Abstract
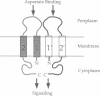
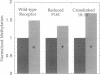
The Escherichia coli aspartate receptor, a dimer of identical subunits, has two transmembrane regions (TM1, residues 7-30; TM2, residues 189-212) of 24 residues each. To study the relative placement and orientation of the regions, cysteine residues were introduced individually into the center of each: at positions 17, 18, and 19 in TM1; and at positions 198, 199, 200, and 201 in TM2. Based on the patterns of disulfide cross-linking observed between subunits in the mutant receptors, there appears to be close contact between the TM1 and TM1' regions at the dimer interface but no such direct interaction between the TM2 and TM2' regions. The cross-linking results are consistent with an alpha-helical structure extending across the transmembrane region up through at least residue 36, which lies on the periplasmic side of TM1. The ability of an 18-18' cross-linked dimer to transmit an aspartate-induced transmembrane signal is also supportive of such an extended helix. The changes in relative rates of disulfide cross-linking provide experimental evidence of a conformational change transmitted through the transmembrane domain during signaling. Once formed, disulfides between the transmembrane regions are unusually resistant to reduction by low molecular weight thiols in the presence of denaturants like SDS. These targeted disulfide cross-links can be used to reveal structural and dynamic aspects of protein function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbach C., Flitsch S. L., Khorana H. G., Hubbell W. L. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989 Sep 19;28(19):7806–7812. doi: 10.1021/bi00445a042. [DOI] [PubMed] [Google Scholar]
- Boyd A., Kendall K., Simon M. I. Structure of the serine chemoreceptor in Escherichia coli. Nature. 1983 Feb 17;301(5901):623–626. doi: 10.1038/301623a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Levitt M., Richardson D. Helix to helix packing in proteins. J Mol Biol. 1981 Jan 5;145(1):215–250. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
- Falke J. J., Dernburg A. F., Sternberg D. A., Zalkin N., Milligan D. L., Koshland D. E., Jr Structure of a bacterial sensory receptor. A site-directed sulfhydryl study. J Biol Chem. 1988 Oct 15;263(29):14850–14858. [PubMed] [Google Scholar]
- Falke J. J., Koshland D. E., Jr Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science. 1987 Sep 25;237(4822):1596–1600. doi: 10.1126/science.2820061. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Mowbray S. L., Jap B. K., Koshland D. E., Jr Purification and characterization of the aspartate chemoreceptor. J Biol Chem. 1985 Sep 25;260(21):11706–11710. [PubMed] [Google Scholar]
- Katz B. A., Kossiakoff A. The crystallographically determined structures of atypical strained disulfides engineered into subtilisin. J Biol Chem. 1986 Nov 25;261(33):15480–15485. [PubMed] [Google Scholar]
- Krueger B. K. Toward an understanding of structure and function of ion channels. FASEB J. 1989 Jun;3(8):1906–1914. doi: 10.1096/fasebj.3.8.2470631. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan D. L., Koshland D. E., Jr Site-directed cross-linking. Establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J Biol Chem. 1988 May 5;263(13):6268–6275. [PubMed] [Google Scholar]
- Mowbray S. L., Foster D. L., Koshland D. E., Jr Proteolytic fragments identified with domains of the aspartate chemoreceptor. J Biol Chem. 1985 Sep 25;260(21):11711–11718. [PubMed] [Google Scholar]
- Mowbray S. L., Koshland D. E., Jr Additive and independent responses in a single receptor: aspartate and maltose stimuli on the tar protein. Cell. 1987 Jul 17;50(2):171–180. doi: 10.1016/0092-8674(87)90213-3. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Rees D. C., DeAntonio L., Eisenberg D. Hydrophobic organization of membrane proteins. Science. 1989 Aug 4;245(4917):510–513. doi: 10.1126/science.2667138. [DOI] [PubMed] [Google Scholar]
- Russo A. F., Koshland D. E., Jr Separation of signal transduction and adaptation functions of the aspartate receptor in bacterial sensing. Science. 1983 Jun 3;220(4601):1016–1020. doi: 10.1126/science.6302843. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988 Nov;13(11):443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]