Abstract
Background
Recent changes to the Food and Drug Administration boxed warning for metformin will increase use in individuals with historical contraindications or precautions. Prescribers must understand clinical outcomes of metformin use in these populations.
Purpose
To synthesize data addressing outcomes of metformin use in populations with type 2 diabetes and moderate-to-severe chronic kidney disease, congestive heart failure, or chronic liver disease with hepatic impairment.
Data Sources
MEDLINE (via PubMed) from January 1994 to September 2016; Cochrane Library, EMBASE, and International Pharmaceutical Abstracts from January 1994 to November 2015.
Study Selection
English-language studies that examined adults with type 2 diabetes and chronic kidney disease with eGFR <60 mL/min/1.73m2, congestive heart failure, or chronic liver disease with hepatic impairment; compared diabetes regimens that included metformin to regimens that did not; and reported all-cause mortality, major adverse cardiovascular events and other outcomes of interest.
Data Extraction
Two reviewers abstracted data and independently rated study quality and strength of evidence.
Data Synthesis
Based on quantitative/qualitative syntheses involving 17 observational studies, metformin use is associated with reduced all-cause mortality in patients with chronic kidney disease, congestive heart failure, and chronic liver disease with hepatic impairment, and reduced heart failure readmission in patients with chronic kidney disease and congestive heart failure.
Limitations
We identified low strength of evidence and sparse data on multiple outcomes of interest. Available studies were observational and had varying follow-up durations.
Conclusions
Metformin use in patients with moderate chronic kidney disease, congestive heart failure, or chronic liver disease with hepatic impairment is associated with improvements in key clinical outcomes. Our findings support recent changes in metformin labeling.
Registration
PROSPERO CRD42016027708
Funding Source
U.S. Department of Veterans Affairs
Introduction
Since its approval by the Food and Drug Administration (FDA) in 1994, metformin has become the recommended initial treatment for type 2 diabetes mellitus in the United States (1). Beyond its glycemic benefits, metformin typically does not cause weight gain or hypoglycemia and may be associated with lower mortality (2,3). Due to concerns about lactic acidosis with phenformin, a related biguanide withdrawn from the market in 1977, the FDA applied a boxed warning to metformin concurrent with its approval (4). This warning cautioned against using metformin in the setting of chronic kidney disease (CKD), which may impair excretion of the drug, and recommended caution with conditions that can promote lactate accumulation (e.g., congestive heart failure [CHF] and chronic liver disease [CLD]) (5).
Despite this warning, recent estimates suggest that 20–30% of metformin users have historical contraindications or precautions to its use (6,7). These findings reflect the fact that many prescribers have found the FDA boxed warning to be excessively restrictive (8,9). Literature reviews indicate no clear association between metformin and lactic acidosis (10), and suggest that the drug is safe for patients with moderate CKD or CHF (11,12). In 2006, the FDA removed CHF as a contraindication to metformin use, though acute or unstable CHF remains a precaution (13,14). In April 2016, the FDA revised its warning regarding metformin use in patients with CKD, switching from a serum creatinine-based definition for renal impairment to more inclusive criteria based on estimated glomerular filtration rate (eGFR) (15). With this change, an estimated one million additional patients with moderate CKD (eGFR 30–<60 mL/min/1.73m2) became eligible for metformin use, though severe CKD (eGFR <30mL/min/1.73m2) remains a contraindication (16).
In the wake of these changes, utilization of metformin will continue increasing in populations with historical contraindications and precautions. Prescribers must therefore fully understand the consequences of metformin use in these groups. In order to promote informed prescribing, we systematically reviewed existing literature regarding the benefits and harms of metformin use (beyond lactic acidosis) among patients with common chronic diseases historically identified by the FDA boxed warning as contraindications or precautions: moderate-to-severe CKD, CHF, and CLD with impaired hepatic function.
Methods
Study Design
This work was part of a Veterans Health Administration (VHA)–funded report. Additional details are available online (www.hsrd.research.va.gov/publications/esp). The present analysis focuses on the question: for patients with type 2 diabetes and a historical contraindication or precaution to metformin use, what are the benefits and harms (beyond lactic acidosis) of treatment with metformin?
This review followed a published protocol for this review (PROSPERO: CRD42016027708), and each step was pilot-tested to train and calibrate investigators.
Data Sources and Study Selection
In consultation with an expert medical librarian, we searched PubMed, the Cochrane Central Register of Controlled Trials, Embase, and the International Pharmaceutical Abstracts in November 2015; our PubMed search was subsequently updated through September 2016. We also searched ClinicalTrials.gov for relevant completed and ongoing studies. Appendix Table 1 contains our exact search strategies. We also screened reference lists of published reviews and queried Bristol-Myers Squibb, the manufacturer of the branded formulation of metformin, for other relevant studies.
Our prespecified inclusion and exclusion criteria are found in Appendix Table 2. We included English-language clinical trials and observational cohort studies that: 1) examined adults with type 2 diabetes and a metformin contraindication/precaution of interest (moderate-to-severe CKD [eGFR <60 mL/min/1.73m2], CHF, or CLD with hepatic impairment); 2) compared antihyperglycemic regimens that included metformin to regimens that did not; and 3) reported all-cause mortality, major adverse cardiovascular events (MACE), glycemic control, lipid control, hypoglycemia, weight gain, or vitamin B12 deficiency. Our VHA stakeholders and technical expert panel provided guidance on outcome selection.
Data Extraction and Quality Assessment of Individual Studies
Two investigators screened all citations for eligibility, and citations considered relevant by either individual advanced to full-text review. Two investigators reviewed all full-text articles and resolved disagreements through discussion or adjudication by a third investigator. Prior to excluding any potentially eligible study whose primary analysis did not explicitly address a population with a metformin contraindication/precaution, we examined the full text for relevant subgroup analyses.
Two investigators independently assessed study quality and resolved disagreements by consensus or arbitration by a third investigator. Using published quality criteria, we developed a customized risk of bias assessment tool designed to address selection, performance, attrition, detection, and reporting biases (Appendix Table 3) (18). We assigned each study a risk of bias score (low, moderate, or high).
Data Abstraction
For each included study, an investigator abstracted data using a customized DistillerSR database (Manotick, ON, Canada); a second investigator independently reviewed these data for accuracy. Relevant data included demographics, study setting, contraindication/precaution definitions, metformin dose, other antihyperglycemic agents, comparator, and outcomes. We treated multiple publications from a single study as a single data point, prioritizing the longest term and most complete results. When critical data were missing or unclear in published reports, we contacted manuscript authors.
Data Synthesis
We developed summary tables to characterize all included studies for each metformin contraindication/precaution of interest. Of note, two studies (19,20) separately compared distinct groups of metformin users—those using metformin monotherapy and those using metformin/sulfonylurea combination therapy—to patients using sulfonylurea monotherapy. In each case, we derived a pooled, weighted hazard ratio (HR) for all metformin users, incorporating an approximation of the correlation resulting from the shared sulfonylurea monotherapy reference group (see Technical Appendix). For another study (21), we estimated HR and variance from the reported frequencies and odds ratio (OR) using an established approach (22,23) (see Technical Appendix).
When ≥3 studies were conceptually similar in terms of design, population, intervention, and outcomes, we performed quantitative synthesis using a random-effects model to generate summary hazard ratios. For analyses with <20 studies, we used the Knapp-Hartung approach to adjust the standard errors of the estimated coefficients (24,25). Where appropriate, we conducted sensitivity analyses by omitting subgroups with greater contraindication/precaution severity (e.g., eGFR <30 mL/min/1.73m2), studies with shorter follow-up duration (<2 years), and studies not using propensity score adjustment. We evaluated statistical heterogeneity using Cochran’s Q and I2 statistics, and for analyses including ≥10 studies, assessed publication bias using funnel plots and Begg and Egger tests (26,27). When there were too few studies to warrant meta-analysis, we performed qualitative synthesis.
We performed all quantitative analyses using R (version 3.1.2), including R package “metafor” (version 1.9–7) for meta-analysis.
Strength of Evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to evaluate the overall strength of evidence (SOE) for outcomes with sufficient data. Utilizing the domains of risk of bias, directness, and consistency/precision of treatment effects, an investigator (JWW) rated SOE as high, moderate, low, or insufficient. We considered the impact of residual confounders, magnitude of effect, and publication bias (28,29).
Role of the Funding Source
This review was funded by the U.S. Department of Veterans Affairs. The funding source had no role in the study design, data collection, analysis, preparation of the manuscript, or the decision to submit the manuscript for publication.
Results
From 4,849 screened citations, we reviewed 523 full-text articles and identified 17 eligible studies (Figure 1). All were observational and addressed populations with moderate-to-severe CKD (n=5), CHF (n=11), or CLD with hepatic impairment (n=3); 3 studies addressed both CKD and CHF. Appendix Table 4 provides details on included studies. Of note, we identified no ongoing studies meeting our inclusion criteria in ClinicalTrials.gov.
Figure 1. Flow of articles through the literature search and screening process.
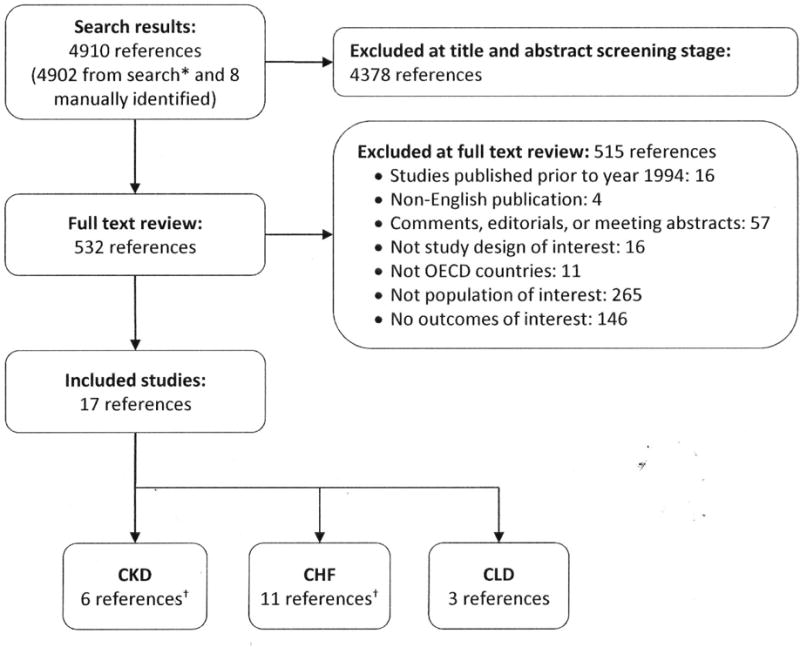
Abbreviations: CHF=congestive heart failure; CKD=chronic kidney disease; CLD=chronic liver disease
*Search results are from Embase (2512), PubMed (2312), Cochrane (17).
†Three references were relevant to both CKD and CHF.
Chronic Kidney Disease
Six observational studies—4 retrospective cohort (30–33), one prospective cohort (34), and one nested case-control derived from a cohort (35)—evaluated metformin’s effect on relevant outcomes in patients with type 2 diabetes and moderate-to-severe CKD. Sample sizes ranged from 1,246 to 11,481 patients with moderate-to-severe CKD, and mean/median age ranged from 65 to 76 years. CKD definitions varied among studies, with 4 reporting eGFR-based definitions (30,31,34,35), and two using serum creatinine-based definitions (32,33). Only one study reported a median daily metformin dose (1100–1900 mg in various subgroups) (31). All studies adjusted for multiple baseline population differences between metformin users and nonusers; 3 utilized propensity scores (30,31,34). Follow-up ranged from one to 3.9 years. Two studies had low risk of bias (ROB) (30,33) and 4 moderate ROB (31,32,34,35).
All-cause mortality
Five studies (n=33,442) examined all-cause mortality, defined using medical records or administrative data in 5 studies (30–33) and prospective assessment in the fifth (34). Rather than comparing metformin to specific alternatives, all studies compared diabetes treatment regimens including metformin to regimens not including metformin. On meta-analysis, the relative chance of dying during follow-up was 22% lower for patients taking metformin than for those not taking metformin (HR 0.78; 95% CI 0.63 to 0.96; Q=29.7 [p<0.001], I2=79.8%) (Figure 2). Sensitivity analyses examining 3 studies (30,31,33) with follow-up duration ≥2 years and 3 (30,31,34) that used propensity score adjustment yielded similar HR point estimates and statistical heterogeneity to our main analysis.
Figure 2. Meta-analysis of all-cause mortality among patients with moderate-to-severe CKD using treatment regimens including metformin versus regimens not including metformin* † ‡.
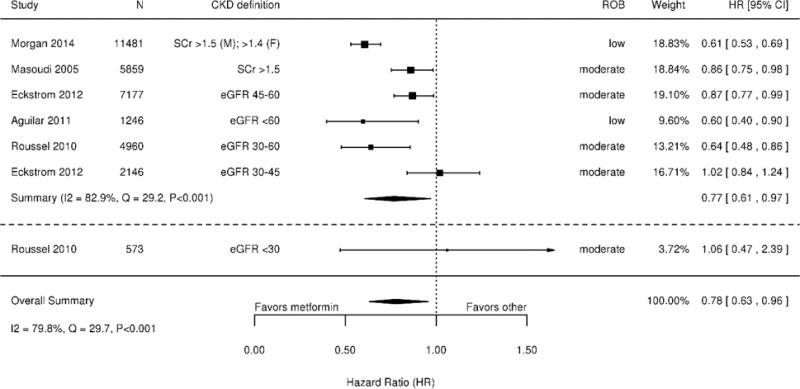
Abbreviations: CI=confidence interval; CKD=chronic kidney disease; eGFR=estimated glomerular filtration rate; HR=hazard ratio; ROB=risk of bias; SCr=serum creatinine
*Studies on the forest plot are ordered by increasing CKD severity.
†Eckstrom, 2012 (31) and Roussel, 2010 (34) stratified their respective populations by eGFR; these eGFR categories are presented separately for these studies.
‡SCr (serum creatinine) > 132.6 μmol/L (1.5mg/dL)
Two studies reported mortality by CKD severity subcategory and suggested that patients with eGFR 30–<45 mL/min/1.73m2 experienced less benefit with metformin than patients with eGFR 45–<60 mL/min/1.73m2 (Figure 2) (31,34). A sensitivity analysis excluding a 573 patients with eGFR <30 mL/min/1.73m2—a level of kidney impairment at which metformin remains contraindicated—produced findings similar to the main meta-analysis (34).
Major adverse cardiovascular events
Two studies (n=14,408) examined MACE with diabetes treatment regimens including metformin versus regimens not including metformin (31,32). One used administrative data to identify MACE-related diagnoses (including myocardial infarction, angina, stroke, and procedures), and found no difference in outcomes with metformin use among patients with eGFR 45–<60 mL/min/1.73m2 (n=6655; HR 0.94; 95% CI 0.84 to 1.05) and 30–<45 mL/min/1.73m2 (n=1894; HR 1.00; 95% CI 0.83 to 1.19) (31). The other study used administrative data to examine readmission for CHF and found that metformin use was significantly associated with slightly lower readmission (n=5859; HR 0.91; 95% CI 0.84 to 0.99) (32).
Hypoglycemia
One study (n=1,644 with eGFR <60 mL/min/1.73m2) used diagnosis codes to examine hypoglycemia with use of metformin, glyburide, or insulin monotherapy (35) With metformin as the reference group, both glyburide (adjusted OR 6.0; 95% CI 3.8 to 9.5) and insulin (adjusted OR 7.9; 95% CI 5.0 to 12.4) were associated with more hypoglycemia. These associations persisted with restriction to patients with eGFR <45 mL/min/1.73m2 (glyburide OR 7.5; 95% CI 3.7 to 15.3; insulin OR 8.9; 95% CI 4.3 to 17.8) and eGFR <30 mL/min/1.73m2 (glyburide OR 4.7; 95% CI 1.5 to 14.1; insulin OR 3.2, 95% CI 1.1 to 9.5).
Congestive Heart Failure
Eleven observational studies—8 retrospective cohort (19,20,30,32,36,37,39,40), two prospective cohort (34,38), and one nested case-control derived from a cohort (21)—evaluated metformin’s effect on relevant outcomes in patients with type 2 diabetes and CHF. The entire population had CHF in 9 studies (19–21,30,32,36,37,39), and we examined CHF subgroups in the remaining two (34, 40). Sample sizes ranged from 346 to 13,930 patients with CHF, and mean/median age ranged from 55 to 77 years. CHF definitions varied widely, with most studies using diagnosis codes. CHF severity was reported variably; 4 studies reported left ventricular ejection fraction (LVEF) (30,32,38,39), two reported New York Heart Association class (both of which also reported LVEF) (38,39), two reported other clinical criteria (19,37), and 5 did not report CHF severity (20,21,34,36,40). No studies reported median metformin dose. All studies adjusted for multiple baseline population differences between metformin users and nonusers; 5 utilized propensity scores (20,21,30,34,38). Follow-up ranged from one to 4.7 years. Two studies had low ROB (30,38) and the others moderate ROB.
All-cause mortality
Eleven studies (n=35,410) examined all-cause mortality, defined using medical records or administrative data in 9 studies (19,21,30,32,36–40), prospective assessment in one (34), and not defined in one (20). Nine studies compared diabetes treatment regimens including metformin to regimens not including metformin, while two compared metformin to sulfonylurea monotherapy (19,20). On meta-analysis, the relative chance of dying during follow-up was 22% lower for patients taking metformin than for those not taking metformin (HR 0.78; 95% CI 0.71 to 0.87; Q=26.6 [p=0.003], I2=62.3%) (Figure 3). Sensitivity analyses examining 7 studies (19,20,30,36,38–40) with follow-up duration ≥2 years and 5 studies (20,21,30,34,38) that used propensity score adjustment yielded similar HR point estimates and statistical heterogeneity to our main analysis. Inspection of a funnel plot showed no clear evidence for publication bias (Appendix Figure 1), nor did Begg (p=0.16) and Egger tests (p=0.09).
Figure 3. Meta-analysis of all-cause mortality among patients with CHF using treatment regimens including metformin versus regimens not including metformin*.
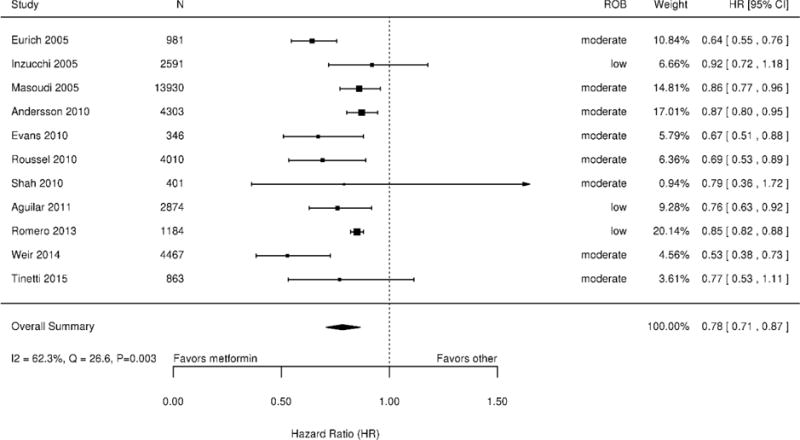
Abbreviations: CI=confidence interval; CHF=congestive heart failure; HR=hazard ratio; ROB=risk of bias
*Studies on the forest plot are ordered chronologically.
Two studies examined mortality by CHF severity. One reported mortality by LVEF category and found no difference with metformin in subgroups with moderate (LVEF 30% to 39%; HR 0.87; 95% CI 0.67 to 1.13) or severe CHF (LVEF <30%; HR 0.87; 95% CI 0.69 to 1.08) (32). The other included only patients with LVEF <40% and found no mortality difference with metformin (HR 0.79; 95% CI 0.36 to 1.71) (39).
Major adverse cardiovascular events
Six studies used medical records or administrative data to examine MACE, with 4 (n=26,510) evaluating CHF readmission (21,30,32,38), and 3 (n=6,468) examining cardiovascular mortality (19,20,38). In all, 4 studies compared diabetes treatment regimens including metformin to regimens not including metformin, while two compared metformin to sulfonylurea monotherapy (19,20). We performed separate meta-analyses for each MACE outcome. On meta-analysis, the relative chance of readmission for CHF during follow-up was 13% lower for patients taking metformin than for those not taking metformin (HR 0.87; 95% CI 0.78 to 0.97; Q=11.7 [p=0.009], I2=74.3%) (Appendix Figure 2). A sensitivity analysis examining 3 studies (21,30,38) that used propensity score adjustment yielded a similar HR point estimate to our main analysis, but reduced statistical heterogeneity (Q=1.6 [p=0.44], I2=0.0%). The summary HR for cardiovascular mortality also favored metformin (Appendix Figure 3) but was not statistically significant (HR 0.77; 95% CI 0.53 to 1.12; Q=7.8 [p=0.02], I2=74.3%).
Chronic Liver Disease
Three observational cohort studies—two retrospective (41,43) and one prospective (42)—evaluated the effect of metformin on relevant outcomes in patients with type 2 diabetes and CLD with cirrhosis defined by histology. We therefore considered all patients to have “impaired hepatic function,” as specified by the FDA boxed warning (5). Individual sample sizes ranged from 82 to 250 patients, and mean/median age ranged from 60 to 61 years. No studies reported the median metformin dose. Follow-up ranged from 4.5 to 5.7 years. One study had low ROB (43) and one moderate ROB (41). The third study was well-designed overall but had high ROB with regard to all-cause mortality (42); the primary outcome was liver-specific mortality, and only unadjusted all-cause mortality rates could be derived.
All-cause mortality
Three studies (n=432) examined all-cause mortality, defined using medical record or administrative data in two studies (41,43) and prospective assessment in one (42). Each study compared diabetes treatment regimens including metformin to regimens not including metformin. All studies adjusted for baseline population differences between metformin users and nonusers for their primary analyses; however, in two studies we could abstract only unadjusted event rates for all-cause mortality (41,42). Because of these differences in outcome reporting, we did not attempt meta-analysis.
The low-ROB study found significantly longer survival associated with metformin use (n=250; HR 0.43; 95% CI 0.24 to 0.78), regardless of cirrhosis severity (Child-Pugh class A: HR 0.47; 95% CI 0.27 to 0.82; B/C: HR 0.46; 95% CI 0.21 to 0.98) (43). On post-hoc subgroup analysis, a positive association between metformin and survival was seen only with cirrhosis secondary to nonalcoholic steatohepatitis (n=142; HR 0.33; 95% CI 0.17 to 0.63), and not in the smaller groups with cirrhosis related to alcohol or viral hepatitis.
Trends toward lower all-cause mortality with metformin use were present in the moderate-ROB study (n=82; 7.3% [3/41] vs. 17.1% [7/41]; p=NR) (41) and high-ROB study (n=100; 7.7% [2/26] vs. 48.6% [36/74]; p=NR) (42).
Other Outcomes
We identified no studies evaluating metformin’s effects on glycemic control, lipid control, weight gain, or B12 deficiency in adults with diabetes and contraindications/precautions of interest. We found no studies evaluating hypoglycemia in adults with diabetes and CHF or CLD, nor MACE in adults with diabetes and CLD.
Study Quality
Most studies had moderate or low ROB (Appendix Table 5). Common quality concerns included: 1) incomplete accounting for baseline population differences and confounding by indication, though some studies did utilize propensity scores; 2) limited assessment of metformin use throughout the study period (e.g., assessment at baseline without accounting for subsequent metformin discontinuation or initiation), though some studies did analyze metformin exposure status in “intervals” to account for this concern; 3) incomplete assessment and description of attrition; and 4) unblinded outcome assessment.
Strength of Evidence
Table 1 summarizes the overall SOE regarding metformin’s effect on all-cause mortality and MACE among patients with moderate-to-severe CKD or CHF. We only assessed SOE for outcomes where the number of studies warranted meta-analysis. For all-cause mortality, there was low SOE for reduced mortality among metformin users with moderate-to-severe CKD or CHF. There was likewise low SOE supporting reduced CHF readmission among metformin users with CHF; the evidence for reduction of cardiovascular mortality in this group was insufficient.
Table 1.
Overall strength of evidence regarding key outcomes associated with metformin use among patients with moderate-to-severe CKD and CHF
| Outcome | # Studies (Patients) | Findings | SOE Rationale by Domain |
|---|---|---|---|
| Patients with moderate-to-severe CKD | |||
| All-cause mortality | 5 observational (33,442) |
HR 0.77 (95% CI 0.61 to 0.97) 48 fewer deaths/1,000 (81 to 6 fewer) |
Low SOE Moderate ROB, Inconsistent, Precise, Direct |
| Patients with CHF | |||
| All-cause mortality | 11 observational (35,410) |
HR 0.78 (95% CI 0.71 to 0.87) 48 fewer deaths/1,000 (64 to 29 fewer) |
Low SOE Moderate ROB, Consistent, Precise, Direct |
| Cardiovascular mortality | 3 observational (6,468) |
HR 0.77 (0.53 to 1.12) 66 fewer deaths/1,000 (136 fewer to 35 more) |
Insufficient SOE Moderate ROB, Consistent, Imprecise, Direct |
| CHF readmission | 4 observational (26,510) |
HR 0.87 (95% CI 0.78 to 0.97) 12 fewer readmissions/1,000 (20 to 3 fewer) |
Low SOE Low ROB, Consistent, Precise, Direct |
Abbreviations: CHF=congestive heart failure; CKD=chronic kidney disease; HR=hazard ratio; ROB=risk of bias; SOE=strength of evidence
Discussion
Following recent FDA labelling changes, metformin use in populations with historical contraindications or precautions will continue to rise. This systematic review sought to inform prescribing by examining clinical outcomes associated with metformin use among adults with type 2 diabetes and comorbid moderate-to-severe CKD, CHF, or CLD with impaired hepatic function. Based on available observational evidence, we found that metformin appears to be associated with reduced all-cause mortality in moderate CKD, CHF, and CLD with impaired hepatic function, reduced CHF readmission among patients with moderate CKD or CHF, and a lower rate of hypoglycemia among patients with moderate CKD.
Clinical and Policy Implications
As the consensus first-line therapy in type 2 diabetes, metformin is the most widely prescribed diabetes drug in the world (44). Beyond its glycemic effects, metformin is appealing because it is weight-neutral, safe, and may be associated with improved long-term outcomes in general diabetes populations (1–3). Although data were limited, we found no evidence to suggest that metformin’s benefits do not extend to patients with moderate CKD, CHF, or CLD with impaired hepatic function. Together with reports regarding the safety of metformin with respect to lactic acidosis (10,11), our findings support FDA’s recent actions.
This analysis adds to existing knowledge about metformin’s effects on mortality outcomes. Based on a meta-analysis of 35 randomized controlled trials (RCTs) reported through October, 2009, Lamanna et al. concluded that metformin monotherapy is likely associated with improved survival (2). In a subsequent analysis of 6 RCTs and 8 observational studies reported between April, 2009, and April, 2015, Bolen et al. reported lower cardiovascular mortality with metformin versus sulfonylureas (risk difference 0.1% to 2.9% in RCTs) (3). In contrast, Palmer et al. conducted a network meta-analysis of 25 comparative monotherapy studies reported through March, 2016, and found that cardiovascular mortality did not differ between diabetes medication classes, including metformin (45); of note, this analysis included only 67 total cardiovascular deaths. Our review differs from these analyses in that we focused on diabetes populations with historical metformin contraindications or precautions. Consequently, we analyzed observational studies with longer follow-up periods, which are in some ways better suited to examine outcomes that require long-term observation (like mortality). Our findings are consistent with those of Eurich et al. (12), who found that metformin is associated with reduced mortality in CHF; our analysis included 3 additional studies (n=6,514) (21,38,40), and excluded another without an active comparator (46).
Beyond providing information for prescribers, this review may help inform revision of clinical guidelines. The 2016 American Diabetes Association guidelines note that “accumulating observational data suggest that metformin may be safely continued down to glomerular filtration rate (GFR) of 45 mL/min/1.73m2/1.73m2 or even 30 mL/min/1.73m2/1.73 m2” (1). Given the apparent mortality reduction associated with metformin use in diabetes patients with moderate CKD and other relevant comorbidities, this review may support strengthening this endorsement.
Limitations
Though we utilized a rigorous, protocol-driven approach, our analysis does have limitations. First, in order to assure relevance for our VHA stakeholders, we limited our search to studies from Organization for Economic Cooperation and Development (OECD) countries (17), which may have excluded potentially relevant articles from non-OECD countries. Second, although we examined numerous outcomes, we did not examine all outcomes of potential interest. Because our objective was informing metformin prescribing, we focused on the most clinically relevant outcomes for our stakeholders.
The observational evidence base warrants additional caution when interpreting our findings. First, registries like ClinicalTtrials.gov do not include observational studies, which limited our ability to assess for publication bias. Second, although most studies adjusted for baseline differences between metformin users and nonusers (sometimes including propensity scores), confounding by indication remains a potential source for unmeasured population differences. For example, many studies did not report outcomes based on contraindication/precaution severity, so unaccounted-for between-group differences in disease severity could potentially have influenced our findings. Of note, sensitivity analyses examining studies using propensity score adjustment yielded similar HR point estimates to our main analyses, but did substantially reduce statistical heterogeneity in one case (readmission in CHF). Third, most included studies analyzed prevalent metformin users, which could introduce bias if the hazards associated with metformin (or comparators) vary with time (47). Fourth, because included studies typically compared diabetes treatment regimens including metformin to regimens not including metformin, intervention and comparator patients alike may have used sulfonylureas, insulin, and other medications. This issue prevented comparisons between metformin and specific alternatives. Fifth, while some studies analyzed outcomes based on time intervals during which patients did or did not receive metformin, most defined metformin use at baseline only. Post-baseline medication changes could therefore have led to misclassification of patients. Sixth, the timing of outcome assessment varied between studies and little information on attrition was typically available, potentially affecting study population composition over time. If the hazards of metformin use are time-varying, pooling data from studies with different follow-up durations could introduce bias (48); however, sensitivity analyses examining studies with follow-up ≥2 years yielded similar results to our main analysis.
All these limitations may have contributed to statistical heterogeneity observed in our quantitative syntheses. However, because most meta-analyzed studies showed metformin to be associated with improved outcomes of interest, this heterogeneity appears related to variance in the precise magnitude of an overall effect consistently favoring metformin. As such, the observed heterogeneity does not invalidate our findings.
Future Research
To date, most metformin trials have excluded patients with moderate-to-severe CKD, CHF, or CLD. As such, the primary evidence gap regarding metformin use for patients with historical contraindications or precautions is the lack of RCTs. Various factors reduce the feasibility of metformin RCTs for these populations; metformin is a generic medication widely viewed as a first-line treatment, and the length of time required for assessment of mortality and MACE may be prohibitive. Even without RCTs, new observational studies could ensure that deleterious outcomes do not become more apparent as metformin prescribing increases in populations with historical contraindications or precautions. Of note, we identified no such ongoing studies meeting our inclusion criteria in ClinicalTrials.gov.
The impact of contraindication/precaution severity on the apparently beneficial effects of metformin remains unclear. For example, although our primary CKD meta-analysis included patients with a range of eGFR values <60 mL/min/1.73m2, additional studies focusing specifically on cohorts with eGFR 30–45 mL/min/1.73m2 or even <30 mL/min/1.73m2 would further inform metformin prescribing and guideline refinement. Data regarding precaution severity in CHF and CLD are sparse, and observational research could address these gaps.
Building on the issue of severity, the possibility of tailoring metformin prescribing based on the severity of historical contraindications/precautions would benefit from further research. Canadian prescribing guidelines have long recommended metformin dose reduction based on eGFR (49), and U.S. thought leaders have suggested a maximum metformin dose of 2550 mg for patients with eGFR ≥60 mL/min/1.73m2, 2000 mg daily for eGFR 45–<60 mL/min/1.73m2, and 1000 mg/day for eGFR 30–<45 mL/min/1.73m2 (11). Given that metformin is excreted unchanged in the urine (50), dose adjustment has a clear rationale, but there are no trial data and limited observational data supporting this approach.
Finally, because diabetes medication classes have varying effects on cardiovascular outcomes (51,52), additional research comparing metformin to specific alternative agents in populations with historical contraindications/precautions would facilitate refinement of prescribing guidelines for these groups.
Conclusions
Based on limited evidence, metformin appears associated with reduced all-cause mortality in patients with moderate CKD, CHF, or CLD with impaired hepatic function. Further, metformin may be associated with reduced CHF readmission in patients with moderate CKD or CHF and reduced hypoglycemia incidence in patients with moderate CKD. Available data provide no evidence that the risks of metformin exceed risks associated with other antihyperglycemic medications in these populations. Our findings support recent FDA labeling changes, point toward areas for future research, and may help inform clinical practice and revision of clinical guidelines.
Supplementary Material
Acknowledgments
This project was supported by the VHA ESP (Project #09–009). MJC is supported by a Career Development Award from VHA Health Services Research and Development (CDA 13–261). CJD is supported by a Mentored Patient-Oriented Research Career Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK099385). The views expressed in this article do not necessarily reflect the position of the Department of Veterans Affairs or Duke University. The authors thank David D’Alessio, MD, for his critical review of this manuscript and Liz Wing, MA, for her editorial assistance.
Footnotes
Statement of Availability
Protocol: PROSPERO (CRD42016027708)
Statistical Code: See methods; analytic dataset (.csv files) and code are available upon request
Data: See appendix and VHA report “Metformin Use in Patients with Historical Contraindications or Precautions” (www.hsrd.research.va.gov/publications/esp)
References
- 1.American Diabetes Association. Standards of Medical Care in Diabetes—2016: Summary of Revisions. Diabetes Care. 2016;39:S4–S5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 2.Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2011;13:221–8. doi: 10.1111/j.1463-1326.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- 3.Bolen S, Tseng E, Hutfless S, Segal JB, Suarez-Cuervo C, Berger Z, et al. Diabetes Medications for Adults With Type 2 Diabetes: An Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 4.Misbin RI. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care. 2004;27:1791–3. doi: 10.2337/diacare.27.7.1791. [DOI] [PubMed] [Google Scholar]
- 5.Metformin hydrochloride. Boxed warning. Available at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=b8004451-7b26-425b-b5ea-cbb1b08e30e3&type=display. Accessed October 6, 2015.
- 6.Tuot DS, Lin F, Shlipak MG, Grubbs V, Hsu CY, Yee J, et al. Potential impact of prescribing metformin according to eGFR rather than serum creatinine. Diabetes Care. 2015;38:2059–67. doi: 10.2337/dc15-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang DL, Abrass IB, Young BA. Medication safety and chronic kidney disease in older adults prescribed metformin: a cross-sectional analysis. BMC Nephrol. 2014;15:86. doi: 10.1186/1471-2369-15-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasisht KP, Chen SC, Peng Y, Bakris GL. Limitations of metformin use in patients with kidney disease: are they warranted? Diabetes Obes Metab. 2010;12:1079–83. doi: 10.1111/j.1463-1326.2010.01295.x. [DOI] [PubMed] [Google Scholar]
- 9.Philbrick AM, Ernst ME, McDanel DL, Ross MB, Moores KG. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017–23. doi: 10.2146/ajhp080330. [DOI] [PubMed] [Google Scholar]
- 10.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010:CD002967. doi: 10.1002/14651858.CD002967.pub4. [DOI] [PubMed] [Google Scholar]
- 11.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–75. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 13.Swift TMM. Metformin use in patients with diabetes and heart failure: cause for concern? Diabetes Spectrum. 2009;22:18–20. doi: 10.2337/diaspect.22.1.18. [DOI] [Google Scholar]
- 14.Bristol-Myers Squibb. Glucophage (metformin hydrochloride). Presecribing information. Available at: http://www.bms.com/products/Pages/home.aspx. Accessed August 8, 2016.
- 15.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed June 16, 2016.
- 16.Flory JH, Hennessy S. Metformin use reduction in mild to moderate renal impairment: possible inappropriate curbing of use based on food and drug administration contraindications. JAMA Intern Med. 2015;175:458–9. doi: 10.1001/jamainternmed.2014.6936. [DOI] [PubMed] [Google Scholar]
- 17.Organization for Economic Cooperation and Development. Available at: http://www.oecd.org/about/membersandpartners/list-oecd-member-countries.htm. Accessed October 20, 2015.
- 18.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. 2012 Mar 8. Available from: http://www.ncbi.nlm.nih.gov/books/NBK91433/. Accessed October 6, 2015. [PubMed] [Google Scholar]
- 19.Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jorgensen CH, et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia. 2010;53:2546–53. doi: 10.1007/s00125-010-1906-6. [DOI] [PubMed] [Google Scholar]
- 20.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–51. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 21.Weir DL, McAlister FA, Senthilselvan A, Minhas-Sandhu JK, Eurich DT. Sitagliptin use in patients with diabetes and heart failure: a population-based retrospective cohort study. JACC Heart Fail. 2014;2:573–82. doi: 10.1016/j.jchf.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z. Converting odds ratio to relative risk in cohort studies with partial data information. Journal of Statistical Software. 2013;55:1–11. [Google Scholar]
- 23.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Available at: http://www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=318. Accessed October 6, 2015. [PubMed] [Google Scholar]
- 29.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circulation: Heart Failure. 2011;4:53–8. doi: 10.1161/circheartfailure.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekström N, Schiöler L, Svensson AM, Eeg-Olofsson K, Jonasson JM, Zethelius B, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: A cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2:4. doi: 10.1136/bmjopen-2012-001076. http://dx.doi.org/10.1136/bmjopen-2012-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–90. doi: 10.1161/01.cir.0000154542.13412.b1. [DOI] [PubMed] [Google Scholar]
- 33.Morgan CL, Mukherjee J, Jenkins-Jones S, Holden SE, Currie CJ. Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: a retrospective, observational study. Diabetes Obes Metab. 2014;16:957–62. doi: 10.1111/dom.12302. [DOI] [PubMed] [Google Scholar]
- 34.Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC, Jr, Goto S, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010;170:1892–9. doi: 10.1001/archinternmed.2010.409. [DOI] [PubMed] [Google Scholar]
- 35.Weir MA, Gomes T, Mamdani M, Juurlink DN, Hackam DG, Mahon JL, et al. Impaired renal function modifies the risk of severe hypoglycaemia among users of insulin but not glyburide: a population-based nested case-control study. Nephrol Dial Transplant. 2011;26:1888–94. doi: 10.1093/ndt/gfq649. [DOI] [PubMed] [Google Scholar]
- 36.Evans JM, Doney AS, AlZadjali MA, Ogston SA, Petrie JR, Morris AD, et al. Effect of Metformin on mortality in patients with heart failure and type 2 diabetes mellitus. Am J Cardiol. 2010;106:1006–10. doi: 10.1016/j.amjcard.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Masoudi FA, Wang Y, Kosiborod M, Foody JM, Setaro JF, et al. Insulin-sensitizing antihyperglycemic drugs and mortality after acute myocardial infarction: insights from the National Heart Care Project. Diabetes Care. 2005;28:1680–9. doi: 10.2337/diacare.28.7.1680. [DOI] [PubMed] [Google Scholar]
- 38.Romero SP, Andrey JL, Garcia-Egido A, Escobar MA, Perez V, Corzo R, et al. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol. 2013;166:404–12. doi: 10.1016/j.ijcard.2011.10.141. [DOI] [PubMed] [Google Scholar]
- 39.Shah DD, Fonarow GC, Horwich TB. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J Card Fail. 2010;16:200–6. doi: 10.1016/j.cardfail.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinetti ME, McAvay G, Trentalange M, Cohen AB, Allore HG. Association between guideline recommended drugs and death in older adults with multiple chronic conditions: population based cohort study. BMJ. 2015;351:h4984. doi: 10.1136/bmj.h4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ampuero J, Ranchal I, Nunez D, Diaz-Herrero Mdel M, Maraver M, del Campo JA, et al. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PloS One. 2012;7:e49279. doi: 10.1371/journal.pone.0049279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601–8. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008–16. doi: 10.1002/hep.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 45.Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis. Jama. 2016;316:313–24. doi: 10.1001/jama.2016.9400. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald MR, Eurich DT, Majumdar SR, Lewsey JD, Bhagra S, Jhund PS, et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care. 2010;33:1213–8. doi: 10.2337/dc09-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 48.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canadian Diabetes Association. Clinical Practice Guidelines: Pharmacotherapy for Type 2 Diabetes. Available at: http://guidelines.diabetes.ca/bloodglucoselowering/pharmacologyt2-(1). Accessed June 30, 2016.
- 50.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–7. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallagher AM, Smeeth L, Seabroke S, Leufkens HG, van Staa TP. Risk of death and cardiovascular outcomes with thiazolidinediones: a study with the general practice research database and secondary care data. PloS One. 2011;6:e28157. doi: 10.1371/journal.pone.0028157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:938–53. doi: 10.1111/dom.12116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


