 15. Harmening D. . Clinical Hematology and Fundamentals of Homeostasis. Philadelphia, PA: FA Davis; 1997: 493– 501. [Google Scholar]
- 16. Beiner JM, Jokl P. . Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res. 2002; 403 suppl: S110– S119. [DOI] [PubMed] [Google Scholar]
- 17. Madigan RR. . Acute compartment syndrome in hemophilia. A case report. J Bone Joint Surg Am. 1982; 64 2: 313. [PubMed] [Google Scholar]
- 18. Coelho JD, Cameron KL. . Hemophilia and resistance training: implications for the strength and conditioning professional. Strength Cond J. 1999; 21 5: 30– 33. [Google Scholar]
- 19. Wittmeier K, Mulder K. . Enhancing lifestyle for individuals with haemophilia through physical activity and exercise: the role of physiotherapy. Haemophilia. 2007; 13 suppl 2: 31– 37. [DOI] [PubMed] [Google Scholar]
- 20. Ogden CL, Carroll MD, Kit BK, Flegal KM. . Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012; 307 5: 483– 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van der Net J, Vos RC, Engelbert RH, van den Berg MH, Helders PJ, Takken T. . Physical fitness, functional ability and quality of life in children with severe haemophilia: a pilot study. Haemophilia. 2006; 12 5: 494– 499. [DOI] [PubMed] [Google Scholar]
- 22. Seuser A, Boehm P, Kurme A, Schumpe G, Kurnik K. . Orthopaedic issues in sports for persons with haemophilia. Haemophilia. 2007; 13 suppl 2: 47– 52. [DOI] [PubMed] [Google Scholar]
- 23. Von Mackensen S. . Quality of life and sports activities in patients with haemophilia. Haemophilia. 2007; 13 suppl 2: 38– 43. [DOI] [PubMed] [Google Scholar]
- 24. Manco-Johnson MJ. . Collision sports and risk of bleeding in children with hemophilia. JAMA. 2012; 308 14: 1480– 1481. [DOI] [PubMed] [Google Scholar]
- 25. Pelletier J. Quinn. David. Greatest Hockey Legends Web site. http://internationalhockeylegends.blogspot.com/2007/12/david-quinn.html. Published December 2007. Accessed September 19, 2014. [Google Scholar]
- 26. Heijnen L, Mauser-Bunschoten EP, Roosendaal G. . Participation in sports by Dutch persons with haemophilia. Haemophilia. 2000; 6 5: 537– 546. [DOI] [PubMed] [Google Scholar]
- 27. Heijnen L, Mauser-Bunschoten E, Roosendaal G. . Inventory of participation in physical activities and sport by 166 Dutch persons with haemophilia. Haemophilia. 1996; 2 suppl 1: 120. [DOI] [PubMed] [Google Scholar]
- 28. Mitten MJ. . When is disqualification from sports justified? Medical judgment vs. patients' rights. Phys Sportsmed. 1996; 24 10: 75– 78. [DOI] [PubMed] [Google Scholar]
- 29. Putukian M. . Return to play: making the tough decisions. Phys Sportsmed. 1998; 26 9: 25– 27. [DOI] [PubMed] [Google Scholar]
- 30. Thomas SB. . College students and disability law. LD Online Web site. http://www.ldonline.org/article/6082. Accessed September 19, 2014. [Google Scholar]
- 31. Americans with Disabilities Act Amendments Act of 2008. US Equal Employment Opportunity Commission Web site. http://www.eeoc.gov//laws/statutes/adaaa_info.cfm. Published September 25, 2008. Accessed September 15, 2014. [Google Scholar]
- 32. The Rehabilitation Act, Public Law 93-112, 87 stat. 355. Internet Archive Web site. https://archive.org/stream/publiclaw931129300unit#page/nl/mode/2up. Accessed October 28, 2016. [Google Scholar]
- 33. Matthews v Nat'l Collegiate Athletic Ass'n, Pac-10 Athletic Conference, 79 F Supp 2d 1199 (Wash 1999). [Google Scholar]
- 34. Cornet J. . Congress passes new laws beneficial to people with bleeding disorders. Hemophilia of Georgia Web site. http://www.hog.org/publications/detail/congress-passes-new-laws-beneficial-to-people-with-bleeding-disorders. Accessed October 7, 2014. [Google Scholar]
- 35. Manco-Johnson MJ, Abshire TC, Shapiro AC, et al. . Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007; 357 6: 535– 544. [DOI] [PubMed] [Google Scholar]
- 36. Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM; . ESPRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the Esprit Study). J Thromb Haemost. 2011; 9 4: 700– 710. [DOI] [PubMed] [Google Scholar]
- 37. Ross C, Goldenberg NA, Hund D, Manco-Johnson MJ. . Athletic participation in severe hemophilia: bleeding and joint outcomes in children on prophylaxis. Pediatrics. 2009; 124 5: 1267– 1272. [DOI] [PubMed] [Google Scholar]
- 38. Tiktinsky R, Kenet G, Dvir Z, et al. . Physical activity participation and bleeding characteristics in young patients with severe haemophilia. Haemophilia. 2009; 15 3: 695– 700. [DOI] [PubMed] [Google Scholar]
- 39. Broderick CR, Herbert RD, Latimer J, et al. . Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012; 308 14: 1452– 1459. [DOI] [PubMed] [Google Scholar]
- 40. Collins PW, Fischer K, Morfini M, Blanchette VS, Bjorkman S; . International Prophylaxis Study Group Pharmacokinetics Expert Working Group. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011; 17 1: 2– 10. [DOI] [PubMed] [Google Scholar]
- 41. Pipe SW. . The hope and reality of long-acting hemophilia products. Am J Hematol. 2012; 87 suppl 1: 533– 539. [DOI] [PubMed] [Google Scholar]