Abstract
Suicide is a significant worldwide public health problem. Understanding the neurobiology is important as it can help us to better elucidate underlying etiological factors and provide opportunities for intervention. In recent years, many lines of research have suggested that the polyamine system may be dysregulated in suicidal behaviors. Initial research in animals provided evidence of a dysfunctional polyamine stress response system, while later work using post-mortem human brain tissue has suggested that molecular mechanisms may be at play in the suicide brain. In this review, we will describe the research that suggests the presence of alterations in the polyamine system in mental disorders and behavioral phenotypes, with particular attention to work on suicide. In addition, we will also describe potential avenues for future work.
Keywords: Epigenetics, Major Depressive Disorder, Neurobiology, Polyamines, Polyamine Stress Response, Spermidine/Spermine-N1-Acetyltransferase, Suicide
1 – Introduction
Suicide, most often associated with major depressive disorder (MDD) [1], is an important cause of premature death around the world [2]. According to the World Health Organization (www.who.int, 2012), approximately one million people die by suicide annually, and the rate of suicide attempts is 20 times greater than that of suicide completion (www.who.int, 2012). As such, suicidal behaviors (SB) are an important source of public health concern. The etiology of suicide is complex [3], and commonly understood as resulting from the interaction of predisposing (distal) and precipitant (proximal) factors [4]. Biological alterations resulting from a combination of genetic and environmental risk factors underlie predisposition to suicide, but the exact molecular mechanisms at play remain largely unclear. As such, it is important that we gain a better understanding of the molecular processes associated with suicide in order to identify new avenues for prevention and intervention.
Over the past several decades, molecular studies of suicide and MDD have focused on the monoamine system. Beginning in the late 1960s, research into the neurobiology of suicide was focused primarily on 5-hydroxyindoleacetic acid, the main metabolite of serotonin and noradrenaline [3]. Over the next decades, dopamine, serotonin, norepinephrine, and other monoamine neurotransmitters and associated receptors were investigated in the neurobiology of suicide and SB. Although important, alterations in this system account for only part of the neurobiological changes associated with MDD and suicide [3].
More recently, there has been growing evidence to support the involvement of a polyamine-mediated stress-response signaling system in the neurobiology of suicide. This evidence includes findings of altered polyamine levels and altered expression of polyamine genes in cortical brain areas of suicide completers [5,6], which are associated with sequence variation and environmental influences on polyaminergic genes [7,8]. In this review, we aim to describe the putative function of polyamines in the neurobiology of suicide and propose avenues for future research.
2 – Polyamines
2.1 – Biosynthesis
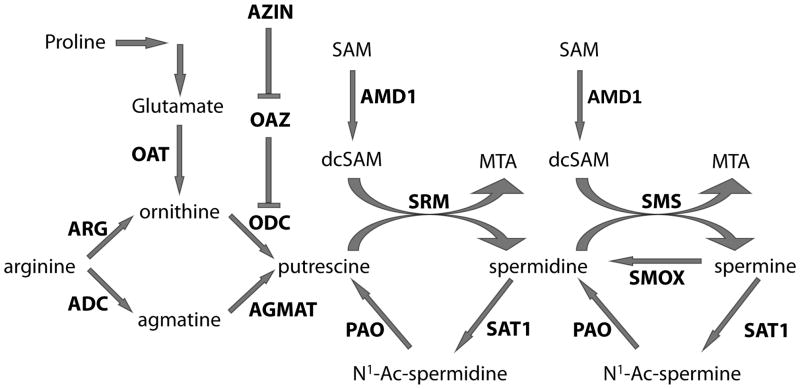
Polyamines are ubiquitous aliphatic molecules containing two, three, and four amine groups. These include agmatine, putrescine, spermine, and spermidine; each of which is incorporated into a highly regulated signaling pathway (fig. 1). The major rate-limiting enzymes of this pathway are ornithine decarboxylase (ODC), spermidine/spermine N1-acetyl transferase (SAT1), and S-adenosylmethionine decarboxylase (AMD1), whose activities are influenced by numerous regulatory proteins as well as the polyamine levels [9]. In addition, not only are polyamines known to influence neurotransmitter systems, such as catecholamines [10,11], gamma-aminobutyric acid (GABA) [12], glutamate [13], and nitric oxide [14], but agmatine is also believed to act as a neurotransmitter [15]. Finally, polyamines are also able to influence the properties of several transmembrane channels, thereby affecting cell excitability [16].
Figure 1. Polyamine biosynthesis pathway.
Enzymes in bold. Abbreviations are as follows:
ARG = Arginase; ADC = arginine decarboxylase; OAT = ornithine aminotransferase; AZIN = Ornithine decarboxylase antizyme inhibitor; OAZ = Ornithine decarboxylase antizyme; ODC = Ornithine decarboxylase; AGMAT = agmatinase; SAM = S-adenosylmethionine; AMD1 = S-adenosylmethionine decarboxylase; dcSAM = decarboxylated S-adenosylmethionine; PAO = polyamine oxidase; SRM = spermidine synthase; MTA = 5′ methylthioadenosine; SAT1 = Spermidine/spermine N1 acetyltransferase; SMS = spermine synthase; SMOX = spermine oxidase
Within the central nervous system (CNS), synthesis and storage occurs both in neuronal and non-neuronal cell types depending on the polyamine. For instance, it is believed that spermine and spermidine are stored in astrocytes and synthesized in neurons. These findings originated from the discovery that ODC, a rate-limiting enzyme responsible for the conversion of ornithine to putrescine, is absent in astrocytes [17]. Recently, however, it has been shown that putrescine may be derived through an alternative pathway that includes agmatine, a compound that is mainly synthesized in glia cells [9] but predominantly found in neurons [18]. This, and the identification of the neuronal localization of spermine synthase and spermidine synthase, provided more concrete evidence of the location of these polyamines.
2.2 – Role of Polyamines in CNS
Due to their limited capacity to cross the blood-brain barrier [19], concentrations of polyamines in the brain are generally in the nanomolar range [20]. In fact, only agmatine and arginine are able to cross the blood-brain barrier [21]. Systemic injection of agmatine has been shown to substantially increase cortical agmatine concentration in rats [22], even though other studies suggest that 99% of the injected agmatine is rapidly metabolized by peripheral tissues [23]. This is interesting as agmatine is believed to function as a CNS neurotransmitter by binding to NMDA receptors [24], as well as acetylcholine, imidazoline, and serotonin receptors [15]. Furthermore, agmatine has been shown to have antidepressant, anxiolytic, anticonvulsant, anti-inflammatory, and neuro-protective properties [25,26,27]. In light of these functions, although much of the agmatine is rapidly degraded, it is possible that systemic injections of agmatine induce endogenous release of this neurotransmitter such that it can exert all of its effects.
Similar to agmatine, putrescine has also been shown to possess antidepressant properties. Hippocampal levels of putrescine, spermidine, and spermine were shown to be significantly decreased in a rat model of depression, while only putrescine levels were reduced in the nucleus accumbens. Administration of S-adenosyl methionine (SAM) successfully restored the levels of putrescine in the nucleus accumbens, while only slightly increasing spermidine and spermine levels in the hippocampus [28]. In a more direct study, it was shown that systemic and central injections of putrescine reduced immobility times in the forced swimming and tail suspension tests, both of which are well-established paradigms for determining antidepressant-like properties. Further, the efficacy of putrescine was comparable to imipramine, a widely available tricyclic anti-depressant [29]. Finally, the antidepressant-like effects of putrescine were attenuated by arcaine, an antagonist of the NMDA receptor polyamine-binding site. Similar results were observed when putrescine was co-administered with agmatine, a more selective antagonist of the NMDA polyamine-binding site [24]. Together, these findings suggest that putrescine’s antidepressant-like effects may be achieved through the inhibition of NMDA receptors.
2.3 – Function of Rate-Limiting Enzymes of Polyamine Biosynthesis
Although not known for its antidepressant or anxiolytic functions, SAT1 has recently been linked to depression and suicide. SAT1 is the rate-limiting enzyme of polyamine catabolism and is responsible for the conversion of spermine to acetyl-spermine and spermidine to acetyl-spermidine. Gene expression studies have shown that a downregulation of SAT1 across multiple brain areas of depressed suicide completers [6,30] is partly associated with genetic variation in the promoter [6,7]. Moreover, a polymorphism in the promoter region was also associated with suicide completion [31]. Not only have these findings been replicated in independent samples [32], but a downregulation of SAT1 gene expression was also observed in monkeys exposed to adult social stress paradigms [33]. This is of importance as exposure to social stress has been shown to lead to depressive-like behaviors in monkey [34]. Although SAT1 has been consistently linked to mood disorders and stress, questions remain regarding the biological mechanisms underlying these effects and the extent to which gene regulatory processes account for this dysregulation.
Unlike polyamine catabolism, the biosynthesis pathway contains two rate-limiting enzymes, both of which have short turnover times in mammalian tissues [9]. ODC is an enzyme that is highly influenced by the ODC antizyme (OAZ) and antizyme inhibitor. Binding of OAZ to ODC disrupts homodimeric ODC and targets it for degradation by the 26S proteasome [35]. Not only do high intracellular polyamine concentrations promote OAZ synthesis, but they also inhibit ubiquitin-mediated OAZ degradation [36]. Another important enzyme in the biosynthesis pathway is AMD1. It is responsible for synthesizing decarboxylated SAM, which is a necessary substrate for the conversion of putrescine to spermidine and spermidine to spermine. Once decarboxylated, SAM is irreversibly committed to the polyamine synthesis pathway. SAM has been recently implicated in MDD as it has been suggested for use as a monotherapy or as an adjunctive treatment for individuals with MDD that do not respond to traditional antidepressant therapies [37]. It is proposed that the antidepressant effect of SAM is mediated by monoamines, as it is a precursor molecule for dopamine, norepinephrine, and serotonin. [38]. Interestingly, however, it is also possible that SAM exerts its antidepressant function by normalizing putrescine, spermine, and spermine levels in the brain [28].
2.4 – Putative Mechanisms of Action of Polyamines
As discussed above, there is substantial evidence suggesting that polyamines and their related enzymes may play a role in mood disorders (Table 1). Their exact mechanisms of action, however, have yet to be elucidated, although putative mechanisms have been explored. Polyamines have been shown to regulate ion fluxes by binding to NMDA, GABA, and nicotinic receptors. They also bind to ion channels, such as potassium and calcium channels, both on the cell membrane and on certain intracellular organelles [16]. Intracellularly, changes in polyamine levels have been associated with the initiation of deoxyribonucleic acid (DNA) synthesis and with the kinetics of the DNA replication fork [9]. In addition, spermine and spermidine stabilize the DNA by forming complexes that neutralize negatively-charged phosphate groups [9]. By these mechanisms, they may also be involved in regulating gene transcription, translation, and degradation.
Table 1.
Polyamine alterations in stress, anxiety, and mood disorders
| Study | Major Finding |
|---|---|
|
| |
| Genedani et al. 2001 | Administration of SAM restores polyamine levels in animal model of depression |
| Gilad and Gilad 2003 | Review on polyamine stress response (PSR) -- stress-related alterations of polyamines in rat models |
| Zomkowski et al. 2006 | Systemic injections of putrescine in mice produces antidepressant-like behaviours |
| Gong et al. 2006 | Agmatine displays anxiolytic effects in rats and mice |
| Karssen et al. 2007 | Decreased SSAT gene expression in monkeys exposed to adult social stress |
| Fiori and Turecki 2008 | Review on polyamines in mental disorders -- extensive list of polyamine-related findings in human and animal models of mental disorders |
| Taksande et al. 2009 | Antidepressant-like effects of SSRIs may be mediated by agmatine |
| Papakostas et al. 2010 | SAM used as both a monotherapy and adjunctive treatment of MDD in humans |
| Sokolowski et al. 2012 | Association between suicide attempts and polyaminergic genetic variants, namely the SAT1 promoter SNP (rs6526342) and SNPs in ODC |
The association between suicide, polyamines, and NMDA receptors is highly intriguing. It has been well established that NMDA receptors are involved in synaptic transmission and plasticity in the CNS [16]. The existence of a natural polyamine-binding site on these receptors [16] lends to the notion that the polyamine system may be involved in the neurobiology of SB by altering synaptic transmission. The binding of polyamines to NMDA receptors prevents the release of magnesium from the channel, which in turn inhibits glutamate transmission. Alternatively, polyamines have been shown to also block both inward-rectifying potassium channels and calcium-permeable AMPA receptors [16]. As such, a decrease in calcium transmission may inhibit proper depolarization of the cell and, consequently, prevent the removal of the magnesium blockade of the NMDA receptor. Certainly, further investigation will be necessary in determining the exact effect of polyamines on synaptic transmission. Nevertheless, it is clear that alterations of the polyamine-glutamatergic system play a role in the complex nature of the suicide brain.
2.5 – Polyamines and mood disorders
Studies using psychological autopsies have confirmed that suicide has a high comorbidity with mood and personality disorders [39,40,41]. In particular, although most people who suffer from MDD do not commit suicide, research suggests that greater than 60% of suicide completers have a history of MDD [39]. This association is of great importance as it lends to the idea that a psychiatric disorder is a necessary predisposing factor to suicide completion, although it may not be sufficient to commit such an act. Interestingly, SAT1 gene expression has been shown to be altered in suicide completers with a history of MDD and has also been demonstrated in stressed monkeys displaying depressive-like behaviors.
In addition to MDD, polyamines have also been implicated in schizophrenia, a disorder that is comorbid with suicide. In fact, a multitude of polyamines are increased in the blood and fibroblasts of schizophrenic patients (summarized in [38]). It appears, however, that increases in polyamine levels in these populations may be caused by neuroleptic treatment [42]. Furthermore, attempts to replicate these findings in postmortem brain tissue have been largely unsuccessful [43]. Although past research may suggest that peripheral polyamine levels are implicated in schizophrenia, significant work is required, both in blood and in brain, to determine the actual role of polyamines in this disorder.
3 – Polyamines and Suicide
3.1 – Polyamine Stress Response
The role of polyamines in MDD and suicide may best be understood in the context of their role in the polyamine stress response (PSR) system. This idea was initially proposed in the mid-1980s, but it was more broadly accepted in the early 1990s when research showed that stressful stimuli rapidly elicited changes in polyamine metabolism [44]. Following physical, emotional, or hormonal stressors, a cascade of second messenger systems activates the PSR. In the CNS, this cascade can be induced independent of the hypothalamic-pituitary axis (HPA) or by the neural circuitry itself, whereas in the periphery, PSR activation relies solely on activation of the HPA axis. Regardless of location, the traditional PSR involves prompt, transient increases in polyamine metabolism.
Initial research suggested that the PSR’s adaptive neuronal mechanisms differ based on the intensity and duration of the stressor [45]. Following acute stress, a seemingly incomplete PSR led to transient increases in ODC and putrescine, while spermidine and spermine levels remained unchanged. This likely occurred as a result of the tightly regulated homeostatic mechanisms that balanced polyamine metabolism with its SAT1-induced catabolic activity. On the contrary, persistent changes in polyamine levels caused by extreme or chronic stressors induced longer-lasting increases in ODC and putrescine. Levels of spermidine and spermine remain unchanged following a single stressful event but gradually increase after repetitive stimuli. Based on these initial findings, it is suggested that not only is putrescine playing a protective role in the CNS, but also the dynamic nature of the PSR is essential in effectively coping with both acute and chronic stressors. Follow-up research into the PSR system primarily used dexamethasone, a synthetic glucocorticosteroid, to induce stressful stimuli in rats. In the brain, stress-induced increases in ODC were inhibited if lithium was given at least 7 days prior to dexamethasone challenge. Similarly, the increases in AMD1 and SAT1 following dexamethasone injection were also prevented by the chronic lithium treatments [46]. Of the polyamines, only the levels of putrescine were attenuated with chronic lithium treatments following dexamethasone challenge, while levels of spermidine and spermine were not significantly altered [47]. Lithium treatment alone, given for 2 weeks, caused major increases in putrescine and minor increases in spermidine and spermine. Interestingly, without a stressor, chronic lithium treatment had no effect on levels of ODC. These studies suggest that lithium treatment may exert its effect by increasing SAT1 expression, thereby increasing polyamine turnover through the SAT1-mediated catabolic pathway rather than an ODC-mediated metabolic pathway.
Repeated stressful events are associated with an increase in the risk of negative mental health outcomes, including depression and suicide. The PSR appears to be an important component in the appropriate response mechanisms. In the brain, the same stress-induced changes in putrescine and ODC are seen after each stressful stimulus, whereas the response to chronic stress of AMD1, spermidine, and spermine are adaptive. Research has shown that long-term lithium treatment could block the PSR when given either prior to or after the application of stressful stimuli. Thus, by imposing an effect on SAT1-mediated polyamine catabolism, lithium may exert its role by taming a hypersensitive PSR.
As has been shown above, a significant interaction exists between stress, polyamines, and glucocorticoids. As such, it is no surprise that polyamines also regulate glucocorticoid receptor (GR) activity. Along with NF-E2-related factor 2, polyamine-modulated factor 1 (PMF-1) has been shown to regulate not only SAT1 expression [48], but also GR activity. It is suggested that PMF-1 binds directly to GR and negatively influences its activity [49]. As such, one can hypothesized that, in addition to its role in reducing the PSR, stress-induced increases in SAT1 may also lead the repressive effects PMF-1 on GR. This link is interesting as decreases in GR gene expression have been associated with suicide completers who suffered childhood abuse [50]. Although, levels of DNA methylation in the promoter of GR have been correlated to these gene expression results [50,51], we should not discount the potential influence of polyamines. Certainly further investigation will be necessary to elucidate the role of PMF-1 on GR expression in suicide completers.
3.3 – Polyamine Gene Expression
The link between polyamine metabolism and suicidal behaviors has gained significant support in recent years, largely sparked by results of a gene expression study identifying downregulated expression of SAT1 across brain regions of suicide completers [6]. As this gene represents one of the main rate-limiting enzymes in polyamine metabolism, these changes in gene expression were theorized to lead to disruptions in polyamine homeostasis. Follow-up studies have indicated that functional genetic variants in the SAT1 promoter region are partially responsible for the observed variation in gene expression [6,7]. Moreover, certain polymorphisms in the promoter region were also associated with suicide in individuals with MDD [31], and DNA methylation had a significant negative effect on SAT1 expression in the brain [8]. Most recently, the SAT1 promoter single nucleotide polymorphism (SNP) rs6526342 originally reported in suicide completers [6,7] was also linked to suicide attempters [52]. In addition to SAT1, other genes active during polyamine catabolism were also investigated. Gene expression levels of SAT2, an acetyltransferase that shares identity and homology with SAT1 [53], and ornithine aminotransferase-like 1, involved in the production of ornithine, were both found to be increased in the hippocampus of suicide completers [54]. In addition, the expression levels of spermine oxidase (SMOX) and spermine synthase (SMS), enzymes responsible, respectively, for the breakdown and synthesis of spermine, were altered in suicide completers. Neither SNPs nor epigenetic modifications were responsible for these changes [55].
Further investigation of the polyamine system showed upregulation of additional polyamine-related genes, including OAZ1, OAZ2, ARG2, and AMD1, in suicide completers with a history of mood disorders [5]. Our group reported an increase in histone H3 trimethylation at lysine 4 (H3K4me3), a marker of open chromatin, in the promoter of OAZ1, and this increase was significantly correlated with the expression of the OAZ1 gene [56]. In addition, we assessed the levels of DNA methylation in the promoter of these four genes and found significant site-specific decreases in ARG2 and AMD1 in suicide completers, which also negatively correlated with their expression levels (unpublished data).
3.4 – Protein Levels of Polyamines
To gain a more complete profile of the relationship between polyamines and suicide, it was essential to investigate not only the gene expression levels of enzymes in the synthesis and degradation pathways, but also the levels of their common substrates and products. While differences in gene expression provide consistent evidence suggesting polyamine system dysregulation in suicide, research has also identified downstream effects of these differences. The analysis of diverse polyamines in postmortem brain tissue was achieved through a series of high-resolution gas chromatography-mass spectrometry (GC-MS) based analytic methods. This technique was specifically developed for the extraction and quantification of spermine, spermidine, putrescine, and agmatine from postmortem brain [57,58,59]. Using GC-MS, it was shown that putrescine and spermidine levels were significantly elevated in the brains of depressed suicide completers [60], thus lending further support to the idea that decreases in SAT1 expression in depressed suicide completers are likely the result of a feedback regulation.
Overall, the findings discussed above (summarized in Table 2) provide complementary evidence linking polyamines to MDD and suicide. Dysregulation of SAT1 gene expression has been the most widespread and consistent result. In addition, other rate-limiting enzymes also show dysregulated gene expression patterns, and differences in protein levels of the polyamines themselves exist as well. It is therefore conceivable that changes to this biosynthetic pathway may be linked to a hypersensitive PSR in individuals with MDD and suicidal behaviors. More specifically, decreases in SAT1 gene expression may act as a compensatory mechanism to counter the increases in putrescine caused by an improper PSR. As such, the changes observed in other higher order polyamines and polyamine-related precursor molecules would therefore occur as a direct result of this compensatory decrease in SAT1 gene expression.
Table 2.
Polyamine alterations in suicide completers
| Study | Major Finding |
|---|---|
|
| |
| Sequeira et al. 2006 | SAT1 downregulation in BA4, 8/9, 11 of depressed suicides relative to controls (French-Canadian males) Association between a SAT1 promoter SNP (rs6526342) and suicide completion |
| Sequeira et al. 2007 | SAT2 and OATL1 upregulation in hippocampus of suicide completers without MDD SMS upregulation in hippocampus of suicide completers, regardless of MDD diagnosis |
| Guipponi et al. 2008 | SAT1 downregulation in ventral prefrontal cortex of suicide completers, irrespective of psychiatric pathology (independent sample) |
| Klempan et al. 2009 | SAT1 downregulation in across multiple brain regions (BA4, 11, 20, 21, 44) of depressed suicides relative to controls (French-Canadian males) Additional replication of findings in BA 11 and 17 of independent sample (German origin) |
| Fiori et al. 2009 | Decreased SAT1 expression associated with haplotype containing the risk allele for rs6526342 |
| Fiori et al. 2010 | Increased frequency of a 15 adenine deletion in suicide completers with low expression haplotype |
| Chen et al. 2010 | Increased protein levels of putrescine and spermidine in post-mortem brain tissue of suicide completers |
| Fiori et al. 2010 | Decreased gene expression of SMOX and increased gene expression of SMS Neither the selected SNPs nor epigenetic modifications were correlated with these changes in gene expression |
| Fiori et al. 2011 | CpG methylation is negatively correlated with decreases SAT1 expression High levels of methylation at the polymorphic CpG site described in Sequeira et al. 2006 |
| Fiori et al. 2011 | Global gene expression profiling identified 14 additional polyamine genes with altered expression levels |
| Fiori et al. 2011 | Increased levels of H3K4me3 in the promoter of OAZ1 in suicide completers is correlated with OAZ1 gene expression |
| Gross et al. (unpublished) | Site-specific decreases in CpG methylation in ARG2 and AMD1 are correlated with increases in their gene expression levels |
| Chen et al. (unpublished) | Decreased protein levels of agmatine in post-mortem brain tissue of suicide completers |
| Squassina et al. (unpublished) | Increased SSAT gene expression in lithium-treated cell lines from healthy controls but not in suicide completers with history of bipolar disorder |
It is important to also note that the polyamine biosynthetic pathway is linked not only to GABA/glutamate neurotransmission, but also to the tricarboxylic acid cycle and to nitric oxide metabolism, each of which have been associated to MDD and suicide. It is therefore possible that alterations in these systems may represent the upstream activators of a hypersensitive PSR, which, in turn, lead to dysregulated polyamine metabolism. Indeed, it remains crucial that we continue to investigate each of these links in order to elucidate and better understand the neurobiology of suicide and MDD.
4 – Future methods to study the effect of polyamines in suicide
4.1 – Next-Generation Sequencing and Epigenetics
Much of the work linking polyamines to suicidal behavior and major depressive disorder has used recent, but relatively low-throughput technologies. For the most part, the research has relied on real-time polymerase chain reaction, gene expression microarrays, and Sanger sequencing to achieve this success. With the advent of next generation sequencing technologies, the ability to accurately sequence samples of interest will soon become standard protocol. In recent years, a significant effort has been placed on reducing costs, increasing accuracy, and achieving higher throughput. Currently, these sequencing technologies are being employed in conjunction with earlier methods, such as DNA and chromatin immunoprecipitation, bisulfite treatment, RNA analyses, etc., which has greatly increased our genetic understanding of diseases and disorders.
As we delve deeper into characterizing the polyamine system and its association with suicide completion, it is evident that we must use sequencing technologies to study the epigenome. To date, epigenetic modifications, such as DNA methylation and post-transalational histone modifications have been suggested in some psychiatric phenotypes, including schizophrenia, bipolar disorder, and suicide [61,62,63]. Of particular interest is DNA methylation, a process in which cystosine nucleotides are methylated with the aid of DNA methyltransferases (DNMTs). DNA methylation does not affect base-pairing, therefore this mark can regulate gene expression without changes to the underlying DNA sequence [64]. The primary function of this mark is the silencing of gene promoters by preventing the binding of transcription factors [65]. Recent literature also suggests an activating role for DNA methylation in gene bodies [66]. Since DNA methylation is maintained through cell division [64], it has become the most well-studied epigentic mark, as it has the potential to be a diagnostic tool or a therapeutic target.
Since its discovery, DNA methylation has often been referred to as the 5th DNA base [67]. Recently, it was discovered that methylated cytosines are converted to 5-hydroxymethylated cytosines (hmC) by the enzyme ten-eleven translocation 1 (TET1) [68]. This finding, along with that of Kriaucionis and Heintz who described the presence of hmC in purkinje neurons [69], stimulated growing interest of research describing the genomic location, the abundance in different tissues, and the putative functions of hydroxymethylation. Even though the vast majority of this research has described hmC in embryonic stem cells, there are a handful of studies on this mark in the brain. Quantifying hmC levels in multiple human tissues, Li and Liu showed that these levels are highest in the brain [70]. Furthermore, Jin and colleagues showed that hmC levels in gene bodies correlate positively with gene expression, whereas there was no correlation when comparing hmC levels at promoters with high CpG content [71]. hmC may indeed act as an epigenetic mark since the conversion of methylcytosine to hmC relies on oxoglutarate and oxygen. As such, it is conceivable that this enzymatic process is highly sensitive to the environment [72], and therefore it is an excellent target for future studies that aim to uncover underlying neurobiological alterations in suicide and MDD.
4.2 – Polyamines of Interest
As we aim to characterize the epigenetic landscapes of the polyamine system through next-generation sequencing technologies, it will be important to expand our search to include genes both related to polyamine biosynthesis and to those associated with arginine and proline metabolism. Given the intricacy and direct links from one system to another, the inclusion of molecules that are fed into the polyamine biosynthetic pathway may provide answers as to how a tightly regulated system can be altered in SB.
The most consistent findings implicating polyamines and suicide have been those related to the downregulation of SAT1 gene expression. Although the preliminary epigenetic research performed to understand the underlying regulatory mechanisms explaining SAT1 and polyamine dysregulation has yet to produce robust results, it is likely that the progressive adoption of high-throughput and high-resolution sequencing technologies will provide more conclusive information.
In addition to SAT1, it will be interesting to investigate the link between SB and arginine, agmatine, and putrescine. These three molecules should be a particular focus as they represent potential biomarkers. Agmatine and putrescine possess anti-depressant properties, and both arginine and agmatine can cross the blood brain barrier. Altering the peripheral circulation levels of arginine and agmatine may lead to changes in the CNS, either directly or by eliciting endogenous release. As such, these polyamines may prove to be potential targets for pharmacological therapies. We must acknowledge, however, that polyamines are ubiquitous molecules involved in a large variety of cellular functions. Therefore, being able to target a specific biomarker will be a daunting task. Nevertheless, this should not stop us from investigating possible solutions, as the potential for therapeutic targets is invaluable.
Concluding statement
Traditionally, the polyamine system has been known for its crucial role in many cellular functions such as growth, proliferation, and division. Over the last 2 decades, it has become clear that, whether it is the polyamines, their enzymes or their related compounds, this system is important in stress-response, and it is likely involved in the neurobiology of MDD and suicide. With alterations in the polyamine system observed in genomic, proteomic, and clinical studies, its role in depression and SB cannot be overlooked. Undoubtedly, significant progress has been made in this field of research. Nevertheless, it is essential that we continue to better understand and characterize previous findings and explore underlying mechanisms that may be etiologically associated with these alterations.
Abbreviations
- ARG2
Arginase 2
- CNS
Central nervous system
- DNA
Deoxyribonucleic acid
- DNMT
DNA methylytransferase
- GABA
Gamma-aminobutyric acid
- GC-MS
Gas chromatography-mass spectrometry
- GR
Glucocorticoid receptor
- hmC
5-hydroxymethylcytosine
- HPA
Hypothalamic-pituitary axis
- MDD
Major depressive disorder
- NMDA
N-methyl-D-aspartate
- ODC
Ornithine decarboxylase
- OAZ
Ornithine decarboxylase antizyme
- PMF-1
Polyamine-modulated factor 1
- PSR
Polyamine stress response
- SAM
S-adenosylmethionine
- AMD1
S-adenosylmethionine decarboxylase
- SNP
Single nucleotide polymorphism
- SAT1
Spermidine/spermine-N1-acetyltransferase
- SB
Suicidal behavior
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Arsenault-Lapierre G, Kim C, Turecki G. Psychiatric diagnoses in 3275 suicides: a meta-analysis. BMC psychiatry. 2004;4:37. doi: 10.1186/1471-244X-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, et al. Suicide and suicidal behavior. Epidemiologic reviews. 2008;30:133–154. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst C, Mechawar N, Turecki G. Suicide neurobiology. Progress in neurobiology. 2009;89:315–333. doi: 10.1016/j.pneurobio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Turecki G, Ernst C, Jollant F, Labonte B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends in neurosciences. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Fiori LM, Bureau A, Labbe A, Croteau J, Noel S, et al. Global gene expression profiling of the polyamine system in suicide completers. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–11. doi: 10.1017/S1461145710001574. [DOI] [PubMed] [Google Scholar]
- 6.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Archives of general psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Fiori LM, Mechawar N, Turecki G. Identification and characterization of spermidine/spermine N1-acetyltransferase promoter variants in suicide completers. Biological psychiatry. 2009;66:460–467. doi: 10.1016/j.biopsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Fiori LM, Turecki G. Epigenetic regulation of spermidine/spermine N(1)-acetyltransferase (SAT1) in Suicide. Journal of psychiatric research. 2011;45:1229–1235. doi: 10.1016/j.jpsychires.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Tabor CW, Tabor H. Polyamines. Annual review of biochemistry. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 10.Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, et al. Sexual dimorphism of ornithine decarboxylase in the mouse adrenal: influence of polyamine deprivation on catecholamine and corticoid levels. American journal of physiology Endocrinology and metabolism. 2007;292:E1010–1017. doi: 10.1152/ajpendo.00316.2006. [DOI] [PubMed] [Google Scholar]
- 11.Ritz MC, Mantione CR, London ED. Spermine interacts with cocaine binding sites on dopamine transporters. Psychopharmacology. 1994;114:47–52. doi: 10.1007/BF02245443. [DOI] [PubMed] [Google Scholar]
- 12.Gilad GM, Gilad VH, Wyatt RJ. Polyamines modulate the binding of GABAA-benzodiazepine receptor ligands in membranes from the rat forebrain. Neuropharmacology. 1992;31:895–898. doi: 10.1016/0028-3908(92)90127-b. [DOI] [PubMed] [Google Scholar]
- 13.de Vera N, Martinez E, Sanfeliu C. Spermine induces cell death in cultured human embryonic cerebral cortical neurons through N-methyl-D-aspartate receptor activation. Journal of neuroscience research. 2008;86:861–872. doi: 10.1002/jnr.21538. [DOI] [PubMed] [Google Scholar]
- 14.Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. The Biochemical journal. 1996;316(Pt 1):247–249. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends in pharmacological sciences. 2000;21:187–193. doi: 10.1016/s0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 16.Williams K. Modulation and block of ion channels: a new biology of polyamines. Cellular signalling. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 17.Krauss M, Langnaese K, Richter K, Brunk I, Wieske M, et al. Spermidine synthase is prominently expressed in the striatal patch compartment and in putative interneurones of the matrix compartment. Journal of neurochemistry. 2006;97:174–189. doi: 10.1111/j.1471-4159.2006.03721.x. [DOI] [PubMed] [Google Scholar]
- 18.Otake K, Ruggiero DA, Regunathan S, Wang H, Milner TA, et al. Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain research. 1998;787:1–14. doi: 10.1016/s0006-8993(97)01200-6. [DOI] [PubMed] [Google Scholar]
- 19.Diler AS, Ziylan YZ, Uzum G, Lefauconnier JM, Seylaz J, et al. Passage of spermidine across the blood-brain barrier in short recirculation periods following global cerebral ischemia: effects of mild hyperthermia. Neuroscience research. 2002;43:335–342. doi: 10.1016/s0168-0102(02)00059-7. [DOI] [PubMed] [Google Scholar]
- 20.Seiler N. Polyamine metabolism and function in brain. Neurochemistry international. 1981;3:95–110. doi: 10.1016/0197-0186(81)90027-9. [DOI] [PubMed] [Google Scholar]
- 21.Piletz JE, May PJ, Wang G, Zhu H. Agmatine crosses the blood-brain barrier. Annals of the New York Academy of Sciences. 2003;1009:64–74. doi: 10.1196/annals.1304.007. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatric research. 2002;52:606–611. doi: 10.1203/00006450-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Luszczki JJ, Czernecki R, Wojtal K, Borowicz KK, Czuczwar SJ. Agmatine enhances the anticonvulsant action of phenobarbital and valproate in the mouse maximal electroshock seizure model. Journal of neural transmission. 2008;115:1485–1494. doi: 10.1007/s00702-008-0046-3. [DOI] [PubMed] [Google Scholar]
- 24.Gibson DA, Harris BR, Rogers DT, Littleton JM. Radioligand binding studies reveal agmatine is a more selective antagonist for a polyamine-site on the NMDA receptor than arcaine or ifenprodil. Brain research. 2002;952:71–77. doi: 10.1016/s0006-8993(02)03198-0. [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, et al. Antidepressant-like effect of agmatine and its possible mechanism. European journal of pharmacology. 2003;469:81–88. doi: 10.1016/s0014-2999(03)01735-7. [DOI] [PubMed] [Google Scholar]
- 26.Gong ZH, Li YF, Zhao N, Yang HJ, Su RB, et al. Anxiolytic effect of agmatine in rats and mice. European journal of pharmacology. 2006;550:112–116. doi: 10.1016/j.ejphar.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 27.Taksande BG, Kotagale NR, Tripathi SJ, Ugale RR, Chopde CT. Antidepressant like effect of selective serotonin reuptake inhibitors involve modulation of imidazoline receptors by agmatine. Neuropharmacology. 2009;57:415–424. doi: 10.1016/j.neuropharm.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Genedani S, Saltini S, Benelli A, Filaferro M, Bertolini A. Influence of SAMe on the modifications of brain polyamine levels in an animal model of depression. Neuroreport. 2001;12:3939–3942. doi: 10.1097/00001756-200112210-00017. [DOI] [PubMed] [Google Scholar]
- 29.Zomkowski AD, Santos AR, Rodrigues AL. Putrescine produces antidepressant-like effects in the forced swimming test and in the tail suspension test in mice. Progress in neuro-psychopharmacology & biological psychiatry. 2006;30:1419–1425. doi: 10.1016/j.pnpbp.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Guipponi M, Deutsch S, Kohler K, Perroud N, Le Gal F, et al. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2009;150B:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- 31.Fiori LM, Turecki G. Association of the SAT1 in/del polymorphism with suicide completion. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2010;153B:825–829. doi: 10.1002/ajmg.b.31040. [DOI] [PubMed] [Google Scholar]
- 32.Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, et al. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2009;150B:934–943. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- 33.Karssen AM, Her S, Li JZ, Patel PD, Meng F, et al. Stress-induced changes in primate prefrontal profiles of gene expression. Molecular psychiatry. 2007;12:1089–1102. doi: 10.1038/sj.mp.4002095. [DOI] [PubMed] [Google Scholar]
- 34.Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PloS one. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurian L, Palanimurugan R, Godderz D, Dohmen RJ. Polyamine sensing by nascent ornithine decarboxylase antizyme stimulates decoding of its mRNA. Nature. 2011;477:490–494. doi: 10.1038/nature10393. [DOI] [PubMed] [Google Scholar]
- 36.Palanimurugan R, Scheel H, Hofmann K, Dohmen RJ. Polyamines regulate their synthesis by inducing expression and blocking degradation of ODC antizyme. The EMBO journal. 2004;23:4857–4867. doi: 10.1038/sj.emboj.7600473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. The American journal of psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 38.Fiori LM, Turecki G. Implication of the polyamine system in mental disorders. Journal of psychiatry & neuroscience: JPN. 2008;33:102–110. [PMC free article] [PubMed] [Google Scholar]
- 39.Dumais A, Lesage AD, Alda M, Rouleau G, Dumont M, et al. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. The American journal of psychiatry. 2005;162:2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- 40.Lesage AD, Boyer R, Grunberg F, Vanier C, Morissette R, et al. Suicide and mental disorders: a case-control study of young men. The American journal of psychiatry. 1994;151:1063–1068. doi: 10.1176/ajp.151.7.1063. [DOI] [PubMed] [Google Scholar]
- 41.Turecki G. Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. Journal of psychiatry & neuroscience: JPN. 2005;30:398–408. [PMC free article] [PubMed] [Google Scholar]
- 42.Das I, Ramchand CN, Gliddon A, Hirsch SR. Nitric oxide, free radicals and polyamines may have a role in the membrane pathology of schizophrenia. Neuropsychobiology. 1998;37:65–67. doi: 10.1159/000026478. [DOI] [PubMed] [Google Scholar]
- 43.Gilad GM, Gilad VH, Casanova MF, Casero RA., Jr Polyamines and their metabolizing enzymes in human frontal cortex and hippocampus: preliminary measurements in affective disorders. Biological psychiatry. 1995;38:227–234. doi: 10.1016/0006-3223(94)00256-3. [DOI] [PubMed] [Google Scholar]
- 44.Gilad GM, Gilad VH. Polyamines in neurotrauma. Ubiquitous molecules in search of a function. Biochemical pharmacology. 1992;44:401–407. doi: 10.1016/0006-2952(92)90428-l. [DOI] [PubMed] [Google Scholar]
- 45.Gilad GM, Gilad VH. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cellular and molecular neurobiology. 2003;23:637–649. doi: 10.1023/A:1025036532672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilad GM, Gilad VH, Wyatt RJ, Casero RA., Jr Chronic lithium treatment prevents the dexamethasone-induced increase of brain polyamine metabolizing enzymes. Life sciences. 1992;50:PL149–154. doi: 10.1016/0024-3205(92)90289-2. [DOI] [PubMed] [Google Scholar]
- 47.Gilad GM, Gilad VH, Casero RA., Jr Lithium exerts a time-dependent and tissue-selective attenuation of the dexamethasone-induced polyamine response in rat brain and liver. Brain research. 1994;636:187–192. doi: 10.1016/0006-8993(94)91016-2. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Devereux W, Stewart TM, Casero RA., Jr Characterization of the interaction between the transcription factors human polyamine modulated factor (PMF-1) and NF-E2-related factor 2 (Nrf-2) in the transcriptional regulation of the spermidine/spermine N1-acetyltransferase (SSAT) gene. The Biochemical journal. 2001;355:45–49. doi: 10.1042/0264-6021:3550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoji Y, Osman W, Zilliacus J. Polyamine-modulated factor 1 represses glucocorticoid receptor activity. Biochemical and biophysical research communications. 2007;361:176–181. doi: 10.1016/j.bbrc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 50.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, et al. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biological psychiatry. 2012;72:41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D. Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: associations and gene-environment interactions with childhood/adolescent physical assault. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.112. [DOI] [PubMed] [Google Scholar]
- 53.Coleman CS, Stanley BA, Jones AD, Pegg AE. Spermidine/spermine-N1-acetyltransferase-2 (SSAT2) acetylates thialysine and is not involved in polyamine metabolism. The Biochemical journal. 2004;384:139–148. doi: 10.1042/BJ20040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, et al. Patterns of gene expression in the limbic system of suicides with and without major depression. Molecular psychiatry. 2007;12:640–655. doi: 10.1038/sj.mp.4001969. [DOI] [PubMed] [Google Scholar]
- 55.Fiori LM, Turecki G. Genetic and epigenetic influences on expression of spermine synthase and spermine oxidase in suicide completers. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13:725–736. doi: 10.1017/S1461145709991167. [DOI] [PubMed] [Google Scholar]
- 56.Fiori LM, Gross JA, Turecki G. Effects of histone modifications on increased expression of polyamine biosynthetic genes in suicide. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–6. doi: 10.1017/S1461145711001520. [DOI] [PubMed] [Google Scholar]
- 57.Chen GG, Turecki G, Mamer OA. A quantitative GC-MS method for three major polyamines in postmortem brain cortex. Journal of mass spectrometry: JMS. 2009;44:1203–1210. doi: 10.1002/jms.1597. [DOI] [PubMed] [Google Scholar]
- 58.Chen GG, Turecki G, Mamer OA. A novel liquid-liquid extraction and stable isotope dilution NCI-GC-MS method for quantitation of agmatine in postmortem brain cortex. Journal of mass spectrometry: JMS. 2010;45:560–565. doi: 10.1002/jms.1742. [DOI] [PubMed] [Google Scholar]
- 59.Chen GG, Fiori LM, Mamer OA, Turecki G. High-resolution capillary gas chromatography in combination with mass spectrometry for quantification of three major polyamines in postmortem brain cortex. Methods in molecular biology. 2011;720:427–436. doi: 10.1007/978-1-61779-034-8_27. [DOI] [PubMed] [Google Scholar]
- 60.Chen GG, Fiori LM, Moquin L, Gratton A, Mamer O, et al. Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:1477–1484. doi: 10.1038/npp.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akbarian S, Ruehl MG, Bliven E, Luiz LA, Peranelli AC, et al. Chromatin alterations associated with down-regulated metabolic gene expression in the prefrontal cortex of subjects with schizophrenia. Archives of general psychiatry. 2005;62:829–840. doi: 10.1001/archpsyc.62.8.829. [DOI] [PubMed] [Google Scholar]
- 62.Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, et al. Altered gene expression of histone deacetylases in mood disorder patients. Journal of psychiatric research. 2010;44:263–270. doi: 10.1016/j.jpsychires.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 63.McGowan PO, Sasaki A, Huang TC, Unterberger A, Suderman M, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PloS one. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schubeler D. Epigenomics: Methylation matters. Nature. 2009;462:296–297. doi: 10.1038/462296a. [DOI] [PubMed] [Google Scholar]
- 65.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nature neuroscience. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 66.Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song CX, He C. The hunt for 5-hydroxymethylcytosine: the sixth base. Epigenomics. 2011;3:521–523. doi: 10.2217/epi.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Liu M. Distribution of 5-hydroxymethylcytosine in different human tissues. Journal of nucleic acids. 2011;2011:870726. doi: 10.4061/2011/870726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin SG, Wu X, Li AX, Pfeifer GP. Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic acids research. 2011;39:5015–5024. doi: 10.1093/nar/gkr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chia N, Wang L, Lu X, Senut MC, Brenner C, et al. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics: official journal of the DNA Methylation Society. 2011;6:853–856. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]