Abstract
Whether dietary vitamin A intake could reduce pancreatic cancer risk is still conflicting. We therefore conducted a meta-analysis to summarize the evidence from epidemiological studies. We searched the databases of PubMed and Web of Knowledge up to July 2016. Random model was used to combine study-specific relative risks (RR) and 95% confidence interval (CI). Publication bias was assessed by Egger regression asymmetry test and Begg's funnel plot. Eleven studies (10 case-control studies and 1 cohort study) involving 2705 pancreatic cancer cases were included in the present study. The RR (95% CI) of pancreatic cancer for highest category of vitamin A intake compared with lowest category was 0.839 (95% CI=0.712–0.988) with low heterogeneity detected (I2=17.8%, Pheterogeneity=0.274). The relationships were also significant for studies designed by case-control [RR=0.808, 95% CI=0.690–0.947], as well as in European population [RR=0.821, 95% CI=0.693–0.972]. No evidence of publication bias was found. This meta-analysis demonstrated that dietary vitamin A intake might inversely associated with the risk of pancreatic cancer.
Keywords: meta-analysis, pancreatic cancer, vitamin A
INTRODUCTION
Pancreatic cancer is a common gastrointestinal cancer. Early diagnosis of pancreatic cancer is difficult because of the lack of clinical symptoms in the early stages, and the 5-year survival rate ranges from 4% to 6% or less [1,2]. Although the incidence and mortality of pancreatic cancer are remarkably high, few treatment options are effective. Therefore, primary prevention of pancreatic cancer is an important matter in the current society.
A recent study had reported that fruit and vegetable intake is associated inversely with the risk of pancreatic cancer [3]. Vitamin A is one of the most common antioxidants from fruits and vegetables, and it may exert chemopreventive effects [4]. Vitamin A could protect cells from oxidative DNA damage, thereby blocking carcinogenesis [5]. Furthermore, antioxidants have their effect on the inflammatory process, and chronic inflammation may play a role in pancreatic carcinogenesis [6]. To date, a number of epidemiologic studies have been published to explore the relationships between dietary vitamin A intake and pancreatic cancer risk. However, the results are not consistent. Therefore, the aim of the present study was to systematically examine whether dietary vitamin A intake could reduce the risk of pancreatic cancer.
MATERIALS AND METHODS
Search strategy and inclusion criteria
A comprehensive search was performed to identify all published studies on dietary vitamin A intake and pancreatic cancer risk. Searches of the PubMed and Web of Knowledge (up to July 2016) were conducted to identify eligible studies, with no language restriction. The key words used for the search strategy were ‘pancreatic’ and ‘cancer’ or ‘carcinoma’ and ‘vitamin A’ or ‘vitamins’ or ‘diet’ or ‘lifestyle’. Handsearching of reference lists of all relevant publications was finally done to identify more studies that could have been omitted from the databases’ search.
Studies were eligible for inclusion if the following predefined criteria were met: (1) using a case-control design or prospective design or cross-sectional design; (2) the association was between vitamin A and pancreatic cancer; (3) published as original articles; (4) contained the minimum information necessary to estimate the effects, i.e. odds ratio (OR) or relative ratio (RR) or hazard ratio (HR), and a corresponding measure of uncertainty, i.e. confidence interval (CI); and (5) the articles were included between 1980 and 2016. Otherwise, we excluded studies that had non-human subjects, duplicated data, those lack of OR or RR or HR, those conducted before 1980 and others that were mainly reviews, viewpoints or editorials. No language restrictions were applied.
Data extraction and quality assessment
Two independent investigators (Tao Zhang and Hongqiang Chen) independently performed the abstract review and subsequent full text review. The key elements related to the study inclusion criteria were collected from each of the included studies. Extracted data will include OR or RR or HR of the outcomes, and their 95% CI, first author's name, study design, country of region, population size and statistical adjustment. The third reviewer (Shiyong Qin) or by contacting content experts were needed until the two reviewers reached a consensus when discrepancies appeared. The quality of each study was assessed using the established form first developed and applied by McShane et al. [7] and Hayes et al. [8]. Studies with scores ≥6 were considered high quality.
Statistical analyses
RR and 95% CI were extracted from each study, which estimated the association for high compared with low vitamin A intake and pancreatic cancer risk. Random-effects model was used to combine the overall and subgroup results. Inconsistency was estimated using the I2 statistics; values of 25, 50 and 75% were considered low, moderate and high inconsistencies respectively [9]. Meta-regression and subgroup analyses (study design, geographic locations) were performed to assess the potentially important covariates that might exert substantial impact on between-study heterogeneity [10]. Sensitivity analysis was conducted to describe how robust the pooled estimator was to removal of individual studies [11]. Publication bias was evaluated using Egger regression asymmetry test [12] and Begg's funnel plot. STATA version 12.0 was used to analyse the results.
RESULTS
Flow of the included studies
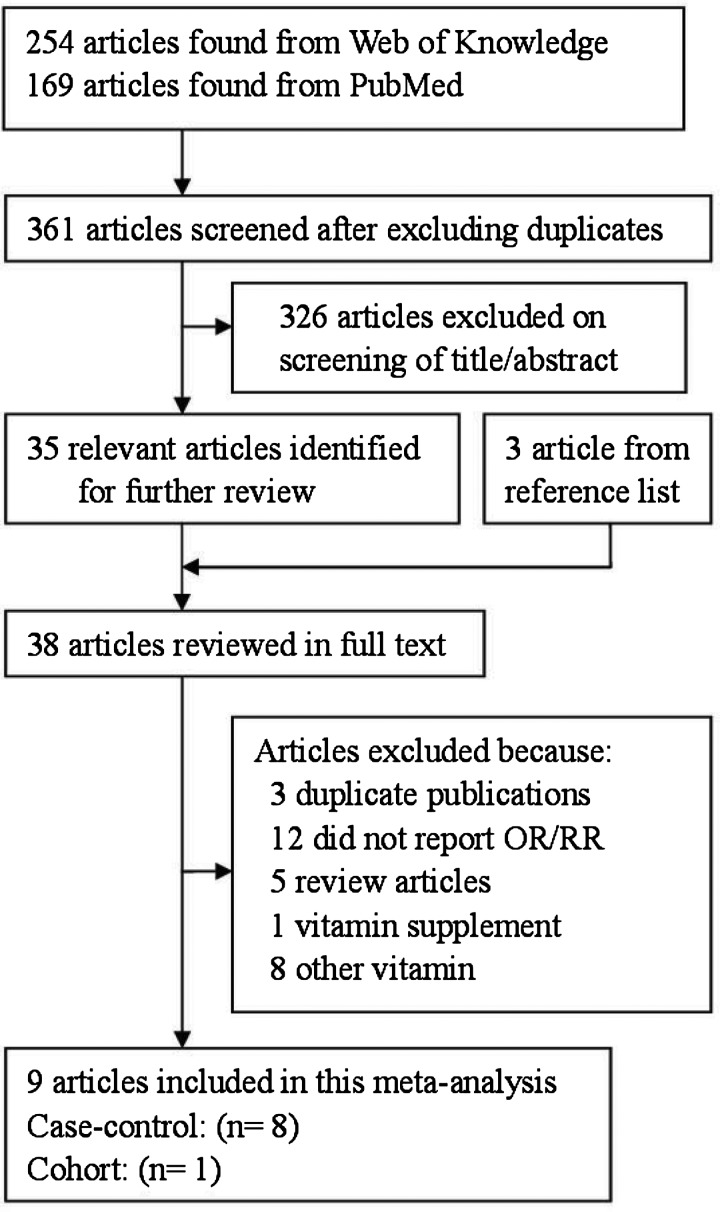
Figure 1 showed a flow chart of studies that were suitable for this analysis. At the end, 11 studies [13–22] with 2705 cases were included in the study. Five studies were come from Europe, five from America and one from Asia. Ten studies were designed in case-control studies (six population-based case-control studies, four hospital-based case-control studies) and one in cohort. The characteristics of these included studies are presented in Table 1.
Figure 1. The flow diagram of screened, excluded and analyzed publications.
Table 1. Characteristics of the included studies.
| Study, year |
Study design | Country | Age (years) | Cases | RR (95% CI) for highest compared with lowest category | Quality scores | Adjustment for covariates |
|---|---|---|---|---|---|---|---|
| Howe et al. (1990) |
PBCC | Canada | 35–79 | 249 | 1.12 (0.67–1.88) | 7 | Adjust for caloric and fibre intake, lifetime cigarette consumption |
| Olsen et al. (1991) |
PBCC | United States | 40–84 | 212 | 1.50 (0.70–3.00) | 7 | Adjusted for total energy, age, cigarette usage, alcohol consumption, respondent-reported history of diabetes mellitus and educational level |
| Zatonski et al. (1991) |
PBCC | Poland | 62.2 | 110 | 0.53 (0.20–1.45) | 8 | Adjust for cigarette lifetime consumption and calories |
| Kalapothaki et al. (1993) |
HBCC | Greece | Not available | 181 | 0.80 (0.65–0.99) | 7 | Adjust for age, gender, hospital, past residence, years of schooling, cigarette smoking, diabetes mellitus and energy intake |
| Stolzenberg–Solomon et al. (2002) | Cohort | Finland | 50–69 | 163 | 1.21 (0.71–2.03) | 8 | Adjust for by the residual method and for age and years of smoking, energy-adjusted folate intake and energy-adjusted saturated fat intake |
| Lin et al. (2005) |
PBCC | Japan | 40–79 | 109 | 1.09 (0.62–1.92) | 6 | Adjust for age, pack-years of smoking and energy intake |
| Bravi et al. (2011) |
HBCC | Italian | 34–80 | 326 | 0.73 (0.44–1.19) | 7 | Adjusted for age, sex and centre, year of interview, education, tobacco smoking and history of diabetes, body mass index and total energy intake |
| Zablotska et al. (2011) |
PBCC | United States | 21–85 | 532 | Male: 0.78 (0.42–1.50) Female: 0.78 (0.36–1.70) |
8 | Adjusted for energy intake by the residual method, body mass index, race, education, smoking, history of diabetes, physical activity and alcohol consumption |
| Jansen et al. (2013) |
HBCC | United States | 31–92 | 983 | 0.55 (0.37–0.81) | 8 | Adjusted for energy, smoking, BMI, age, sex and drinks of alcohol per week |
| Jeurnink et al. (2015) |
PBCC | European | 63.1 | 446 | 0.84 (0.50–1.40) | 7 | Adjusted for smoking status, duration and intensity of smoking, cotinine levels, waist circumference and diabetes status |
Vitamin A and pancreatic cancer
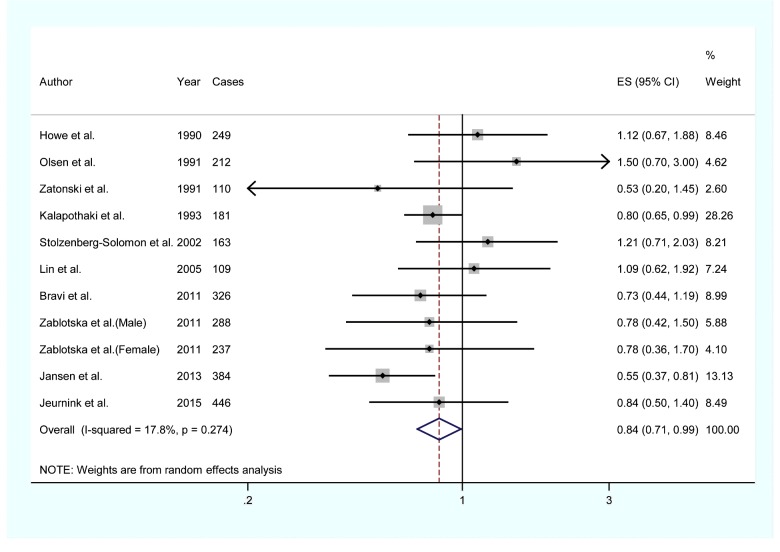
Two individual studies reported an inverse association between dietary vitamin A intake and pancreatic cancer risk, whereas nine studies found no significant association between vitamin A intake and the risk of pancreatic cancer. Figure 2 presents the each RR with their 95% CI for all 11 studies assessing the association between vitamin A intake and risk of pancreatic cancer. The pooled RR of 0.839 (95% CI=0.712–0.988) shows that higher category of dietary vitamin A intake was associated with reduced risk of pancreatic cancer, with low heterogeneity detected (I2=17.8%, Pheterogeneity=0.274).
Figure 2. The forest plot between highest compared with lowest categories of dietary vitamin A intake and pancreatic cancer risk.
Meta-regression and subgroup analyses
As shown in Figure 2, low category of heterogeneity (I2=17.8%, Pheterogeneity=0.274) was found in the pooled results. Thus, the univariate meta-regression was not performed.
Ten of 11 included articles were designed in case-control studies, so subgroup analysis for case-control studies was performed. Significant inverse association was found between dietary vitamin A intake and pancreatic cancer in case-control studies [summary RR=0.808, 95% CI=0.690–0.947]. Besides, we also extracted the detailed information for hospital based case-control studies (HBCC) or population bases case-control studies. And the significant associations were found in the hospital bases case-control studies [summary RR=0.745, 95% CI=0.632–0.878], but not in population based case-control studies (PBCC) [summary RR=0.985, 95% CI=0.753–1.288]. Studies were also stratified by geographic area, the RR were 0.821 (95% CI=0.693–0.972) for studies conducted in Europe, 0.852 (95% CI=0.588–1.233) for studies in America. There is only one study designed in cohort design and one study conducted in Japan. Thus, the subgroup analysis for them was not conducted. The detailed results are summarized in Table 2.
Table 2. Summary risk estimates of the association between dietary vitamin A intake and pancreatic cancer risk.
| Subgroups | Cases | Studies | RR (95% CI) | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|
| All studies | 2705 | 11 | 0.839 (0.712–0.988) | 17.8 | 0.274 |
| Study design | |||||
| Case-control | 2542 | 10 | 0.808 (0.690–0.947) | 10.0 | 0.351 |
| PBCC | 1205 | 6 | 0.985(0.753–1.288) | 0.0 | 0.548 |
| HBCC | 1337 | 4 | 0.745(0.632–0.878) | 0.0 | 0.398 |
| Cohort | _ | _ | _ | _ | _ |
| Geographic locations | |||||
| America | 1370 | 5 | 0.852 (0.588–1.233) | 50.1 | 0.091 |
| Europe | 1226 | 5 | 0.821 (0.693–0.972) | 0.0 | 0.537 |
Sensitivity analyses and publication bias
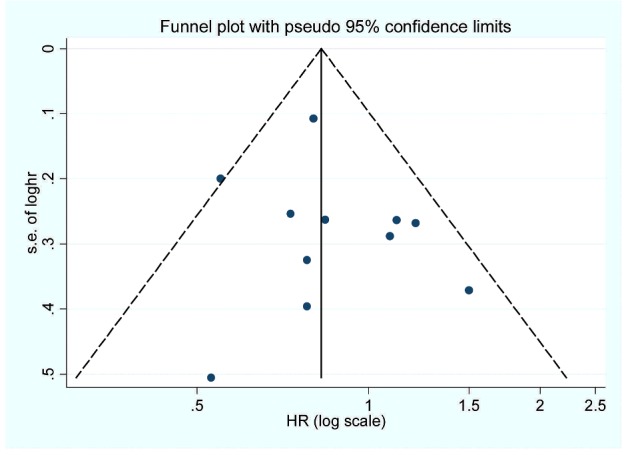
Sensitivity analysis showed that no individual study had excessive influence on the association of vitamin A intake and pancreatic cancer risk. Egger regression asymmetry test (P=0.483) and funnel plot (Figure 3) showed no evidence of significant publication bias between vitamin A intake and pancreatic cancer risk.
Figure 3. The funnel plot of the association between dietary vitamin A intake and pancreatic cancer risk.
DISCUSSION
The present study provided convincing evidence that dietary vitamin A intake is associated with a reduced risk of pancreatic cancer. This relationship between dietary vitamin A intake and pancreatic cancer risk was apparent and consistent in case-control studies and in the subgroup analysis of Europe populations.
A recent meta-analysis had been conducted to assess the association between vitamin E intake and the risk of pancreatic cancer [23]. The results in that meta-analysis found an inverse association between vitamin E intake and pancreatic cancer risk. For antioxidants, such as vitamins A and vitamin E, there are several plausible biological mechanisms by which they might prevent pancreatic cancer, including inactivating free radicals and reducing oxidative DNA damage, stimulating immune function [24]. Furthermore, dietary antioxidants have been shown in laboratory studies to enhance growth restriction of cancer cells in general and cancer cells in particular [25,26]. Vitamin A and vitamin E as antioxidant nutrients may influence this process due to their being free radical scavengers, suggesting that dietary antioxidants may play an important role in prevention.
As a meta-analysis of published studies, our findings showed some advantages. First, a highlight of the present study was that we found an inverse association between dietary vitamin A intake and the risk of pancreatic cancer with low between-study heterogeneity. Second, the present study included a large number of cases and participants; this may derive a more precise estimation of the relationship between vitamin A intake and pancreatic cancer risk. Third, no significant publication bias was detected in this meta-analysis. Fourth, the quality scores of each study are assessed with high quality. Fifth, between-study heterogeneity is common in the meta-analysis, and our study found no evidence of between-study heterogeneity in the pooled analysis and subgroup analysis.
There are some limitations in this meta-analysis should be concerned. First, our meta-analysis included 10 case-control studies and one cohort study. For the case-control studies, some recall or selection bias may be inherent in the original studies. Although prospective studies can allow a much greater possibility of reaching reasonable conclusions, the case-control study is an important method in aetiology research. Therefore, more original studies especially with prospective design are wanted in the future studies. Second, the association was only significant in the hospital-based case-controls, but not in the population-based case-controls. This may be caused by the little cases included in the population-based case-controls. Third, we did not do a dose-response analysis for vitamin A intake and the risk of pancreatic cancer because of the limited data in the reported articles. Further studies with detailed dose for each category are wanted to assess this association. Fourth, other unpublished literatures on relevant pharmaceutical websites were not searched and only studies in English were included, which may lead to a potential publication bias, although no significant publication bias was found by Egger's test and funnel plot. Finally, we only found significant association in the European populations, but not in the America and other populations. Therefore, more studies conducted in America and other areas are wanted to assess the relationship between dietary vitamin A intake and pancreatic cancer.
In summary, results from the present study showed clearly that dietary vitamin A intake can significantly reduce the risk of pancreatic cancer.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HBCC
hospital based case-control studies
- HR
hazard ratio
- OR
odds ratio
- PBCC
population based case-control studies
- RR
relative risks
AUTHOR CONTRIBUTION
Tao Zhang and Hongqiang Chen designed the experiments; Shiyong Qin, Minghai Wang and Xin Zhang collected the data; Tao Zhang and Shuguang Zhang analyse the data; Tao Zhang and Fei Liu wrote the main manuscript text and all authors reviewed the manuscript.
References
- 1.Klapman J., Malafa M.P. Early detection of pancreatic cancer: why, who, and how to screen. Cancer Control. 2008;15:280–287. doi: 10.1177/107327480801500402. [DOI] [PubMed] [Google Scholar]
- 2.Karim-Kos H.E., de Vries E., Soerjomataram I., Lemmens V., Siesling S., Coebergh J.W. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur. J. Cancer. 2008;44:1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Wu Q.J., Wu L., Zheng L.Q., Xu X., Ji C., Gong T.T. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur. J. Cancer Prev. 2015;25:196–205. doi: 10.1097/CEJ.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 4.Mahdavi R., Faramarzi E., Seyedrezazadeh E., Mohammad-Zadeh M., Pourmoghaddam M. Evaluation of oxidative stress, antioxidant status and serum vitamin C levels in cancer patients. Biol. Trace Elem. Res. 2006;130:1–6. doi: 10.1007/s12011-008-8309-2. [DOI] [PubMed] [Google Scholar]
- 5.Pathak S.K., Sharma R.A., Steward W.P., Mellon J.K., Griffiths T.R., Gescher A.J. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur. J. Cancer. 2005;41:61–70. doi: 10.1016/j.ejca.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Algul H., Treiber M., Lesina M., Schmid R.M. Mechanisms of disease: chronic inflammation and cancer in the pancreas–a potential role for pancreatic stellate cells? Nat. Clin. Pract. Gastroenterol. Hepatol. 2007;4:454–462. doi: 10.1038/ncpgasthep0881. [DOI] [PubMed] [Google Scholar]
- 7.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. Reporting recommendations for tumour marker prognostic studies (REMARK) Br. J. Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes D.F., Ethier S., Lippman M.E. New guidelines for reporting of tumor marker studies in breast cancer research and treatment: REMARK. Breast Cancer Res. Treat. 2006;100:237–238. doi: 10.1007/s10549-006-9253-5. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P., Thompson S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 11.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata. Tech. Bull. 1999;47:15–17. [Google Scholar]
- 12.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe G.R., Jain M., Miller A.B. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int. J. Cancer. 1990;45:604–608. doi: 10.1002/ijc.2910450405. [DOI] [PubMed] [Google Scholar]
- 14.Olsen G.W., Mandel J.S., Gibson R.W., Wattenberg L.W., Schuman L.M. Nutrients and pancreatic cancer: a population-based case-control study. Cancer Causes Control. 1991;2:291–297. doi: 10.1007/BF00051668. [DOI] [PubMed] [Google Scholar]
- 15.Zatonski W., Przewozniak K., Howe G.R., Maisonneuve P., Walker A.M., Boyle P. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. Int. J. Cancer. 1991;48:390–394. doi: 10.1002/ijc.2910480314. [DOI] [PubMed] [Google Scholar]
- 16.Kalapothaki V., Tzonou A., Hsieh C.C., Karakatsani A., Trichopoulou A., Toupadaki N., Trichopoulos D. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer Causes Control. 1993;4:383–389. doi: 10.1007/BF00051342. [DOI] [PubMed] [Google Scholar]
- 17.Stolzenberg-Solomon R.Z., Pietinen P., Taylor P.R., Virtamo J., Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am. J. Epidemiol. 2002;155:783–792. doi: 10.1093/aje/155.9.783. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y., Tamakoshi A., Hayakawa T., Naruse S., Kitagawa M., Ohno Y. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. J. Gastroenterol. 2005;40:297–301. doi: 10.1007/s00535-004-1537-0. [DOI] [PubMed] [Google Scholar]
- 19.Bravi F., Polesel J., Bosetti C., Talamini R., Negri E., Dal Maso L., Serraino D., La Vecchia C. Dietary intake of selected micronutrients and the risk of pancreatic cancer: an Italian case-control study. Ann. Oncol. 2011;22:202–206. doi: 10.1093/annonc/mdq302. [DOI] [PubMed] [Google Scholar]
- 20.Zablotska L.B., Gong Z., Wang F., Holly E.A., Bracci P.M. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control. 2011;22:91–100. doi: 10.1007/s10552-010-9678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen R.J., Robinson D.P., Stolzenberg-Solomon R.Z., Bamlet W.R., de Andrade M., Oberg A.L., Rabe K.G., Anderson K.E., Olson J.E., Sinha R., Petersen G.M. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J. Gastrointest. Cancer. 2013;44:152–161. doi: 10.1007/s12029-012-9441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeurnink S.M., Ros M.M., Leenders M., van Duijnhoven F.J., Siersema P.D., Jansen E.H., van Gils C.H., Bakker M.F., Overvad K., Roswall N., et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: a nested case-control study. Int. J. Cancer. 2015;136:E665–E676. doi: 10.1002/ijc.29175. [DOI] [PubMed] [Google Scholar]
- 23.Peng L., Liu X., Lu Q., Tang T., Yang Z. Vitamin E intake and pancreatic cancer risk: a meta-analysis of observational studies. Med. Sci. Monit. 2015;21:1249–1255. doi: 10.12659/MSM.893607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meydani S.N., Meydani M., Blumberg J.B., Leka L.S., Siber G., Loszewski R., Thompson C., Pedrosa M.C., Diamond R.D., Stollar B.D. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. J. Am. Med. Assoc. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 25.D'Archivio M., Santangelo C., Scazzocchio B., Varì R., Filesi C., Masella R., Giovannini C. Modulatory effects of polyphenols on apoptosis induction: relevance for cancer prevention. Int. J. Mol. Sci. 2008;9:213–228. doi: 10.3390/ijms9030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoshyomn S., Nathan D., Manske G.C., Osler T.M., Penar P.L. Synergistic effect of genistein and BCNU on growth inhibition and cytotoxicity of glioblastoma cells. J. Neurooncol. 2002;57:193–200. doi: 10.1023/A:1015765616484. [DOI] [PubMed] [Google Scholar]