Abstract
The endogenous deoxynucleoside triphosphate (dNTP) pool includes deoxyadenosine triphosphate (dATP), deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP), and thymidine triphosphate (TTP). The endogenous dNTP pool is regulated by complex enzymatic pathways that can be targeted by drugs, such as antimetabolites. In addition, these components compete with antiviral nucleos(t)ide analog triphosphates, contributing to the mechanism of pharmacologic action. This communication describes the development and validation of a sensitive method to quantify the intracellular dNTP pool in cellular lysates. The extraction process was optimized for dNTPs using an indirect strategy—the separation of mono-, di-, and triphosphate moieties by strong anion exchange, dephosphorylation of target fractions to molar equivalent nucleosides—followed by sensitive quantitation using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The validated analytical range was 50 to 2500 fmol/sample for each dNTP. The assay was used to quantify dNTPs in peripheral blood mononuclear cells (PBMC) from forty clinical research participants (n=279 samples). Median (interquartile range) concentrations were 143 (116, 169) for dATP, 737 (605, 887) for dCTP, 237 (200, 290) for dGTP, and 315 (220, 456) for TTP, respectively, in femtomole per million cells. This method allows for future studies of endogenous dNTP disposition in subjects receiving antimetabolites, nucleos(t)ide analogues, or for other clinical scenarios.
Keywords: endogenous dNTP, intracellular, LC-MS/MS, pharmacokinetics, pharmacodynamics
Introduction
The endogenous deoxynucleoside triphosphate (dNTP) pool includes deoxyadenosine triphosphate (dATP), deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP), and thymidine triphosphate (TTP). The endogenous dNTP pool components are the building blocks of DNA, and they play important roles in cell division, proliferation, and differentiation. The balanced concentrations of the four components in the dNTP pool, as well as the overall pool sizes, maintain these essential functions of nucleated cells.
The endogenous dNTP pool is regulated by complex enzymatic pathways that can be targeted by drugs. For example, antimetabolites as chemotherapy agents, affect the enzymes involved in the anabolism of dNTPs, systematically suppressing the dNTP pool and thereby cancer cell proliferation. Fluorouracil is an example of a suicide inhibitor for thymidylate synthase that depletes thymidine nucleotides (Bai, et al. 2015, Wu, et al. 2016). Also, methotrexate competitively inhibits dihydrofolate reductase, suppressing folate and eventually the de novo synthesis of purine and pyrimidine bases (Rajagopalan, et al. 2002), whereas, mercaptopurine interferes with hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and phosphoribosylpyrophosphate (PRPP) amidotransferase, eventually suppressing purine nucleotide biosynthesis (Wojtuszkiewicz, et al. 2014). Given that these drugs target the dNTP pool, the quantitation of these moieties can help inform antimetabolite biological effects.
Additionally, numerous nucleos(t)ide analogs (NA) are marketed as antivirals and these agents compete with dNTPs (due to their similar structures) and inhibit virus genetic material replication. For example, aciclovir, an anti-herpes antiviral, has an active metabolite that competes with deoxyguanosine triphosphate at the binding site of HSV DNA polymerase (Elion 1982). And, tenofovir—a HIV drug used for HIV infection pre-exposure prophylaxis (PrEP) and treatment—competes with deoxyadenosine triphosphate at the HIV reverse transcriptase (RT) active site. If the NA is incorporated this results in HIV-DNA chain termination as these moieties lack the 3′ hydroxyl needed for chain elongation (Mayer, et al. 2015). For these and other nucleos(t)ide analogs, the ratios between nucleos(t)ide analog and the corresponding dNTP define their pharmacologic efficacy (Anderson and Fletcher 2001). Thus, it is important to have methodologies to investigate the dNTP disposition in individuals receiving nucleos(t)ide analogues.
Historically, the quantitation of the dNTP pool presented challenges because of sensitivity needs, chromatography problems, and matrix issues for endogenous compounds. Traditional detectors such as UV (Shewach 1992, Decosterd, et al. 1999) and diode array (Revich, et al. 1984, Rimerman, et al. 1993) are generally not sensitive enough to quantify dNTPs in clinical samples, which contain limited cell numbers. The high polarity and chemical similarities of dNTP (and NTP) require ion-pairing or similar approaches (Cohen, et al. 2009), which in our hands, increase run times, limit sensitivity, and introduce chromatography variability (King, et al. 2006). Here, we report a novel LC-MS/MS quantitative method of each individual dNTP (dATP, dCTP, dGTP, and TTP) from cellular lysate, which circumvents many of these challenges.
Experimental
Chemicals and materials
Chemicals were acquired from Sigma-Aldrich Chemical, St. Louis, MO: A deoxynucleotide set, 10 mM (deoxyadenosine triphosphate: Product#: D6920, MW: 535.15; deoxycytidine triphosphate: Product#: D7045, MW:511.12; deoxyguanosine triphosphate: Product#: D7170, MW: 573.13; and thymidine triphosphate: Product#: T7791, MW: 504.15). Internal standards were purchased from Cambridge Isotope Laboratories, Inc. Andover, MA including 2′-deoxyadenosine-15N5 (dA-IS, Cat#: PR-15211, MW: 256.21), 2′-deoxycytidine-15N3 (dC-IS, Cat#: NLM-3897-0, MW: 230.19), 2′-deoxyguanosine:H2O-15N5 (dG-IS, Cat#: NLM-3899-CA-0, MW: 290.22), and Thymidine-13C10, 15N2 (T-IS, Cat#: CNLM-3902-0, MW: 254.14). Analytical grade reagents were obtained from: Fisher Scientific, Fairlawn, NJ: methanol, 2-propanol, acetic acid, and potassium chloride; Sigma-Aldrich Chemical: alkaline phosphatase; and JT Baker, Phillipsburg, NJ: acetonitrile. Ultrapure (UP) water was prepared in house from deionized water with a Barnstead Nanopure System (Thermo Fisher Scientific, Waltham, MA). Consumables included Waters Sep-Pak Accell Plus QMA Cartridge, 3cc/500 mg (Water Corporation, Milford, MA); Phenomenex Strata-X-CW 33 μm Polymeric Weak Cation Mixed Mode Cartridge 3cc/200mg (Phenomenex, Inc., Torrance, CA); and blood products for the lysed cellular matrix (Bonfils, Denver, CO).
Analytical Approach
Standard and QC stocks were created in a concentration of 50 pmol/μL (stored at −80°C) in ultrapure water from Sigma® Deoxynucleotide Set 10mM (dATP, dCTP, dGTP, and TTP). Purity and potency of the compounds were assessed, as previously described (King, et al. 2006) and were further diluted in 70:30 methanol: water (70% methanol), which was also called the cell lysis solution, to working standards ranging between 50 and 2500 fmol/sample, which were aliquoted and stored at −80°C. QC preparation stocks were also created in ultrapure water at a concentration of 50 pmol/μL, then diluted to concentrations of 150, 600, and 2000 fmol/sample in 70% methanol. Sample was defined as 200 μL in cell lysis solution. These solutions did not include cells because cellular lysate matrix contains endogenous dNTPs, which would interfere with quantitation.
To help qualify analytical runs, this method also included a quality assurance (QA) sample in each run. This was a blank lysed PBMC matrix sample (from Bonfils, Denver, CO) with an aliquot assayed in each run to demonstrate consistent results, similar to an incurred sample reanalysis (ISR). A combined lot of PBMC lysate in 70% methanol was processed resulting in “stock” solution consisting of 10×106 cells/mL. This solution was then aliquoted into one million cell samples and all were stored at −80°C. One aliquot was quantified in each run to assess the assay reproducibility. The average value and %CV were assessed, with acceptance criteria of ≤15%.
For each analytical run, two 70% methanol solutions (as blank samples), standards, QCs, and the QA were extracted. Separation of intracellular monophosphate (MP), diphosphate (DP), and triphosphate (TP) fractions was accomplished using a potassium chloride concentration gradient (MP: 5mL × 75mM KCl, DP: 7mL × 90mM KCl, TP: 2mL × 1M KCl) with Waters QMA Solid Phase Extraction (SPE) cartridges. The triphosphate fraction was then dephosphorylated using excess phosphatase. Both alkaline and acid phosphatase from Sigma® were assessed. Peak area responses from 10, 15, 30, or 60-minute incubations were tested for completion of dephosphorylation. Following dephosphorylation, internal standard (IS) working stock solution (20 μL) was added to all except the blank without IS sample. Samples were desalted and concentrated using Phenomenex Strata-X-CW SPE cartridges, using an extraction process optimized for deoxynucleosides. The Strata-X-CW SPE was prepared with 1 × 2.0mL methanol and 1 × 2.0 mL ultrapure water with 1 min × 200 g centrifugation. Samples were then applied to the cartridge, and centrifuged for 3 min × 100 g. The cartridge was then washed with 2 × 2.0 mL ultrapure water at 1 min × 200 g centrifugation plus a 1 × 0.25 mL methanol addition (this latter step significantly facilitated the drying process). Analytes were then eluted using 3 × 0.5 mL methanol for 1 min × 200 g centrifugation. Samples were dried for 30 minutes under nitrogen at 50°C in a Zymark TurboVap (Zymark Corp., Hopkinton, MA). The sample was reconstituted using 100 μL ultrapure water, vortex mixed, and transferred to a 150 μL low volume insert. 20 μL of reconstituted solution was injected onto the ultrahigh pressure liquid chromatography (UHPLC-MS/MS) system. Figure 1 briefly summarizes the extraction process, and shows the extra quality assurance (QA) sample application.
Figure 1.
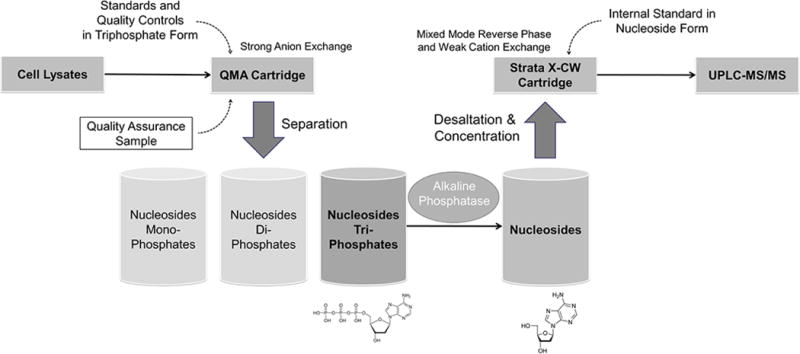
A Thermo Quantum Ultra® triple quadrupole mass spectrometer coupled with a HESI II® probe was used for detection. The Waters Acquity® UHPLCsystem (Waters corporation, Milford, MA) included Binary Solvent Manager (BSM) and Sample Manager (SM). SM used a 250 μL sample syringe, 50 μL sample loop, and data were captured with Xcalibur™ 2.2 SP1.48. A 100 × 2.1 mm Phenomenex Kinetex 2.6μ PFP 100Å analytical column (Phenomenex, Torrance, CA) was used for chromatographic separations. The mobile phase consisted of 2% isopropanol and 0.1% acetic acid in ultrapure water at an isocratic flow of 400 μL/min. The column temperature was 50°C, and the sample temperature was 15°C. Each injection was followed by a strong (70:20:10 Methanol: Water: isopropanol) and weak needle wash (50:50 acetonitrile: water), to eliminate carry over. The source was operated in positive ionization mode. The spray voltage was 3500 V, vaporizer temperature 400°C, sheath gas (nitrogen) 60 arbitrary units, aux gas (nitrogen) 20 arbitrary units, capillary temperature 175°C, chromfilter peak width 6.0 s, collision gas (argon) pressure 1.0 mTorr, T lens (V) were 150, 159, 144, and 173 for dA, dC, dG, and T. Collision energy (V) were 10, 13, 11, and 11 for dA, dC, dG, and T. Resolution was set at 0.7 FWHM for both Q1/Q3, scan width 0.002 m/z, scan time 0.035 s, and centroid data type collected. The run time was three minutes. The SRM precursor/product transitions (m/z) were as follows: dA (252.092/136.100), dA-IS (257.210/141.000), dC (228.112/112.081), dC-IS (231.200/115.100); dG (268.081/152.100), dG-IS (273.102/157.100); and T (243.081/127.085), T-IS (255.081/134.085).
Validation
This method was validated using FDA guidance, with acceptance criteria of ±15% (LLOQ: ±20%) for both accuracy (compared to nominal as % difference (%diff)) and precision (as % coefficient of variation (%CV)) determinations at all concentrations. Accuracy and precision were determined by replicate analysis (n=6) at each QC level described above, plus a QC prepared at the LLOQ level (50 fmol/sample), in three separate analytical runs. Standard curve performance in these runs was also assessed. Matrix effect (ME), recovery (RE), and processing efficiency (PE) were determined following the experiments described by Matuszewski et al (Matuszewski, et al. 2003). Since the biological matrix (cellular lysate) already contained the analytes (endogenous dNTPs), to investigate the ME, RE, and PE of the desaltation and concentration process, we used the internal standards peak area (stable labeled isotopes of each analyte) as previously described by Machon et al (Machon, et al. 2014). Six different lots of PBMC lysate matrix were extracted, and three levels of stable labeled internal standard working solutions were prepared at concentrations of 150, 600, and 2000 fmol/sample. The slopes %CV (calculated from six lots of matrix) of internal standard (dNTP-IS) peak areas from post extraction spike sample (set 2) and extracted sample (set 3) were used for assessment (an analyte:IS ratio was not available).
Minimization of Contamination, Specificity and Selectivity
The endogenous dNTP pool measurement at an ultra-sensitive level (lower limit of quantitation: 50 fmol/sample) requires minimizing the risk of environmental contamination, which is particularly important for method specificity and selectivity. A separate set of glassware and reagents were exclusively used for this assay. All solutions were either freshly made with an expiration day less than 72 hours (e.g. potassium chloride solution), or aliquoted and frozen at −20 °C (e.g. enzyme buffer solution). Consumables such as tubes, pipette tips, transfer tips, and vials were disposed of immediately after use. Gloves were frequently changed during extraction process. Centrifuges that had been shared with other personnel was wiped with deionized water before use.
Specificity was determined by injecting extracted 70% methanol lysate solution and monitoring for dNTPs. The high standard (2500 fmol/sample) with no internal standard and blank with internal standard were used to evaluate cross-talk between dNTP and dNTP internal standard. Carry over was evaluated by assessing signal in a blank water injection following the cross-talk samples. Additionally, blank and blank internal standards were included with each analytical run to monitor for specificity and selectivity.
Conditional Stability
Conditional dNTP stability in 70% methanol was determined by assessing freeze/thaw stability and room temperature stability using triplicate quality control (QC) samples at 150 and 2000 fmol/sample (QL and QH). Freeze thaw stability in three cycles, from −80°C to ambient, was assessed, and room temperature stability was investigated by maintaining triplicate QC samples at room temperature for up to 24 hours prior to extraction. Result mean values were compared to triplicate control sample mean values. Extracted sample stability was determined by assessing six replicates of extracted sample using QL and QH. Samples were tested by maintaining in the auto-sampler (15°C) for five days prior to reinjection. Result mean values were compared to the first day injection data averages. Long term stability was assessed using triplicate QC samples in 300 fmol/sample, which were maintained at −80°C for up to 2 years. Result mean values were compared to triplicate fresh extracted control sample averages.
Conditional dNTP stability in water was determined for prep stock solutions. Freeze/thaw (3 cycles) stability, room temperature stability up to 24 hours were assessed. Dilution were made prior to analysis, and result averages from triplicated extracted samples were compared to the nominal value (2000 fmol/sample).
Conditional dNTP stability in biological matrix was assessed using the quality assurance sample (QA, one combined lot of PBMC lysate stored at −80°C). Freeze/thaw (3 cycles) stability, room temperature stability at a longer time period up to 9 days, and long term stability (−80°C) up to 2 years were evaluated. Triplicate treated sample averages were compared to mean values from 22 separate analytical runs (see section 3.5).
Results from conditional stability were assessed with the acceptance criteria of ≤15%.
Method Application
This method was used to determine repeated measurements of the deoxynucleoside triphosphate pool in peripheral blood mononuclear cell lysates from 40 subjects (n=279 total samples). The clinical protocol was approved by the institutional review broad (IRB) of the University of Colorado, and participants provided informed consent. Among these subjects, 19 were infected with HIV, 21 were HIV-negative. Participants were receiving daily co-formulated 300 mg Tenofovir disoproxil fumarate (TDF) and 200 mg Emtricitabine (FTC), plus daily 600 mg Efavirenz in HIV-infected group, as part of a pharmacokinetic study. Typically, 1.5 million cells were assayed.
Results
Accuracy and Precision
Standard curves were best fitted by quadratic regression (y=ax2+bx+c) with 1/concentration weighting, and ranged from 50 to 2500 fmol/sample. Standard performance is shown in table 1a. Accuracy was within ±13.0% and precision ≤ 14.3% for back-calculated standards. The calibration curve r2 were ≥0.9965.
Table 1a.
Accuracy and precision of calibration standards from n=3 analytical runs.
| Nominal Value (fmol/sample) | 50 | 100 | 250 | 500 | 750 | 1000 | 1500 | 2500 | |
|---|---|---|---|---|---|---|---|---|---|
| dATP | %CV | 5.0 | 6.0 | 10.2 | 2.9 | 5.8 | 3.1 | 2.0 | 0.7 |
| %dev | 5.9 | −5.2 | −2.9 | −0.4 | −2.2 | 3.5 | −0.3 | −0.2 | |
|
| |||||||||
| dCTP | %CV | 4.7 | 10.5 | 7.9 | 3.0 | 8.2 | 5.3 | 3.0 | 1.0 |
| %dev | 5.3 | −0.6 | −5.3 | −1.7 | −1.0 | 3.0 | 0.6 | −0.5 | |
|
| |||||||||
| dGTP | %CV | 3.8 | 14.3 | 9.2 | 4.6 | 7.0 | 2.0 | 3.2 | 0.8 |
| %dev | 4.7 | 0.1 | −3.9 | −1.7 | −4.1 | 4.3 | 1.1 | −0.6 | |
|
| |||||||||
| TTP | %CV | 7.0 | 7.5 | 3.2 | 1.6 | 5.6 | 6.3 | 1.5 | 0.2 |
| %dev | 12.0 | −13.0 | −2.2 | −4.6 | −0.5 | 3.6 | 1.0 | −0.6 | |
Intra- and inter-assay accuracy and precision based on the QCs are shown in table 1b. Intra-assay accuracy was within ±12.3% and precision within 15.2% for LLOQ, and accuracy within ±8.6% and precision within 11.1% for other QCs. Inter-assay accuracy was within ±9.3% and precision within 13.2% for all QC levels, including the LLOQ.
Table 1b.
Inter- and intra-assay accuracy and precision of quality control samples prepared at known concentrations.
| Nominal Value (fmol/sample) | Inter-assay Statistics, n=18 | Intra-assay Statistics (min, max), n=3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 | 150 | 600 | 2000 | 50 | 150 | 600 | 2000 | ||
| dATP | %CV | 8.1 | 7.2 | 5.4 | 2.7 | 2.9, 7.4 | 5.0, 6.4 | 2.5, 4.5 | 2.1, 3.4 |
| %dev | 5.5 | −2.1 | 3.6 | 0.7 | −2.5, 12.4 | −8.0, 3.4 | −2.0, 7.4 | −0.7, 1.8 | |
|
| |||||||||
| dCTP | %CV | 13.2 | 9.0 | 5.5 | 2.2 | 6.9, 15.2 | 4.8, 10.4 | 4.1, 5.9 | 1.1, 1.9 |
| %dev | 1.0 | −2.6 | 0.9 | 1.9 | −7.9, 3.4 | −6.2, 3.8 | −3.2, 2.9 | −0.2, 4.1 | |
|
| |||||||||
| dGTP | %CV | 7.8 | 7.8 | 6.9 | 2.9 | 7.0, 7.9 | 2.2, 8.6 | 3.0, 6.2 | 2.3, 3.9 |
| %dev | 9.3 | −0.3 | 2.6 | −1.3 | 4.0, 12.3 | −8.2, 8.6 | −4.8, 6.9 | −2.3, −0.3 | |
|
| |||||||||
| TTP | %CV | 13.0 | 11.2 | 5.9 | 4.3 | 5.4, 16.1 | 9.1, 11.1 | 4.5, 5.9 | 2.4, 4.1 |
| %dev | 4.2 | −1.3 | 3.1 | 2.2 | −0.5, 12.8 | −6.3, 6.9 | −0.8, 7.4 | −1.2, 5.9 | |
Matrix Effect, Recovery, and Process Efficiency
The recovery of the endogenous deoxynucleoside triphosphate moiety off the Waters QMA SPE were previously determined to be ≥95.2%(Bushman, et al. 2011). Acid phosphatase from Sigma® was assessed and found to be containing deoxynucleosides, therefore, alkaline phosphatase was adopted to dephosphorylate the nucleotide fractions. Peak area responses were equivalent during incubation ranging from 10 to 60 minutes, demonstrating complete dephosphorylation. A 15-minute incubation was adopted.
Matrix effect, recovery, and process efficiency are detailed in table 2. The slopes %CV (n=6) from post extraction spike samples (set 2) were 2.3% for dATP, 3.9% for dGTP, 3.9% for TTP, and 4.1% for dCTP; while from extracted samples (set 3) were 2.9% for dATP, 3.8% for dGTP, 3.8% for TTP, and 49.3% for dCTP. The %CV was high in dCTP set 3 samples but not in set 2 samples, which was because the IS peak areas were used instead of analyte/IS ratios (see validation section). The dCTP recovery is lowered by the addition of the 1 × 0.25 mL methanol wash prior to elution to facilitate drying process in Strata-X-CW process (see section 2.2). The variation in the dCTP recovery caused high %CV in slopes for set 3 samples, however, in practice, the analyte/IS ratio will correct for the variability of recovery.
Table 2.
Metrix effect (ME), recovery (RE), and process efficiency (PE).
| % | dATP | dCTP | dGTP | TTP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Nominal Value (fmol/sample) | ME | RE | PE | ME | RE | PE | ME | RE | PE | ME | RE | PE |
| 150 | 104.1 | 98.8 | 102.8 | 64.3 | 53.1 | 34.2 | 101.7 | 97.7 | 99.3 | 67.8 | 100.4 | 68.1 |
| 600 | 102.9 | 97.1 | 99.9 | 62.4 | 48.9 | 30.5 | 104.3 | 103.9 | 108.5 | 63.8 | 97.5 | 62.2 |
| 2000 | 101.7 | 96.2 | 97.8 | 65.3 | 46.0 | 30.1 | 104.4 | 98.5 | 102.9 | 67.8 | 96.1 | 65.1 |
| Mean | 102.9 | 97.4 | 100.2 | 64.0 | 49.4 | 31.6 | 103.5 | 100.1 | 103.5 | 66.5 | 98.0 | 65.1 |
Data shown are averages from six matrix lots
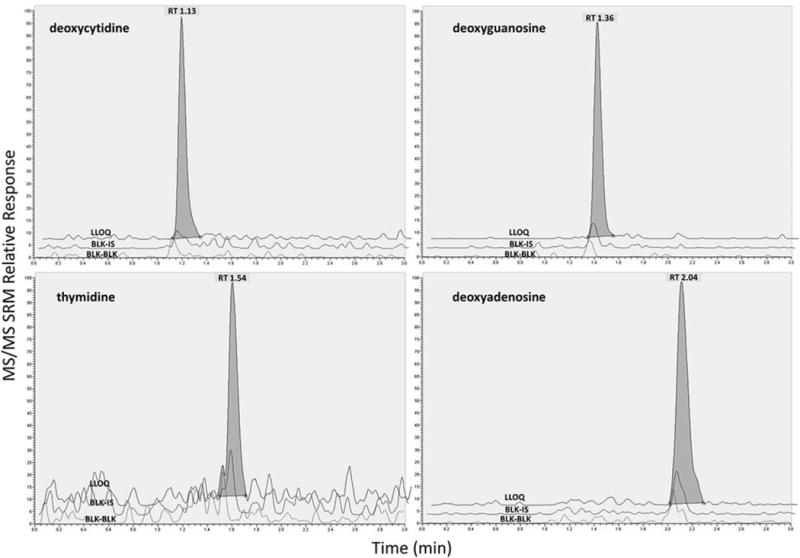
Specificity and Selectivity
The overlay chromatographs of blank, blank and internal standard, and LLOQ sample is shown in figure 2. No more than 20% (IS: 5%) of the LLOQ level (50 fmol/sample) was found in the blank lysate solution matrix samples (≤12%), nor was any significant cross-talk or carry over observed for any dNTP or the internal standard (≤0.94%), showing the method to be specific and selective for dNTPs and dNTPs internal standard.
Figure 2.
Analyte Stability
The stability of dNTP pool components in 70% methanol, water, and cellular lysate is shown in table 3. Treated samples were within ±13.2% of control for all tested conditions, except for dGTP QL samples after 24-hour room temperature treatment (−18.5% of control), suggesting QC samples require freezer storage. The dNTP room temperature stabilities were up to 6 hours for QCs in 70% methanol, 24 hours for prep stocks in water, and 9 days for QA samples (cellular lysate). Both QC and QA samples were stable for up to 2 years at −80C.
Table 3.
Accuracy of samples evaluated for conditional stability.
| %difference | dATP | dCTP | dGTP | TTP | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| QC in 70% Methanol | QL | QH | QL | QH | QL | QH | QL | QH | |
| Freeze/Thaw | 3 cycles | −5.8 | −4.4 | 1.3 | −2.9 | −3.5 | −4.6 | −13.2 | −5.0 |
| Room Temp. | 3 hours | −3.4 | −5.8 | −9.6 | −5.4 | 6.0 | −2.7 | −4.9 | 5.5 |
| Room Temp. | 6 hours | −5.3 | −4.9 | −5.9 | −5.1 | −2.1 | −0.1 | −1.1 | 8.1 |
| Room Temp. | 24 hours | −12.2 | −2.7 | −9.7 | −5.5 | −18.5 | −9.0 | −13.4 | −5.7 |
| Autosampler | 5 days | 3.6 | 2.7 | −6.9 | −4.9 | −7.2 | −4.7 | −0.1 | −2.0 |
| Long Term* | 2 years | 1.4 | −0.7 | 0.9 | 6.1 | ||||
|
| |||||||||
| Prep. Stocks in Water | Compared to nominal value (2000 fmol/sample) | ||||||||
| Freeze/Thaw | 3 cycles | 8.8 | −2.0 | 0.4 | 5.3 | ||||
| Room Temp. | 24 hours | 4.7 | −3.5 | 0.2 | 4.7 | ||||
|
| |||||||||
| QA Sample: PBMC Lysate | Compared to fresh extracted control sample | ||||||||
| Freeze/Thaw | 3 cycles | −7.7 | −8.8 | −10.0 | −8.5 | ||||
| Room Temp. | 24 hours | −1.3 | 1.2 | −4.2 | −6.8 | ||||
| Room Temp. | 3 days | 2.0 | 2.7 | −4.1 | −3.1 | ||||
| Room Temp. | 6 days | 3.9 | 6.2 | −2.2 | −0.1 | ||||
| Room Temp. | 9 days | 2.6 | 4.9 | 0.0 | −1.1 | ||||
| Long Term | 2 years | −4.0 | 2.2 | −4.3 | 11.6 | ||||
Compared to nominal value (300 fmol/sample)
Clinical Application
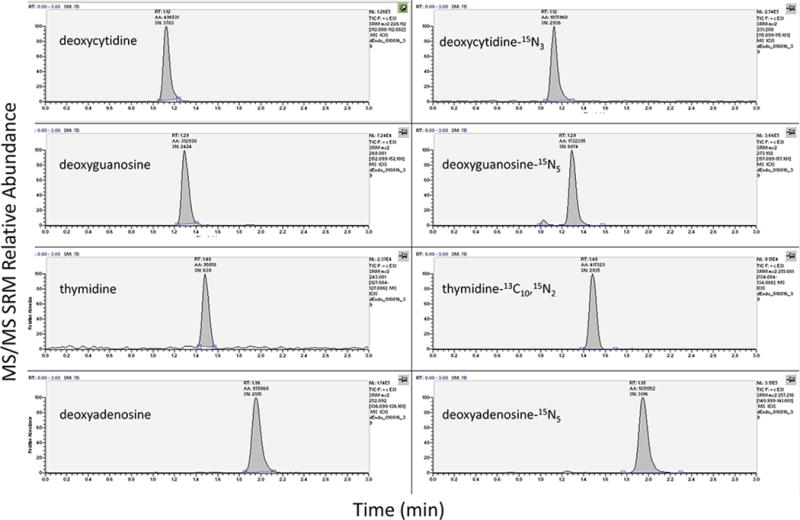
Median (interquartile range) concentrations in peripheral blood mononuclear cells from study participants were 143 (116, 169) for dATP, 737 (605, 887) for dCTP, 237 (200, 290) for dGTP, and 315 (220, 456) for TTP, respectively, in femtomole per million cells (see figure 3). Typical chromatographs for a clinical research sample is shown in figure 4.
Figure 3.
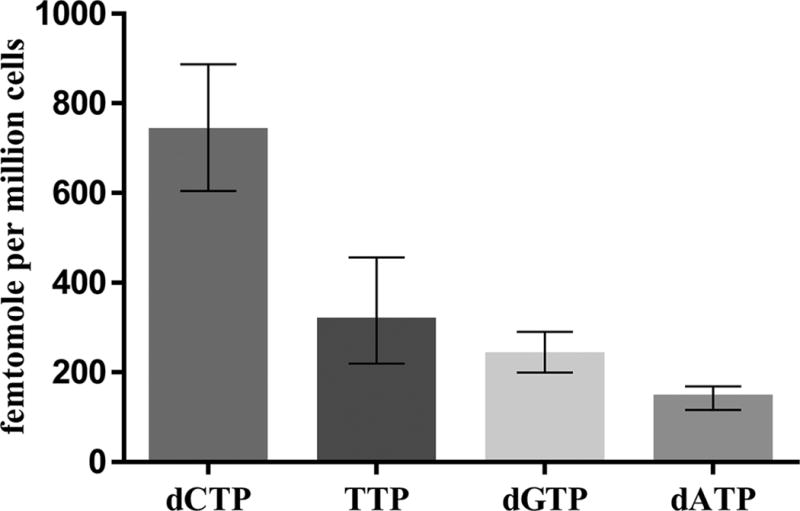
Figure 4.
For the QA sample, for which one million cells were extracted from a single PBMC pool, a total of 22 separate analytical runs were analyzed. The average level of dATP, dCTP, dGTP, and TTP in femtomole per million cells was 270, 313, 152, and 198, and the %CVs were 7.67, 5.15, 9.17, and 8.84.
Discussion
Currently, most quantitative methodologies of the dNTP pool adopted a traditional strategy: measurement of the target analytes directly using ion-pairing (Cohen, et al. 2009, Decosterd, et al. 1999, Wu, et al. 2015), anion exchange (Revich, et al. 1984, Rimerman, et al. 1993, Shewach 1992) chromatographic techniques, or periodate oxidation procedure (to remove NTP) (Hennere, et al. 2003, Shewach 1992) for separation. Our experiences with ion-pairing technologies led to carryover issues likely from residual ion-pairing binding on the system (King, et al. 2006). Thus, we took a different strategy using an indirect approach: first, we separated mono-, di-, and triphosphate moieties using solid phase extraction; next, by collecting and dephosphorylating the triphosphate fractions, we acquired molar equivalent deoxynucleosides; then, another solid phase extraction was optimized exclusively for deoxynucleosides; last, a sensitive liquid chromatography-tandem mass spectrometry quantitative method was developed for these deoxynucleosides. This method requires a longer extraction process, however, compared to other methodologies that measure triphosphates directly, an indirect strategy has several advantages. We were able to simplify the liquid chromatography by separating deoxynucleosides, facilitating a faster, sharper, and cleaner chromatography with isocratic mobile phase. We were able to achieve excellent sensitivity represented by lower LLOQ (50 fmol/sample), compared with other LC-MS/MS methodologies: LLOQ=0.25 pmol/sample reported by Cohen et al. (Cohen, et al. 2009), and LLOQ=0.3–0.4 pmol/sample reported by Hennere et al (Hennere, et al. 2003). Potential interferences were minimized from similar compounds such as dGTP and ATP (and nucleoside analog drugs such as zidovudine-triphosphate), which have the same molecular weight and major transition product (King, et al. 2006). This approach is also able to quantify monophosphates and diphosphates with similar advantages. This indirect quantitative method can be used for cellular lysate samples of different cell types, as previously described (Bushman, et al. 2011).
For an endogenous compound analytical method, the development and validation require special considerations, such as how to handle the biological matrix and minimize the environmental contamination. In this case, biological matrix (cellular lysates) contains the endogenous dNTP pool, so it cannot be used for matrix effect assessment or quality control sample preparation. For this reason, we used lysis solution as the assay matrix and evaluated peak areas of stable labeled internal standards to assess matrix effects (Machon, et al. 2014). To further assess the overall extraction performance, we introduced a quality assurance sample (an aliquot from one combined lot of PBMC lysate). These samples assessed performance of dNTP standards and QCs in every run. Finally, environmental contamination was a problem that may affect the assay accuracy and precision, introducing systematic bias, particularly at the lower concentrations. Multiple procedural steps were used in this method to prevent contamination.
The assay was used to quantify the dNTP pool in peripheral blood mononuclear cell lysate from study participants. Median (interquartile range) concentrations were 143 (116, 169) for dATP, 739 (606, 889) for dCTP, 238 (201, 291) for dGTP, and 315 (220, 458) for TTP, respectively, in femtomole per million cells. These results generally agree with previous with results from Hawkins et al who reported a median (interquartile range) concentration in femtomole per million cells of 154 (10, 474) for dATP, and 111 (53, 359) for dGTP, in HIV-infected patients (Hawkins, et al. 2011). Other studies reported higher concentrations such as Hennere et al. who reported a TTP level in femtomole per million cells range from 1800 to 6700, and dCTP from 550 to 3970 in patients receiving d4T, AZT, and 3TC (Hennere, et al. 2003), as well as Goicoechea et al who reported medians range 3238–4638 for dATP and 2464–4026 for dGTP in femtomole per million cells (Goicoechea, et al. 2010). The reasons for these differences might due to the analytical methodologies, underscoring the need for additional research in this area.
On the benchtop, the dGTP quality control did not pass at 24 hours. This sample type was prepared in 70% methanol suggesting that pure triphosphate solutions may degrade at room temperature. Importantly, the QA sample, which represents a biological cell lysate in 70% methanol, was stable at room temperature for 9 days, suggesting more robust stability in the biological matrix. Both sample types were stable long term at −80°C.
Conclusion
In conclusion, the quantitation of dNTPs is challenging. The described quantitative method addressed many of these challenges and was used to quantify deoxycytidine triphosphate, deoxyguanosine triphosphate, thymidine triphosphate, and deoxyadenosine triphosphate concentrations in cellular lysates from clinical research individuals. This method will be highly useful for in vivo studies of the endogenous dNTP pool disposition, such as in subjects who receive antimetabolites or nucleos(t)ide analogues.
Acknowledgments
This study was supported by NIH U01 AI84735 and U01 AI106499, NIH/NCATS Colorado CTSA UL1 TR001082, and Colorado Clinical Translational Sciences Institute (CCTSI) TL1 TR001081. We would also like to thank all of the participants in the study. Gilead Sciences provided study drug for clinical participants.
References
- Anderson PL, Fletcher CV. Clinical Pharmacologic Considerations for HIV-1 Protease Inhibitors. Curr Infect Dis Rep. 2001;3(4):381–387. doi: 10.1007/s11908-001-0079-3. [DOI] [PubMed] [Google Scholar]
- Bai W, Wu Y, Zhang P, Xi Y. Correlations between expression levels of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase, and efficacy of 5-fluorouracil-based chemotherapy for advanced colorectal cancer. Int J Clin Exp Pathol. 2015;8(10):12333–45. [PMC free article] [PubMed] [Google Scholar]
- Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng JH, Ray ML, Anderson PL. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56(2):390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Megherbi M, Jordheim LP, Lefebvre I, Perigaud C, Dumontet C, Guitton J. Simultaneous analysis of eight nucleoside triphosphates in cell lines by liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(30):3831–40. doi: 10.1016/j.jchromb.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Decosterd LA, Cottin E, Chen X, Lejeune F, Mirimanoff RO, Biollaz J, Coucke PA. Simultaneous determination of deoxyribonucleoside in the presence of ribonucleoside triphosphates in human carcinoma cells by high-performance liquid chromatography. Anal Biochem. 1999;270(1):59–68. doi: 10.1006/abio.1999.4066. [DOI] [PubMed] [Google Scholar]
- Elion GB. Mechanism of action and selectivity of acyclovir. Am J Med. 1982;73(1A):7–13. doi: 10.1016/0002-9343(82)90055-9. [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Jain S, Bi L, Kemper C, Daar ES, Diamond C, Ha B, Flaherty J, Sun S, Richman D, Louie S, Haubrich R, California Collaborative Treatment G Abacavir and tenofovir disoproxil fumarate co-administration results in a nonadditive antiviral effect in HIV-1-infected patients. AIDS. 2010;24(5):707–16. doi: 10.1097/QAD.0b013e32833676eb. [DOI] [PubMed] [Google Scholar]
- Hawkins T, Veikley W, Durand-Gasselin L, Babusis D, Reddy YS, Flaherty JF, Ray AS. Intracellular nucleotide levels during coadministration of tenofovir disoproxil fumarate and didanosine in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55(4):1549–55. doi: 10.1128/AAC.00910-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennere G, Becher F, Pruvost A, Goujard C, Grassi J, Benech H. Liquid chromatography-tandem mass spectrometry assays for intracellular deoxyribonucleotide triphosphate competitors of nucleoside antiretrovirals. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789(2):273–81. doi: 10.1016/s1570-0232(03)00099-0. [DOI] [PubMed] [Google Scholar]
- King T, Bushman L, Anderson PL, Delahunty T, Ray M, Fletcher CV. Quantitation of zidovudine triphosphate concentrations from human peripheral blood mononuclear cells by anion exchange solid phase extraction and liquid chromatography-tandem mass spectroscopy; an indirect quantitation methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831(1–2):248–57. doi: 10.1016/j.jchromb.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Machon C, Jordheim LP, Puy JY, Lefebvre I, Dumontet C, Guitton J. Fully validated assay for the quantification of endogenous nucleoside mono- and triphosphates using online extraction coupled with liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(12):2925–41. doi: 10.1007/s00216-014-7711-1. [DOI] [PubMed] [Google Scholar]
- Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Analytical Chemistry. 2003;75(13):3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Hosek S, Cohen S, Liu A, Pickett J, Warren M, Krakower D, Grant R. Antiretroviral pre-exposure prophylaxis implementation in the United States: a work in progress. J Int AIDS Soc. 2015;18(4 Suppl 3):19980. doi: 10.7448/IAS.18.4.19980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan PT, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci U S A. 2002;99(21):13481–6. doi: 10.1073/pnas.172501499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revich GG, Hillebrand GG, Beattie KL. High-performance liquid chromatographic purification of deoxynucleoside 5′-triphosphates and their use in a sensitive electrophoretic assay of misincorporation during DNA synthesis. J Chromatogr. 1984;317:283–300. doi: 10.1016/s0021-9673(01)91667-x. [DOI] [PubMed] [Google Scholar]
- Rimerman RA, Prorok GD, Cordel KL, Shahwan AM, Vaughan WP. Improved high-performance liquid chromatographic analysis of intracellular deoxyribonucleoside triphosphate levels. J Chromatogr. 1993;619(1):29–35. doi: 10.1016/0378-4347(93)80443-8. [DOI] [PubMed] [Google Scholar]
- Shewach DS. Quantitation of deoxyribonucleoside 5′-triphosphates by a sequential boronate and anion-exchange high-pressure liquid chromatographic procedure. Anal Biochem. 1992;206(1):178–82. doi: 10.1016/s0003-2697(05)80030-2. [DOI] [PubMed] [Google Scholar]
- Wojtuszkiewicz A, Barcelos A, Dubbelman B, De Abreu R, Brouwer C, Bokkerink JP, de Haas V, de Groot-Kruseman H, Jansen G, Kaspers GL, Cloos J, Peters GJ. Assessment of mercaptopurine (6MP) metabolites and 6MP metabolic key-enzymes in childhood acute lymphoblastic leukemia. Nucleosides Nucleotides Nucleic Acids. 2014;33(4–6):422–33. doi: 10.1080/15257770.2014.904519. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang Y, Wiegand R, Wang J, Bepler G, Li J. Quantitative analysis of intracellular nucleoside triphosphates and other polar metabolites using ion pair reversed-phase liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1006:167–78. doi: 10.1016/j.jchromb.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Lai YH, Yang YC, Wu WC, Hong SJ. 5-Fluorouracil-Induced Apoptosis Changes in Cultured Corneal Epithelial Cells. J Ocul Pharmacol Ther. 2016;32(3):155–62. doi: 10.1089/jop.2015.0109. [DOI] [PubMed] [Google Scholar]


