Abstract
Child maltreatment is associated with increased risk for psychiatric disorders, and a range of health problems later in life. The aim of this paper is to review emerging data on the role of epigenetic mechanisms in the etiology of stress-related psychiatric disorders with a focus on future avenues of investigation. Epigenetic processes are described, key findings in the field presented, clinical implications of the research discussed, methodological issues, and future avenues of research considered. Research suggests that adverse early experiences can lead to changes in gene expression through epigenetic mechanisms that can alter stress reactivity, brain function, and behavior. While these changes are frequently long lasting, they can be reversed through pharmacological and environmental manipulations. The complexity of the epigenome is not fully understood. Future studies should investigate epigenetic marks other than methylcytosine, and assess the efficacy of interventions to reverse epigenetic processes associated with early-life adversity.
Child abuse and neglect are associated with increased risk for depression, anxiety, posttraumatic stress disorder, and substance use disorders 1, and a range of physical health problems including obesity, diabetes, cardiovascular disease, and cancer 2. Emerging data suggest that epigenetic mechanisms may help explain the association between adverse childhood experiences and later health problems. The goal of this paper is to briefly review epigenetic processes, highlight key findings in the field, discuss their clinical implications, and consider future avenues of research.
Epigenetic Processes
Although the same DNA is found in every cell of our body, cells differentiate into specific cell types and synthesize different proteins to perform diverse functions. The process that makes brain cells distinct from cells found in the heart and other organs is primarily coordinated by epigenetic mechanisms that contribute to the establishment and the maintenance of cell identity 3. Epigenetics refers to chemical and physical modifications of the DNA molecule and its chromatin environment that functionally regulate the activity of the genome without making any changes to the DNA sequence 4. While the epigenetic changes that lead to cell differentiation are hardwired, more recently it has become apparent that the social environment can also regulate genomic function through epigenetic processes5, 6. As such, epigenetic regulation of gene function can be viewed as the adaptation of the genome to environmental influences, a process that has been referred to as genomic plasticity. Thus, it is possible to conceptualize the epigenome as an interface through which the environment can influence genetic processes and, as a result, regulate behavior at least partially in response to environmental needs 5, 7–9.
There are three main types of epigenetic mechanisms: 1) DNA methylation (Figure 1); 2) histone modifications (Figure 2); and 3) post-translational regulation of gene expression via non-coding RNA species. In this review, we will focus on the two first epigenetic mechanisms, as they have been primarily investigated as possible mediators of the long term effects of early life stressors. DNA methylation is a post-transcriptional modification that refers mainly to the transfer of a methyl group (CH3) to the 5′ carbon of a cytosine base. DNA methylation occurs mostly, but not exclusively, at sequences composed of cytosines followed by guanines (CpG). Cytosine methylation in the promoter region of the gene is generally associated with transcriptional repression that occurs by interference with the recruitment and binding of transcription factors 6. DNA methylation also occurs within the gene body and within intergenic regions of the DNA with variable function. Recently, additional epigenetic marks resulting from the oxidation of methylated cytosines have been identified (Figure 1). Of particular interest is 5-hydroxymethylcytosine (5hmC) because there is accumulating evidence suggesting that this mark has a unique functional role, given its differential anatomical and genomic distribution, with increased levels in the brain, and preferential localization in promoter, and intragenic sequence 10, enhancers and actively transcribed regions 11–13. Moreover, 5-hmC is an intermediate in DNA demethylation and its levels are positively correlated with transcription 14. It plays a role in neurodevelopment, as there are significant developmental differences in 5hmC brain levels in regulatory regions of the genome 12, 15. The other products in the oxidation of methylcytosines are 5-formylcytosine and 5-carboxylcytosine 16. While these modifications have different biochemical properties than methylcytosine and hydroxymethylcytosine, and thus could have different functional roles, their levels are very low and practically undetectable in normal physiological conditions 16.
Figure 1.
DNA methylation and demethylation. Cytosine (C); 5-methylcytosine (5-mC); 5-hydroxymethylcytosine (5-hmC); 5-formylcytosine (5-fC); 5-carboxylcytosine (5-caC); DNA methyltransferase (DNMT); ten-eleven translocation enzyme (TET); active modification-passive dilution (AM-PD); thymine DNA glycosylase (TDG).
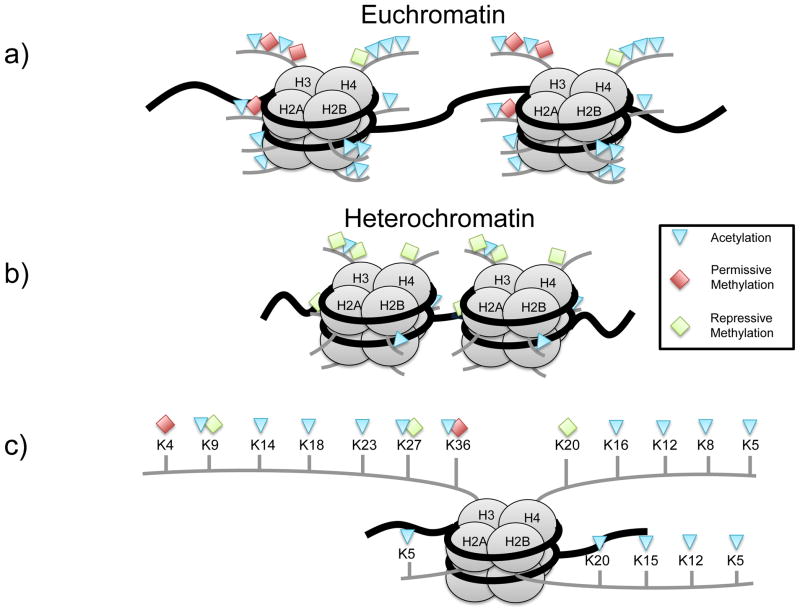
Figure 2.
(a) Euchromatin is associated with active transcription, whereas (b) heterochromatin with transcriptional repression. (c) Each histone has N-termini tails that can be modified, activating or inhibiting transcription.
Histone proteins act as spools around which DNA winds, and play a role in gene regulation via epigenetic mechanisms (Figure 2). Histones proteins have N-terminal tails that contain a number of residues. Post-translational modifications of histone tails are associated with transcriptionally active or inactive chromatin states. In general, high histone acetylation predicts active transcription, whereas high histone methylation, with important exceptions, is associated with more condensed chromatin states and gene repression 17. DNA methylation and histone modifications frequently act synergistically to regulate gene expression.
Early experience can alter the regulation of genes involved in stress reactivity and synaptic plasticity; genes implicated in the etiology of depression, PTSD, and other stress related psychiatric disorders
The last decade has seen marked interest in understanding the regulation of the epigenome by the social environment. Animal studies have consistently suggested that variation in the early environment promotes stable expression changes in genes important for key behavioral and stress responses. These models have provided empirical support for the notion of genomic plasticity9. While several models have been used in the animal literature, one of the best investigated is a model of maternal care in rats, which is based on naturally occurring variations in quantifiable maternal behaviors, including licking and grooming (LG). Low-LG has been found to have significant and stable effects on endocrine and behavioral responses in the offspring. Specifically, offspring of low-LG dams have been found to have lifelong increased stress reactivity and anxiety-like behaviors 18. Subsequent studies in animals have shown that differential DNA methylation of the glucocorticoid receptor (GR) gene in the hippocampus mediate these findings.
The hippocampus is a key structure involved in putting the brakes on the hypothalamic pituitary adrenal (HPA) axis stress response, as glucocorticoids (e.g., corticosteroids in rats; cortisol in humans) provide inhibitory feedback to the stress axis via the hippocampus. In rats, the GR gene is preceded by 10 non-coding exons and by 14 in humans 19, 20. The expression of the non-coding exon 17 in rats and the human homologue 1F are more prominent in the hippocampus 19. Each of the untranslated exon 1 variants has its own promoter and multiple transcription factor binding sites, including nerve growth factor-inducible protein A (NGFI-A), have been identified in GR promoter sequences 19. NGFI-A, also known as egr-1 or zif-268 is a transcription factor that belongs to the early growth response family of proteins, and is highly expressed in the brain. It is activated by a number of stimuli, including neurotransmitters and cellular stimulation, and plays a role in neuronal plasticity 21. The early-life environment regulates methylation patterns in the exon 17 promoter in rats. More specifically, in offspring raised by low-LG rat mothers, CpG methylation levels in the exon 17 promoter region are significantly increased at almost all CpGs compared to offspring raised by high-LG mothers, and importantly, one CpG located in the 5′ end of a NGFI-A binding site is methylated in almost 100% of offspring raised by low-LG mothers whereas it is almost not methylated in offspring from high-LG mothers. Offspring of high-LG have increased acetylation at the lysine 9 residue (K9) of H3 histone 5, and increased trimethylation of H3K4 22 as compared to offspring of low L-G mothers. Enhanced methylation, altered histone modification and NGFI-A binding then results in fewer GR receptors and reduced inhibitory control of the HPA axis stress response 5, 22.
Adult offspring of low-LG mothers also exhibit impaired hippocampal-dependent learning and memory and reduced hippocampal long-term potentiation (LTP), a cellular model for synaptic plasticity that appears to underlie learning and memory 23. These effects appear to be mediated by reduced Glutamate Receptor, Metabotropic 1 (Grm1) gene expression, which encodes for the group I metabotropic glutamate receptor (mGluR). Sustained difference in hippocampal Grm1 expression appears to be due to both DNA methylation and histone modifications. Offspring of low-LG mothers have enhanced methylation in the promoter of Grm1, and reduced histone post-translational modifications in the gene region showing differences in methylation, and a region near the transcriptional start site of Grm1. Moreover, these epigenetic changes associate with decreased Grm1 transcription 23.
In mouse, Murgatroyd et al. (2009) showed that early-life stress associates with hypomethylation of the arginine vasopressin gene (Avp) enhancer and increased gene expression 7. Another study reported changes in DNA methylation of the Brain derived neurotrophic factor (Bdnf) gene in rats exposed to maltreatment during the first postnatal week 8. A broad epigenomic response to maternal care, including alterations in H3K9 acetylation, DNA methylation and gene expression, in multiple regions was also reported 24, Moreover, early life stress can also modify the epigenetic machinery, such as the expression of histone deacetylase 25 and DNA methyltransferase 26. Are these epigenetic differences in gene expression, stress reactivity, synaptic plasticity, and behavior truly due to differences in early maternal care and not genetic differences or differences in prenatal or in utero factors? Cross-fostering studies verify that variations in maternal care account for these differences 5. Offspring of high-LG dams raised by low-LG dams have reduced GR number, enhanced stress reactivity, and phenotypically resemble the biological offspring of low-LG dams.
Clinical Implications
Encouraged by the exciting findings observed in animal studies, over the last few years there has been an exponential increase in the number of epigenetic studies of behavioral phenotypes in humans. Building on the animal findings suggesting hippocampal GR expression and DNA methylation changes associated with early-life environmental variation, and the increased HPA reactivity observed in humans exposed to childhood maltreatment, McGowan and colleagues translated the rodent findings to humans and provided the first evidence for an effect of early-life adversity on the human epigenome 27. Subsequent studies by independent investigators (reviewed in 28) provided additional evidence suggesting that increased GR promoter methylation associates with early life adversity 29. In addition to the GR gene findings, recent studies have suggested that other genes and biological pathways are epigenetically differentially regulated. For instance, studies have implicated additional genes interacting with the HPA axis 30, genes involved in neuroplasticity 31, cognition 32, neoplastic processes 2 and several other biological pathways 33. Some of these studies also observed that epigenetic processes may mediate the interaction between environmental exposure and genetic polymorphisms on the risk of developing stress related psychiatric disorders 30.
Collectively, these data suggest that early-life adversity induces epigenetic regulation of several genes in the brain involved in the regulation of different biological processes. These biological characteristics may help explain clinical heterogeneity in diagnostic categories where a portion of affected individuals have histories of early-life adversity. Accordingly, there is growing consensus that patients with depression, anxiety, and substance use disorders with a history of maltreatment represent a clinically and biologically distinct subtype with earlier age at onset, greater symptom severity, more comorbidity, a greater risk for suicide, and poorer treatment response than non-maltreated individuals with the same diagnoses 1. Maltreated individuals also differ from other individuals with the same diagnoses on a number of neurobiological measures, leading to the recommendation that treatment guidelines and algorithms may be enhanced if maltreated and non-maltreated individuals with the same diagnostic labels are differentiated 1. More work, however, is needed in this area.
Methodological Issues
As additional epigenetic studies are planned, there is a need to discuss important methodological questions arising from our experience in behavioral epigenetics thus far. The validity of studies based on the use of peripheral samples to infer brain molecular processes, the relevance of using tissue homogenates rather than single cell population samples, utility of current molecular strategies characterized by focus on single epigenetic marks, variability on analytical approaches used by different studies and lack of consistency in reporting results are among the many important methodological questions that should be debated. Among these, arguably the most pressing question is the validity of studying methylation and other epigenetic processes using peripheral samples to infer brain-based molecular processes.
Access to brain tissue samples from living subjects is not usually possible and sources of well-characterized postmortem brain tissue are limited. Whereas many studies have been conducted using peripheral tissues, particularly whole blood or saliva, to what extent results observed using peripheral tissues can be used to inform CNS phenomena is unclear. Epigenetic processes are essential in the differentiation of cells and tissues and developmental regulation of genes, and as such, predictably, there is higher variability when comparing epigenetic patterns between tissues of the same individual than when comparing the same tissue of different individuals 34. However, there is also some evidence of within-individual epigenetic variant correlation across tissues 34. It is still early to make definite conclusions about the value of epigenetic studies using samples obtained from peripheral tissues to understand brain processes. Before we engage in large scale studies investigating associations between peripheral epigenetic marks and psychiatric phenotypes, we need to conduct additional studies with single-base resolution, incorporating likely sources of variation such as age, gender and cell composition into our analytical models. Such studies will provide us with much needed insight into the extent to which epigenetic studies using peripheral samples are informative of brain epigenetic processes.
While questions remain about the validity of peripheral samples as proxy of brain tissue to study epigenetic associations with mental illnesses, there are no doubts about the utility of peripheral samples to investigate dynamic epigenetic changes associated with disease that are systemic and may be detected peripherally. For instance, well known systemic factors that present peripheral variation and are associated with psychiatric phenotypes are certain components of the HPA axis, neurotrophic factors, and the immune-inflammatory systems and cytokines. Peripheral samples are also likely to be informative in studies investigating epigenetic factors associated with pharmacological treatment, given that both peripheral as well as central processes are involved in the individual response to treatment. Peripheral factors such as pharmacokinetic, pharmacodynamic and metabolizing factors influence blood levels of the drug, side-effect profile, and treatment response, and differentially regulate peripheral mRNA and ncRNA levels. Methylation studies of peripheral and central tissues of animals treated with haloperidol support this approach 35 and human studies of histone mark changes in peripheral samples of depressed patients in relationship with antidepressant response are consistent with animal findings 36.
Future Avenues of Research
Although we have just begun to understand the complexity of the epigenome, and to study epigenetic factors associated with psychopathology, preliminary results are encouraging. A critical question derived from these findings, however, is whether or not these epigenetic changes that are laid down early in development are reversible later in life. While we have learned over the last few years that DNA methylation is more dynamic than initially thought, DNA methylation is in general a fairly stable process, involved in long-term gene silencing, including X-chromosome inactivation, as well as suppression of alternative promoters and tissue-specific gene regulation. A recent study conducting an in-depth methylation analysis of the whole genome in diverse human tissue types revealed that only approximately 20% the autosomal CpGs are dynamic 37. These dynamic CpGs, which are more distal to the regions commonly investigated in most methylation studies conducted to date, seem to be part of lineage-specific regulators, i.e., genomic elements involved in tissue-type differentiation. It remains unclear to what extent CpGs regulated by the environmental experiences and associated with psychopathological phenotypes are dynamic, i.e., it is unclear to what degree CpGs methylated by adverse environmental experiences may become unmethylated as a result of treatment, psychotherapy or another type of intervention. Some insight into this question comes from animal studies. Adult offspring of low-LG and high-LG dams, when given intracerebroventricular infusions of substances that globally alter the levels of DNA methylation (methionine) or the levels of histone acetylation (trichostatin A, which is a histone deacetylase (HDAC) inhibitor) show reversal of methylation patterns, expression differences and phenotypes between groups 38. This is encouraging and suggests that, in spite of the stability of methylation marks, they can be potentially modified. Therefore, there is active interest in the development of clinically useful interventions that may directly influence DNA methylation to treat stress related psychiatric disorders 39. Currently available drugs that alter epigenetic marks, however, are generally unselective, and many associate with numerous potentially harmful and uncontrollable side effects.
There is also animal data indirectly suggesting that environmental changes may help reverse epigenetic marks associated with unfavorable behavioral phenotypes. For instance, environmental enrichment, which is the exposure of laboratory animals to larger cages with running wheels and other toys, can ameliorate the phenotypes and several of the gene expression changes associated with adverse effects of negative environments in early life. However, studies have yet to prove that phenotypic and molecular changes associated with environmental enrichment are mediated by reversal of epigenetic changes in systems found to mediate early-life stressors. Future studies should address these important questions, which have tremendous clinical relevance.
Apart from a few exceptions, the bulk of the literature investigating epigenetic marks in phenotypes associated with early-life adversity has been focused on DNA methylation. This may be explained by the strong animal data suggesting that DNA methylation mediates environmental effects on psychiatric phenotypes and the role of these studies promoting behavioral epigenetics. In addition, it is methodologically simpler to study DNA methylation, and studies can be easily conducted with readily available DNA samples collected as part of banking efforts for numerous genetic variation projects. Future studies should investigate the potential role of other epigenetic marks on behavioral phenotypes associated with early-life adversity. Of particular interest are novel DNA modifications resulting from the oxidation of methylated cytosine that have been recently identified, and particularly hydroxymethylcytosine, given its high levels in the brain. Similarly, exciting new data suggests that non CpG methylation accumulates in neurons during developmental periods of synaptogenesis 15. This interesting discovery suggests that, in addition to CpG methylation, we should look at the potential role of non CpG methylation mediating environmental influences on behavioral development. In addition, the recent conclusion of the ENCODE (Encyclopedia of DNA elements) project has shed light on a large amount of ncRNA species that regulate important genomic processes. Of these, both short and long noncoding RNAs are promising avenues of investigation. Recent evidence suggests that micro RNAs, which are RNA molecules that average 22 nucleotides and are very stable, circulate peripherally in vesicles called exosomes and may act as “hormones”, involved in communication between cells 40. Long noncoding RNAs are molecules larger than 200 nucleotides that are highly expressed in the CNS, and there is accumulating evidence suggesting they play a critical role in brain plasticity. In sum, epigenetic mechanisms other than methylcytosine are promising epigenetic marks that may mediate the effect of the early-life environment on brain function. They should be the focus of future research investigating behavioral and emotional phenotypes associated with early-life adversity.
Most of the studies conducted so far have used a candidate-gene approach with significant attention paid to the GR gene. However, as genome-wide approaches became technically more accessible and less costly, there has been an increase in the number of genome-wide studies. Technical platforms have changed quickly, and with them, the level of resolution of the studies conducted. As sequencing costs decrease, future studies of the epigenome investigating psychiatric phenotypes should not limit their focus exclusively to promoters and upstream regulatory sequences, and use single-base resolution techniques, such as reduced-representation bisulfite sequence or whole genome bisulfite sequence.
In conclusion, convergent animal and human data suggest that adverse early-life experiences can lead to changes in gene expression through epigenetic mechanisms that can alter stress reactivity, brain function, and behavior. While findings from previous studies are encouraging, we have just begun to understand the complexity of the epigenome. Future studies should use alternative designs, investigate epigenetic marks other than methylcytosine, and assess the efficacy of interventions to reverse epigenetic processes associated with early-life adversity.
Acknowledgments
GT is supported by grants from the Canadian Institute of Health Research (CIHR) # MOP119429, MOP119430 and by the Fonds de Recherche du Québec – Santé (FRQS) through a Chercheur National salary award and through the Réseau québécois sur le suicide, les troubles de l’humeur et les troubles associés.
Footnotes
Contributors: GT and JK contributed to the initial draft, VKO collaborated on designing the figures and revisions, and all authors then revised the final draft.
Conflict of Interest Statement. JK has provided consultation to Pfizer and Merck Pharmaceutical Company to train investigators to assess bipolar disorder in youth.. The other authors have no conflicts of interest to disclose.
Contributor Information
Gustavo Turecki, McGill Group for Suicide Studies, Douglas Mental Health Institute. McGill University. Address: 6875 LaSalle blvd, Montreal, QC H4H 1R3, Canada.
Vanessa Kiyomi Ota, McGill Group for Suicide Studies, Douglas Mental Health Institute; Interdisciplinary Laboratory of Clinical Neurosciences, Federal University of São Paulo (UNIFESP). Genetics Division, Department of Morphology and Genetics, UNIFESP. Address: Rua Botucatu, 740, Genetica, São Paulo, CEP 04023-900, Brazil.
Sintia Iole Belangero, Interdisciplinary Laboratory of Clinical Neurosciences, UNIFESP. Department of Psychiatry, UNIFESP. Genetics Division, Department of Morphology and Genetics, UNIFESP. Address: Rua Botucatu, 740, Genetica, São Paulo, CEP 04023-900, Brazil.
Andrea Jackowski, Interdisciplinary Laboratory of Clinical Neurosciences, UNIFESP. Department of Psychiatry, UNIFESP. Address: Rua Pedro de Toledo, 669 - 3° andar fundos, São Paulo, CEP 04039-032, Brazil.
Joan Kaufman, Yale University School of Medicine. Address: 301 Cedar Street - Rm. 221, PO Box 208098, New Haven, CT, 06520, USA.
References
- 1.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. The American journal of psychiatry. 2013;170(10):1114–33. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang BZ, Zhang H, Ge W, Weder N, Douglas-Palumberi H, Perepletchikova F, et al. Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med. 2013;44(2):101–7. doi: 10.1016/j.amepre.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nature reviews Genetics. 2014;15(2):93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consortium EP. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 6.Lutz PE, Turecki G. DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience. 2014;264:142–56. doi: 10.1016/j.neuroscience.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature neuroscience. 2009;12(12):1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 8.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological psychiatry. 2009;65(9):760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turecki G, Ernst C, Jollant F, Labonte B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35(1):14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473(7347):394–7. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen L, Li X, Yan L, Tan Y, Li R, Zhao Y, et al. Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain. Genome biology. 2014;15(3):R49. doi: 10.1186/gb-2014-15-3-r49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Pan Q, Lin L, Szulwach KE, Song CX, He C, et al. Genome-wide DNA hydroxymethylation changes are associated with neurodevelopmental genes in the developing human cerebellum. Human molecular genetics. 2012;21(26):5500–10. doi: 10.1093/hmg/dds394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Liu M. Distribution of 5-hydroxymethylcytosine in different human tissues. Journal of nucleic acids. 2011;2011:870726. doi: 10.4061/2011/870726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestor CE, Ottaviano R, Reddington J, Sproul D, Reinhardt D, Dunican D, et al. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome research. 2012;22(3):467–77. doi: 10.1101/gr.126417.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502(7472):472–9. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 19.Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35(2):283–92. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- 20.McCormick JA, Lyons V, Jacobson MD, Noble J, Diorio J, Nyirenda M, et al. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Mol Endocrinol. 2000;14(4):506–17. doi: 10.1210/mend.14.4.0438. [DOI] [PubMed] [Google Scholar]
- 21.Veyrac A, Gros A, Bruel-Jungerman E, Rochefort C, Kleine Borgmann FB, Jessberger S, et al. Zif268/egr1 gene controls the selection, maturation and functional integration of adult hippocampal newborn neurons by learning. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(17):7062–7. doi: 10.1073/pnas.1220558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang TY, Labonte B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38(1):111–23. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, Diorio J, et al. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17200–7. doi: 10.1073/pnas.1204599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PloS one. 2011;6(2):e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine A, Worrell TR, Zimnisky R, Schmauss C. Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiology of disease. 2012;45(1):488–98. doi: 10.1016/j.nbd.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anier K, Malinovskaja K, Pruus K, Aonurm-Helm A, Zharkovsky A, Kalda A. Maternal separation is associated with DNA methylation and behavioural changes in adult rats. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2014;24(3):459–68. doi: 10.1016/j.euroneuro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 27.McGowan P, Sasaki A, D’Alessio A, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12(3):342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turecki G. The epigenetic basis of behavioral phenotypes: is there reason for continued optimism? Depress Anxiety. 2013;30(12):1147–50. doi: 10.1002/da.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature neuroscience. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labonte B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69(7):722–31. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labonte B, Suderman M, Maussion G, Lopez JP, Navarro-Sanchez L, Yerko V, et al. Genome-wide methylation changes in the brains of suicide completers. The American journal of psychiatry. 2013;170(5):511–20. doi: 10.1176/appi.ajp.2012.12050627. [DOI] [PubMed] [Google Scholar]
- 33.Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(20):8302–7. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome biology. 2012;13(6):R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aberg KA, Xie LY, McClay JL, Nerella S, Vunck S, Snider S, et al. Testing two models describing how methylome-wide studies in blood are informative for psychiatric conditions. Epigenomics. 2013;5(4):367–77. doi: 10.2217/epi.13.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, Berlim MT, et al. Epigenetic regulation of BDNF expression according to antidepressant response. Mol Psychiatry. 2013;18(4):398–9. doi: 10.1038/mp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziller MJ, Gu H, Muller F, Donaghey J, Tsai LT, Kohlbacher O, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500(7463):477–81. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder M, Hillemacher T, Bleich S, Frieling H. The epigenetic code in depression: implications for treatment. Clin Pharmacol Ther. 2012;91(2):310–4. doi: 10.1038/clpt.2011.282. [DOI] [PubMed] [Google Scholar]
- 40.Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nature reviews Endocrinology. 2013;9(9):513–21. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]