Abstract
Naphthalene, phenanthrene, and biphenyl and their derivatives having different ethynyl, propynyl, butynyl, and propargyl ether substitutions were examined for their interaction with and oxidation by cytochromes P450 (P450) 2A13 and 2A6. Spectral interaction studies suggested that most of these chemicals interacted with P450 2A13 to induce Type I binding spectra more readily than with P450 2A6. Among the various substituted derivatives examined, 2-ethynylnaphthalene, 2-naphthalene propargyl ether, 3-ethynylphenanthrene, and 4-biphenyl propargyl ether had larger ΔAmax/Ks values in inducing Type I binding spectra with P450 2A13 than their parent compounds. P450 2A13 was found to oxidize naphthalene, phenanthrene, and biphenyl to 1-naphthol, 9-hydroxyphenanthrene, and 2- and/or 4-hydroxybiphenyl, respectively, at much higher rates than P450 2A6. Other human P450 enzymes including P450s 1A1, 1A2, 1B1, 2C9, and 3A4 had lower rates of oxidation of naphthalene, phenanthrene, and biphenyl than P450s 2A13 and 2A6. Those alkynylated derivatives that strongly induced Type I binding spectra with P450s 2A13 and 2A6 were extensively oxidized by these enzymes upon analysis with HPLC. Molecular docking studies supported the hypothesis that ligand-interaction energies (U values) obtained with reported crystal structures of P450 2A13 and 2A6 bound to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, indole, pilocarpine, nicotine, and coumarin are of use in understanding the basis of possible molecular interaction of these xenobiotic chemicals with active sites of P4502A13 and 2A6 enzymes. In fact, the ligand-interaction energies with P450 2A13 4EJG bound to these chemicals were found to relate to their induction of Type I binding spectra.
Graphical Abstract
INTRODUCTION
Naphthalene, phenanthrene, and biphenyl are aromatic hydrocarbon contaminants found in the environment, and the former two chemicals belong to the class of polycyclic aromatic hydrocarbons (PAHs) that include toxic and carcinogenic compounds such as benzo[a]pyrene, 7,12-dimethylbenz[a]anthracene, and benzo[c]phenanthrene.1–5 These carcinogenic PAHs have been shown to require metabolic activation by so-called xenobiotic-metabolizing enzymes, such as cytochrome P450 (P450), to evoke their toxic and carcinogenic responses in laboratory animals as well as in humans.6–8
Naphthalene has been shown to cause toxic responses in several animal species and is known to require bioactivation by P450 2F2 in mice to elicit toxicity.9,10 Hu et al. (2013)11 have also reported that P450 2A5 plays an important role in olfactory mucosal toxicity in mice. Metabolism of naphthalene by different P450 enzymes has been reported in humans12,13 and also in Rhesus monkeys.14 Metabolism of phenanthrene and biphenyl has also been reported in various laboratory animals;15–22 however, detailed studies on the roles of human P450s 2A13 and 2A6 in the metabolism of naphthalene, phenanthrene, and biphenyl have not been reported, except that Fukami et al. showed that P450 2A13 can catalyzes oxidation of naphthalene in humans.13
Our previous studies have shown that naphthalene, phenanthrene, and biphenyl and their substituted derivatives induced Type I binding spectra with P450s 2A13 and 2A6, suggesting that these chemicals may be oxidized by these P450 enzymes.23 In this study, we studied how these chemicals are oxidized by P450s 2A13 and 2A6, and whether or not there are structure-function relationships for interacting with and biotransformation by these enzymes, based on analysis with HPLC. We used naphthalene and six substituted derivatives, phenanthrene and six substituted derivatives, and biphenyl and nine substituted derivatives for spectral interaction with and metabolism by P450s 2A13 and 2A6, Other P450 enzymes—human 1A1, 1A2, 1B1, 2C9, and 3A4—were also used to study the oxidation of the parent compounds naphthalene, phenanthrene, and biphenyl. Docking simulation of interaction of P450s 2A13 and 2A6 and these chemicals was also examined to visualize how these chemicals interact with these P450 enzymes.
EXPERIMENTAL PROCEDURES
Chemicals
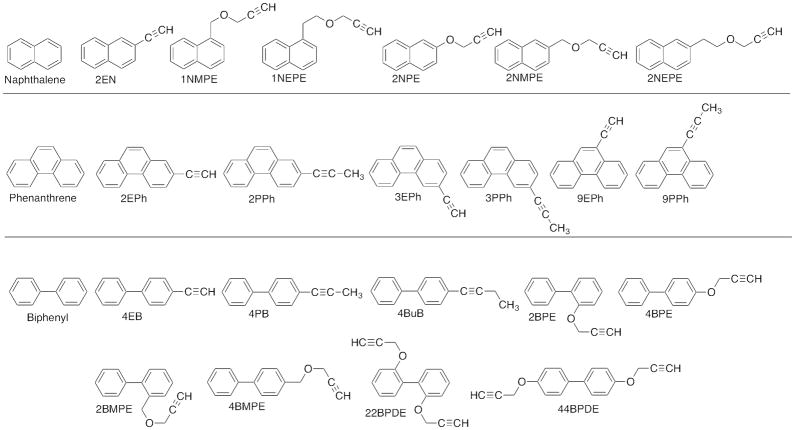
Naphthalene, phenanthrene, and biphenyl were obtained from Sigma Aldrich (St. Louis, MO) or Wako Pure Chemical (Osaka, Japan) (Figure 1). 1-Naphthol, 4-naphthol, 9-hydroxyphenanthrene, and 2- and 4-hydroxybiphenyl were also obtained from Wako Pure Chemical. Acetylenic PAHs and biphenyl derivatives, including 2-ethynylnaphthalene (2EN), 1-naphthalene methyl propargyl ether (1NMPE), 1-naphthalene ethyl propargyl ether (1NEPE), 2-naphthalene propargyl ether (2NPE), 2-naphthalene methyl propargyl ether (2NMPE), 2-naphthalene ethyl propargyl ether (2NEPE), 2-ethynylphenanthrene (2EPh), 3-ethynylphenanthrene (3EPh), 9-ethynylphenanthrene (9EPh), 2-(1-propynyl)phenanthrene (2PPh), 3-(1-propynyl)phenanthrene (3PPh), 9-(1-propynyl)phenanthrene (9PPh), 4-ethynylbiphenyl (4EB), 4-propynylbiphenyl (4PB), 4-butynylbiphenyl (4BuB), 2-biphenyl propargyl ether (2BPE), 4-biphenyl propargyl ether (4BPE), 2-biphenyl methyl propargyl ether (2BMPE), 4-biphenyl methyl propargyl ether (4BMPE), 2,2′-biphenyl dipropagyl ether (22BDPE), and 4,4′-biphenyl dipropargyl ether (44BDPE) (Figure 1) were synthesized as described previously.23–30 These substituted chemicals have been used for the studies of mechanisms of inhibition of human P450 enzymes, including P450 2A13 and 2A6.23–30
Figure 1.
Structures of chemicals used in this study.
Other chemicals and reagents used in this study were obtained from the sources described previously or were of the highest quality commercially available.23–25
Enzymes
Human P450s, NADPH-P450 reductase, and cytochrome b5 enzymes used in this study were expressed in Escherichia coli as described previously.23–25 Bacterial bicistronic P450s 1A2, 1B1 (1B1.3, L432V), 2A6, 2A13, 2C9, and 3A4 co-expressing human NADPH-P450 reductase were also prepared, and the E. coli membranes were suspended in 10 mM Tris-HCl buffer (pH 7.4) containing 1.0 mM EDTA and 20% glycerol (v/v). Human P450s 1A1, 2A6, 2A13, and NADPH-P450 reductase were purified from membranes of recombinant E. coli as described elsewhere.23–25 Catalytic activities (nmol product formed/min/nmol P450) for these eight P450 enzymes were determined using typical model substrates and were found to be: 35, 3.2, and 14 min−1 for 7-ethoxyresorufin O-deethylation by P450s 1A1, 1A2, and 1B1, respectively; 3.3 and 1.4 min−1 for coumarin 7-hydroxylation by P450s 2A6 and 2A13, respectively; 3.5 min−1 for flurbiprofen 4′-hydroxylation by P450 2C9; 3.2 and 6.5 min−1 for midazolam 1′- and 4′-hydroxylation, respectively, by P450 3A4.
Spectral Binding Titrations
Purified P450 2A13 and 2A6 were diluted to 1.0 μM in 0.10 M potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v), and binding spectra were recorded with subsequent additions of chemicals in a JASCO V-550 or OLIS-Aminco DW2a spectrophotometer (On-Line Instrument Systems, Bogart, GA) as described previously.23–25 Briefly, the chemicals were added to the buffer with or without the P450 and the spectra were recorded between 350 nm and 500 (or 700) nm. The substrate binding spectra were obtained by subtracting the blank spectra (in the absence of the P450) from the P450 spectra (in the presence of the P450). Spectral dissociation constants (Ks) were estimated using GraphPad Prism software (GraphPad Software, San Diego, CA), either using hyperbolic plots or quadratic fits in the cases of tight binding.
Oxidation of Naphthalene
Oxidative metabolism of naphthalene by P450s 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4 was determined in a standard incubation mixture (0.25 mL) containing 25 pmol P450 (in bicistronic membranes), 50 μM naphthalene, and an NADPH-generating system according to the method described by Fukami et al. (2008).13 (The chemicals were dissolved in (CH3)2SO as 10 mM stock solutions and diluted, with the organic solvent concentration ≤0.5%, v/v.). In reconstitution experiments, P450 membranes were replaced by purified P450 1A1 (25 pmol), NADPH-P450 reductase (50 pmol), and L-α-dilauroyl-syn-glycero-3-phosphocholine (50 μg) as described previously.23–25 Incubation was carried out at 37 °C for 20 min, following a preincubation time of 1 min. Reactions were terminated by adding 100 μL of CH3CN, and following centrifugation the upper layer was subjected to HPLC using a Mightsil RP-18 C18 GP column. (Note: naphthalene and its metabolites are volatile under a nitrogen stream after these chemicals are extracted with organic solvents.) The mobile phase used was 30% CH3CN (v/v, in H2O) containing 0.01% H3PO4 (w/v).
Oxidation of Phenanthrene and Biphenyl and Their Derivatives and Naphthalene Derivatives
Oxidative metabolism of phenanthrene and biphenyl and their derivatives (and also naphthalene derivatives) was determined in a standard reaction mixture (final volume of 0.25 mL) as described above. After incubation at 37 °C for 20 min, extraction was done by adding 0.25 ml of cold CH3OH, and then 0.5 mL of mixture of a CHCl3 and ethyl acetate mixture (1:1, v/v). After centrifugation, the lower organic layer was recovered and the extraction was repeated once. The extracts were combined and concentrated under a nitrogen stream. HPLC separation was done with a JASCO system (Tokyo, Japan) equipped with a Wakopack Navi C18-5 octadecylsilane column (2.0 mm × 150 mm) (Wako Pure Chem.) with UV detection at 254 nm and fluorescence detection with an excitation wavelength of 242 nm and emission wavelength at 380 nm. Elution of chemicals and their metabolites utilized a linear gradient from 20% to 100% CH3OH (v/v, in H2O) for 25 min and then held at 100% CH3OH for 5 min, with a flow rate of 0.2 mL/min.
Other Enzyme Assays
Coumarin 7-hydroxylation, 7-ethoxyresorufin O-deethylation, flurbiprofen 4-hydroxylation, and midazolam 1′- and 4′-hydroxylation activities were determined using bicistronic bacterial membrane or reconstituted monooxygenase systems as described previously.23–25,31–33
Docking Simulations into Human P450 Enzymes
Crystal structures of P450 2A13 bound to NNK (PDB 4EJH),34 indole (PDB 2P85),35 pilocarpine (PDB 3T3S),36 and nicotine (PDB 4EJG)34 have been reported and were used for these studies. Structures of P450 2A6 bound to coumarin (PDB 1Z10),37 pilocarpine (PDB 3T3R),36 and nicotine (PDB 4EJJ)34 have also been reported. Simulations were carried out after removing each ligand from these P450 structures using the MMFF94x force field described in the MOE software (ver. 2015.10, Computing Group, Montreal, Canada).23–25 Ligand-interaction energies (U values) were obtained by use of ASEdock program in MOE. Lower U values are the indication of higher interaction between a chemical and the enzyme.
Kinetic Analysis
Kinetic parameters were estimated by nonlinear regression analysis using the program Kaleida-Graph (Synergy Software, Reading, PA) or Graphpad Prism (Graphpad Software, La Jolla, CA).
RESULTS
Spectral Interactions of Naphthalene, Phenanthrene, and Biphenyl and Their Derivatives with P450s 2A13 and 2A6
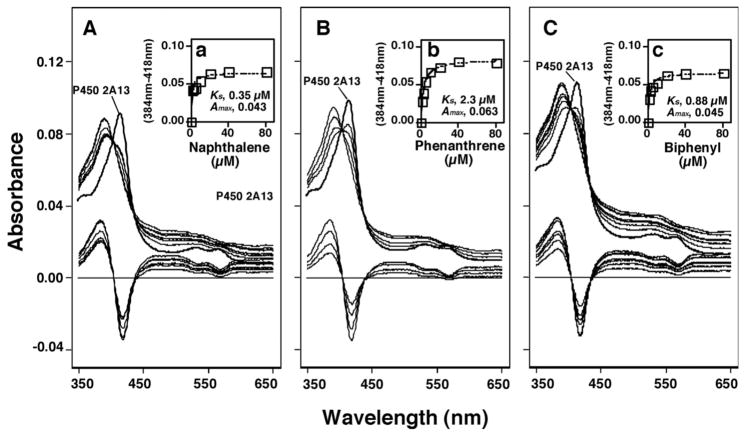
As has been reported previously23, naphthalene, phenanthrene, and biphenyl interacted with P450 2A13 in inducing Type I binding spectral changes (Figure 2). The Ks values obtained with these three chemicals were determined to be 0.35, 2.3, and 0.88 μM, respectively, and the ΔAmax values obtained were between 0.043, 0.063, and 0.045, respectively (Figure 2).
Figure 2.
Spectral changes produced by interaction of naphthalene (A), phenanthrene (B), and biphenyl (C) with P450 2A13. The lower portion of each figure indicates difference spectra obtained.
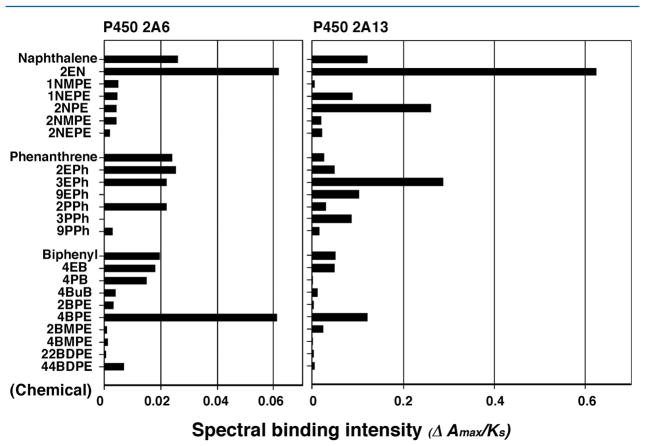
Naphthalene and its six derivatives, phenanthrene and its six derivatives, and biphenyl and its nine derivatives were also compared for their abilities (ΔAmax/Ks ratio) to induce spectral changes with P450 2A6 as well as P450 2A13 (Figure 3). It should be pointed out that the scales for the ΔAmax/Ks ratio on the x-axis were different for P450s 2A13 and 2A6, indicating that the former enzyme showed higher spectral changes than the latter. 2EN induced Type I binding spectra with P450 2A13 to much higher extents than the parent naphthalene did, and the ΔAmax/Ks ratio for interaction of 2EN with P450 2A13 was about 10-fold of that with P450 2A6 (Figure 3). 2NPE, 3EPh, and 4BPE were also found to show higher intensities of interaction with P450 2A13 than the parent compounds, naphthalene, phenanthrene, and biphenyl. 4BPE was also found to induce spectral changes with P450 2A6 at higher level than biphenyl.
Figure 3.
Comparison of spectral changes (spectral binding efficiency, ΔAmax/Ks ratio) of interaction of various chemicals with P450s 2A6 and 2A13.
Oxidation of Naphthalene, Phenanthrene, and Biphenyl by Human P450 Enzymes
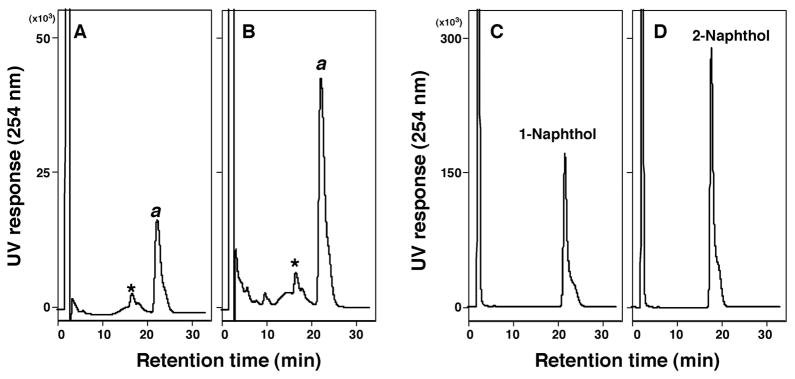
Oxidation of naphthalene was determined with P450s 2A13 and 2A6 according to the method of Fukami et al.13 P450s 2A13 and 2A6 produced one major peak that corresponded to a standard 1-naphthol, but not 2-naphthol, in the retention time on HPLC chromatograms, with P450 2A13 showing more activity than P450 2A6 (Figure 4). Since we did not extract naphthalene and its metabolites and concentrate for HPLC analysis due to their volatile nature, it is not known whether or not small peaks seen in the chromatograms are due to 2-naphthol and other metabolites.
Figure 4.
Oxidation of naphthalene by P450 2A6 (A) and P450 2A13 (B) by analysis with HPLC. Standard 1-naphthol (C) and 2-naphthol (D) were also analyzed. Formation of 1-naphthol from naphthalene by P450s 2A6 and 2A13 was shown as “a”. An asterisk (*) indicates minor peaks of products that were not present in the absence of an NADPH-generating system.
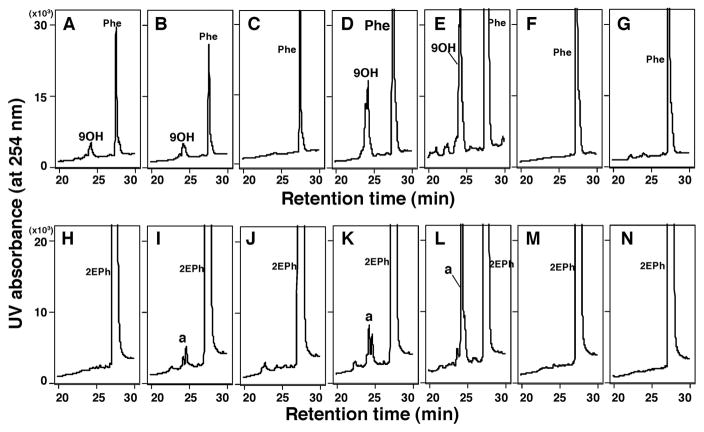
P450 2A13 was also active in oxidizing phenanthrene to 9-hydroxyphenanthrene (Supporting information Figure S1). The standard 9-hydroxyphenanthrene and a metabolite produced by P450 2A13 showed similar profiles showing two peaks on HPLC chromatograms (it is not known at present why two peaks appear for 9-hydroxyphenanthrene or if it arises by an experimental artifact possible to detector saturation). When the scale of a vertical line was increased (zoomed in), several minor peaks appeared only in the presence of an NADPH-generating system reacted with P450 2A13 (Supporting information Figure S1b). We also found very similar, but different, chromatographic profiles of product formation for the metabolism of 2EPh and phenanthrene by P450 2A13 (Supporting information Figure S1C, S1D, S1c, and S1d). We do not have a standard 2EPh metabolite, but the major peak (*) seems to be similar in nature to 9OH-phenanthrene.
In order to compare product formations for the metabolism of phenanthrene and 2EPh, we determined activities with seven human P450 enzymes, P450s 1A1, 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4 (Figure 5). The results showed that P450 2A13 was the most active in oxidizing phenanthrene (Figure 5E) and 2EPh (Figure 5L) to 9-hydroxyphenanthrene and metabolite a, respectively, followed by P450 2A6 (Figure 5D and 5K). Other P450 enzymes examined—P450s 1A1, 1A2, and 1B1—were active in producing 9-hydroxyphenanthrene from phenanthrene, and P450s 1A2 and 1B1.3 oxidized 2EPh at lower levels than P450s 2A13 and 2A6.
Figure 5.
Oxidation of phenanthrene (Figure 6A–6H) and 2EPh (Figure 6I–6P) by P450 1A1 (A and H), 1A2 (B and I), 1B1 (C and J), 2A6 (D and K), 2A13 (E and L), 2C9 (F and M), and 3A4 (G and N). Standard incubation conditions were used for analysis with HPLC and duplicate determinations in each incubation showed very similar formation of products of these chemicals.
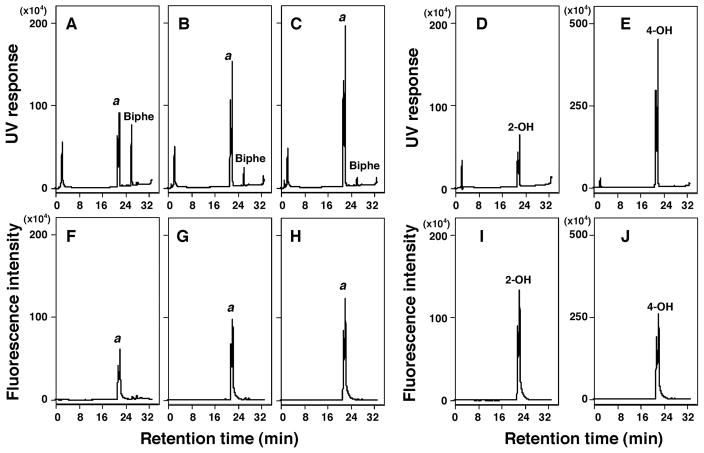
P450 2A13 oxidized biphenyl to 2- and/or 4-hydroxybiphenyl on analysis with HPLC (Figure 6). Our HPLC assay did not separate 2- and 4-hydroxylated biphenyl products.. It should also be noted that both standard 2- and 4-hydroxybiphenyl produced two peaks as in the case of 9OH-phenanthrene as described above. Increasing the P450 concentration from 0.025 μM to 0.1 μM caused increases in the formation of metabolite(s) (both with UV and fluorescence detection).
Figure 6.
Oxidation of biphenyl by P450 2A13 using analysis with HPLC with UV detection (A, B, and C) and fluorescence detection (F, G, and H). HPLC chromatograms of incubations of biphenyl (50 μM) with P450 2A13 (concentration of 0.025 μM) (A and F), 0.05 μM (B and G), and 0.1 μM (C and H), and chromatograms of standard 2-hydroxybiphenyl (2.5 nmol; D and I) and standard 4-hydroxybiphenyl (E and J). Detection was by UV absorbance (A–E) or fluorescence intensity (F–J).
P450s 1A1, 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4 were compared for oxidation of naphthalene, phenanthrene, and biphenyl (P450 1A1 was measured in reconstituted monooxygenase systems containing DLPC and NADPH-P450 reductase as described under Experimental Procedures). P450 2A13 was found to be the most active enzyme in oxidizing these chemicals, followed by P450 2A6 (Table 1). With other P450s examined, naphthalene hydroxylation activities were detected with P450s 1A2, 1B1, and 3A4, in that order, but were not detected in P450s 1A1 and 2C9. Phenanthrene 9-hydroxylation activities were detected with P450s 1A1, 1A2, 3A4, and 1B1.3, but not P450 2C9. Oxidation of biphenyl was detected with P450s 1A1 and 1A2, but not with P450s 1B1, 2C9, and 3A4.
Table 1.
Oxidation of naphthalene, phenanthrene, and biphenyl by human P450 enzymes
| P450 | naphthalenea | phenanthrenea | biphenyla |
|---|---|---|---|
| 1-Naphtholb | 9-OH-phenanthreneb | 2- & 4-OH-biphenylb | |
| (nmol/min/nmol P450) | |||
| P450 1A1 | <0.01 | 0.33 ± 0.034 | 0.29 ± 0.033 |
| P450 1A2 | 0.91 ± 0.11 | 0.23 ± 0.024 | 0.24 ± 0.027 |
| P450 1B1 | 0.41 ± 0.062 | 0.02 ± 0.01 | <0.1 |
| P450 2A6 | 2.6 ± 0.44 | 1.69 ± 0.22 | 2.3 ± 0.31 |
| P450 2A13 | 6.1 ± 0.88 | 3.14 ± 0.35 | 3.1 ± 0.19 |
| P450 2C9 | <0.01 | <0.01 | <0.1 |
| P450 3A4 | 0.04 ± 0.013 | 0.05 ± 0.02 | <0.1 |
Oxidations of naphthalene, phenanthrene, and biphenyl by P450 enzymes were determined by methods described in Experimental Procedures.
Substrate used;
product measured.
Data shown are means of three or four samples determined ± S.D.
Oxidation of Various Substituted Derivatives of Naphthalene, Phenanthrene, and Biphenyl by P450s 2A13 and 2A6
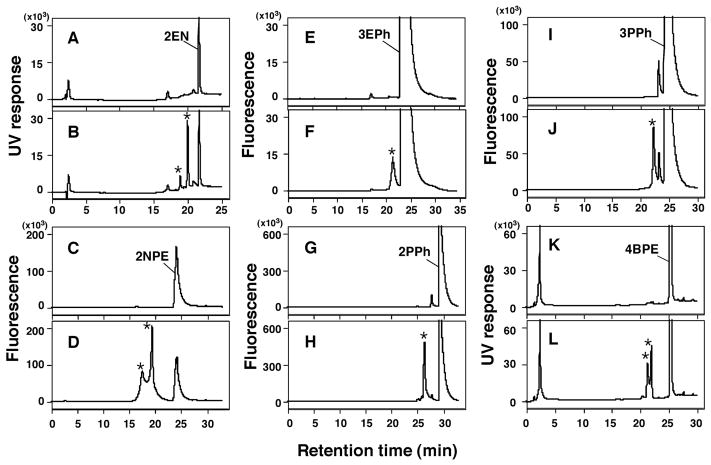
Since our results suggested that P450s 2A13 and 2A6 are the major enzymes in oxidizing naphthalene, phenanthrene, and biphenyl, we extended our study to include the metabolism of a number of substituted derivatives of these three compounds by these enzymes. We first determined oxidation of six acetylenic derivatives, 2EN, 2NPE, 3EPh, 2PPh, 3PPh, and 4BMPE by P450 2A13 in the absence and presence of an NADPH-generating system (Figure 7). These six chemicals were found to be metabolized by P450 2A13 to product(s) which were not present in the absence of an NADPH-generating system in the reaction mixture, although detailed examinations to identify these products were not done (Figure 7). LC-MS analysis suggested the formation of oxygenated products of 2EPh, 2NPE, and 3EPh (results not shown).
Figure 7.
Metabolism of 2EN (A and B), 2NPE (C and D), 3EPh (E and F), 2PPh (G and H), 3PPh (I and J), and 4BPE (K and L) by P450 2A13 in the absence (A, C, E, G, I, and K) and presence (B, D, F, H, J, and L) of an NADPH-generating system, using analysis with HPLC. Possible formation of metabolite(s) from these chemicals was denoted as “*” in the figures.
We further examined the oxidation of 21 substituted derivatives, as well as naphthalene, phenanthrene, and biphenyl, by P450 2A13 and compared spectral binding constant (Ks) of these chemicals with the enzyme (Table 2). P450 2A6 was also used to determine oxidation of some of the derivatives, and for comparison of its spectral binding constants with these chemicals. P450 2A13 oxidized 2EN, 1NEPE, 2NPE, 2EPh, 3EPh, 2PPh, 3PPh, 2BPE, and 4BPE as well as the three parent compounds. Product formation was not detected for the other chemicals with P450 2A13 in our HPLC assay condition. P450 2A6 oxidized 2EN, 2EPh, 3EPh, and 4BPE as well as the parent compounds. However, product formation (peak height of products) was always lower in case of P450 2A6 than P450 2A13 (results not shown).
Table 2.
Spectral binding constants and oxidation results observed for the studied chemicals with P450s 2A13 and 2A6
| chemical | P450 2A13 | P450 2A6 | ||
|---|---|---|---|---|
| Type I spectra a Ks (μM) |
metabolism b (HPLC) | Type I spectra a Ks (μM) |
metabolism b (HPLC) | |
| naphthalene | 0.35 ± 0.02 | yes | 2.9 ± 0.4 | yes |
| 2EN | 0.080 ± 0.04 | yes | 1.0 ± 0.1 | yes |
| 1NMPE | 13.3 ± 3.5 | not detected | 9.3 ± 1.8 | not done |
| 1NEPE | 1.1 ± 0.1 | yes | 11.5 ± 2.3 | not done |
| 2NPE | 0.42 ± 0.10 | yes | 33 ± 9 | not done |
| 2NMPE | 4.9 ± 0.6 | not detected | 21 ± 0 | not done |
| 2NEPE | 2.7 ± 0.6 | not detected | 19 ± 6 | not done |
| phenanthrene | 2.3 ± 0.2 | yes | 2.8 ± 0.4 | yes |
| 2EPh | 1.1 ± 0.3 | yes | 3.3 ± 0.4 | yes |
| 3EPh | 0.45 ± 0.08 | yes | 3.3 ± 0.9 | yes |
| 9EPh | 0.95 ± 0.08 | not detected | ND (10 μM) | not done |
| 2PPh | 3.9 ± 1.2 | yes | 1.6 ± 0.2 | yes |
| 3PPh | 1.2 ± 0.4 | yes | ND (10 μM) | not done |
| 9PPh | 6.4 ± 1.0 | not detected | 13 ± 3 | not done |
| biphenyl | 0.88 ± 0.16 | yes | 2.7 ± 0.5 | yes |
| 4EB | 1.9 ± 0.2 | not detected | 5.4 ± 0.5 | not detected |
| 4PB | 28 ± 12 | not detected | 7.0 ± 1.9 | not done |
| 4BuB | 3.4 ± 1.1 | not detected | 12 ± 2 | not done |
| 2BPE | 15.4 ± 1.7 | yes | 15 ± 2 | not done |
| 4BPE | 0.48 ± 0.06 | yes | 0.75 ± 0.16 | yes |
| 2BMPE | 1.7 ± 0.3 | not detected | 17 ± 3 | not done |
| 4BMPE | 66 ± 19 | not detected | 43 ± 33 | not done |
| 22BDPE | 11 ± 6 | not detected | 32 ± 9 | not done |
| 44BDPE | 6.5 ± 1.6 | not detected | 2.6 ± 0.8 | not detected |
Data reported are means and S.E. Analysis (HPLC) was used for monitoring metabolism. When peak(s) were detected only in the presence of an NADPH-generating system as described in Figure 8, metabolism results were marked as “Yes”. When no metabolite peaks were detected under these assay conditions, results were marked as “not detected”. Several of the chemicals were not examined with P450 2A6 (marked as “not done”).
Molecular Interactions of the chemicals studied with P450s 2A13 and 2A6
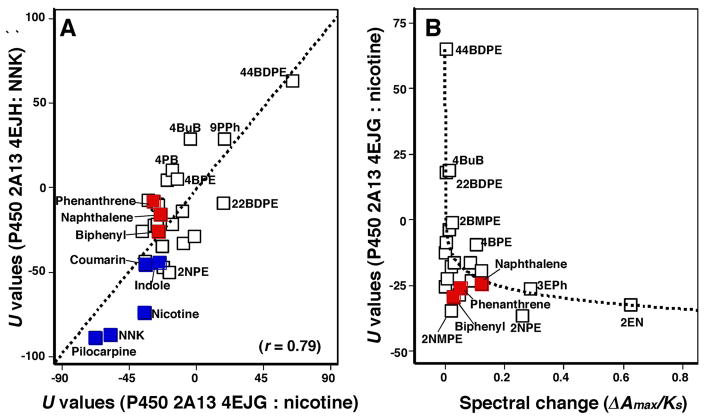
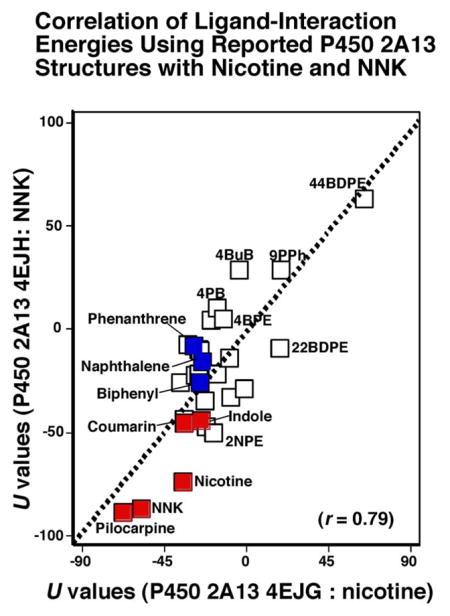
Molecular docking analysis of these chemicals with P450 2A13 were performed using reported structures of P450 2A13 (4EJH)34, 2A13 (2P85)35, 2A13 (3T3S)36, and 2A13 (4EJG)34 bound to NNK, indole, pilocarpine, and nicotine, respectively. Since all biological units of crystal structures of P450 2A13 4EJH, 2P85, and 4EJG are reported as octamer and P450 2A13 3T3S as hexamer, we selected one of defalt biological unit for the docking analysis. Docking analysis was also carried out with reported structures of P450 2A6 (1Z10),36 2A6 (3T3R),37 and 2A6 4EJJ).34 Before performing simulation studies, the ligands were removed from the monomer structures of P450 2A13, and these ligand free structures were used for the docking analysis (Supporting information Figure S2). We first determined optimal U values (ligand-interaction energy) (on analysis with MMFF94x force field) using P450 4EJG and found that U values for nicotine, naphthalene, phenanthrene, and biphenyl were −35.2, −24.3, −29.2, and −25.7, respectively (Supporting information Figure S2), and that of coumarin was −34.4 (results not shown).
There was good relationship between U values of P450 2A13 4EJG (nicotine-type) and 4EJH (NNK-type) with these chemicals (r = 0.79, p<0.01), and we found that the parent compounds, naphthalene, phenanthrene, biphenyl, and coumarin had U values comparable to those of NNK, indole, pilocarpine, nicotine, and coumarin (Figure 8). Compounds that were oxidized by P450 2A13 on HPLC analysis had small U values in this assay condition (Figure 8A). When spectral changes (ΔAmax/Ks values) and U values (using P450 2A13 4EJG for the docking) were compared, some relationship was found between these values when analyzed with logarithmic curve fitting (Cricket Graph version) (Figure 8B). However, we did not find any positive correlation when it was compared U values (with P450 2A13 4EJG) and spectral binding constants (Ks values).
Figure 8.
Correlations of ligand-interaction energies (U values) using molecular docking with P450s 2A13 4EJG (nicotine-type) and 4EJH (NNK-type) with 24 chemicals used in this study and NNK, indole, pilocarpine, nicotine, and coumarin. These latter five chemicals indicated with blue squares and naphthalene, phenanthrene, and biphenyl are marked with red squares (Figure 8A). Effects of spectral binding intensities (ΔAmax/Ks ratio) on ligand binding energies (U values) using P450 2A13 4EJG (nicotine-type) (Figure 8B).
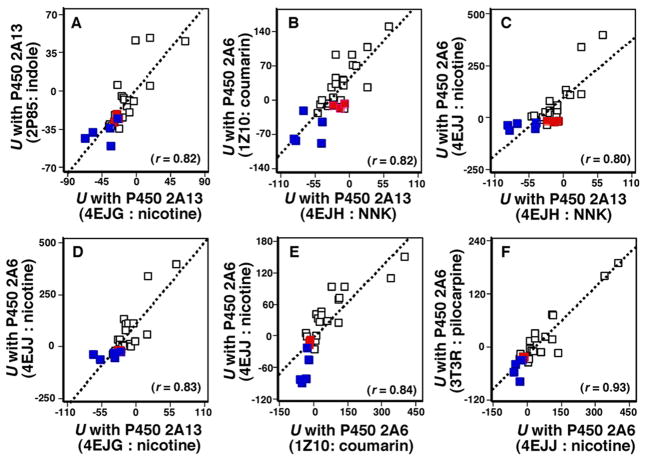
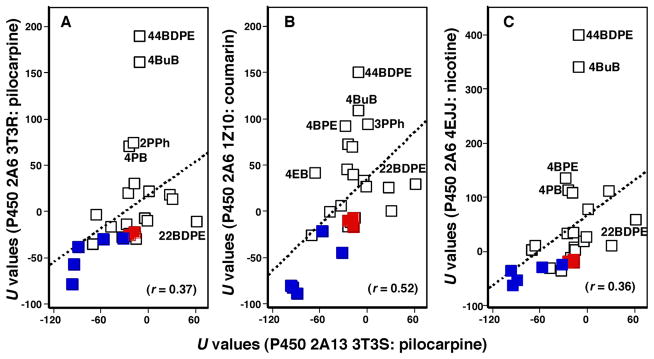
Correlation of U values using P450 2A13 4EJH, 2P85, 3T3S, and 4EJG bound to NNK, indole, pilocarpine, and nicotine, respectively, and P450 2A6 3T3R, 4EJJ, and 1Z10 bound to pilocarpine, nicotine, and coumarin, respectively, was determined with all of the chemicals used in this study as well as with NNK, indole, pilocarpine, nicotine, and coumarin (Supporting information Table S1). As in a case of comparison between U values of P450 2A13 4EJG (nicotine-type) and 4EJH (NNK-type) shown above, there were also good corralations in combinations of these U values examined in these chemicals and the parent compounds, naphthalene, phenanthrene, biphenyl, and coumarin (marked in red) had similar U values comparable to those of NNK, indole, pilocarpine, nicotine, and coumarin (marked in blue) (Figure 9). However, we found relatively low correlation coefficients in comparison of U values of P450 2A13 3T3S bound to pilocarpine and P450 2A6 3T3R (r = 0.37), 1Z10 (r = 0.52), and 4EJJ (r = 0.36) bound to pilocarpine, coumarin, and nicotine (Figure 10), indicating that the crystal structure of P450 2A13 with pilocarpine may be different from those with other compounds. 44BDPE and 4BuB and some other chemicals in P450 2A6 structures were out of place in the line.
Figure 9.
Correlation of ligand-interaction energy obtained with P450 2A13 4EJG (nicotine-type) and P450 2A13 2P85 (indole-type) (A), P450 2A13 4EJH (NNK-type) and P450 2A6 1Z10 (coumarin-type) (B), P450 2A13 4EJH (NNK-type) and P450 2A6 4EJJ (nicotine-type) (C), P450 2A13 4EJG (nicotine-type) and P450 2A6 4EJJ (nicotine-type) (D), P450 2A6 1Z10 (coumarin-type) and P450 2A6 4EJJ (nicotine-type) (E), and P450 2A6 4EJJ (coumarin-type) and P450 2A6 3T3R (pilocarpine-type).
Figure 10.
Correlation of ligand-interaction energy obtained between P450 2A13 3T3S (pilocarpine-type) and P450 2A6 3T3R (pilocarpine-type) (A), P450 2A6 1Z10 (coumarin-type) (B), and P450 2A6 4EJJ (nicotine-type) (C).
Discussion
Present studies showed that P450 2A13 plays very important roles in oxidizing naphthalene, phenanthrene, and biphenyl and several of their substituted derivatives in humans, and that P450 2A6 also participates in metabolizing several of these chemicals. Other human P450s including P450s 1A1, 1A2, 1B1, 2C9, and 3A4 used in this study had lower activities in oxidizing naphthalene, phenanthrene, and biphenyl than P450s 2A13 and 2A6. Turnover rates (nmol product formed/min/nmol P450) of oxidation of naphthalene, phenanthrene, and biphenyl to 1-naphthol, 9-hydroxyphenanthrene, and 2- and/or 4-hydroxybiphenyl by P450 2A13 were 6.1 min−1, 3.1 min−1, and 3.2 min−1, respectively, and catalytic activities by P450 2A6 were always lower than those by P450 2A13. Naphthalene, phenanthrene, and biphenyl induced Type I binding spectra with P450 2A13 with Ks values of 0.35 μM, 2.3 μM, and 0.88 μM, respectively, and these Ks values were lower than those (2.9 μM, 2.8 μM, and 2.7 μM, respectively) with P450 2A6, suggesting possible relationships between spectral binding intensities and metabolism rates of these chemicals by P450 2A13 and 2A6. It should be mentioned that we did not determine formation of epoxide intermediates during metabolism of the above three chemicals by P450 enzymes in this study, although ther are reports to be suggested to produce epoxides of these chemicals by P450 enzymes in laboratory animals.10, 12, 16, 20, 21
Other naphthalene, phenanthrene, and biphenyl derivatives were also analyzed for their spectral binding abilities with P450s 2A13 and 2A6 and their oxidation by these enzymes. The results showed that compounds such as 2EN, 1NEPE, 2NPE, 2EPh, 3EPh, 2PPh, 3PPh, and 4BPE whose spectral binding constant (Ks value) and intensities (ΔAmax/Ks ratio) with P450 2A13 were comparable with those of parent compounds, naphthalene, phenanthrene, and biphenyl were found to be oxidized by this enzyme similarly in the presence and absence of an NADPH-generating system. We also found the formation of oxygenated products of 2EPh, 1NEPE, 2NPE, and 3EPh by P450 2A13 on analysis with LC-MS. In a similar way, 2EN, 2EPh, 3EPh, 2PPh, and 4BPE (having spectral binding intensities with P450 2A6 comparable to the parent compounds) were oxidized by this enzyme, although the product formation (peak height of products) was always lower for P450 2A6 than for P450 2A13, as in the cases of naphthalene, phenanthrene, and biphenyl., P450 2A13 has 94% amino acid sequence similarity to P450 2A6, and the volume of the active site cavity of P450 2A13 (307 Å3) is known to be larger than that of P450 2A6 (260 Å3).34–37 The higher catalytic activities of P450 2A13 compared to P450 2A6 towards these chemicals may be due to the size and shape of substrate binding pocket at the P450 active sites.
In this study, it was also found that there were exceptions in relationships between spectral binding intensities and catalytic activities for several chemicals with P450s 2A13 and 2A6 (Table 2). For example, 2NMPE, 2NEPE, 9EPh, 9PPh, 4EB, 4BuB, 2BMPE, and 44BDPE (for P450 2A13) and 4EB and 44BDPE (for P450 2A6), whose Ks values were ≤10 μM, were found not to be oxidized by these P450 enzymes under our standard assay conditions. Most of these acetylenic derivatives have previously been synthesized to study the mechanisms of inhibition of P450 enzymes, particularly P450 1 and 2 family enzymes.27–31 Our previous studies indicated that most of these substituted chemicals, as well as their parent compounds naphthalene, phenanthrene, and biphenyl, are able to inhibit 7-ethoxyresorufin O-deethylation catalyzed by P450 1B1 and coumarin 7-hydroxylation by P450s 2A13 and 2A6.23,26 In addition, 2EN has been reported to be a mechanism-based inhibitor of P450s 2B1, 2B4, 2B6, and 2B1139–41 and our previous findings indicated that 2EN inhibited P450 2A13- and 2A6-catalyzed coumarin 7-hydroxylation with IC50 values of 1.8 and 8.8 μM. These results suggest that the abilities of some of these compounds to be metabolized by P450 enzymes may be influenced by their inhibition of the enzymes.
Our molecular docking simulation studies indicated that ligand-binding energies (U values) correlate for all of the 24 chemicals examined when comparing the crystal structures of P450 2A13 4EJH (NNK ligand) and P450 2A13 4EJG (nicotine ligand) (r = 0.79). Interestingly, the plotted positions on Figure 8A for naphthalene, phenanthrene, and biphenyl were at relatively similar locations to those of NNK, indole, pilocarpine, nicotine, and coumarin, which are typical ligands for P450 2A13.34–37 Such good correlations were also found when U values were compared using P450 2A13 4EJH (NNK-type), 2P85 (indole-type), 3T3S (pilocarpine-type), and 4EJG (nicotine-type) and P450 2A6 3T3R (pilocarpine-type), 4EJJ (nicotine-type), and 1A10 (coumarin-type) structures with the chemicals used in this study. These results indicated that the structures of substrate binding pocket of P450 2A6 (reported as co-crystals with coumarin, nicotine, or pilocarpine) are almost the same as with those of P450 2A13 with NNK, nicotine, or indole. However, there were some exceptions, with relatively low correlation coefficients when comparing U values of P450 2A13 3T3S bound to pilocarpine and P450 2A6 3T3R (r = 0.37), 1Z10 (r = 0.52), and 4EJJ (r = 0.36) bound to pilocarpine, coumarin, and nicotine (Figure 10). Several chemicals (e.g., 44BDPE, 4BuB, and others) that were not good ligands in interacting with and substrates for oxidation by P450 2A13 and 2A6 are likely to cause such weak correlations. In addition, some other compounds (e.g 4BuB, 2BMPE, 22BDPE, and 44BDPE) that were not oxidized by P450 2A13 showed higher ligand-binding energies on analysis using reported P450 2A13 4EJH, 2P85, 3T3S, and 4EJG structures bound to NNK, indole, pilocarpine, and nicotine. These results indicate the usefulness of molecular docking analysis in predicting possible relationships between interaction of chemicals with active sites of P450 enzymes and their susceptibilities to be metabolized by these enzymes. Comparison of ligand-binding energies (using P450 2A13 4EJG) and spectral binding intensities with these 24 chemicals is of interest, because correlations were observed in these cases when logarithmic extrapolation was employed (Figure 8B). Those compounds having higher spectral binding intensities, (e.g., 2EN, 3EPh, and 2NPE) showed lower U values, while such compounds as 44BDPE, 4BuB, 22BDPE, and 2BPE—which showed less spectral binding intensities—had higher U values. However, it should be noted that we could not detect a positive correlation when spectral binding constants (Ks) were used for comparisons with U values. It is necessary to do more studies to establish the relationships between biological responses and molecular docking results to understand selectivity of P450 reactions.
Human P450s 2A13 and 2A6 are known to catalyze the activation and detoxication of environmental carcinogens such as tobacco-related nitrosamines (e.g., NNK and N-nitrosonornicotine), and to metabolize different kinds of chemicals including coumarin, nicotine, phenacetin, naphthalene, 4-aminobiphenyl, and styrene.13,35,42–46 P450 2A13 is mainly expressed in the respiratory tract, while P450 2A6 is primarily found in the liver.47–49 We have previously shown that P450 2A13 plays a more significant role than P450 2A6 in interacting with and metabolizing diverse environmental chemicals including PAHs.23,25 Our past and present studies also support the importance of P450s 2A13 and 2A6 in metabolizing acenaphthene and acenaphthylene40, pyrene, 1-hydroxypyrene, 1-nitropyrene, and 1-acethylpyrene51, and in the present studies, naphthalene, phenanthrene, biphenyl, and their alkynyl derivatives.
A role of P450 2A13 in the hydroxylation of naphthalene was reported previously by Fukami et al. (2008)13 who showed that naphthalene is oxidized by this enzyme to form a major metabolite, 1-naphthol, and a minor metabolite, 2-naphthol, through the suggested formation of naphthalene 1,2-epoxide. They also showed that these metabolic activities of P450 2A13 were higher than those observed with P450s 1A1 and 1A2. Cho et al. (2006)12 also reported in vitro metabolism of naphthalene by human liver microsomal P450 enzymes and showed that several P450 enzymes—including P450s 1A2, 2A6, and 2B6—catalyze formation of 1-naphthol and 2-naphthol on analysis with HPLC but did not examine metabolism by P450 2A13 in their work. Naphthalene has been shown to be bioactivated by mouse P450 2F2, goat P450 2F3, and rat P450 2F4 to lung toxicants in these species;9,11 however, roles of human P450 2F1 (which is reported to be present in lung and other tissues) in the metabolic activation of naphthalene and other chemicals including styrene, 3-methylindole, benzene, and trichloroethylene are not known at present due to difficulties in expression of P450 2F1 in heterogeneous systems such as E. coli.52 Mouse P450 2A5 has been reported to play important roles in olfactory mucosal toxicity but not in lung toxicity.11
Urinary hydroxylated metabolites of phenanthrene have been used as biomarkers to determine exposure of humans to environmental PAHs in gasoline, diesel fuel, tobacco smoke, and diet.53–55 Several metabolites—including 1-, 4-, and 9-hydroxyphenanthrene—have been identified in human urine,53–55 and in vitro studies have suggested that a number of P450 enzymes are involved in the formation of several hydroxylated and dihydrodiol metabolites of phenanthrene in humans.18,56 Our present results support the conclusion that 9-hydroxyphenanthrene is a major metabolite during incubation of phenanthrene with P450s 2A13 and 2A6.
Little is known about metabolism of biphenyl by human P450 enzymes. Creaven et al. (1965)19 have reported that liver microsomes from several animal species including rats, mice, rabbits, and other species produce more 4-hydroxybiphenyl than 2-hydroxybiphenyl from biphenyl. Other studies have suggested that 3-hydroxybiphenyl (as well as 2- and 4-hydroxybiphenyls) is formed with liver microsomes from rats, hamsters, mice, and rabbits and that the major metabolite is 4-hydroxybiphenyl in all animal species examined.20,21 Our present studies identified 2- and/or 4-hydroxybiphenyl in incubations of human P450s 2A13 and 2A6 with biphenyl, but the two products were not separated in this study. Human P450s 1A1 and 1A2 produced biphenyl metabolite(s) but P450s 1B1.1, 1B1.3, 2C9, and 3A4 did not.
In conclusion, our present studies showed that P450s 2A13 and 2A6 are important enzymes in oxidizing naphthalene, phenanthrene, biphenyl, and several of their alkynyl derivatives. Other human P450 enzymes—including P450s 1A1, 1A2, 1B1, 2C9, and 3A4—had some roles in several oxidation pathways of these chemicals. Molecular docking analysis showed correlations between ligand-interaction energies (U values) for these 24 chemicals with the active sites of P450 2A13 and 2A6 and the susceptibilities of these chemicals to be oxidized by the enzymes. The results also support the usefulness of molecular docking analysis in understanding the basis of molecular interaction of xenobiotic chemicals with active sites of P450 proteins and possibly other enzymes.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported in part by grants from the Ministry of Education, Science, and Culture of Japan, the Ministry of Health and Welfare of Japan (T.S., S.T., K.K., N.M., H.Y., M.K.), NIH grant S06 GM08008 and DOE grant DE-FC26-00NT40843 (M.K.F.), and NIH grants R37 CA090426 and P30 ES000267 (F.P.G.).
ABBREVIATIONS were used for cytochrome
- P450
cytochrome P450
- PAHs
polycyclic aromatic hydrocarbons
- 2EN
2-ethynylnaphthalene
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- 1NMPE
1-naphthalene methyl propargyl ether
- 1NEPE
1-naphthalene ethyl propargyl ether
- 2NPE
2-naphthalene propargyl ether
- 2NMPE
2-naphthalene methyl propargyl ether
- 2NEPE
2-naphthalene ethyl propargyl ether
- 2EPh
2-ethynylphenanthrene
- 3EPh
3-ethynylphenanthrene
- 9EPh
9-ethynylphenanthrene
- 2PPh
2-(1-propynyl)phenanthrene
- 3PPh
3-(1-propynyl)phenanthrene
- 9PPh
9-(1-propynyl)phenanthrene
- 4EB
4-ethynylbiphenyl
- 4PB
4-propynylbiphenyl
- 4BuB
4-butynylbiphenyl
- 2BPE
2-biphenyl propargyl ether
- 4BPE
4-biphenyl propargyl ether
- 2BMPE
2-biphenyl methyl propargyl ether
- 4BMPE
4-biphenyl methyl propargyl ether
- 22BDPE
2,2′-biphenyl dipropargyl ether
- 44BDPE
4,4′-biphenyl dipropargyl ether
Footnotes
Notes
The authors declare no competing financial interests.
Correlation coefficients (r values) in ligand-interaction energies (U values) using reported structures of P450 2A13 and 2A6; oxidation of phenanthrene and 2EPh by P450 2A13 in the absence and presence of an NADPH-generating system (HPLC with UV detection) and docking simulation of interaction of nicotine, naphthalene, phenanthrene, and biphenyl with P450 2A13 (4EJG). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Anttila S, Raunio H, Hakkola J. Cytochrome P450-mediated pulmonary metabolism of carcinogens: regulation and cross-talk in lung carcinogenesis. Am J Respir Cell Mol Biol. 2011;44:583–590. doi: 10.1165/rcmb.2010-0189RT. [DOI] [PubMed] [Google Scholar]
- 2.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 3.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 4.Baum M, Amin S, Guengerich FP, Hecht SS, Köhl W, Eisenbrand G. Metabolic activation of benzo[c]phenanthrene by cytochrome P450 enzymes in human liver and lung. Chem Res Toxicol. 2001;14:686–693. doi: 10.1021/tx000240s. [DOI] [PubMed] [Google Scholar]
- 5.Wood AW, Chang RL, Levin W, Ryan DE, Thomas PE, Croisy-Delcey M, Ittah Y, Yagi H, Jerina DM, Conney AH. Mutagenicity of the dihydrodiols and bay-region diol-epoxides of benzo[c]phenanthrene in bacterial and mammalian cells. Cancer Res. 1980;40:2876–2883. [PubMed] [Google Scholar]
- 6.Rendic S, Guengerich FP. Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol. 2012;25:1316–1383. doi: 10.1021/tx300132k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Wei Y, Van Winkle L, Zhang QY, Zhou X, Hu J, Xie F, Kluetzman K, Ding X. Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. J Pharmacol Exp Ther. 2011;339:62–71. doi: 10.1124/jpet.111.184671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, Plopper C. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol. 1995;47:74–81. [PubMed] [Google Scholar]
- 11.Hu J, Sheng L, Li L, Zhou X, Xie F, D’Agostino J, Li Y, Ding X. Essential role of the cytochrome P450 enzyme CYP2A5 in olfactory mucosal toxicity of naphthalene. Drug Metab Dispos. 2014;42:23–27. doi: 10.1124/dmd.113.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho TM, Rose RL, Hodgson E. In vitro metabolism of naphthalene by human liver microsomal cytochrome P450 enzymes. Drug Metab Dispos. 2006;34:176–183. doi: 10.1124/dmd.105.005785. [DOI] [PubMed] [Google Scholar]
- 13.Fukami T, Katoh M, Yamazaki H, Yokoi T, Nakajima M. Human cytochrome P450 2A13 efficiently metabolizes chemicals in air pollutants: naphthalene, styrene, and toluene. Chem Res Toxicol. 2008;21:720–725. doi: 10.1021/tx700325f. [DOI] [PubMed] [Google Scholar]
- 14.Buckpitt A, Morin D, Murphy S, Edwards P, Van Winkle L. Kinetics of naphthalene metabolism in target and non-target tissues of rodents and in nasal and airway microsomes from the Rhesus monkey. Toxicol Appl Pharmacol. 2013;270:97–105. doi: 10.1016/j.taap.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Boyland E, Sims P. Metabolism of polycyclic compounds. 20. The metabolism of phenanthrene in rabbits and rats: mercapturic acids and related compounds. Biochem J. 1962;84:564–570. doi: 10.1042/bj0840564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyland E, Sims P. Metabolism of polycyclic compounds. 21. The metabolism of phenanthrene in rabbits and rats: dihydrodihydroxy compounds and related glucosiduronic acids. Biochem J. 1962;84:571–582. doi: 10.1042/bj0840571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturapit S, Holder GM. Studies on the hepatic microsomal metabolism of (14C) phenanthrene. Biochem Pharmacol. 1978;27:1865–1871. doi: 10.1016/0006-2952(78)90034-5. [DOI] [PubMed] [Google Scholar]
- 18.Shou M, Korzekwa KR, Krausz KW, Crespi CL, Gonzalez FJ, Gelboin HV. Regio- and stereo-selective metabolism of phenanthrene by twelve cDNA-expressed human, rodent, and rabbit cytochromes P-450. Cancer Lett. 1994;83:305–313. doi: 10.1016/0304-3835(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 19.Creaven PJ, Parke DV, Williams RT. A fluorimetric study of the hydroxylation of biphenyl in vitro by liver preparations of various species. Biochem J. 1965;96:879–885. doi: 10.1042/bj0960879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen DA. Biphenyl metabolism by rat liver microsomes: regioselective effects of inducers, inhibitors, and solvents. Drug Metab Dispos. 1981;9:212–218. [PubMed] [Google Scholar]
- 21.Billings RE, McMahon RE. Microsomal biphenyl hydroxylation: the formation of 3- hydroxybiphenyl and biphenyl catechol. Mol Pharmacol. 1978;14:145–154. [PubMed] [Google Scholar]
- 22.Burke MD, Mayer RT. Inherent specificities of purified cytochromes P-450 and P-448 toward biphenyl hydroxylation and ethoxyresorufin deethylation. Drug Metab Dispos. 1975;3:245–253. [PubMed] [Google Scholar]
- 23.Shimada T, Kim D, Murayama N, Tanaka K, Takenaka S, Nagy LD, Folkman LM, Foroozesh MK, Komori M, Yamazaki H, Guengerich FP. Binding of diverse environmental chemicals with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition. Chem Res Toxicol. 2013;26:517–528. doi: 10.1021/tx300492j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada T, Tanaka K, Takenaka S, Murayama N, Martin MV, Foroozesh MK, Yamazaki H, Guengerich FP, Komori M. Structure-function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chem Res Toxicol. 2010;23:1921–1935. doi: 10.1021/tx100286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada T, Murayama N, Tanaka K, Takenaka S, Guengerich FP, Yamazaki H, Komori M. Spectral modification and catalytic inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2A6, and 2A13 by four chemopreventive organoselenium compounds. Chem Res Toxicol. 2011;24:1327–1337. doi: 10.1021/tx200218u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada T, Tanaka K, Takenaka S, Foroozesh MK, Murayama N, Yamazaki H, Guengerich FP, Komori M. Reverse type I binding spectra of human cytochrome P450 1B1 induced by flavonoid, stilbene, pyrene, naphthalene, phenanthrene, and biphenyl derivatives that inhibit catalytic activity: a structure-function relationship study. Chem Res Toxicol. 2009;22:1325–1333. doi: 10.1021/tx900127s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foroozesh M, Primrose G, Guo Z, Bell LC, Guengerich FP, Alworth WL. Propynylaryl acetylenes as mechanism-based inhibitors of cytochrome P450 1A1, 1A2, and 2B1 enzymes. Chem Res Toxicol. 1997;10:91–102. doi: 10.1021/tx960064g. [DOI] [PubMed] [Google Scholar]
- 28.Kelley AT, Mesbah JY, McKendall ME, Smith TP, Foroozesh M. Synthesis of a family of naphthyl propargyl ethers as potential cytochrome P450 inhibitors. J Undergrad Chem Res. 2004;3:103–106. [Google Scholar]
- 29.Zhu N, Lightsey K, Foroozesh M, Alworth W, Chaudhary A, Willett KL, Klein Stevens CLK. Naphthoflavone propargyl ether inhibitors of cytochrome P450. J Chem Crystallogr. 2006;36:289–296. [Google Scholar]
- 30.Bowman B, Lightsey D, McKendall M, Smith T, Zhu N, Stevens CLK, Foroozesh M. Synthesis of a family of biphenylpropagyl ethers as potential inhibitors of P450 enzymes. X-ray crystal structure of 2,2′-biphenyldipropargyl ether. J Undergrad Chem Res. 2005;2:57–61. [Google Scholar]
- 31.Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP, Gillam EM. Recombinant human cytochrome P450 1B1 expression in Escherichia coli. Arch Biochem Biophys. 1998;357:111–120. doi: 10.1006/abbi.1998.0808. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki H, Nakajima M, Nakamura M, Asahi S, Shimada N, Gillam EM, Guengerich FP, Shimada T, Yokoi T. Enhancement of cytochrome P450 3A4 catalytic activities by cytochrome b5 in bacterial membranes. Drug Metab Dispos. 1999;27:999–1004. [PubMed] [Google Scholar]
- 33.Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, Yokoi T. Roles of NADPH-P450 reductase and apo- and holo-cytochrome cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif. 2002;24:329–337. doi: 10.1006/prep.2001.1578. [DOI] [PubMed] [Google Scholar]
- 34.DeVore NM, Scott EE. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes. J Biol Chem. 2012;287:26576–26585. doi: 10.1074/jbc.M112.372813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith BD, Sanders JL, Porubsky PR, Lushington GH, Stout CD, Scott EE. Structure of the human lung cytochrome P450 2A13. J Biol Chem. 2007;282:17306–17313. doi: 10.1074/jbc.M702361200. [DOI] [PubMed] [Google Scholar]
- 36.DeVore NM, Meneely KM, Bart AG, Stephens ES, Battaile KP, Scott EE. Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine. FEBS J. 20112;279:1621–1631. doi: 10.1111/j.1742-4658.2011.08412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano JK, Hsu MH, Griffin KJ, Stout CD, Johnson EF. Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen. Nat Struct Mol Biol. 2005;12:822–823. doi: 10.1038/nsmb971. [DOI] [PubMed] [Google Scholar]
- 38.Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochromes P450 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–1056. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- 39.Roberts ES, Pernecky SJ, Alworth WL, Hollenberg PF. A role for threonine 302 in the mechanism-based inactivation of P450 2B4 by 2-ethynylnaphthalene. Arch Biochem Biophys. 1996;331:170–176. doi: 10.1006/abbi.1996.0295. [DOI] [PubMed] [Google Scholar]
- 40.Roberts ES, Hopkins NE, Foroozesh M, Alworth WL, Halpert JR, Hollenberg PF. Inactivation of cytochrome P450s 2B1, 2B4, 2B6, and 2B11 by arylalkynes. Drug Metab Dispos. 1997;25:1242–1248. [PubMed] [Google Scholar]
- 41.Cheng D, Harris D, Reed JR, Backes WL. Inhibition of CYP2B4 by 2-ethynylnaphthalene: evidence for the co-binding of substrate and inhibitor within the active site. Arch Biochem Biophys. 2007;468:174–182. doi: 10.1016/j.abb.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang HC, Wang CY, Lee HL, Tsou TC. Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK-induced gene mutation-a mammalian cell-based mutagenesis approach. Toxicol Appl Pharmacol. 2011;253:145–152. doi: 10.1016/j.taap.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Wong HL, Murphy SE, Hecht SS. Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem Res Toxicol. 2005;18:61–69. doi: 10.1021/tx0497696. [DOI] [PubMed] [Google Scholar]
- 44.Wong HL, Zhang X, Zhang QY, Gu J, Ding X, Hecht SS, Murphy SE. Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem Res Toxicol. 2005;18:913–918. doi: 10.1021/tx0500777. [DOI] [PubMed] [Google Scholar]
- 45.DeVore NM, Smith BD, Wang JL, Lushington GH, Scott EE. Key residues controlling binding of diverse ligands to human cytochrome P450 2A enzymes. Drug Metab Dispos. 2009;37:1319–1327. doi: 10.1124/dmd.109.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeVore NM, Smith BD, Urban MJ, Scott EE. Key residues controlling phenacetin metabolism by human cytochrome P450 2A enzymes. Drug Metab Dispos. 2008;36:582–5890. doi: 10.1124/dmd.108.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su T, Bao Z, Zhang Q-Y, Smith TJ, Hong J-Y, Ding X. Human cytochrome P450 CYP2A13: Predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- 48.Chiang HC, Wang CK, Tsou TC. Differential distribution of CYP2A6 and CYP2A13 in the human respiratory tract. Respiration. 2012;84:319–326. doi: 10.1159/000339591. [DOI] [PubMed] [Google Scholar]
- 49.Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, Wang SL, Yang CS, He XY, Hong JY. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos. 2006;34:1672–1676. doi: 10.1124/dmd.106.011049. [DOI] [PubMed] [Google Scholar]
- 50.Shimada T, Takenaka S, Murayama N, Kramlinger VM, Kim JH, Kim D, Liu J, Foroozesh MK, Yamazaki H, Guengerich FP, Komori M. Oxidation of pyrene, 1-hydroxypyrene, 1-nitropyrene and 1-acetylpyrene by human cytochrome P450 2A13. Xenobiotica. 2016;46:211–224. doi: 10.3109/00498254.2015.1069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimada T, Takenaka S, Murayama N, Yamazaki H, Kim JH, Kim D, Yoshimoto FK, Guengerich FP, Komori M. Oxidation of acenaphthene and acenaphthylene by human cytochrome P450 enzymes. Chem Res Toxicol. 2015;28:268–278. doi: 10.1021/tx500505y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behrendorff JB, Moore CD, Kim KH, Kim DH, Smith CA, Johnston WA, Yun CH, Yost GS, Gillam EM. Directed evolution reveals requisite sequence elements in the functional expression of P450 2F1 in Escherichia coli. Chem Res Toxicol. 2012;25:1964–74. doi: 10.1021/tx300281g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lintelmann J, Hellemann C, Kettrup A. Coupled-column high-performance liquid chromatographic method for the determination of four metabolites of polycyclic aromatic hydrocarbons, 1-, 4- and 9-hydroxyphenanthrene and 1-hydroxypyrene, in urine. J Chromatogr B Biomed. 1994;660:67–73. doi: 10.1016/0378-4347(94)00267-3. [DOI] [PubMed] [Google Scholar]
- 54.Kuusimäki L, Peltonen Y, Mutanen P, Peltonen K, Savela K. Urinary hydroxy-metabolites of naphthalene, phenanthrene and pyrene as markers of exposure to diesel exhaust. Int Arch Occup Environ Health. 2004;77:23–30. doi: 10.1007/s00420-003-0477-y. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Zhong Y, Carmella SG, Hochalter JB, Rauch D, Oliver A, Jensen J, Hatsukami DK, Upadhyaya P, Hecht SS, Zimmerman CL. Phenanthrene metabolism in smokers: use of a two-step diagnostic plot approach to identify subjects with extensive metabolic activation. J Pharmacol Exp Ther. 2012;342:750–760. doi: 10.1124/jpet.112.194118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schober W, Pusch G, Oeder S, Reind H, Behrendt H, Buters JT. Metabolic activation of phenanthrene by human and mouse cytochromes P450 and pharmacokinetics in CYP1A2 knockout mice. Chem Biol Interact. 2010;183:57–66. doi: 10.1016/j.cbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.