Abstract
Suicide ranks among the leading causes of death around the world, and takes a heavy emotional and public health toll on most societies. Both distal and proximal factors contribute to suicidal behaviour. Distal factors — such as familial and genetic predisposition, as well as early-life adversity — increase the lifetime risk of suicide. They alter responses to stress and other processes through epigenetic modification of genes and associated changes in gene expression, and through the regulation of emotional and behavioural traits. Proximal factors associate with the precipitation of a suicidal event and include alterations in key neurotransmitter systems, inflammatory changes and glial dysfunction in the brain. This Review explores the key molecular changes associated with suicidality, and presents some promising avenues for future research.
Introduction
In most developed countries, suicide ranks among the leading causes of death for individuals of all ages and is the leading or the second most common cause of death for young people1. Due to the existence of effective treatments for suicidality and associated psychiatric disorders, death by suicide is often avoidable2. However, suicide remains, to a large degree, charged with prejudice and stigma, characteristics that do not encourage individuals who experience suicidal ideation or exhibit suicidal behaviour to reach out and seek help. Despite the extent of its public health impact, there is generally little societal awareness about suicide’s toll, which has granted suicide the title of “the quiet epidemic”3.
Suicide completion is commonly regarded as the extreme end of a continuum that also includes suicide attempts and suicidal ideation4. Forthe purpose of this Review, suicidality will be used to refer to both suicidal ideation and suicidal behaviours, and suicidal behaviours will be considered as a single entity. However, there is a debate about the relationship between these phenotypes, and disagreement about the inclusion of other presentations of self-harm, such as non-suicidal self-injurious behaviours, in this continuum5. Considerable variability exists between and within suicidality categories in terms of level of intent to self-harm, severity of self-harm outcome and associated psychopathology, among others; this variability probably reflects the underlying aetiological heterogeneity of suicidality and related psychopathology. Most individuals presenting with suicidal behaviour are affected by a psychiatric disorder6,7, an association that is strongest among suicide completers (approximately 90% of individuals who die by suicide were affected by a psychiatric disorder before death, notably by major depressive disorder (MDD), schizophrenia, substance-related disorders, and/or personality disorders7). Of note, suicidal behaviour disorder (SBD) was recently proposed as a diagnosis for further consideration for the DSM8. Other variables partially explain the observed variability in each phenotypic suicidality group. Among suicide completers, differences in psychopathology, personality traits and suicide intent between individuals may be age related; for example, impulsive-aggressive traits are more strongly linked to suicide in younger subjects9,10. Sex also contributes to phenotypic variability in each category: male and female suicide attempters and suicide completers show clear differences in the methods used, their underlying psychopathology and comorbid diagnoses11,12. Thus, models of suicidal behaviours, including biological factors predisposing individuals to and mediating these phenotypes, will probably explain only a fraction of the total phenotypic variability that is observed. Nevertheless, they are helpful as they provide important conceptual frameworks that enhance our understanding of suicide-spectrum behaviours and can guide us toward new hypotheses.
Of the many existing models of suicide risk13, most are conceptually related to the initial model proposed by Moscicki14 and its refined version presented by Mann15, and are based on the premise that suicide results from the interaction of risk factors acting distally to the suicidal event, which increase predisposition to such an event, with those acting proximally to the suicidal event, which precipitate the suicidal crisis. In this Review, I describe the relationships between such distal and proximal factors, focusing on molecular events that predispose individuals to suicidality, mediate suicidal ideation and behaviour and precipitate suicidal crises, rather than on the neural circuitry of suicidal behaviour.
Distal or predisposing factors
Distal factors confer risk of suicide but have a distant temporal relationship to the suicidal crisis. As such, these factors act by increasing predisposition to suicide rather than by precipitating suicide crises. Among distal risk factors for suicide, family history, genetic variation and early-life adversity(ELA) are the best investigated.
Familial and genetic predisposition
A large number of studies examining family history of suicidal behaviour, including a recent large-registry study that comprised the total population of Sweden16, as well as studies directly interviewing relatives of probands with suicidal behaviour, have shown that suicidal behaviour runs in families, with relatives of suicide probands estimated to have a 3- to 10-fold greater risk of suicidal behaviour than relatives of controls16–18. As suicidal behaviour is strongly associated with psychopathology (see below), and psychiatric disorders also run in families, considerable effort has been made to understand to what extent the liability to suicidal behaviour is different from the liability to psychiatric disorders. In contrast to suicidal behaviour, suicidal ideation does not appear to be as clearly linked to family history of suicidal ideation19–21, although a family history of suicide attempt or completion does predict a higher frequency of suicidal ideation (2.41–5.1 times greater risk)21,22. Studies have shown that familial transmission of suicide and psychopathology, although partially overlapping, are distinct20,23,24. Thus, familial aggregation of suicide is not explained by transmission of psychopathology24. Evidence that the familial aggregation of suicidal behaviour is at least partly due to genetic factors has been provided by several twin studies, yielding variable estimates of heritability, but consistently pointing to increased concordance of such behaviour in monozygotic twins compared with dizygotic twins16,17; similarly, adoption studies have demonstrated concordant results, with biological relatives of suicide adoptees having a higher rate of suicide than relatives of control adoptees16,25 or alternatively, with parental suicide having similar risk of recurrence in adopted away and non-adopted offspring26.
Genetic variation
Guided by the genetic epidemiological data suggesting that genes increase predisposition to suicidal behaviour, many molecular genetic studies have been conducted over the past two decades to identify genetic variants that may explain liability to suicide. As for other psychiatric phenotypes, most studies used a candidate gene association approach, and particularly focused on genes coding for components of the serotonergic system, given the evidence suggesting that suicidal behaviour is associated with decreased serotonergic neurotransmission27,28. In particular, the genes coding for the serotonin transporter, tryptophan hydroxylase and monoamine oxidase A have been widely studied in molecular analyses owing to their well-defined roles in serotonin metabolism27,29. Unfortunately, given the lack of consistent findings between studies, the experience from candidate gene-based association studies told us little about the contributions of specific genes. More recently, genome-wide association studies (GWASs) of suicidal behaviour or of treatment-emergent suicidal events have been conducted to identify novel loci (Supplementary information S1 (table))30–35. Although these have produced mostly inconclusive or inconsistent results, it is notable that such approaches permit an unbiased analysis of molecular pathways implicated in disease and are an important avenue to explore. For instance, one GWAS identified 14 single-nucleotide polymorphisms (SNPs) that were associated with treatment-emergent suicidal ideation in patients treated with antidepressants31, and another study examining suicide completers identified seven genes that were differentially expressed in suicide but not in mood disorders36. However, most of the GWASs used relatively small samples that lacked the power to detect effects other than major gene effects and their results require confirmation. In many of the large-scale GWASs conducted to date, SNPs that have been identified to be of interest in the discovery phase of studies have not been identified as such in replication cohorts32–35,37.
Early-life adversity
Several lines of evidence support a strong relationship between ELA — such as experiences of sexual or physical abuse during childhood and parental neglect — and lifetime risk of suicidal behaviour. This evidence comes from prospective cohort studies that have investigated epidemiologically representative samples6,38 and retrospective cross-sectional studies that have investigated epidemiological and clinical samples39,40. Maltreatment during early development is also among the strongest predictors of psychiatric pathology and of a severe clinical course for psychiatric disorders, including early onset of illness, poor treatment response and increased comorbidity41–43. However, the specific association between ELA and lifetime risk of suicidal behaviour is robust and is not solely explained by the link between psychiatric disorders and childhood maltreatment44,45.
Between 10 and 73% of individuals who manifest suicidal behaviour have a history of childhood abuse (the rate depends on the type and frequency of the abuse, and the form of suicidal behaviour)38,46–48. Thus, this association only explains a portion, albeit a notable to substantial one, of the total variance in risk of suicidality. A few important factors determine the strength of the relationship between childhood abuse and suicidal behaviour, including the sex of the abused, the identity of the abuser and the frequency of the abuse. For instance, the risk of suicidal behaviour is higher among individuals who have experienced abuse by close caregivers than individuals who have experienced abuse by extended family members or unrelated individuals45, and frequent abuse increases the risk of suicidal behaviour45. During development, immediate caregivers are responsible for the establishment of adequate attachment styles and appropriate emotional responses to environmental and stress-related stimuli throughout the life of the individual49,50. Attachment styles define the ways in which individuals relate to others and can be broadly divided into secure and insecure attachments, with insecure attachments being characterized by anxiety or avoidance51. Thus, frequent exposure to maltreatment by those who are supposed to protect and provide care may signal that an environment is hostile. These signals in turn may prompt the developing brain to adjust stress-response circuits to adapt to this hostile environment by increasing levels of alertness. In support of this hypothesis, studies suggest a relationship between poor parent-child attachment, maladjustment in the parental role and childhood abuse, and suicidal behaviour38,51.
Biological systems affected by early-life adversity
Biological embedding refers to the effects of early-life experiences on the differential regulation of biological systems and development52. Important insight into systems and pathways that undergo biological embedding and the underlying molecular mechanisms has come from animal studies that clearly suggest that variation in the early-life environment associates with stable expression changes in genes that are important for key behavioural and stress responses. Indeed, animal studies have shown that epigenetic processes, particularly DNA methylation of various genes in different brain structures, mediate biological embedding (Box 1).
Box 1. Biological embedding through DNA methylation.
Biological embedding refers to the effects of early-life experience on the regulation of biological systems52. Animal studies have provided important insight into mechanisms explaining biological embedding. One of the best-investigated models is the maternal care model in rats, which is based on naturally occurring variations in quantifiable behaviours (licking and grooming (LG); arch back-nursing) in nursing rat mothers. Variations in maternal care have marked and stable effects on endocrine and behavioural responses in the offspring, for example increased tactile stimulation by mothers dampens the hormonal response to stress203, and reduced behavioural fearfulness104. Other animal models, such as the induced maternal abusive behaviours in rats75 or early-life stress in mice204 have shown similar effects. Subsequent studies in animals have shown that epigenetic processes, particularly DNA methylation involving different genes and brain structures, mediate biological embedding205.
DNA methylation refers to the addition of a methyl group (CH3) to the 5′ carbon of a cytosine base. DNA methylation occurs commonly, but not exclusively, at sequences composed of cytosines followed by guanines (CpG dinucleotides). In general, cytosine methylation associates with transcriptional repression, which occurs by interference with the recruitment and binding of transcriptional machinery to gene regulatory regions206. However, in studies identifying DNA methylation within the gene body, it has been associated with transcriptional activation and alternative transcript selection207. DNA methylation is theoretically dynamic, but this is only the case for a minority of CpGs208, and thus DNA methylation is generally a stable process involved in long-term gene silencing, including X-chromosome inactivation, suppression of alternative promoters and tissue-specific suppression of gene regulation.
The exciting findings from animal studies have led to a growing interest in the investigation of epigenetic factors — primarily DNA methylation — as possible mediators of biological embedding resulting from ELA in humans. Although theoretically appealing, studying methylation changes caused by ELA in humans and associating them with behavioural and emotional phenotypes poses a number of operational and logistical challenges. Epigenetic changes are tissue- and cell-type specific, but sampling brain tissue from living subjects is impossible. Thus, epigenetic studies on human brains are limited to post-mortem specimens. Although post-mortem studies are informative, it is difficult to establish temporal relationships between epigenetic marks and behavioural phenotypes from such studies. By contrast, peripheral samples are less challenging to obtain, but the extent to which they are informative about processes occurring in the CNS is currently under debate. In spite of these challenges, several epigenetic studies have been conducted investigating both CNS and peripheral samples of individuals exposed to ELA.
Of particular interest in the context of suicidal behaviour is the regulation of the hypothalamus-pituitary-adrenal (HPA) axis. The activation of the HPA axis leads to cortisol release, which is a steroid hormone secreted in response to stress that promotes arousal and attention, among other physiological changes53. Cortisol also binds to the glucocorticoid receptor in the hippocampus to inhibit further stimulation of the HPA axis in a negative feedback loop. The HPA is overactive in individuals with ELA, who are more likely than controls to display heightened stress responsiveness54. As such, NR3C1, which encodes the glucocorticoid receptor, has been widely studied. Several animal studies have suggested that the early environment regulates Nr3c1 through DNA methylation changes (Box 2). The first evidence for an effect of ELA on the human epigenome indicated that childhood abuse or severe neglect in humans regulates both the patterns and levels of NR3C1 methylation in the hippocampus, which can lead to increased HPA reactivity in humans exposed to childhood maltreatment55. Individuals who died by suicide and had histories of severe childhood abuse had a marked decrease in the level of both total NR3C1 mRNA and untranslated exon 1F variant NR3C1 mRNA, compared with individuals who died by suicide and did not have histories of childhood abuse and non-suicide controls. This decreased mRNA expression was associated with increased methylation in the exon 1F promoter, particularly in a region where the transcription factor nerve growth factor-induced protein A (NGFI-A) binds, a finding in line with evidence from rats (Box 1). In vitro studies suggested that this increased methylation observed in suicide completers with histories of abuse could interfere with NGFI-A binding of the NR3C1 promoter, resulting in decreased glucocorticoid receptor expression55.
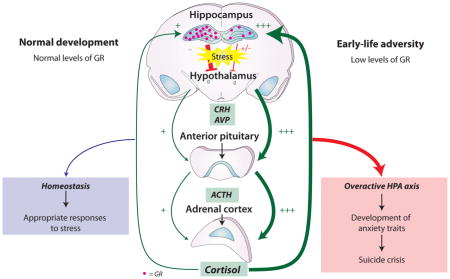
Box 2. Effect of early-life adversity on the hypothalamus-pituitary-adrenal axis.
The hypothalamus-pituitary-adrenal (HPA) axis is activated by stress. Upon activation, the hypothalamus secretes corticotropin releasing hormone (CRH) and arginine vasopressin (AVP), which act on the anterior pituitary gland to trigger the release of adrenocorticotropic hormone (ACTH). This stimulates the adrenal cortex to produce cortisol, which heightens alertness. Cortisol-mediated activation of glucocorticoid receptors in the hippocampus exerts a negative feedback on HPA activity. The glucocorticoid receptor-encoding gene, NR3C1, is downregulated in the hippocampus of individuals exposed to ELA, leading to ineffective inhibition of CRH secretion and an overactive HPA. This overactive HPA may be associated with the development of anxiety traits, which in turn are mediators of suicidal behaviour risk. In humans, NR3C1 mRNA may contain one of11 untranslated splice variants of exon 1, encoded by 7 different exon 1s, each with its own promoter, and each mRNA variant has a unique tissue-specific distribution209. There is remarkable homology between the human and rat exon 1 structure209,210, and mRNAs containing exon 17 (in rats) and 1F (in humans) are highly expressed in the hippocampus209. CpG methylation levels in the exon 17 promoter region are markedly increased at almost all CpGs in offspring raised by low LG rat mothers, whose increased physical attention to pups results in attenuated stress responses (Box 1), compared with offspring raised by high LG mothers. Importantly, one CpG located in the 5′ end of the binding site of the transcription factor, nerve growth factor-induced protein A (NGFI-A), is methylated in almost 100% of offspring raised by low LG mothers, whereas it is almost never methylated in offspring from high LG mothers205.
Other animal studies on the effects of early life environments on behavioural phenotypes have shown comparable results in genes coding for components of the HPA axis or other key gene systems. For instance, in mice, maternal deprivation (placement in a cleaned cage, devoid of maternal odour, for three hours per day for the first ten days of life) produces sustained hyperactivity of the HPA axis that is characterized by increases in stress-induced corticosteroid secretion and in the expression of pro-opiomelanocortin (POMC) in the pituitary, and hypertrophy of the adrenals204. These changes in POMC expression associate with sustained POMC promoter hypomethylation211. These mice also present increased despair-like behaviour and memory deficits, phenotypes that are mediated through arginine vasopressin (AVP) signalling and epigenetic adaptations at an AVP enhancer locus. Accordingly, mice subject to early life stress present a persistent increase in AVP expression in the hypothalamic paraventricular nucleus (PVN), which associates with decreased DNA methylation of an AVP enhancer element located in the intergenic region between AVP and oxytocin, where methyl CpG binding protein 2 (MeCP2) binds. As a result, MeCP2 occupancy of this AVP regulatory sequence is decreased, leading to sustained increased AVP expression204. Such overexpression of AVP can result in HPA hyperactivity and altered coping mechanisms resembling depression in humans. In adult animals, decreased AVP promoter methylation in the amygdala is associated with active coping mechanisms after exposure to stressful stimuli212, and AVP expression is associated with aggressive behaviour213–215. Collectively, animal studies on early life environmental variation75,205 indicate that the early environment epigenetically regulates diverse genomic loci that are important in the regulation of emotional and behavioural traits of relevance to depression and suicide.
Since the initial study linking glucocorticoid receptor methylation in the brain with childhood abuse55 was published, several independent studies have been conducted to investigate the relationship between NR3C1 exon 1 methylation, ELA and suicidal behaviour or psychopathology, including phenotypes associated with altered parental care56–61 (Supplementary information S2 (table 2)). Although these studies were conducted using different study designs, measures of adversity, and different tissue samples, there is a remarkable consistency in their findings, which largely indicate increased levels of methylation in NR3C1 exon 1F among individuals exposed to less favourable environmental experiences during early life.
Epigenetic processes are essential in the differentiation of cells and tissues, as well as in the developmental regulation of genes. Thus, there is a predictably higher variability when comparing epigenetic patterns between tissues from the same individual than when comparing the same tissue from different individuals62. However, there is also evidence of within-individual epigenetic variant correlation across tissues62. The consistency in the NR3C1 methylation findings observed using different tissues suggests that social adversity may activate systemic signals such as steroids or other hormones that may have an impact on epigenetic patterns in multiple tissues.
A different line of evidence suggesting that ELA regulates the activity of the HPA axis through differential methylation comes from studies investigating FK506-binding protein 5 (FKBP5). This protein regulates the glucocorticoid receptor by decreasing its capacity to bind glucocorticoids, which hampers the ensuing translocation of the glucocorticoid receptor complex to the nucleus. Certain sequence variants of FKBP5 put individuals at increased risk of depression and posttraumatic stress disorder63,64 and of exhibiting suicidal behaviour65–67 or experiencing suicidal events in the course of antidepressant treatment68,69, and the link with suicidality is particularly strong for subjects with a history of ELA70,71. Interestingly, one study reported that the interaction between FKBP5 genotype and ELA occurs through decreased methylation affecting functional glucocorticoid response elements located in intron 7 of FKBP5, resulting in a downstream increase in FKBP5 expression and glucocorticoid receptor resistance72.
ELA also differentially regulates genes and pathways involved in neuronal plasticity. For instance, genome-wide methylation studies in hippocampal tissue from individuals who died by suicide and had histories of ELA indicated that ELA was associated with differential methylation of genes involved in neuronal growth and neuroprotection58,73. Studies in peripheral samples from living subjects also suggested that a history of ELA was linked to differential methylation of neuronal plasticity genes74. These findings are concordant with animal studies that support early-life environmental regulation of plasticity genes, such as the gene coding for brain-derived neurotrophic factor (BDNF). Experiments conducted in rodents showed that when gestating females were put under conditions of stress (introduction to a new environment with inadequate bedding material), the expression of Bdnf was dysregulated. Specifically, Bdnf mRNA expression was decreased in the prefrontal cortex, and this decreased expression correlated with hypermethylation of the Bdnf promoters in exons IV and IX75. Similar effects have been reported for the Bdnf exon IV promoter in the dorsal hippocampus in an adult rat model of post-traumatic stress disorder76.
Another neurotrophic factor, glial-cell derived neurotrophic factor (GDNF), is also epigenetically regulated by environmental experience in rodents through chromatin modification and DNA methylation of its gene promoter, and these changes associate with behavioural responses to chronic stress77. However, these methylation changes in Gdnf were observed in adult mice, thus suggesting that the epigenetic programming of neurotrophic factors such as GDNF may not be limited to developmental periods. It remains unclear whether the epigenetic changes occurring as a result of ELA are of a similar quality, intensity and stability if they occur during childhood or later in life(Box 3).
Box 3. Stability of DNA methylation changes over time.
In well-characterized brain circuits, brain plasticity is considerably more pronounced during childhood than in later years216, and clinical studies suggest that abusive experiences during early-life are more likely to result in pervasive behavioural phenotypes than similar events experienced in adulthood217–219. However, the relationship between timing of the stressor, epigenetic impact and behavioural consequences remains one of the most critical questions in epigenetics studies of mental health phenotypes.
Recent publications have begun exploring longitudinal analyses of methylation in relation to the subject’s environment. Although the stability of epigenetic changes over time has not yet been clearly demonstrated, a study evaluating methylation at a wide range of loci indicates that DNA methylation is likely to be stable over long periods (11–20 years)220. Although this finding requires confirmation, it is interesting to note that the authors specifically examined genes involved in the stress response (NR3C1 and CRH) and found their methylation status to be stable over time. Additionally, emotional adversity during childhood effects long-term changes in methylation. In a study evaluating monozygotic twins at 5 and 10 years of age, Ouellet-Morin and colleagues showed that children who had experienced bullying exhibited increased methylation at the serotonin transporter gene (SERT) compared with their non-bullied monozygotic twin221. Interestingly, this study also showed that individuals with increased SERT methylation exhibited decreased cortisol response to stressful situations, pointing to a link between the serotonergic response and the hypothalamus-pituitary-adrenal axis. Lasting changes in DNA methylation also appear to occur into adulthood, as shown by recent studies on post-stroke depression. In patients having suffered a stroke, those experiencing post-stroke depression or worsening depression during the year following their stroke had increased methylation of BDNF and SLC6A4222,223. Such examples of long-lasting epigenetic changes help to understand how distal events, such as early-life adversity, can contribute to increased suicide risk, and how proximal events, such as stressful life events, can precipitate suicidal acts by altering gene expression profiles in the brain that have a profound impact on behaviour.
BDNF and the gene encoding its high-affinity receptor, tropomyosin-receptor-kinase B (TrkB), have received significant attention in human studies of suicidal behaviour, although most of these studies have not examined whether the participants had a history of ELA. Some of these studies have revealed that suicide completers have decreased mRNA and/or protein levels of BDNF, TrkB or both in the prefrontal cortex78–81, temporal cortex82 and hippocampus78,83. Moreover, others have reported differential methylation patterns in regulatory regions of BDNF and TRKB80,83. Specifically, in the Wernicke area of suicide completers, four CpG sites located downstream of the BDNF promoter IV transcription initiation site were more highly methylated in suicide completers than in controls, and this increased methylation was associated with significantly lowered BDNF expression, which is consistent with the expected repressive effects of methylation in promoter sequences on transcription84. In line with these findings, recent studies have reported that living depressed patients display differential methylation of the BDNF promoter in peripheral samples85–87. In addition, studies have revealed that suicide completers exhibit differential methylation in the promoter80 and in a transcript-specific 3′UTR81 of TRKB in the prefrontal cortex.
In addition to hypothesis-driven studies, a growing number of genome-wide studies have investigated changes in the methylome associated with ELA and/or suicide. In spite of the inherent sources of variation when measuring methylation levels88, and the relatively small sample size of these studies, particularly in comparison to those of GWASs, it is noteworthy that epigenome-wide association studies have identified methylation differences at several genomic loci. Studies using peripheral samples74,89 and brain samples58,73 have been conducted and have pointed to differential methylation in gene pathways involved in stress, neural plasticity and cognitive processes. Studies that investigated brain tissue and conducted cell sorting58,73 found that these differences were mostly accounted for by methylation changes in neuronal DNA. These strong associations between ELA and DNA methylation point to long-lasting changes in the regulation of gene expression that are triggered by early environment experiences, and these changes are strongly linked to suicidal behaviour 90,91.
Mediators of suicide risk
Distal factors affect biological systems and, in turn, the resulting changes in these systems can increase the risk of suicide. Thus, distal factors do not directly cause suicidal behaviour. Indeed, if they did, these factors would have been subject to selective pressures and progressively eliminated. Moreover, if distal factors had a direct link with suicide risk, ELA would have a more specific relationship with suicidal behaviour, instead of being linked to an increased risk of several negative mental health outcomes. Distal factors are therefore thought to act indirectly, through developmental traits, which may act as intermediate phenotypes, or endophenotypes, for suicidality92,93.
Although it is unclear what specific genes contribute to the total genetic variance increasing predisposition to suicidal behaviour, family studies have consistently indicated that familial aggregation for suicidal behaviour is partially explained by transmission of impulsive-aggressive traits. Familial clustering of suicidal behaviour is increased in families in which probands have higher levels of impulsive-aggressive traits than in those in which probands have lower levels of such traits20. Moreover, compared with controls, relatives of suicide completers have elevated levels of impulsive-aggressive traits and are more likely themselves to have histories of suicidal behaviour23,24. In addition, impulsive-aggressive traits show evidence of familial loading in families of suicide completers and mediate the relationship between family history and suicidal behaviour24. Thus, the transmission of behavioural traits (or endophenotypes92) such as impulsive-aggressive behaviours is probably explained by familial, and possibly genetic, susceptibility for suicidal behaviour.
In studies examining the link between endophenotypes and suicidality, anxiety, impulsivity and aggressive traits correlate with suicidal behaviour24,38,94. These findings have been supported by clinical and epidemiological studies on samples that are representative and non-representative of the general population95,96. Although they are more difficult to conduct than cross-sectional studies, longitudinal studies using multiple observation points offer stronger evidence of causal relationships and allow temporal relationships between different variables to be examined. A series of longitudinal and trajectory studies have investigated predictors of suicidal behaviour38,45,46,97–99. Collectively, this work shows that high levels of childhood anxiety and disruptive behaviour (characterized by impulsivity, aggression, hyperactivity and oppositional behaviour) are linked to suicidal behaviour in adulthood94. Supporting these findings, cross-sectional studies and two-point longitudinal studies have shown that stable anxiety and impulsive-aggressive behaviour are associated with suicidality in adulthood100–102.
ELA is likely to act through developmental dysregulation of behavioural and emotional traits. In animal studies, changes in the early-life environment correlate with stable behavioural phenotypes103,104. For instance, in non-human primates, social deprivation leads to sustained aggressive behaviour103. In humans, individuals with histories of ELA frequently display dysregulation of emotional and behavioural traits such as internalizing and externalizing disorders105, and the personality trait phenotypes of individuals with histories of ELA share characteristics with those seen among individuals with suicidal behaviour38,106. However, this association is mitigated by other variables, such as sex and underlying disorders. High-anxiety trajectories fully mediate the relationship between ELA and suicide attempts among individuals with externalizing disorders, such as attention deficit and hyperactivity, oppositional-defiant and conduct disorders, whereas among individuals without externalizing disorders, this mediation, although still marked, is partial91. The relationships between these mechanisms may explain the link between ELA and development of high-anxiety trajectories (Fig. 1). Similarly to high-anxiety trajectories, highly disruptive trajectories, which are characterized by high levels of impulsive-aggressive traits, hyperactivity and oppositional behaviour, partially mediate the relationship between ELA and suicide attempts among females, but not among males91.
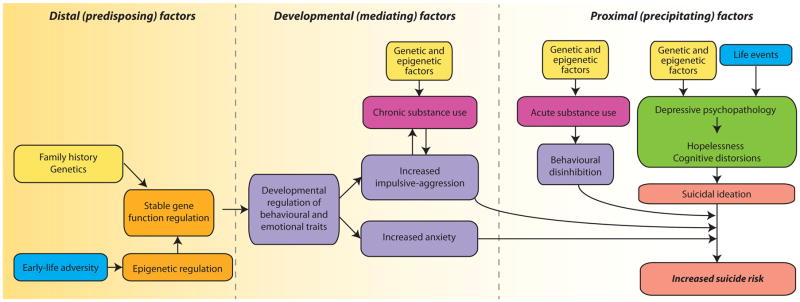
Figure 1. Overview of the contributors to suicidal behaviour.
Suicidal behaviour is regulated by a number of different factors that can be broadly categorized as distal (predisposing), developmental (mediating) or proximal (precipitating) factors. The model proposed here takes into account the classic psychosocial and genetic risk factors for suicide, and integrates newer findings on epigenetic changes associated with suicidality. Predisposing factors include family history of suicide, and associated genetic predisposition; and early-life adversity and associated epigenetic changes. In both cases, predisposing factors lead to long-term effects on gene expression and regulation. Predisposing factors do not directly trigger suicidal events, but are linked to increased suicide risk through the effects of mediating factors. Mediating factors, which may directly result from the gene changes that occur as a consequence of predisposing factors, or may be associated with other factors such as chronic substance abuse, increase the risk of suicide by accentuating traits linked with suicidality. Specifically, family disposition and early-life events can shape behavioural and emotional traits such as impulsive-aggressive behaviour and anxiety traits, which increase the risk of acting on suicidal ideation, a common feature of depressive psychopathology and hopelessness. Proximal risk factors, such as depressive psychopathology and acute substance abuse, can also be associated with genetic and epigenetic factors and are often triggered by life events.
Approximately 40% of individuals who die by suicide have lifetime histories of alcohol dependence or abuse and around 25% have histories of illicit substance dependence or abuse9. Exact rates vary globally and between studies7, but there is a robust association between chronic substance use disorders and suicide. Impulsive traits strongly associate with substance use disorders, and although it is difficult to establish in human studies whether impulsivity is a cause or an effect of the chronic use of substances, animal experiments suggest that chronic substance use leads to increased impulsivity34. Interestingly, suicide cases that were associated with a high number of distal (predisposing) risk factors had particularly high levels of comorbidity with substance use disorders107,108. Thus, chronic use of substances may further increase the levels of impulsive-aggressive traits among individuals at risk of suicide. Consistent with this hypothesis, suicide completers with comorbid substance use disorders had higher levels of impulsive-aggressive behavioural traits than those without such disorders 109.
To date, the molecular factors underlying or correlating with mediators have not been well characterized, but there are a few promising leads. Impulsive-aggressive behaviours are associated with low levels of serotonin110, as discussed below, and suicidal behaviour is associated with low levels of cholesterol25,111–113. Overall, studies exploring the link between cholesterol levels and suicidal behaviour have indicated that subjects with low serum cholesterol levels are at higher risk of suicidal behaviour, increased impulsive-aggressive behaviour, violence, and violent methods of suicide attempt (reviewed in REF25). The mechanisms underlying these relationships are currently unclear, but brain cholesterol is essential for continued neural plasticity, and it is tempting to speculate that predatory behaviours such as impulsive aggression should be modulated to some degree by dietary needs, which correlate with serum lipid levels.
Proximal or precipitating factors
Suicidal crises are typically triggered by recent life events114 and strongly associate with the onset of episodes of psychopathology. Thus, approximately 90% of individuals who die by suicide meet criteria for a psychiatric disorder in the last six months of life, according to a large number of retrospective, proxy-based studies (known as psychological autopsies)7, epidemiological and registry-based studies115, and studies in clinical at-risk populations116. Although only an average of 50% of individuals who die by suicide meet the criteria for MDD (a proportion that increases with age) at the time of death7, virtually all individuals who are suicidal, regardless of their main psychiatric diagnosis, present some degree of suicidal ideation, hopelessness, or other similar cognitive distortions that are characteristic of depressive states. When these cross-nosological depressive symptoms drastically alter the individual’s problem-solving capacity and judgment, they may be interpreted as precipitants of suicidal crises. These depressive states associate with molecular changes that are likely to underlie proximal risk factors for suicide and the most important are reviewed below.
Serotonergic alterations
Decreased serotonergic neurotransmission mediates depressive states. Almost four decades ago, Asberg and colleagues suggested that, among patients with depression, those with lower cerebrospinal fluid (CSF) levels of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), are more likely to exhibit suicidal behaviour117. Subsequently, important postmortem studies pointed to altered serotonergic binding in the frontal cortex of suicide completers118,119, and since then, researchers have extensively investigated serotonergic changes in suicide and related behaviours (reviewed in REF15). A detailed discussion of this extensive body of work is beyond the scope of this paper. One of the most consistent findings suggests that responses to challenges with fenfluramine, a potent serotonergic agonist, is blunted among living subjects who attempted suicide110. Additionally, post-mortem work using the brain cortex of suicide completers revealed decreased availability of the serotonin transporter118, and both post-mortem binding studies and in vivo studies using PET have reported altered 5HT1A and 5HT2A receptor availability in suicidal brains, suggesting that insufficient serotonin in certain areas of the brain may be linked to suicidality27,28.
Two important questions remain in relation to the association between serotonin and suicide. First, to what extent are serotonergic changes in suicide explained by depressive psychopathology? Although there is some evidence suggesting that serotonin alterations may be specific to suicidal behaviour120,121, these alterations partially overlap with those observed in the depressed brain. For example, the more pronounced serotonergic changes observed in suicide might be a function of more intense hopelessness and suicidal ideation. This key point has not been adequately addressed, particularly in post-mortem brain studies, in large part due to the methodological challenges of assessing subjective and non-behavioural symptoms of depression by means of proxy-based interviews, which is the process whereby post-mortem brain studies obtain clinical information on the subjects they investigate. Another challenge is obtaining brain tissue from individuals who were affected by depression of equivalent severity to that presented by suicides, but who died from other causes. The second question is to what extent are serotonergic changes associated with suicide state markers of suicidality or depression, or trait markers of predisposition? There is evidence that lower serotonergic neurotransmission also associates with behaviours that increase suicide risk, such as impulsive-aggressive traits122, and that serotonergic genes have both shared and unique contributions to the risk for depression and suicidal behaviour123. However, it is also clear that changes in serotonin levels associate with onset of depressive and suicidal states in euthymic individuals who had previous depressive episodes124, and in individuals at risk for depression125. Thus, serotonergic changes underlie proximal depressive and suicide risk factors.
The polyamine stress response
Our group and others have studied changes in the polyamine stress response (PSR) system in the context of suicide. The PSR, like the HPA axis, is implicated in physiological responses to physical, emotional and hormonal stressors, including glucocorticoids (Box 4). The mRNA and protein levels of several components of the polyamine system are altered in cortical and subcortical brain regions of suicide completers126–129, as well as in peripheral samples from suicide attempters130,131 and psychiatric patients132. In addition, non-human primate models of depression suggest that stress induces changes in gene expression that lead to altered brain polyamine levels133. Spermidine/spermine N1-acetyl-transferase (SAT1), which is the rate-limiting enzyme in the catabolism of polyamines, is decreased in the cortex of suicide completers126, and may even act as a peripheral biomarker of suicidality. Epigenetic regulation of some key polyaminergic genes, involving different epigenetic processes in both biosynthetic and catabolic polyamine genes, has been reported in suicide cases134–137. Although the evidence suggests differential epigenetic regulation of several polyaminergic genes in suicide, further studies are necessary to understand the external validity of these findings, their functional impact on the PSR, and their relationship to peripheral markers.
Box 4. The polyamine stress response system.
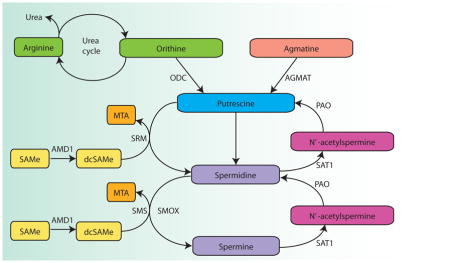
Polyamines are ubiquitous aliphatic molecules containing two or more amine (NH2) groups. This group of molecules primarily comprises putrescine, spermidine, spermine and agmatine, whose presence in mammalian brains was discovered fairly recently224. Polyamine synthesis is highly regulated, with several rate-limiting enzymes, like ornithine decarboxylase (ODC), S-adenosylmethionine (SAM) decarboxylase (AMD1) and spermidine/spermine N1-acetyl-transferase (SAT1). Their activities are tightly controlled with polyamine-mediated feedback loops. Other enzymes, such as spermidine synthase (SRM) and spermine synthase (SMS) may be induced in some conditions but otherwise exhibit constant activity levels, whereas polyamine oxidase (PAO) activity is controlled by substrate availability (see the Figure; reviewed in 223). Ornithine is produced through the metabolism of arginine in the urea cycle, which is itself controlled by substrate-limited enzymes.
Polyamines have a multitude of functions, including regulation of gene transcription and posttranscriptional modifications, in addition to modulating the activities of several proteins225. Polyamines regulate the expression or release of several neurotransmitters, including catecholamines226, glutamate227, GABA228 and nitric oxide229, and agmatine itself is thought to act as a neurotransmitter230. They also interact with several transmembrane channels and can thus influence the properties of excitable cells227,231.
In the mammalian brain, stressful stimuli such as physical, emotional and hormonal stressors, including glucocorticoids, elicit the polyamine stress response (PSR)232. Activation of the PSR results in elevated levels of putrescine and agmatine in both the brain and peripheral tissues. The magnitude of the PSR is related to the intensity of the stressor, and correlates with levels of behavioural and physiological responsiveness under stressful conditions232. The PSR can be pharmacologically manipulated233,234, and there is strong evidence showing that elevated agmatine and putrescine levels in the brain are beneficial: numerous animal studies have shown that these compounds display both anxiolytic and antidepressant effects235, and polyamine depletion can produce altered emotional reactivity and anxiety-like behaviours236. Similarly, there are some encouraging pilot data in humans suggesting that agmatine has antidepressant properties237, and in agreement with these results, studies investigating the effects of antidepressants indicate a role for the polyamine system in the antidepressant response, particularly through agmatine or putrescine allosteric binding to NMDA receptors 235.
SAM: S-adenosylmethionine; AMD1: S-adenosylmethionine decarboxylase; dcSAM: decarboxylated SAM; MTA: 5′methylthioadenosine; SMS: spermine synthase; SMOX: spermine oxidase; SRM: spermidine synthase; SAT1: spermidine/spermine N1-acetyltransferase; PAO: polyamine oxidase; ODC: ornithine decarboxylase; AGMAT: agmatinase
Glutamatergic and GABAergic alterations
Several studies have been conducted over the past decade investigating genome-wide transcriptomic changes associated with depression and suicide138,139. These studies typically used mRNA microarrays to compare post-mortem brain tissue from individuals who were diagnosed with MDD and died by suicide and individuals with good mental health who did not die by suicide. They reported dysregulation of genes involved in glutamatergic and GABAergic signalling in diverse cortical and subcortical regions. In particular, for GABAergic signalling, genes encoding GABA type A receptor subunits and their associated binding proteins were consistently found to be upregulated, notably in the prefrontal cortex, hippocampus and anterior cingulate138,139. Among genes related to glutamatergic signalling, those encoding AMPA and NMDA glutamatergic receptor subunits were upregulated in the anterior cingulate and dorsolateral prefrontal cortex, whereas the glutamine synthase gene (GLUL), which is implicated in glutamate recycling, and the genes encoding the solute carriers SLC1A2 and SLIC1A3 were downregulated in these cortical areas and the amygdala138,139. GLUL and the solute carriers are of particular interest, as these are primarily expressed in glial cells, which are altered in depression and suicide (see below). Although glutamatergic receptors have multiple functions and these expression differences may signal global expression changes that may not be specific to the underlying psychopathological processes taking place in the suicidal brain, they are consistent with growing evidence of the efficacy of glutamatergic agents as rapid-acting antidepressants that are both useful in the treatment of depressed mood140 and acute suicidal ideation141,142.
Inflammatory factors
Large amounts of data produced over the past few decades support a relationship between inflammation and depressive states143. Notably, such states have been linked to increased levels of proinflammatory cytokines, particularly tumour necrosis factor (TNF)-α and interleukin (IL)-6144–147. Furthermore, high levels of comorbidity have been observed between inflammatory autoimmune illnesses and depression, and a substantial proportion of patients undergoing therapy with cytokines, such as interferon (IFN)-α, have been found to develop depression148–151. In contrast to the clear evidence indicating an association between changes in inflammatory markers and depressive states, there is only sparse evidence linking these markers and suicidal behaviour, but the existing data seem to support an association. There are several reports of an association between suicidal behaviour, irrespective of depression status, and inflammatory cytokines (reviewed in REF152), with the most consistent results suggesting that patients with suicidal behaviour have an increase in IL-6 and a decrease in IL-2. Other inflammatory factors have been described to be altered in such individuals, including reports of decreased vascular endothelial growth factor (VEGF)153, increased quinolinic acid154 and increased kynurenic acid in patients with major depressive disorder with a history of attempted suicide155,156. Although most studies investigating inflammatory markers in suicidal behaviour have investigated blood samples, some studies were conducted in CSF154,157 or in post-mortem brain tissue158–161, and the results of these studies have led to the suggestion that suicide is associated with low-grade inflammation in the brain. Glucocorticoids can blunt immune and inflammatory responses, but recent studies have shown that they can also stimulate immune responses in certain circumstances, such as during acute cellular damage or the death of brain tissue (reviewed in REF162). Glucocorticoid-mediated regulation of the immune system could lead to lasting effects of ELA on immunomodulation, as has been suggested by others163, and this is supported by the observation that victims of childhood maltreatment exhibit heightened immune activation as adults164.
Glial and astrocytic dysfunction
Traditionally, psychiatric phenotypes have been understood to result from dysfunction in brain neurons, and glia have been considered primarily for their role in supporting neuronal function. As neuroscience has gained increased insight into the diversity of functions that glial cells perform in the brain, more interest has been paid to glial cells, and particularly to astrocytes, in psychopathology. Studies investigating animal models of depression have reported glial cell alterations, such as impaired glial function165,166, and post-mortem brain studies have suggested that glial cell counts are decreased in cortical grey matter from patients with depression167, and that astrocytes are hypertrophied in cortical white matter from depressed individuals who died by suicide168. These results are in agreement with the findings from several mRNA expression studies using genome-wide microarrays in post-mortem brain tissue from depressed individuals who committed suicide, which consistently showed alterations in the expression of a number of astrocytic genes138,169,170. Among the most pronounced findings, these studies have pointed to a marked downregulation of the expression of connexin 30 (Cx30) and Cx43 — two genes coding for gap-junction proteins that in the brain are almost exclusively expressed in astrocytes. Interestingly, Cx30 and Cx43 single knock-out mice exhibit altered reactivity to novel environments and important changes in brain neurotransmitters, including serotonin171,172. Other astrocyte-specific genes have been reported as differentially expressed in suicide completers. For example, expression of the gene encoding the truncated splice variant of TrkB, TrkB.T1, which lacks the catalytic activity of the full-length protein173, is reduced in the frontal cortex of suicide completers compared to controls80. This is also consistent with results from work in mice indicating that mice overexpressing TrkB.T1 have an increased sensitivity to chronic social stress, resulting in consistent social avoidance174.
Neuroendocrine dysfunction in suicide
As discussed above, the HPA axis is dysregulated in individuals having experienced ELA. In suicide attempters and completers, there is also evidence of HPA dysregulation, regardless of ELA history. Studies investigating suicide attempters have shown that HPA axis dysregulation correlates with the violence of the suicide attempt175. There is also evidence that HPA axis alterations may be more closely linked to suicidal behaviour than to individual psychopathologies, since the ability to suppress dexamethasone (a synthetic glucocorticoid used to measure HPA response) predicts suicide completion176 and suicidal behaviour in patients with MDD177. Post-mortem studies of suicide completers have revealed that such individuals have increased corticotrophin-releasing hormone (CRH) activity in the paraventricular nucleus (PVN)178–180, increased CRH expression in the CSF181, fewer CRH binding sites in the frontal cortex182, decreased glucocorticoid receptor expression in the hippocampus55 and increased POMC in the pituitary183, indicating altered HPA function in the brains of suicide completers. Concordant with these findings is the observation that suicide completers have increased adrenal weight and cortical hypertrophy184,185, and relatives of suicide completers also exhibit altered HPA responses in experimental evaluations of stress response186.
Considerations for future directions
Suicide is a complex behaviour that results from the interaction of different factors. This Review examined evidence focusing on molecular changes associated with distal factors that increase the lifetime predisposition to suicide; the molecular and behavioural changes that are associated with factors mediating suicide risk; and, finally, those changes that are associated with depressive states that function as precipitants of a suicidal crisis (Fig. 1). Although this Review discusses these factors as if ‘suicidal behaviour’ were a single phenotype, this is not the case. There is only partial overlap between suicide attempts and suicide completion, and there is clear clinical, phenomenological and likely aetiological heterogeneity in and between each of these phenotypes. Thus, aetiological models, such as the one discussed in this paper, are helpful as they provide theoretical relationships that can be tested, but they should not be taken at face value. In addition, many of the studies discussed here focused on depression as a precursor to suicide, yet other psychiatric conditions, such as schizophrenia, bipolar disorder and personality disorders, are important contributors to suicidality, both in terms of suicidal ideation and suicidal behaviour7,102. In this regard, the recently proposed diagnosis of suicidal behaviour disorder should be considered in future research, as proposed in the DSM-5. This practice will help us better separate neurobiological factors associated with suicidal behaviour from those related to psychopathology.
One avenue of investigation that may shed some light into the molecular basis of suicidality is the study of the molecular basis of treatment-emergent suicidal events and mechanisms of action of pharmacological agents that act on suicidality. For instance, recent advances in clarifying the mechanism of action of lithium suggest that it functions by modulating dopaminergic, glutamatergic and GABAergic pathways, as well as by upregulating neuroprotective factors, such as BDNF and BCL-2, and downregulating apoptotic factors187. Lithium is effective at reducing the risk of suicide in patients with mood disorders, an effect that seems to be independent of its mood-stabilizing effect188, and it may also decrease aggression and impulsivity189. As such, further exploring lithium activity in suicidal patients may identify new genes involved in suicidality. Similarly, studies investigating dynamic molecular changes associated with treatment-emergent suicidal events or with fast cessation of suicidal ideation, such as seen with rapid-acting glutamatergic agents141, may point to new molecular pathways of interest to understand suicide.
The studies discussed above have led to increased interest in the investigation of epigenetic factors associated with both ELA and molecular processes mediating suicidality. However, before embarking on large sequencing-based studies, the field should consider a number of adjustments to the current approaches that could enhance the quality of the results generated. First, consortium-based initiatives, such as those seen in genetic variation studies of schizophrenia, should be promoted as they have recently produced encouraging results190,191,192. Second, alternative designs focusing on related phenotypes should be considered. For instance, investigating simple Mendelian conditions like Lesch-Nyhan disease193, in which the genetic architecture is reasonably well understood and phenotypes of interest, such as self-injurious behaviours, are manifested, should help us identify gene pathways of interest. Third, a major focus of upcoming research initiatives should be to improve comparability of the data. Standardizing the way methodologies are reported (for example, by including exact sequences of DNA regions investigated, detailed descriptions of probes or specific stimuli and parameters controlled for) will improve comparability and help to avoid some of the drawbacks linked to genetic variability.
Other methodological aspects to consider for studies of suicide are the use of peripheral tissues to study epigenetic changes linked to suicidality and the use of tissue homogenates. Although epigenetic studies conducted on peripheral tissues are feasible and cost-effective, their scientific value has not been well characterized, and we do not know to what extent epigenetic modifications in peripheral tissues are representative of those in the CNS. Epigenetic regulation is, to a large degree, cell- and tissue- specific194–196, with greater epigenetic variability existing between different tissues of a single individual than between similar tissues of different individuals62,197–199. There is therefore a need to clearly establish the relationship between peripheral epigenetic marks and CNS counterparts, a task that should become easier once reference epigenomic maps are generated from different brain regions and cellular fractions and compared to similar reference maps from peripheral tissues. In addition, studying single cell types can be facilitated through the use of laser capture microdissection200 or fluorescence-activated cell sorting201. Alternatively, computational methods allowing for estimation of cell populations within tissue homogenates202 could be used to refine results from studies using whole-tissue homogenates. Finally, epigenetic studies to date in suicide research have mainly focused on DNA methylation. However, other mechanisms of epigenetic regulation have been uncovered and should be further explored in the coming years (Box 5).
Box 5. Rethinking epigenetic mechanisms in suicidal behaviour.
Most epigenetic research investigating suicidal behaviours to date has focused on DNA methylation. As we gain insight into which different epigenetic marks are enriched in the brain, we should investigate the role of other epigenetic mechanisms that may be associated with suicidal behaviours, namely the activity of intermediaries of cytosine methylation, histone modifications and the effects of noncoding RNAs.
Epigenetic modifications of cytosine residues leads to the formation of intermediate products of methylcytosine oxidation, such as hydroxymethylcytosine, formylcytosine and carbocytosine, all of which are enriched in brain tissue. These intermediaries are likely functional, and their potential contribution to the regulation of gene expression should be explored further. Cytosine methylation outside of CpG dinucleotides is also more prevalent in brain tissue than in other tissues. Most studies to date have focused on CpG methylation, and investigating the contribution of non-CpG methylation to the regulation of gene expression in brain tissue may be a promising avenue of research in suicide studies.
Histones are essential protein complexes that regulate the accessibility of DNA to transcriptional machinery by modulating the level of chromatin compaction. Post-translational modifications of the N-terminal tails of histones change their impact on chromatin structure and activity. Specific genomic loci are linked to distinct histone modification profiles, for example active promoters are associated with histone 3 lysine 4 (H3K4) dimethylation and trimethylation and histone 3 lysine 27 (H3K27) acetylation and H3K4 monomethylation in enhancer regions, whereas repressed promoters are associated with H3K9 and H3K27 dimethylation and trimethylation. Specific contributions of histone modification to the emergence of suicide-related phenotypes have yet to be fully explored, but histone modification represents a ubiquitous mechanism for regulating gene transcription.
Another new aspect of epigenetic regulation of gene expression is the role of non-coding RNA. A large portion of the transcriptome is composed of regulatory RNAs that do not encode protein and instead regulate mRNA transcription, function and availability, and interact directly with DNA, regulatory proteins and enzymes. Among non-coding RNA species, long ncRNAs are of particular interest as they are highly expressed in the brain and are less evolutionarily conserved than other RNA species, and thus may associate with human brain processes that could be relevant to suicide research.
Such methodological improvements would allow us to confidently draw conclusions from studies on epigenetic modifications linked to suicide and might accelerate the identification of useful biomarkers for increased risk of suicide. In the long-term, this would translate into better management of high-risk patients and improved health outcomes.
Supplementary Material
Acknowledgments
Preparation of this review was supported by grants from the Canadian Institute of Health Research (CIHR) # MOP119429, MOP119430, and by the Fonds de Recherche du Québec – Santé (FRQS) through a Chercheur National salary award to the author and through support to the Réseau québécois sur le suicide, les troubles de l’humeur et les troubles associés. The author is indebted to Sylvanne Daniels for expert and essential help in the preparation of this review.
Glossary
- Suicidality
This broad term encompasses all forms of suicidal behaviour and suicidal ideation.
- Suicidal ideation
This term describes the wish to die, including thoughts of actively ending one’s life.
- Suicidal behaviour
This term describes behaviours that result in self-injury, and is generally used to refer to suicide attempts and suicide completion.
- Self-harm
This broad term includes suicidal behaviour and non-suicidal self-injurious behaviours.
- Non-suicidal self-injurious behaviour
Deliberate self-injury, often in the form of superficial skin cuts that are made with the intent to decrease emotional pain, rather than to die.
- Suicidal behaviour disorder
This disorder has recently been proposed as a condition for further study in the DSM-5, and is defined by the occurrence of at least one suicide attempt with some intent to die within the last 24 months. Conditions for further study in the DSM are disorders that should be investigated and considered for its future versions.
- Suicidal crisis
Period when suicidal ideation becomes acute, which is often associated with emotional instability.
- Distal risk factors
Predisposing factors that occur or are expressed temporally distant from the onset of the phenotype.
- Early-life adversity
Acts by a parent or caregiver that result in physical, sexual and/or psychological abuse of a child or that lead to neglect of essential physical or psychological needs of childhood.
- Treatment-emergent suicidal events
This term describes suicidal ideation or suicidal behaviour that occurs in association with treatment, and is typically used for clinical trials with antidepressants.
- Childhood sexual abuse
Any completed or attempted sexual act, or exposure to sexual interactions, with or without physical contact, with a child by a caregiver.
- Childhood physical abuse
The intentional use of physical force against a child that results in, or has the potential to result in, physical injury.
- Parental neglect
Failure to meet a child’s basic physical, emotional, medical or dental, or educational needs; or a failure to ensure a child’s safety.
- Attachment styles
Stereotypical interpersonal styles that are rooted in early-life interactions with caregivers.
- Mediators
Mediators are variables that fully or partially explain the relationship between a predictor and a dependent variable.
- Biological embedding
The effects of early-life experiences on the differential regulation of biological systems and development.
- Endophenotypes
These are traits that associate with an illness in the population, are heritable, state-independent, co-segregate with the condition investigated, and are present in non-affected family members of affected individuals at a higher rate than in the general population.
- Impulsive-aggressive behaviours
The tendency to react with animosity or overt hostility without consideration of the possible consequences, when piqued or under stress.
- State marker
Biological, psychological, behavioural or clinical markers associated with a given phenotype.
- Trait marker
Biological, psychological, behavioural or clinical markers that indicate a predisposition to or risk of a given phenotype.
- Proximal risk factors
Precipitating factors that occur or are expressed temporally close to the onset of the phenotype.
Biography
Gustavo Turecki is Professor of Psychiatry at McGill University, Montreal, Canada, where he directs the McGill Group for Suicide Studies and the Depressive Disorders Program. He received his MD from UNIFESP, Brazil, where he also completed his psychiatry residency, and obtained his PhD from McGill University. His research focuses on molecular mechanisms of depression and suicide.
References
- 1.World Health Organization. WHO Press; Geneva, Switzerland: 2013. [Google Scholar]
- 2.Mann JJ, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294:2064–74. doi: 10.1001/jama.294.16.2064. [DOI] [PubMed] [Google Scholar]
- 3.Duffy AMI. The Ottawa Citizen B1 - Front. Infomart, a division of Postmedia Network Inc; Ottawa, Ont: 2003. [Google Scholar]
- 4.Silverman MM. The language of suicidology. Suicide Life Threat Behav. 2006;36:519–32. doi: 10.1521/suli.2006.36.5.519. [DOI] [PubMed] [Google Scholar]
- 5.Kapur N, Cooper J, O’Connor RC, Hawton K. Non-suicidal self-injury v. attempted suicide: new diagnosis or false dichotomy? Br J Psychiatry. 2013;202:326–8. doi: 10.1192/bjp.bp.112.116111. [DOI] [PubMed] [Google Scholar]
- 6.Brezo J, et al. Identifying correlates of suicide attempts in suicidal ideators: a population-based study. Psychol Med. 2007;37:1551–62. doi: 10.1017/S0033291707000803. [DOI] [PubMed] [Google Scholar]
- 7.Arsenault-Lapierre G, Kim C, Turecki G. Psychiatric diagnoses in 3275 suicides: a meta-analysis. BMC Psychiatry. 2004;4:37. doi: 10.1186/1471-244X-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- 9.Dumais A, et al. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. Am J Psychiatry. 2005;162:2116–24. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- 10.McGirr A, et al. Impulsive-aggressive behaviours and completed suicide across the life cycle: a predisposition for younger age of suicide. Psychol Med. 2008;38:407–17. doi: 10.1017/S0033291707001419. [DOI] [PubMed] [Google Scholar]
- 11.Beautrais AL. Suicide and serious suicide attempts in youth: a multiple-group comparison study. Am J Psychiatry. 2003;160:1093–9. doi: 10.1176/appi.ajp.160.6.1093. [DOI] [PubMed] [Google Scholar]
- 12.Dalca IM, McGirr A, Renaud J, Turecki G. Gender-specific suicide risk factors: a case-control study of individuals with major depressive disorder. J Clin Psychiatry. 2013;74:1209–16. doi: 10.4088/JCP.12m08180. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor RC. In: International handbook of suicide prevention : research, policy and practice. O’Connor RC, Platt S, Gordon J, editors. John Wiley & Sons; Chichester; Malden, MA: 2011. p. xv.p. 677. [Google Scholar]
- 14.Moscicki EK. Gender differences in completed and attempted suicides. Ann Epidemiol. 1994;4:152–8. doi: 10.1016/1047-2797(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 15.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–28. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 16.Tidemalm D, et al. Familial clustering of suicide risk: a total population study of 11.4 million individuals. Psychol Med. 2011;41:2527–34. doi: 10.1017/S0033291711000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldessarini RJ, Hennen J. Genetics of suicide: an overview. Harv Rev Psychiatry. 2004;12:1–13. doi: 10.1080/10673220490425915. [DOI] [PubMed] [Google Scholar]
- 18.Turecki G. Suicidal Behavior: Is There a Genetic Predisposition? Bipolar Disorders. 2001;3:335–349. doi: 10.1034/j.1399-5618.2001.30608.x. [DOI] [PubMed] [Google Scholar]
- 19.Brent D. What family studies teach us about suicidal behavior: implications for research, treatment, and prevention. Eur Psychiatry. 2010;25:260–3. doi: 10.1016/j.eurpsy.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Brent DA, Bridge J, Johnson BA, Connolly J. Suicidal behavior runs in families. A controlled family study of adolescent suicide victims. Arch Gen Psychiatry. 1996;53:1145–52. doi: 10.1001/archpsyc.1996.01830120085015. [DOI] [PubMed] [Google Scholar]
- 21.Lieb R, Bronisch T, Hofler M, Schreier A, Wittchen HU. Maternal suicidality and risk of suicidality in offspring: findings from a community study. Am J Psychiatry. 2005;162:1665–71. doi: 10.1176/appi.ajp.162.9.1665. [DOI] [PubMed] [Google Scholar]
- 22.Blum R, Sudhinaraset M, Emerson MR. Youth at risk: suicidal thoughts and attempts in Vietnam, China, and Taiwan. J Adolesc Health. 2012;50:S37–44. doi: 10.1016/j.jadohealth.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Kim CD, et al. Familial aggregation of suicidal behavior: a family study of male suicide completers from the general population. Am J Psychiatry. 2005;162:1017–9. doi: 10.1176/appi.ajp.162.5.1017. [DOI] [PubMed] [Google Scholar]
- 24.McGirr A, et al. Familial Aggregation of Suicide Explained by Cluster B Traits: A Three-Group Family Study of Suicide Controlling for Major Depressive Disorder. Am J Psychiatry. 2009 doi: 10.1176/appi.ajp.2009.08111744. [DOI] [PubMed] [Google Scholar]
- 25.Ernst C, Mechawar N, Turecki G. Suicide neurobiology. Prog Neurobiol. 2009;89:315–33. doi: 10.1016/j.pneurobio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.von Borczyskowski A, Lindblad F, Vinnerljung B, Reintjes R, Hjern A. Familial factors and suicide: an adoption study in a Swedish National Cohort. Psychol Med. 2011;41:749–58. doi: 10.1017/S0033291710001315. [DOI] [PubMed] [Google Scholar]
- 27.Mann JJ. The serotonergic system in mood disorders and suicidal behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120537. doi: 10.1098/rstb.2012.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bach H, Arango V. In: The Neurobiological Basis of Suicide. Dwivedi Y, editor. CRC Press; Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- 29.Brezo J, Klempan T, Turecki G. The genetics of suicide: a critical review of molecular studies. Psychiatr Clin North Am. 2008;31:179–203. doi: 10.1016/j.psc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Laje G, et al. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. 2009;19:666–74. doi: 10.1097/FPC.0b013e32832e4bcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menke A, et al. Genome-wide association study of antidepressant treatment-emergent suicidal ideation. Neuropsychopharmacology. 2012;37:797–807. doi: 10.1038/npp.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perlis RH, et al. Genome-wide association study of suicide attempts in mood disorder patients. Am J Psychiatry. 2010;167:1499–507. doi: 10.1176/appi.ajp.2010.10040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perroud N, et al. Genome-wide association study of increasing suicidal ideation during antidepressant treatment in the GENDEP project. Pharmacogenomics J. 2012;12:68–77. doi: 10.1038/tpj.2010.70. [DOI] [PubMed] [Google Scholar]
- 34.Schosser A, et al. Genomewide Association Scan of Suicidal Thoughts and Behaviour in Major Depression. PLoS One. 2011;6:e20690. doi: 10.1371/journal.pone.0020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willour VL, et al. A genome-wide association study of attempted suicide. Mol Psychiatry. 2012;17:433–44. doi: 10.1038/mp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galfalvy H, et al. A pilot genome wide association and gene expression array study of suicide with and without major depression. World J Biol Psychiatry. 2013;14:574–82. doi: 10.3109/15622975.2011.597875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perlis RH, Ruderfer D, Hamilton SP, Ernst C. Copy number variation in subjects with major depressive disorder who attempted suicide. PLoS One. 2012;7:e46315. doi: 10.1371/journal.pone.0046315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fergusson DM, Woodward LJ, Horwood LJ. Risk factors and life processes associated with the onset of suicidal behaviour during adolescence and early adulthood. Psychol Med. 2000;30:23–39. doi: 10.1017/s003329179900135x. [DOI] [PubMed] [Google Scholar]
- 39.Angst J, Degonda M, Ernst C. The Zurich Study: XV. Suicide attempts in a cohort from age 20 to 30. Eur Arch Psychiatry Clin Neurosci. 1992;242:135–41. doi: 10.1007/BF02191561. [DOI] [PubMed] [Google Scholar]
- 40.Afifi TO, et al. Population attributable fractions of psychiatric disorders and suicide ideation and attempts associated with adverse childhood experiences. Am J Public Health. 2008;98:946–52. doi: 10.2105/AJPH.2007.120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilbert R, et al. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 42.Collishaw S, et al. Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse & Neglect. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Lansford JE, et al. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics and Adolescent Medicine. 2002;156:824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanous AH, Prescott CA, Kendler KS. The prediction of thoughts of death or self-harm in a population-based sample of female twins. Psychol Med. 2004;34:301–12. doi: 10.1017/s0033291703008857. [DOI] [PubMed] [Google Scholar]
- 45.Brezo J, et al. Predicting suicide attempts in young adults with histories of childhood abuse. Br J Psychiatry. 2008;193:134–9. doi: 10.1192/bjp.bp.107.037994. [DOI] [PubMed] [Google Scholar]
- 46.Brezo J, et al. Natural history of suicidal behaviors in a population-based sample of young adults. Psychol Med. 2007;37:1563–74. doi: 10.1017/S003329170700058X. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Castroman J, et al. Suicidal phenotypes associated with family history of suicidal behavior and early traumatic experiences. J Affect Disord. 2012;142:193–9. doi: 10.1016/j.jad.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Castroman J, et al. Early childhood sexual abuse increases suicidal intent. World Psychiatry. 2013;12:149–54. doi: 10.1002/wps.20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: a clinical perspective. Monogr Soc Res Child Dev. 1994;59:73–100. [PubMed] [Google Scholar]
- 50.Malatesta CZ. The role of emotions in the development and organization of personality. Nebr Symp Motiv. 1988;36:1–56. [PubMed] [Google Scholar]
- 51.Smith PN, et al. The relationships of attachment style and social maladjustment to death ideation in depressed women with a history of childhood sexual abuse. J Clin Psychol. 2012;68:78–87. doi: 10.1002/jclp.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hertzman C. Putting the concept of biological embedding in historical perspective. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17160–7. doi: 10.1073/pnas.1202203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 54.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–90. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 55.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8:1321–9. doi: 10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perroud N, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Labonte B, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69:722–31. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Knaap LJ, et al. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Transl Psychiatry. 2014;4:e381. doi: 10.1038/tp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perroud N, et al. Childhood maltreatment and methylation of the glucocorticoid receptor gene NR3C1 in bipolar disorder. Br J Psychiatry. 2014;204:30–5. doi: 10.1192/bjp.bp.112.120055. [DOI] [PubMed] [Google Scholar]
- 61.Melas PA, et al. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int J Neuropsychopharmacol. 2013;16:1513–28. doi: 10.1017/S1461145713000102. [DOI] [PubMed] [Google Scholar]
- 62.Davies MN, et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta D, et al. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch Gen Psychiatry. 2011;68:901–10. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willour VL, et al. Family-based association of FKBP5 in bipolar disorder. Mol Psychiatry. 2009;14:261–8. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Supriyanto I, et al. Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:252–6. doi: 10.1016/j.pnpbp.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Leszczynska-Rodziewicz A, et al. Possible association between haplotypes of the FKBP5 gene and suicidal bipolar disorder, but not with melancholic depression and psychotic features, in the course of bipolar disorder. Neuropsychiatr Dis Treat. 2014;10:243–8. doi: 10.2147/NDT.S54538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brent D, et al. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Am J Psychiatry. 2010;167:190–7. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perroud N, et al. Clinical and genetic correlates of suicidal ideation during antidepressant treatment in a depressed outpatient sample. Pharmacogenomics. 2011;12:365–77. doi: 10.2217/pgs.10.189. [DOI] [PubMed] [Google Scholar]
- 70.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–83. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. 2012;46:72–9. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Labonte B, et al. Genome-wide methylation changes in the brains of suicide completers. Am J Psychiatry. 2013;170:511–20. doi: 10.1176/appi.ajp.2012.12050627. [DOI] [PubMed] [Google Scholar]
- 74.Weder N, et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J Am Acad Child Adolesc Psychiatry. 2014;53:417–24. e5. doi: 10.1016/j.jaac.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–26. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uchida S, et al. Epigenetic Status of Gdnf in the Ventral Striatum Determines Susceptibility and Adaptation to Daily Stressful Events. Neuron. 2011;69 doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 78.Dwivedi Y, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–15. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 79.Pandey GN, et al. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in postmortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047–61. doi: 10.1017/S1461145708009000. [DOI] [PubMed] [Google Scholar]
- 80.Ernst C, et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch Gen Psychiatry. 2009;66:22–32. doi: 10.1001/archpsyc.66.1.22. [DOI] [PubMed] [Google Scholar]
- 81.Maussion G, et al. Functional DNA methylation in a transcript specific 3′UTR region of TrkB associates with suicide. Epigenetics. 2014;9 doi: 10.4161/epi.29068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keller S, et al. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–67. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- 83.Banerjee R, Ghosh AK, Ghosh B, Bhattacharyya S, Mondal AC. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin Med Insights Pathol. 2013;6:1–11. doi: 10.4137/CPath.S12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell. 2006;126:1189–201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Fuchikami M, et al. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011;6:e23881. doi: 10.1371/journal.pone.0023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang HJ, et al. BDNF promoter methylation and suicidal behavior in depressive patients. J Affect Disord. 2013;151:679–85. doi: 10.1016/j.jad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Kim JM, et al. Association of BDNF Promoter Methylation and Genotype with Suicidal Ideation in Elderly Koreans. Am J Geriatr Psychiatry. 2014 doi: 10.1016/j.jagp.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Michels KB, et al. Recommendations for the design and analysis of epigenome-wide association studies. Nat Methods. 2013;10:949–55. doi: 10.1038/nmeth.2632. [DOI] [PubMed] [Google Scholar]
- 89.Yang BZ, et al. Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med. 2013;44:101–7. doi: 10.1016/j.amepre.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turecki G, Ernst C, Jollant F, Labonte B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Wanner B, Vitaro F, Tremblay RE, Turecki G. Childhood trajectories of anxiousness and disruptiveness explain the association between early-life adversity and attempted suicide. Psychol Med. 2012:1–10. doi: 10.1017/S0033291712000438. [DOI] [PubMed] [Google Scholar]
- 92.Courtet P, Gottesman I, Jollant F, Gould TD. The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies? Transl Psychiatry. 2011;1:e7. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mann JJ, et al. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65:556–63. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brezo J, et al. Childhood trajectories of anxiousness and disruptiveness as predictors of suicide attempts. Arch Pediatr Adolesc Med. 2008;162:1015–21. doi: 10.1001/archpedi.162.11.1015. [DOI] [PubMed] [Google Scholar]
- 95.Brent DA, et al. Familial transmission of mood disorders: convergence and divergence with transmission of suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2004;43:1259–66. doi: 10.1097/01.chi.0000135619.38392.78. [DOI] [PubMed] [Google Scholar]
- 96.Gureje O, et al. Parental psychopathology and the risk of suicidal behavior in their offspring: results from the World Mental Health surveys. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brezo J, Paris J, Turecki G. Personality traits as correlates of suicidal ideation, suicide attempts, and suicide completions: a systematic review. Acta Psychiatr Scand. 2006;113:180–206. doi: 10.1111/j.1600-0447.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 98.Brezo J, et al. Broad and narrow personality traits as markers of one-time and repeated suicide attempts: a population-based study. BMC Psychiatry. 2008;8:15. doi: 10.1186/1471-244X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fergusson DM, Beautrais AL, Horwood LJ. Vulnerability and resiliency to suicidal behaviours in young people. Psychol Med. 2003;33:61–73. doi: 10.1017/s0033291702006748. [DOI] [PubMed] [Google Scholar]
- 100.Herba CM, Ferdinand RF, van der Ende J, Verhulst FC. Long-term associations of childhood suicide ideation. J Am Acad Child Adolesc Psychiatry. 2007;46:1473–81. doi: 10.1097/chi.0b013e318149e66f. [DOI] [PubMed] [Google Scholar]
- 101.Sourander A, et al. Childhood predictors of completed and severe suicide attempts: findings from the Finnish 1981 Birth Cohort Study. Arch Gen Psychiatry. 2009;66:398–406. doi: 10.1001/archgenpsychiatry.2009.21. [DOI] [PubMed] [Google Scholar]
- 102.Sareen J, et al. Anxiety Disorders and Risk for Suicidal Ideation and Suicide Attempts: A Population-Based Longitudinal Study of Adults. Arch Gen Psychiatry. 2005;62:1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- 103.Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Foundation symposium. 1991;156:171–183. doi: 10.1002/9780470514047.ch11. discussion 183. [DOI] [PubMed] [Google Scholar]
- 104.Caldji C, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Vegt EJ, van der Ende J, Ferdinand RF, Verhulst FC, Tiemeier H. Early childhood adversities and trajectories of psychiatric problems in adoptees: evidence for long lasting effects. J Abnorm Child Psychol. 2009;37:239–49. doi: 10.1007/s10802-008-9272-2. [DOI] [PubMed] [Google Scholar]
- 106.Johnson JG, et al. Childhood adversities, interpersonal difficulties, and risk for suicide attempts during late adolescence and early adulthood. Arch Gen Psychiatry. 2002;59:741–9. doi: 10.1001/archpsyc.59.8.741. [DOI] [PubMed] [Google Scholar]
- 107.Kim C, et al. Patterns of co-morbidity in male suicide completers. Psychol Med. 2003;33:1299–309. doi: 10.1017/s0033291703008146. [DOI] [PubMed] [Google Scholar]
- 108.Seguin M, et al. Life trajectories and burden of adversity: mapping the developmental profiles of suicide mortality. Psychol Med. 2007:1–9. doi: 10.1017/S0033291707000955. [DOI] [PubMed] [Google Scholar]
- 109.Chachamovich E, Ding Y, Turecki G. Levels of aggressiveness are higher among alcohol-related suicides: Results from a psychological autopsy study. Alcohol. 2012 doi: 10.1016/j.alcohol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Coccaro EF, et al. Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry. 1989;46:587–99. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- 111.Freemantle E, Chen GG, Cruceanu C, Mechawar N, Turecki G. Analysis of oxysterols and cholesterol in prefrontal cortex of suicides. Int J Neuropsychopharmacol. 2013;16:1241–9. doi: 10.1017/S1461145712001587. [DOI] [PubMed] [Google Scholar]
- 112.Lalovic A, et al. Cholesterol metabolism and suicidality in Smith-Lemli-Opitz syndrome carriers. Am J Psychiatry. 2004;161:2123–6. doi: 10.1176/appi.ajp.161.11.2123. [DOI] [PubMed] [Google Scholar]
- 113.Lalovic A, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10:159–66. doi: 10.1017/S1461145706006663. [DOI] [PubMed] [Google Scholar]
- 114.Heikkinen M, Aro H, Lonnqvist J. Recent life events and their role in suicide as seen by the spouses. Acta Psychiatr Scand. 1992;86:489–94. doi: 10.1111/j.1600-0447.1992.tb03303.x. [DOI] [PubMed] [Google Scholar]
- 115.Qin P, Agerbo E, Bo Mortensen P. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360:1126. doi: 10.1016/S0140-6736(02)11197-4. [DOI] [PubMed] [Google Scholar]
- 116.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–81. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 117.Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V. Serotonin depression -- a biochemical subgroup within the affective disorders. Science. 1976;191:478–80. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- 118.Stanley M, Virgilio J, Gershon S. Tritiated imipramine binding sites are decreased in the frontal cortex of suicides. Science. 1982;216:1337–9. doi: 10.1126/science.7079769. [DOI] [PubMed] [Google Scholar]
- 119.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–6. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 120.Arango V, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 121.Miller JM, et al. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74:287–95. doi: 10.1016/j.biopsych.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151–69. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- 123.Brezo J, et al. Differences and similarities in the serotonergic diathesis for suicide attempts and mood disorders: a 22-year longitudinal gene-environment study. Mol Psychiatry. 2010;15:831–43. doi: 10.1038/mp.2009.19. [DOI] [PubMed] [Google Scholar]
- 124.Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–9. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- 125.Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–97. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 126.Sequeira A, et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 127.Fiori LM, et al. Global gene expression profiling of the polyamine system in suicide completers. Int J Neuropsychopharmacol. 2011;14:595–605. doi: 10.1017/S1461145710001574. [DOI] [PubMed] [Google Scholar]
- 128.Klempan TA, et al. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:934–43. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- 129.Chen GG, et al. Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology. 2010;35:1477–84. doi: 10.1038/npp.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guipponi M, et al. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- 131.Le-Niculescu H, et al. Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dahel K-d, Al-Saffar N, Flayeh K. Polyamine Oxidase Activity in Sera of Depressed and Schizophrenic Patients after ECT Treatment. Neurochemical Research. 2001;26:415–418. doi: 10.1023/a:1010959300545. [DOI] [PubMed] [Google Scholar]
- 133.Karssen AM, et al. Stress-induced changes in primate prefrontal profiles of gene expression. Mol Psychiatry. 2007;25:25. doi: 10.1038/sj.mp.4002095. [DOI] [PubMed] [Google Scholar]
- 134.Fiori LM, Gross JA, Turecki G. Effects of histone modifications on increased expression of polyamine biosynthetic genes in suicide. Int J Neuropsychopharmacol. 2012;15:1161–6. doi: 10.1017/S1461145711001520. [DOI] [PubMed] [Google Scholar]
- 135.Fiori LM, Turecki G. Epigenetic regulation of spermidine/spermine N1-acetyltransferase (SAT1) in suicide. J Psychiatr Res. 2011;45:1229–35. doi: 10.1016/j.jpsychires.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 136.Gross JA, Fiori LM, Labonte B, Lopez JP, Turecki G. Effects of promoter methylation on increased expression of polyamine biosynthetic genes in suicide. J Psychiatr Res. 2013;47:513–9. doi: 10.1016/j.jpsychires.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lopez JP, et al. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int J Neuropsychopharmacol. 2014;17:23–32. doi: 10.1017/S1461145713000941. [DOI] [PubMed] [Google Scholar]
- 138.Sequeira A, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Choudary PV, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–8. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014;31:291–6. doi: 10.1002/da.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 142.Price RB, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31:335–43. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 145.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 146.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 147.Shelton RC, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–62. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–24. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 149.Musselman DL, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 150.Raison CL, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–8. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Constant A, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–7. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 152.Serafini G, et al. The role of inflammatory cytokines in suicidal behavior: a systematic review. Eur Neuropsychopharmacol. 2013;23:1672–86. doi: 10.1016/j.euroneuro.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 153.Isung J, et al. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry. 2012;2:e196. doi: 10.1038/tp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Erhardt S, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–52. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sublette ME, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272–8. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carlborg A, Jokinen J, Jonsson EG, Erhardt S, Nordstrom P. CSF kynurenic acid and suicide risk in schizophrenia spectrum psychosis. Psychiatry Res. 2013;205:165–7. doi: 10.1016/j.psychres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 157.Bay-Richter C, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 158.Pandey GN, et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tonelli LH, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 161.Steiner J, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–7. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 162.Bellavance MA, Rivest S. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38:1850–7. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Danese A, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–15. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Czeh B, Fuchs E, Flugge G. Altered glial plasticity in animal models for mood disorders. Curr Drug Targets. 2013;14:1249–61. doi: 10.2174/1389450111314110005. [DOI] [PubMed] [Google Scholar]
- 166.Banasr M, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–77. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 168.Torres-Platas SG, et al. Astrocytic Hypertrophy in Anterior Cingulate White Matter of Depressed Suicides. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ernst C, et al. Dysfunction of Astrocyte Connexins 30 and 43 in Dorsal Lateral Prefrontal Cortex of Suicide Completers. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 170.Bernard R, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–46. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dere E, et al. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur J Neurosci. 2003;18:629–38. doi: 10.1046/j.1460-9568.2003.02784.x. [DOI] [PubMed] [Google Scholar]
- 172.Frisch C, et al. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur J Neurosci. 2003;18:2313–8. doi: 10.1046/j.1460-9568.2003.02971.x. [DOI] [PubMed] [Google Scholar]
- 173.Rose CR, et al. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–8. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 174.Razzoli M, et al. A role for BDNF/TrkB signaling in behavioral and physiological consequences of social defeat stress. Genes Brain Behav. 2011;10:424–33. doi: 10.1111/j.1601-183X.2011.00681.x. [DOI] [PubMed] [Google Scholar]
- 175.Roy A. Hypothalamic-pituitary-adrenal axis function and suicidal behavior in depression. Biol Psychiatry. 1992;32:812–6. doi: 10.1016/0006-3223(92)90084-d. [DOI] [PubMed] [Google Scholar]
- 176.Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158:748–53. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- 177.Pfeffer CR, Stokes P, Shindledecker R. Suicidal behavior and hypothalamic-pituitary-adrenocortical axis indices in child psychiatric inpatients. Biol Psychiatry. 1991;29:909–17. doi: 10.1016/0006-3223(91)90057-s. [DOI] [PubMed] [Google Scholar]
- 178.Raadsheer FC, et al. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–6. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 179.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–44. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 180.Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13:786–99. 741. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- 181.Nemeroff CB, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 182.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–9. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 183.Lopez JF, et al. Localization and quantification of pro-opiomelanocortin mRNA and glucocorticoid receptor mRNA in pituitaries of suicide victims. Neuroendocrinology. 1992;56:491–501. doi: 10.1159/000126266. [DOI] [PubMed] [Google Scholar]
- 184.Dumser T, Barocka A, Schubert E. Weight of adrenal glands may be increased in persons who commit suicide. Am J Forensic Med Pathol. 1998;19:72–6. doi: 10.1097/00000433-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 185.Szigethy E, Conwell Y, Forbes NT, Cox C, Caine ED. Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry. 1994;36:374–80. doi: 10.1016/0006-3223(94)91212-2. [DOI] [PubMed] [Google Scholar]
- 186.McGirr A, et al. Dysregulation of the sympathetic nervous system, hypothalamic-pituitary-adrenal axis and executive function in individuals at risk for suicide. J Psychiatry Neurosci. 2010;35:399–408. doi: 10.1503/jpn.090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27:135–53. doi: 10.1007/s40263-013-0039-0. [DOI] [PubMed] [Google Scholar]
- 188.Goodwin FK, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290:1467–73. doi: 10.1001/jama.290.11.1467. [DOI] [PubMed] [Google Scholar]
- 189.Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 190.Ripke S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Purcell SM, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lesch M, Nyhan WL. A Familial Disorder of Uric Acid Metabolism and Central Nervous System Function. Am J Med. 1964;36:561–70. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- 194.Meissner A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–70. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mikkelsen TS, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lister R, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liang P, et al. Genome-wide survey reveals dynamic widespread tissue-specific changes in DNA methylation during development. BMC Genomics. 2011;12:231. doi: 10.1186/1471-2164-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xin Y, et al. Genome-wide divergence of DNA methylation marks in cerebral and cerebellar cortices. PLoS One. 2010;5:e11357. doi: 10.1371/journal.pone.0011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xin Y, et al. Role of CpG context and content in evolutionary signatures of brain DNA methylation. Epigenetics. 2011;6:1308–18. doi: 10.4161/epi.6.11.17876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nakamura N, et al. Laser capture microdissection for analysis of single cells. Methods Mol Med. 2007;132:11–8. doi: 10.1007/978-1-59745-298-4_2. [DOI] [PubMed] [Google Scholar]
- 201.Jiang Y, Matevossian A, Huang HS, Straubhaar J, Akbarian S. Isolation of neuronal chromatin from brain tissue. BMC Neurosci. 2008;9:42. doi: 10.1186/1471-2202-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guintivano J, Aryee MJ, Kaminsky ZA. A cell epigenotype specific model for the correction of brain cellular heterogeneity bias and its application to age, brain region and major depression. Epigenetics. 2013;8:290–302. doi: 10.4161/epi.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 204.Murgatroyd C, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 205.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 206.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 207.Maunakea AK, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–7. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ziller MJ, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–81. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35:283–92. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- 210.McCormick JA, et al. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Mol Endocrinol. 2000;14:506–17. doi: 10.1210/mend.14.4.0438. [DOI] [PubMed] [Google Scholar]
- 211.Wu Y, Patchev AV, Daniel G, Almeida OF, Spengler D. Early-life stress reduces DNA methylation of the pomc gene in male mice. Endocrinology. 2014;155:1751–62. doi: 10.1210/en.2013-1868. [DOI] [PubMed] [Google Scholar]
- 212.Bowen MT, et al. Active coping towards predatory stress is associated with lower corticosterone and progesterone plasma levels and decreased methylation in the medial amygdala vasopressin system. Horm Behav. 2014 doi: 10.1016/j.yhbeh.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 213.Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- 214.Wersinger SR, Caldwell HK, Christiansen M, Young WS., 3rd Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 2007;6:653–60. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–84. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 216.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–94. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fisher HL, et al. The varying impact of type, timing and frequency of exposure to childhood adversity on its association with adult psychotic disorder. Psychol Med. 2010;40:1967–78. doi: 10.1017/S0033291710000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jun HJ, et al. Child abuse and smoking among young women: the importance of severity, accumulation, and timing. J Adolesc Health. 2008;43:55–63. doi: 10.1016/j.jadohealth.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Blaauw E, Arensman E, Kraaij V, Winkel FW, Bout R. Traumatic life events and suicide risk among jail inmates: the influence of types of events, time period and significant others. J Trauma Stress. 2002;15:9–16. doi: 10.1023/A:1014323009493. [DOI] [PubMed] [Google Scholar]
- 220.Talens RP, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 2010;24:3135–44. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- 221.Ouellet-Morin I, et al. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: a longitudinal study of discordant monozygotic twins. Psychol Med. 2013;43:1813–23. doi: 10.1017/S0033291712002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim JM, et al. A longitudinal study of SLC6A4 DNA promoter methylation and poststroke depression. J Psychiatr Res. 2013;47:1222–7. doi: 10.1016/j.jpsychires.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 223.Kim JM, et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J Affect Disord. 2013;149:93–9. doi: 10.1016/j.jad.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 224.Fiori LM, Turecki G. Implication of the polyamine system in mental disorders. J Psychiatry Neurosci. 2008;33:102–10. [PMC free article] [PubMed] [Google Scholar]
- 225.Pegg AE, Casero RA., Jr Current status of the polyamine research field. Methods Mol Biol. 2011;720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bastida CM, et al. Sexual dimorphism of ornithine decarboxylase in the mouse adrenal: influence of polyamine deprivation on catecholamine and corticoid levels. Am J Physiol Endocrinol Metab. 2007;292:E1010–7. doi: 10.1152/ajpendo.00316.2006. [DOI] [PubMed] [Google Scholar]
- 227.Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 228.Brackley P, Goodnow R, Jr, Nakanishi K, Sudan HL, Usherwood PN. Spermine and philanthotoxin potentiate excitatory amino acid responses of Xenopus oocytes injected with rat and chick brain RNA. Neurosci Lett. 1990;114:51–6. doi: 10.1016/0304-3940(90)90427-b. [DOI] [PubMed] [Google Scholar]
- 229.Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J. 1996;316(Pt 1):247–9. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci. 2000;21:187–93. doi: 10.1016/s0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 231.Doyle KM, Kirby BP, Murphy D, Shaw GG. Effect of L-type calcium channel antagonists on spermine-induced CNS excitation in vivo. Neurosci Lett. 2005;380:247–51. doi: 10.1016/j.neulet.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 232.Gilad GM, Gilad VH. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell Mol Neurobiol. 2003;23:637–49. doi: 10.1023/A:1025036532672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hayashi Y, Tanaka J, Morizumi Y, Kitamura Y, Hattori Y. Polyamine levels in brain and plasma after acute restraint or water-immersion restraint stress in mice. Neurosci Lett. 2004;355:57–60. doi: 10.1016/j.neulet.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 234.Lee M, Wynder C, Schmidt D, McCafferty D, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chemistry & biology. 2006;13:563–570. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 235.Piletz JE, et al. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18:880–93. doi: 10.1016/j.drudis.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 236.Gupta N, Zhang H, Liu P. Behavioral and neurochemical effects of acute putrescine depletion by difluoromethylornithine in rats. Neuroscience. 2009;161:691–706. doi: 10.1016/j.neuroscience.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 237.Shopsin B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: a pilot study. Acta Neuropsychiatrica. 2013;25:113–118. doi: 10.1111/j.1601-5215.2012.00675.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.