Abstract
Environmental reward-predictive stimuli can retrieve from memory a specific reward expectation that allows them to motivate action and guide choice. This process requires the basolateral amygdala (BLA), but little is known about the signaling systems necessary within this structure. Here we examined the role of the neuromodulatory opioid receptor system in the BLA in such cue-directed action using the outcome-specific Pavlovian-to-instrumental transfer (PIT) test in rats. Inactivation of BLA mu-, but not delta-opioid receptors was found to dose-dependently attenuate the ability of a reward-predictive cue to selectively invigorate the performance of actions directed at the same unique predicted reward (i.e. to express outcome-specific PIT). BLA mu-opioid receptor inactivation did not affect the ability of a reward itself to similarly motivate action (outcome-specific reinstatement), suggesting a more selective role for the BLA mu-opioid receptor in the motivating influence of currently unobservable rewarding events. These data reveal a new role for BLA mu-opioid receptor activation in the cued recall of precise reward memories and the use of this information to motivate specific action plans.
Keywords: Basolateral Amygdala, Pavlovian-to-instrumental transfer, Memory, Motivation, Rat
Graphical Abstract
Using local pharmacological manipulations, we found that activation of basolateral amygdala mu-, but not delta-opioid receptors is required for a reward-predictive cue to selectively invigorate the performance of actions directed at the same unique predicted reward. These data reveal a new role for basolateral amygdala mu-opioid receptor activation in the cued recall of precise reward expectations and the use of this information to motivate specific action plans.
Environmental stimuli that signal forthcoming reward can motivate reward seeking, influence action planning, and guide choice. Typically this is adaptive, but disruptions can lead to the cognitive symptoms underlying myriad psychiatric disorders. One primary way reward cues direct action is by triggering the recall of a precise memory of their specific predicted reward. This reward expectation biases choice towards and selectively motivates performance of those actions that earn the same unique reward (Kruse et al., 1983; Colwill & Motzkin, 1994; Corbit & Balleine, 2015). The basolateral amygdala (BLA) is required for this cognitive process (Blundell et al., 2001; Corbit & Balleine, 2005; Ostlund & Balleine, 2008; Malvaez et al., 2015), but little is known about the signaling systems necessary within this structure.
The neuromodulatory endogenous opioid system has long been implicated in reward-related behavior (Le Merrer et al., 2009) and all three opioid receptor subtypes are expressed in the BLA (Mansour et al., 1994). Delta- and mu-opioid receptors have been especially implicated and shown to make dissociable contributions (Laurent et al., 2015). Indeed, reward-predictive cues are unable to selectively motivate action in mice with a global knockout of the delta-opioid receptor, while mu-knockout mice have no such deficit, though are impaired in using changes in the value of anticipated rewards to guide choice (Laurent et al., 2012). Therefore, here we tested the hypothesis that BLA delta- and mu-opioid receptor activation are differentially involved in cue-directed action by evaluating the influence of BLA delta- or mu-opioid receptor inactivation on outcome-specific Pavlovian-to-instrumental transfer (PIT).
In this task, rats are trained to associate two auditory stimuli (conditioned stimuli; CSs) with two distinct food rewards and then to earn each of those two rewards by responding on independent levers. In the critical PIT test, both levers are available and CS presentation will selectively enhance performance of the action with which it shares a rewarding outcome. Because the CSs are never associated with the instrumental actions, this test assesses the rats’ ability to, upon CS presentation, retrieve a stored memory of the specific predicted reward and use this expectation to guide and motivate reward-seeking actions. Under these conditions the expected reward is not observable, but rather must be cognitively represented by the subject. Data from the PIT test were, therefore, compared to choice performance influenced by presentation of a fully observable reward using the outcome-specific reinstatement task.
MATERIALS AND METHODS
Subjects
Male, Long Evans rats (Experiment 1: n=35, Experiment 2: n=8, Charles River Laboratories, Wilmington, MA) weighing between 300–360 g were pair housed with no additional enrichment in a temperature (68–79 °F) and humidity-regulated (30–70%) vivarium. Training and testing took place during the dark phase of the 12:12 hr reverse dark:light cycle. Rats had ad libitum access to filtered tap water in the home cage and were maintained on a food-deprived schedule whereby they received 12–14 g of their maintenance diet (Lab Diet, Brentwood, MO) daily to maintain ~85–90% free-feeding body weight. All procedures were conducted in accordance with the NIH Guide for the Care and use of Laboratory Animals and approved by the UCLA Institutional Animal Care and Use Committee.
Behavioral training
Subjects were handled for 3 days prior to training. Training and testing took place in a set of 16 Med Associates (East Fairfield, VT) operant chambers, described previously (Wassum et al., 2016).
Pavlovian training
Each of the 8 daily sessions consisted of 8 tone (1.5 kHz) and 8 white noise CS presentations (75 db, 2-min duration), during which either sucrose solution (20%, 0.1 ml/delivery) or grain pellets (45 mg; Bio-Serv Frenchtown, NJ), were delivered on a 30-s random-time schedule into the food-delivery port, resulting in an average of 4 stimulus-reward pairings per trial. For half the subjects, tone was paired with sucrose and noise with pellets, with the other half receiving the opposite arrangement. CSs were delivered pseudo-randomly with a variable inter-trial interval (2–4 min, mean=3 min). Entries into the food-delivery port were recorded for the entire session. Comparison of anticipatory entries during the CS-probe periods (interval between CS onset and first reward) to entries during baseline periods (2-min period prior to CS onset) provided a measure of Pavlovian conditioning.
Instrumental training
Rats were given 11 days of instrumental training, receiving 2 separate training sessions per day, one with the lever to the left of the food-delivery port and one with the right lever. Each action was reinforced with a different outcome, either grain pellets or sucrose solution (counterbalanced with respect to the Pavlovian contingencies). Each session terminated after 30 outcomes had been earned or 30 min had elapsed. Actions were continuously reinforced on the first day, and then escalated to a random-ratio 20 schedule. The rate of responding on each lever was measured throughout training.
Surgery
After training, rats were implanted with guide cannula (22-gauge, 7 mm-length, stainless steel, Plastics One, Roanoke, VA) targeted bilaterally 1 mm above the BLA (AP −3.0 mm, ML ±5.1 mm, V −7.0 mm relative to bregma). Standard aseptic surgical procedures were used under isoflurane anesthesia (5% induction, 1–2% maintenance). The nonsteroidal anti-inflammatory agent Carprofen was administered pre- and post-operatively to minimize pain and discomfort. Following surgery rats were individually housed and allowed to recover for ~5–7 days.
Experiment 1: Pavlovian-to-instrumental transfer
After recovery, rats received 2 retraining sessions for each instrumental association (2 sessions/day for 2 days) and then one Pavlovian retraining session. On the day prior to each PIT test rats were given a single 30-min extinction session during which both levers were available, but pressing was not reinforced to establish a low level of responding. Rats were also given this retraining between each PIT test.
Rats were split into two groups, one (n=20) group receiving bilateral infusions of 0, 0.5, or 1 μg/side of the selective delta-opioid receptor antagonist naltrindole into the BLA and another (n=15) receiving 0, 0.5, or 1 μg/side of the selective mu-opioid receptor antagonist CTOP, immediately prior to the onset of the PIT test. Each rat was given 3 total PIT tests to allow within-subject drug dose comparisons (test order counterbalanced). During each PIT test, both levers were continuously present, but pressing was not reinforced. After 5 min of extinction, each 2-min CS was presented separately 4 times each in pseudorandom order, separated by a fixed 4-min inter-trial interval. No rewards were delivered during CS presentation. The 2-min prior to each CS presentation served as the baseline control period.
Experiment 2: Outcome-specific reinstatement
Following recovery and retraining, each rat was given two reinstatement tests, one each following intra-BLA infusion of CTOP (1 μg/side) or vehicle, with intervening retraining. During each reinstatement test, both levers were continuously present, but pressing was never reinforced. After 5 min of extinction, rewards were presented in 8 separate reward-presentation periods (4 sucrose, 4 pellet periods, in pseudorandom order) separated by a fixed 4-min inter-trial interval. Each reward presentation period was 2-min in duration and began with 2 deliveries of the appropriate reward, separated by 6 s. The 2-min period prior to each reward-delivery period served as the baseline.
Drug administration
Naltrindole (Tocris Bioscience, Sterling Heights, MI) and CTOP (Tocris Bioscience; Sigma-Aldrich, St. Louis, MO) were chosen based on their selective affinities for the delta- and mu-opioid receptor, respectively (Pelton et al., 1986; Portoghese et al., 1988; Hyytia & Kiianmaa, 2001). The dose range for each drug was selected based on relative affinities and on previous research demonstrating an influence on reward-related behavior when infused into the BLA (Hyytia & Kiianmaa, 2001; Wassum et al., 2011; Wassum et al., 2016).
Drugs were dissolved in sterile saline and infused in a volume of 0.5 μl as described previously (Malvaez et al., 2015; Wassum et al., 2016). Previous work in which infusions were made into the adjacent amygdala central nucleus suggests that these infusion parameters restrict diffusion to the BLA (Wassum et al., 2009). Testing commenced within 5 min following infusion.
Data Analysis
Data were processed with Microsoft Excel (Redmond, WA) then analyzed with GraphPad Prism (La Jolla, CA) and SPSS (IBM Corp, Chicago, IL). For all hypothesis tests, the α level for significance was set to P<0.05. Analyses included repeated-measures ANOVAs (Geisser-Greenhouse correction) with Bonferroni and Dunnets post-hoc analyses used to clarify main effects and interactions, post-hoc linear regression, and Bayes factor analysis for use in supporting a null hypothesis (Gallistel, 2009; Rouder et al., 2009).
For both experiments, data were analyzed for the rate of both lever pressing and entries into food-delivery port. All data were averaged across trials. For the results of Experiment 1, lever presses during the baseline period was collapsed across levers because there was no significant effect of Lever (Delta Group: F1,19=1.15, P=0.30; Mu Group: F1,14=0.25, P=0.63), or Lever x Drug dose interaction (Delta Group: F2,38=0.70, P=0.50; Mu Group: F2,28=0.64, P=0.54) on baseline press rate. This baseline pressing was compared to pressing during the CS periods, which was separated by presses on the lever that, during training, earned the same outcome as the cue predicted (i.e., CS-Same presses) versus those on the other available lever (i.e., CS-Different presses). Initial analyses detected no significant effects of either Cue-reward pairing, Lever-reward pairing, or Test order (Delta Group: F’s=0.01–1.16, P’s=0.93–0.34; Mu Group: F’s=0.10–1.00, P’s=0.79–0.57) and no significant interaction between these variables and Drug dose (Delta Group: F’s=0.25–2.06, P’s=0.73–0.17; Mu Group: F’s=0.10–5.06, P=0.80–0.16) on lever pressing during the PIT test, so these variables were not included in the primary analyses presented below. To focus on the selective elevation in responding induced by CS presentation, in an additional analysis a difference score was computed by subtracting the baseline response rate (thereby normalizing for local response tendencies) from lever pressing during the CS period. These data were then compared across action.
The results of Experiment 2 were analyzed similarly, with reward-period presses separated for those on the lever that previously earned the same outcome as the presented reward (i.e., Reinstated presses) versus those on the alternate lever (i.e., Non-reinstated). Baseline response rates did differ slightly between levers during for this experiment (main effect of Lever: F1,8=21.65, P=0.002), but, importantly, this did not differ between drug conditions (no Lever x Drug interaction: F1,8=0.43, P=0.53). During the baseline period, responding was lower on the to-be-reinstated lever than the to-be-non-reinstated lever for both the Vehicle (Non-reinstated baseline: 15.49±4.47 s.e.m.; Reinstated baseline: 12.21±3.64) and CTOP (Non-reinstated baseline: 11.06±2.55; Reinstated baseline: 5.06±1.47) conditions. Again we detected no main effect of Lever-reward pairing or Test order (F’s=0.06–0.13, P’s=0.81–0.74) and no significant interaction between these variables and Drug (F’s=0.11–4.42, P=0.76–0.09) on lever pressing during the reinstatement test and so did not include these variables in the primary analysis.
Histology
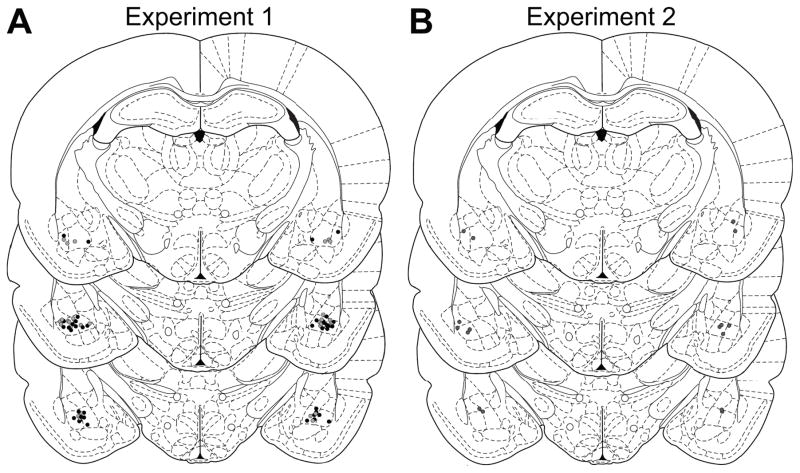
Histological verification of infusion locations was conducted as described previously Wassum et al., 2016) and is presented in Figure 1. Three subjects were removed from Experiment 1 and 4 from Experiment 2 due to cannula misplacement and/or tissue damage.
Figure 1. Histological verification of BLA cannula placements.
Schematic representation of microinfusion injector tips for Experiment 1 (A; black, delta group; gray, mu group) or Experiment 2 (B). Line drawings of each section taken from (Paxinos & Watson, 1998) −2.8 – 3.3 mm posterior from bregma.
RESULTS
Effect of BLA mu- or delta-opioid receptor inactivation on Pavlovian-to-instrumental transfer
Pavlovian conditioning was used to pair each of two distinct auditory stimuli with delivery of one of two unique, but relatively equally valued, food rewards. During the final Pavlovian session rats entered the food-delivery port significantly more during the CS probe period (Delta group: average entry rate 26.16±1.38 s.e.m.; Mu group: 28.31±2.47) than during the baseline period (Delta group: 14.68±0.99; Mu group: 15.32±1.97) and this did not differ between future drug groups (CS: F1,33=152.9, P<0.001; Group: F1,33=0.40, P=0.53; Group x CS: F1,33=0.58, P=0.45). Rats were then trained to instrumentally earn those same food rewards by responding on independent levers. There were also no pre-existing group differences in final average press rate (Delta group: 43.01±2.81; Mu group: 44.20±2.95; t33=0.29, P=0.77).
At the PIT test, both levers were simultaneously present and lever pressing was not rewarded. Each CS was presented 4 times in pseudorandom order (also without accompanying reward), with intervening CS-free, baseline periods. In this test, CS presentation triggers retrieval of a stored memory of the specific predicted reward, which then guides and motivates action performance in the novel choice scenario. Rats were given 3 PIT tests, one each following bilateral intra-BLA infusion of either 0 (vehicle), 0.5, or 1 μg of the selective delta-opioid receptor antagonist naltrindole (Delta group) or the selective mu-opioid receptor antagonist CTOP (Mu group).
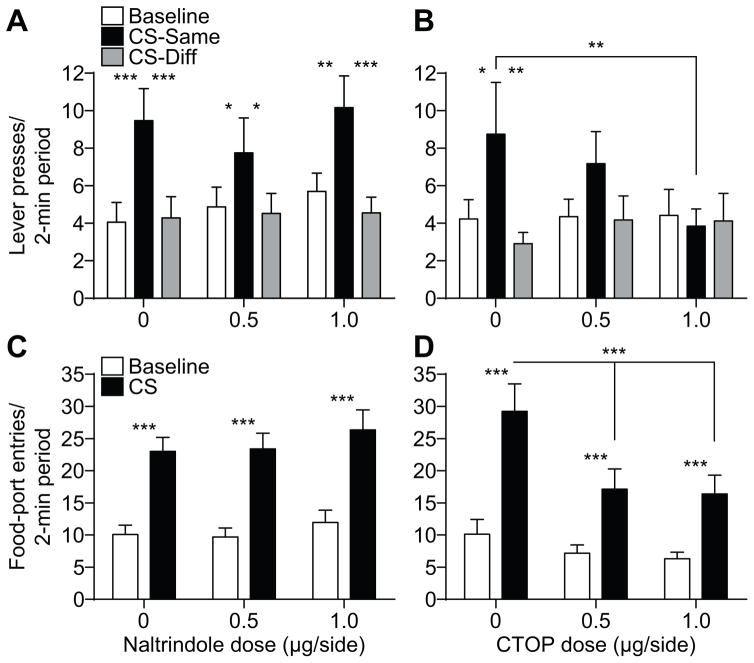
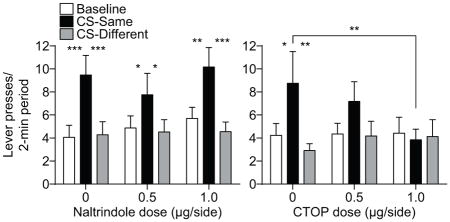
As is clear from Figures 2A and B, we detected differential effects of BLA delta- and mu-opioid receptor blockade on the selective-invigorating influence of the reward-predictive cues over instrumental activity (i.e., expression of outcome-specific PIT). Inactivation of BLA delta-opioid receptors did not significantly alter PIT performance (Figure 2A). ANOVA on these data detected a significant main effect of CS (F2,38=13.68, P<0.0001), with neither an effect of Naltrindole dose (F2,38=0.37, P=0.69), nor a Dose x CS interaction (F4,76=0.76, P=0.55). Corrected post-hoc comparisons revealed that under each drug dose CS presentation elevated press rate selectively on the lever that, in training, earned the same predicted reward (CS-Same) relative to both baseline press rate and pressing during the CS on the alternate available lever (CS-Different; P<0.05-0.001). Blockade of BLA mu-opioid receptors did, however, disrupt expression of outcome-specific PIT (Figure 2B). ANOVA on these data detected a significant effect of CS (F2,28=5.36, P=0.01), no effect of CTOP dose (F2,28=0.39, P=0.68), and a marginally not significant Dose x CS interaction (F4,56=2.14, P=0.09). Robust PIT was demonstrated under vehicle control conditions; the CS elevated performance of the CS-Same action relative to both baseline (P<0.05) and CS-Different responding (P<0.01). This effect was not apparent following intra-BLA CTOP (P>0.05, in all cases) and CS-Same responding was lower following infusion of the high dose of CTOP relative to vehicle control (P<0.01). Indeed, isolated analysis of PIT performance following vehicle v. the high dose of CTOP detected a significant Drug x CS period interaction (F2,28=3.81, P=0.048), with no main effect Drug (F1,14=0.49, P=0.49) and a marginally not significant effect of CS Period (F2,28=2.57, P=0.09). Bayesian analysis further supported the lack of specific PIT expression following the high CTOP dose; the null hypotheses of no difference between CS-Same pressing and either baseline or CS-Different pressing was found to be 3.46 and 3.76 times more likely, respectively, than the alternate hypothesis.
Figure 2. Effect of BLA delta- or mu-opioid receptor inactivation on Pavlovian-to-instrumental transfer.
A, B. Trial-averaged lever presses per 2-min period averaged across both levers during the baseline periods compared to pressing during the CS separated for presses on the lever that, in training, delivered the same outcome as predicted by the CS (CS-Same) and pressing on the other available lever (CS-Diff) for the delta- (A) or mu-opioid receptor antagonist (B) group. C, D. Trial-averaged entries into the food-delivery port during the baseline and CS periods for the delta- (C) or mu-opioid receptor antagonist (D) group. Error bars ±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
Under conditions of either BLA delta- or mu-opioid receptor blockade rats were able to show Pavlovian conditioned food-port approach responding. Entries into the food-delivery port were significantly elevated during the CS relative to the baseline period at all Naltrindole doses (Figure 2C). ANOVA on these data detected a significant main effect of CS (F1,19=89.04; P<0.0001), with neither an effect of Naltrindole dose (F2,38=0.64; P=0.53), nor a Dose x CS interaction (F2,38=0.27; P=0.76). For the Mu group, ANOVA detected a main effect of CS (F1,14=53.49; P<0.0001) on food-port entries, as well as an effect of CTOP dose (F2,28=3.71; P=0.04) and a Dose x CS interaction (F2,28=5.92; P=0.007; Figure 2D). Food-port entries were elevated during the CS relative to the baseline period in all conditions (P<0.001, in all cases), but were lower during the CS following intra-BLA CTOP infusion, relative to vehicle control (P<0.001).
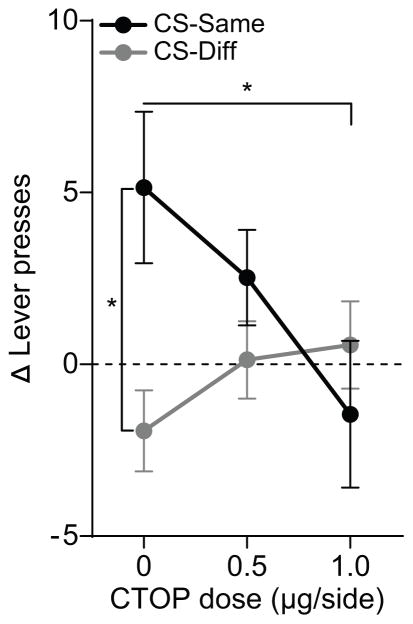
To further clarify the effect of CTOP on the selective elevation of instrumental responding produced by the reward-predictive cues, we computed the CS-induced change in pressing by subtracting baseline press rate from both CS-Same and CS-Different pressing (Figure 3). ANOVA on these data exposed a main effect of Action (Same v. Different: F1,14=4.29, P=0.057), no effect of CTOP dose (F2,28=1.17, P=0.32), but an Action x Dose interaction (F2,28=3.20, P=0.056). CS presentation caused an elevation in responding on Action Same relative to Action Different (P<0.05) following vehicle infusion, but this was blocked by intra-BLA infusion of CTOP at the highest dose (P<0.05). Highlighting the effect of drug dose, there was a significant downward linear trend for the change in Action Same performance (R2=0.12, P=0.02) with increasing CTOP dose, which was not detected for Action Different (R2=0.05, P=0.18).
Figure 3. Effect of BLA mu-opioid receptor inactivation on cue-induced change in lever pressing during Pavlovian-to-instrumental transfer.
CS-induced change (CS – Baseline) in lever pressing on action Same v. Different. Dashed line indicates no change from baseline. Error bars ±s.e.m. *P<0.05.
Effect of BLA mu-opioid receptor inactivation on outcome-specific reinstatement
The data show that blockade of BLA mu-, but not delta-opioid receptors disrupts the ability of a reward-predictive cue to selectively invigorate the performance of actions directed at the same unique reward. This phenomenon requires that the cue is able to retrieve from memory an expectation of its specific predicted reward, information that is currently unobservable. BLA mu-opioid receptor activation may, therefore, participate in this cognitive representation of specific rewards. Conversely, the BLA mu-opioid receptor may simply be needed for a reward, whether observable or not, to motivate action performance. To test between these possibilities, we evaluated the effect of intra-BLA CTOP infusion on outcome-specific reinstatement.
A separate group of rats was trained to instrumentally earn one of two unique, but relatively equally valued food rewards by responding on independent levers (average press rate: 43.26±1.62). During the reinstatement test, both levers were simultaneously present and lever pressing was never rewarded. Each reward was non-contingently presented 4 times in pseudorandom order, with intervening baseline periods. In this task, reward presentation will selectively reinstate performance of the action that earns the same reward. Each rat was tested twice, once following intra-BLA infusion of vehicle and once following infusion of CTOP (1 μg). If BLA mu-opioid receptor activation is selectively required for the motivating influence of cue-elicited expectations of unobservable rewards, then BLA mu-opioid receptor inactivation should have little effect. If however, BLA mu-opioid receptor activation is required for a reward to direct action regardless of its physical presence, then inactivation of this receptor should impair performance.
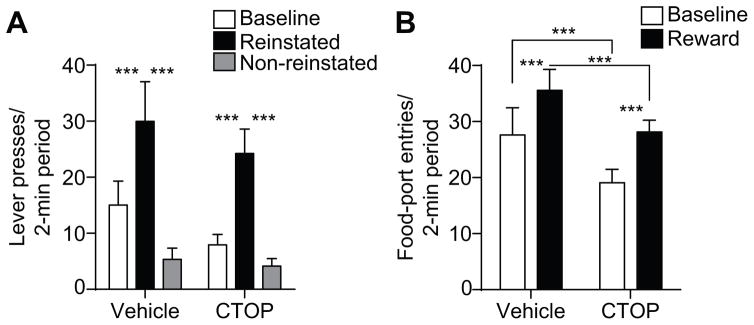
The data provide support for the former. As can be seen in Figure 4A, BLA mu-opioid receptor inactivation was without effect on reinstatement performance. ANOVA on these data detected a significant main effect of Reward delivery (F2,14=16.63, P<0.001), with neither an effect of Drug (F1,7=1.57, P=0.25), nor a Reward x Drug interaction (F2,14=0.94, P=0.41). Following intra-BLA infusion of either vehicle or CTOP reward presentation selectively elevated press rate on the lever that, in training, earned the same unique reward (Reinstated) relative to both baseline press rate and pressing on the alternate available lever (Non-reinstated; P<0.001, in all cases). Entries into the food-delivery port were also elevated by reward delivery under both conditions, though there was an overall attenuation of this behavior following intra-BLA mu-opioid receptor blockade, similar to that detected during the PIT test (Figure 4B). ANOVA on these data detected significant main effects of Reward delivery (F1,7=8.38, P=0.02) and of Drug (F1,7=8.02, P=0.03), with no interaction between these factors (F1,7=0.4, P=0.55). Food-port entries were elevated during the reward period relative to the baseline period in all conditions (P<0.001, in both cases), but lower following intra-BLA CTOP infusion relative to vehicle control (P<0.001, in both cases).
Figure 4. Effect of BLA mu-opioid receptor inactivation on outcome-specific reinstatement.
A. Trial-averaged lever presses per 2-min period averaged across both levers during the baseline periods compared to pressing during the 2-min periods following reward delivery, separated for presses on the lever that, in training, delivered the same outcome as the presented reward (Reinstated) and pressing on the other available lever (Non-reinstated). B. Trial-averaged entries into the food-delivery port during the baseline and reward periods. Error bars ±s.e.m. ***P<0.001.
DISCUSSION
One major source of reward-seeking motivation is the cognitive expectation of specific rewards, information that is often provided by environmental cues. Here we show that endogenous activation of mu-, but not delta-opioid receptors in the BLA is required for a reward-predictive cue to selectively invigorate the performance of actions directed at the same unique predicted reward. Though we note that these effects should be considered in the context on the high variability in PIT performance under control conditions. BLA mu-opioid receptor activation was found not to be required for a reward itself to similarly motivate action. These data reveal a new role for BLA mu-opioid receptor activation in the cued recall of precise reward memories and the use of this information to motivate the execution of specific action plans.
The data demonstrate differential roles for BLA delta- and mu-opioid receptor activation in the expression of outcome-specific PIT. Surprisingly, this was in the opposite direction to that expected based on behaviors observed after globally knocking out these receptors. Delta-opioid receptor knockout mice are unable to show PIT, an effect that has been localized to the nucleus accumbens shell (Laurent et al., 2012), and shown here not to require BLA delta-opioid receptor activity. Conversely, mu-opioid receptor knockout leaves PIT intact (Laurent et al., 2012). The current finding of attenuated PIT following blockade of BLA mu-opioid receptors suggests, therefore, the presence of compensatory mechanisms for this behavior in the mu-knockout mouse, or perhaps differing functions for mu-opioid receptor activation across brain regions.
The BLA is required for the selective motivation of action elicited either by reward-predictive cues (Blundell et al., 2001; Corbit & Balleine, 2005; Ostlund & Balleine, 2008) or by physically present rewards (Ostlund & Balleine, 2008). The data here reveal that BLA mu-opioid receptor activation is only needed for the former. BLA mu-opioid receptor activation was required when the subject had to, upon cue presentation, retrieve a specific reward expectation from memory, information that was previously observed, but was not presently observable, and was not required when this information was fully observable. Disruption of the retrieval of specific reward memories could also explain the slight attenuation of goal-approach responding in both tasks, which may have to a more limited extent been motivated by such information. The lack of an impairment in outcome-specific reinstatement also suggests that BLA mu-opioid receptor activation is not required for rats to access knowledge of the specific consequences of their instrumental actions. It is also unlikely that the BLA or mu-opioid receptor activation therein is required for the decision-making process itself. Were this the case, BLA mu-opioid receptor inactivation would have resulted in a non-specific CS-induced increase in instrumental responding, indicating an inability to select between actions on the basis of the CS-provided specific reward expectation. Instead, BLA mu-opioid receptor activation only attenuated the selective motivating influence of CSs, similar to the effect of BLA inactivation (Corbit & Balleine, 2005; Ostlund & Balleine, 2008; Malvaez et al., 2015).
The BLA is thought to encode motivationally-salient, precise reward memories (Wassum & Izquierdo, 2015). Indeed, neither the BLA (Corbit & Balleine, 2005), nor BLA mu-opioid receptor activation (Mahler & Berridge, 2012) is needed for the expression of the more general form of PIT, in which less precise, more gist-like reward memories can non-discriminately motivate action. Interestingly, BLA mu-opioid receptor activation is also required when the memory of a specific reward is modified to encode a positive shift in value (Wassum et al., 2009; Wassum et al., 2011). Together, these data suggest that BLA mu-opioid receptor activation may regulate access to these specific reward memories, perhaps by modulating GABAergic inputs onto BLA projection cells (Finnegan et al., 2006), thereby altering their response to the incoming glutamate signals shown previously to encode these precise reward memories (Malvaez et al., 2015). This speculation is consistent with the proposed function of the GABAergic, mu-expressing intercalated cells to gate the influence of afferent sensory input over BLA projections (Millhouse, 1986; Likhtik et al., 2008; Asede et al., 2015). Intra-BLA CTOP infusion here likely disrupted activity at both these mu receptors and those expressed, albeit more sparsely, in the BLA itself (Ding et al., 1996; Zhang et al., 2015).
In summary, these findings support a role for BLA mu-opioid receptor activation in use of cue-recalled precise reward expectations to motivate specific action plans. Deficits in this cognitive process have been associated with several psychiatric disorders, including depression, schizophrenia, and drug addiction (Seymour & Dolan, 2008; Hogarth et al., 2013; Morris et al., 2015). These data, therefore, have implications for the understanding and treatment of these and related conditions. They may also help to explain the clinical efficacy of naltrexone, an opioid receptor antagonist with affinity for mu-opioid receptors in humans (Toll et al., 1998) that has been shown to reduce cue-induced urges to use drug in smokers (Hutchison et al., 1999) and alcoholics (Monti et al., 1999; Rohsenow et al., 2000; O’Malley et al., 2002).
Acknowledgments
This research was supported by grant DA035443 from NIDA, a Hellman Foundation Fellowship, and a UCLA Faculty Career Development award to KMW as well as a Dr. Ursula Mandel Scholarship and UCLA Graduate Research Mentorship fellowship to NTL. The authors would like to thank Dr. Alicia Izquierdo for helpful discussions regarding these data.
ABBREVIATIONS
- BLA
basolateral amygdala
- CS
Conditioned stimulus
Footnotes
AUTHOR CONTRIBUTIONS
NTL and KMW designed the research, analyzed, and interpreted the data and wrote the manuscript. NTL conducted the research.
COMPETING INTERESTS
The authors declare no competing financial interests.
References
- Asede D, Bosch D, Lüthi A, Ferraguti F, Ehrlich I. Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron. 2015;86:541–554. doi: 10.1016/j.neuron.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Motzkin DK. Encoding of the unconditioned stimulus in Pavlovian conditioning. Animal Learning & Behavior. 1994;22:384–394. [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Learning and Motivational Processes Contributing to Pavlovian-Instrumental Transfer and Their Neural Bases: Dopamine and Beyond. Curr Top Behav Neurosci. 2015 doi: 10.1007/7854_2015_388. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, Pan HL. Mu opioid receptor activation inhibits GABAergic inputs to basolateral amygdala neurons through Kv1.1/1.2 channels. J Neurophysiol. 2006;95:2032–2041. doi: 10.1152/jn.01004.2005. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The importance of proving the null. Psychol Rev. 2009;116:439–453. doi: 10.1037/a0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Balleine BW, Corbit LH, Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci. 2013;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, Niaura RS, Sirota AD. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl) 1999;142:139–143. doi: 10.1007/s002130050872. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Kruse H, Overmier J, Konz W, Rokke E. Pavlovian conditioned stimulus effects upon instrumental choice behavior are reinforcer specific. Learn Motiv. 1983;14:165–181. [Google Scholar]
- Laurent V, Leung B, Maidment N, Balleine BW. μ- and δ-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J Neurosci. 2012;32:1875–1883. doi: 10.1523/JNEUROSCI.4688-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Morse AK, Balleine BW. The role of opioid processes in reward and decision-making. Br J Pharmacol. 2015;172:449–459. doi: 10.1111/bph.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221:407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Greenfield VY, Wang AS, Yorita AM, Feng L, Linker KE, Monbouquette HG, Wassum KM. Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci Rep. 2015;5:12511. doi: 10.1038/srep12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Morris RW, Quail S, Griffiths KR, Green MJ, Balleine BW. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol Psychiatry. 2015;77:187–195. doi: 10.1016/j.biopsych.2014.06.005. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol. 2000;109:738–742. [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, Maidment NT. Mu opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. Journal of Neuroscience. 2011;31:1583–1599. doi: 10.1523/JNEUROSCI.3102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Greenfield VY, Linker KE, Maidment NT, Ostlund SB. Inflated reward value in early opiate withdrawal. Addict Biol. 2016;21:221–233. doi: 10.1111/adb.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Muller JF, McDonald AJ. Mu opioid receptor localization in the basolateral amygdala: An ultrastructural analysis. Neuroscience. 2015;303:352–363. doi: 10.1016/j.neuroscience.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]