Abstract
Mucopolysaccharidosis IVA (MPS IVA; Morquio A: OMIM 253000) is a lysosomal storage disease with an autosomal recessive trait caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase. Deficiency of this enzyme leads to accumulation of specific glycosaminoglycans (GAGs): chondroitin-6-sulfate (C6S) and keratan sulfate (KS). C6S and KS are mainly produced in the cartilage. Therefore, the undegraded substrates are stored primarily in cartilage and in its extracellular matrix (ECM), leading to a direct impact on cartilage and bone development, and successive systemic skeletal dysplasia. Chondrogenesis, the earliest phase of skeletal formation, is maintained by cellular interactions with the ECM, growth and differentiation factors, signaling pathways, and transcription factors in a temporal-spatial manner. In patients with MPS IVA, the cartilage is disrupted at birth as a consequence of abnormal chondrogenesis and/or endochondral ossification. The unique skeletal features are distinguished by a disproportional short stature, odontoid hypoplasia, spinal cord compression, tracheal obstruction, pectus carinatum, kyphoscoliosis, platyspondyly, coxa valga, genu valgum, waddling gait, and laxity of joints. In spite of many descriptions of these unique clinical features, delay of diagnosis still happens. The pathogenesis and treatment of systemic skeletal dysplasia in MPS IVA remains an unmet challenge. In this review article, we comprehensively describe historical aspect, property of GAGs, diagnosis, screening, pathogenesis, and current and future therapies of MPS IVA.
Keywords: Mucopolysaccharidosis IVA, Keratan sulfate, Chondroitin-6-sulfate, Skeletal dysplasia, N-acetylgalactosamine-6-sulfate sulfatase
1. Introduction: historical aspect of mucopolysaccharidosis IVA
Mucopolysaccharidosis IVA (MPS IVA, Morquio A syndrome; OMIM 253000) is an autosomal recessive disorder caused by deficiency of a lysosomal enzyme, N-acetylgalactosamine-6-suIfate sulfatase (GALNS). This enzyme deficiency leads to accumulation of glycosaminoglycans (GAGs), chondroitin-6-sulfate (C6S) and keratan sulfate (KS), in the body.
In 1929, L. Morquio, a pediatrician in Uruguay, first reported four Swedish siblings with unique skeletal dysplasia, so-called, Morquio or Morquio-Brailsford syndrome [1]. The clinical features included prominent forehead, abnormal face with a large mandible, short neck, pectus carinatum, flaring of the rib cage, genu valgum, hypermobile joints, disproportionately short-trunk dwarfism, and pes planus, as described by Morquio; however, corneal clouding and aortic valve disease were not documented [1]. In the same year, J.F. Brailsford, a radiologist in England, also described a patient with similar skeletal manifestations [2]. In 1962, Pedrini et al. reported urinary excretion of KS in three patients with Morquio syndrome, showing that this metabolic disorder differs from Hurler syndrome [3]. McKusick et al. classified Morquio to MPS IVA [4]. In 1971, Orii et al. reported a milder (intermediate) form of MPS IVA [5]. At 5 months of age, adduction of both thumbs was noticed, and at 18 months of age, a chest abnormality was recognized. At the age of 3 years, kyphosis was present and the patient had an abnormal gait by the age of 5 years old. He had keratosulfaturia and was diagnosed enzymatically as MPS IVA at age 15. At the age of 18 years, the patient was 135 cm tall and manifested the milder skeletal deformities like pectus carinatum, genu valgum, hypermobile joints, and corneal clouding.
In 1981, Orii et al. described a very mild form of MPS IVA in two siblings [6]. Their initial symptom was hip joint pain at the age of 8 years, and both underwent femoral osteotomy at the age 13 years. Both cases had keratosulfaturia, corneal clouding and mild thorax changes, but they did not have the pectus carinatum, genu valgum, excessive joint laxity, or facial changes that characterize the severe forms of MPS IVA. Initial radiographic studies on both cases revealed mild platyspondyly, slight anterior wedging of the lumbar vertebra, and minimal odontoid hypoplasia, as well as subtle capital femoral epiphyses. However, when the two brothers were aged 18 and 22, the ossified femoral heads had disappeared with erosion and widening of the femoral necks. These signs were more marked in the older brother. These siblings were not diagnosed as MPS IVA until they were aged 25 and 29 years, respectively, and their final heights were 147 and 157 cm. Their clinical status is stable now that they are 52 and 57 years old. GALNS activity in patient fibroblasts was about 10% of that seen in cells from normal healthy controls [7] (Table 1).
Table 1.
Historical aspect of Morquio A syndrome.
| Year | Description | Reference |
|---|---|---|
| 1929 | First description of Morquio A patients in Uruguay and England |
Brailsford, 1929; Morquio, 1929 |
| 1962 | Isolation and identification of Keratosulfaturia |
Pedrini et al., 1962 |
| 1971 | Intermediate type of Morquio A patient | Orii et al., 1971 |
| 1976 | Identification of defective enzyme in Morquio A |
Dorfman et al., 1976 |
| 1978 | Assay method of N-acetyl-galactosamine-6-sulfate sulfatase |
Glossl and Kresse, 1978 |
| 1978 | Defect of galactosamine-6-sulfate sulfatase |
Di Ferrante et al., 1978 |
| 1981 | Mild type of Morquio A patients | Orii et al., 1981 |
| 1991 | Purification of GALNS and preparation of the antibody |
Bielicki and Hopwood, 1991; Masue et al., 1991 |
| 1991 | Cloning of human cDNA | Tomatsu et al., 1991 |
| 1992 | First identification of mutations in classical and mild types |
Fukuda et al., 1992 |
| 1992 | Chromosomal localization | Tomatsu et al., 1992 |
| 1994 | Cloning of human genomic gene | Nakashima et al., 1994 |
| 1995 | Common mutation | Tomatsu et al., 1995a |
| 1998 | Establishment of International Morquio Registry |
Montano et al., 2007 |
| 2000 | Cloning of mouse gene | Montano et al., 2000 |
| 2000 | Tertiary structure of GALNS | Sukegawa et al., 2000 |
| 2001 | Retroviral gene therapy in vitro | Toietta et al., 2001 |
| 2003 | Morquio A murine model | Tomatsu et al., 2003 |
| 2004 | KS assay by ELISA | Tomatsu et al., 2004 |
| 2005 | Educational CD for Morquio | Carol Ann Foundation |
| 2005 | Mutation update | Tomatsu et al., 2005 |
| 2007 | Recombinant human GALNS produced in CHO |
Tomatsu et al., 2007 |
| 2007 | KS assay by tandem mass spectrometry | Oguma et al., 2007 |
| 2007 | Description of International Morquio Registry |
Montano et al., 2007 |
| 2008 | ERT on adult Morquio A KO mouse | Tomatsu et al., 2008 |
| 2008 | Growth chart of Morquio A patients | Montano et al., 2008 |
| 2010 | ERT with bone-targeting on Morquio A mouse |
Tomatsu et al., 2010 |
| 2010 | AAV gene therapy in vivo | Almeciga-Diaz et al., 2010 |
| 2013 | Autopsied case report | Yasuda et al., 2013 |
| 2014 | Approval of enzyme replacement therapy in human |
Hendriksz et al., 2014 |
| 2014 | Case report of successful hematopietic stem cell therapy |
Chinen et al., 2014 |
| 2014 | C6S assay by tandem mass spectrometry | Shimada et al., 2014 |
| 2015 | Assessment of tracheal obstruction | Tomatsu et al., 2015 |
| 2016 | Successful surgical intervention for tracheal obstruction |
Pizarro et al., 2016 |
Thus, MPS IVA varies from severe systemic bone dysplasia to a lesser form of the disease that includes only mild bone dysplasia [5–10]. Patients with the severe form of the disease have a shortened lifespan and do not usually survive past their second or third decade. For mild MPS IVA, lifespan has been noted to be as long as 70 years.
The sulfatse that cleaves off the sulfate from N-acetylgalactosamine and galactose residues is deficient in patients with MPS IVA. In 1974, Matalon et al. identified the enzyme and its deficiency by use of oligosaccharide substrates that had been prepared from chondroitin sulfate [11]. The responsible enzyme was named chondroitin sulfate N-acetylhexosamine sulfate sulfatase. As the substrate included sulfated N-acetylgalactosamine, the enzyme was subsequently named “N-acetylgalactosamine 6-sulfate sulfatase, GALNS” [11–13]. In 1978, Di Ferrante demonstrated that extracts of fibroblasts derived from patients with MPS IVA lack or have greatly reduced activities for both the 6-sulfated N-acetylgalactosamine residues of C6S and the 6-sulfated galactose residues of KS [14]. At present, diagnosis of MPS IVA is determined by reduced levels of GALNS activity [15,16].
Molecular analysis and development of innovative therapies for MPS IVA started with purification of human GALNS in 1999 by two groups [17,18]. Subsequently, Orii and Tomatsu’s group has achieved cloning and characterization of the human GALNS gene [19,20], identification of mutations [21,22], and cloning of the mouse gene [23]. Production of recombinant human GALNS in CHO cells allowed purification of human GALNS [24], establishment of the 3D structure of GALNS [25] and experimental ERT [26]. Three mouse models for MPS IVA [27–29] were established to study pathogenesis, experimental ERT [26,30] and gene therapy [31–33]. Development of KS assays using ELISA or liquid chromatography/tandem mass spectrometry (LC-MS/MS) have facilitated diagnosis and screening [34–40]. The International Morquio Registry [41] has provided growth charts for MPS IVA patients [42,43].
Orthopedic surgical interventions for spinal cord compression and deformities of hip, leg, and knee have improved the quality of life for MPS IVA patients [32,33,44–52]. Two therapies, ERT and HSCT, are clinically available for MPS IVA [32,33,50,51,53–57]. Management of difficult airway and anesthesia procedures have also improved the lives of these patients [32,51,58,59]. In 2016, Pizarro et al. described that a new surgical procedure for tracheal obstruction without tracheostomy should reduce the mortality rate of patients with MPS IVA by improving narrowing and difficult airway [60,61].
In this review, we describe clinical manifestations, diagnosis, related GAGs, mouse models, pathogenesis, therapies, and management for MPS IVA. The whole picture of MPS IVA should provide a better understanding of the underlying disease of MPS IVA and consequently should lead to development of better therapies for MPS IVA.
2. Diagnosis
2.1. Clinical diagnosis and phenotype
Deficiency of the GALNS enzyme results in a progressive accumulation of the GAGs: C6S and KS. These GAGs are produced mainly in cartilage and accumulated primarily in lysosomes of chondrocytes, associated ligaments, and the neighboring ECM [9,62–65]. Accumulation of these GAGs leads to systemic skeletal dysplasia, including short stature, cervical instability with spinal cord compression, pectus carinatum, kyphoscoliosis, genu valgum, hypermobile joints, narrowing airway, and an abnormal gait [10,59,66]. Clinical features in patients with a severe form also include prominent forehead, short neck, pectus carinutum with sternal bulging, flaring of the rib cage, flat feet, corneal clouding, and heart valve involvement. In general, most signs and symptoms of MPS IVA do not appear at birth. Symptoms that can be seen at birth include gibbus and abnormal findings in the lumbar vertebral spine in X-rays [67]. By the age of 2 years, C6S and KS are built up in the body and start to cause skeletal problems. The first noticeable symptoms that usually show are oddly shaped bones, genu valgum, spine curvature, pectus carinatum, and rib flaring. As the patients get older, more serious functional issues start to show up: waddling gait, spinal cord compression, laxity of joints, and airway obstruction (Fig. 1).
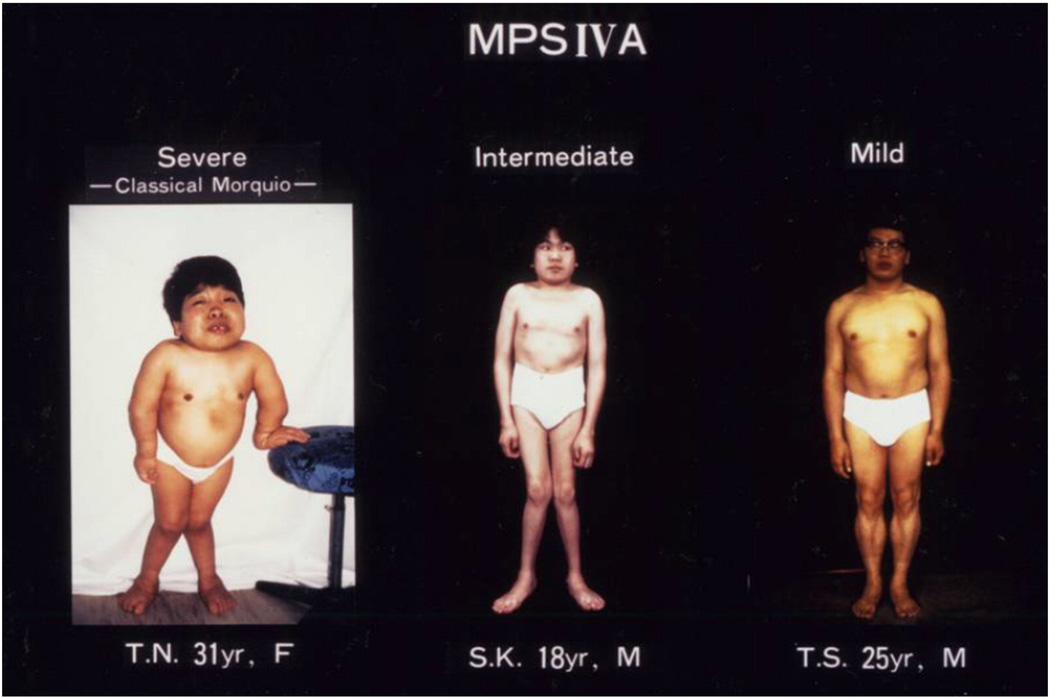
Fig. 1.
Clinical pictures of MPS IVA patients (adapted from Educational CD for Morquio and permitted by Carol Ann Foundation). Left panel; this is 31 year old female patient with a severe form of MPS IVA. She is 90 cm tall, showing skeletal deformities including pectus carinatum, bilateral genu valgum, diffuse corneal clouding, atlantoaxial subluxation, and hyperlaxity of joints. The genotype is c.1288_1289delCA/c.1288_1289delCA Middle panel; this is 18 year old male patient with an intermediate form of disease. He is 135 cm tall with the milder skeletal deformities of pectus carinatum, bilateral genu valgum, and hyperlaxity of joints and corneal clouding. The genotype is p.R94G/p.N204K. Right panel; this is 25 year old male patient with a mild form of disease. He is 157 cm tall and has milder skeletal deformities of thoracolumbar gibbus, mild platyspondyly and anterior wedging of the bodies in X-rays but no genu valgum. The genotype is pN204K/pN204K. All three patients have normal intelligence.
There is a high frequency of tracheal narrowing in children and young adults with MPS IVA which increases in severity with age. Patients with severe tracheal obstruction in MPS IVA are at risk of dying of sleep apnea and related complications. Tracheal obstruction also leads to life-threatening complications during anesthesia as a result of the difficulty in managing the upper airway due to factors inherent to the MPS IVA, compounded by the difficulty in intubating the trachea. A detailed description of the obstructive pathology of the trachea is not available in the literature probably due to lack of a homogenous group of MPS IVA patients to study at any one particular center. Tracheal narrowing, often due to impression from the crossing tortuous brachiocephalic artery, increases with age in MPS IVA patients. Greater attention to the narrowing trachea is needed when evaluating cervical spine MRIs and CTs as well as other clinical investigations, with the goal of establishing a timely treatment protocol to reduce the mortality rate in this patient population. Morquio A
22. Genetic diagnosis and genotype
MPS IVA is an autosomal recessive lysosomal storage disorder (LSD) caused by a deficiency of GALNS [14,15]. The GALNS enzyme has been purified to homogeneity [18]. The GALNS gene, located on chromosome 16q24.3, contains 14 exons spanning 50 kb and encodes a 1566 bp cDNA, which encodes a 522-amino acid protein, including a signal peptide of 26 residues [19,20]. GALNS has been purified from human placenta as an oligomer of 40 and 15 kDa polypeptides [18], with the oligomers inter-linked by disulfide bonding. Mature human GALNS enzyme is stabilized in a complex with two other lysosomal enzymes (β-galactosidase and α-neuraminidase) and the protective protein cathepsin A [67]. The assembly of four components is necessary for correct posttranslational processing and stability of the component enzymes and the efficient catabolism of KS. Molecular analysis of different ethnic populations has revealed over 200 different GALNS mutations until 2014 [68,69]. As of October 2016, 328 mutations in the GALNS gene have been reported [70]. The most common GALNS gene alterations are missense point mutations and small deletions [69]. This extensive allelic heterogeneity of GALNS gene mutations were consistent with the broad spectrum of clinical phenotypes observed in MPS IVA patients. Genotype-phenotype correlation has been used to predict clinical severity in some groups of MPS IVA patients [21,22,37,41,69,71–80]. In silico analysis of mutations occurring in GALNS proved that it was feasible and necessary to compare the three-dimensional structure of the enzyme with its mutants to better understand the change in function [81].
2.3. Radiographic diagnosis
2.3.1. Chest and spine
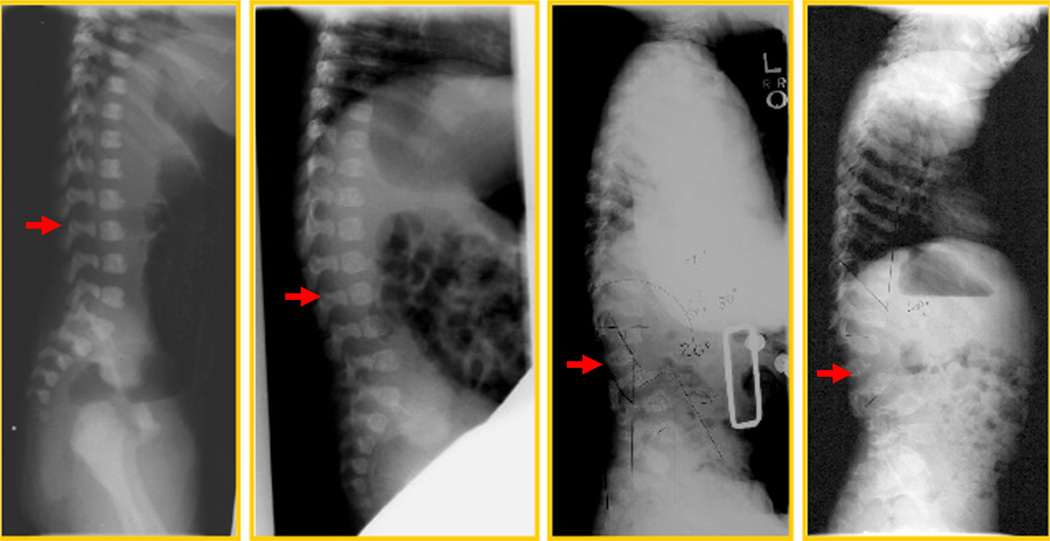
The ribs are wide anteriorly and laterally and overconstricted in their paravertebral portions. Scoliosis contributes to the abnormal and small ribcage. Morquio-specific radiographic changes, before clinical phenotypic changes have been observed (Fig. 2). Lateral view of progressive changes of the spine with age in a patient is shown. At one day old, a sacral dimple was noted at the delivery and the suspected anterior beaking at the level of L1 was seen, although this is common in normal newborns. At two months, the anterior beaking was more prominent at the level of L1 with kyphosis. At 15 months, unusual kyphosis in the thoracic spine and the gibbus at L1 were prominent with the anterior beaking. The L1 vertebral body was compressed. The flaring of the anterior lateral ribs was observed. At 32 months, the accentuated dorsal thoracolumbar kypholordosis with the gibbus deformity was noted remarkably. The advanced platyspondyly, irregularity, and anterior beaking of vertebral bodies characteristic of MPS IVA were observed [82].
Fig. 2.
Lateral view of progressive changes of spine with age in a patient (adapted from Educational CD for Morquio and permitted by Carol Ann Foundation). 1 day; a sacral dimple was noted at the delivery and the suspected anterior beaking at the level of L1. It could also be seen in normal baby. 2 months; the anterior beaking was more prominent at the level of L1 with kyphosis. 15 months; unusual kyphosis in the thoracic spine and the gibbus at L1 were prominent with the anterior beaking. L1 vertebral body was compressed. The flaring of the anterior lateral ribs was observed. 32 months; the accentuated dorsal thoracolumbar kypholordosis with the gibbus deformity was noted remarkably. The advanced platyspondylia, irregularity, and anterior bealdng of vertebral bodies are characteristic of MPS IVA.
Odontoid hypoplasia is a critical skeletal feature recognized in patients with MPS IVA. Less ossification of odontoid process in combination with ligamentous laxity and extradural GAG deposition leads to atlantoaxial subluxation, with consequential quadriparesis or even death. Another potential complication includes cervical myelopathy. A history of exercise intolerance in patients with MPS IVA often predicts the presence of occult cervical myelopathy. This myelopathy also causes bowel and bladder dysfunction and compression of the spinal cord, resulting in weakness or paralysis. Mortality and morbidity rates are mainly associated with the atlantoaxial instability, subsequent cervical myelopathy, and difficulty in anesthesia. Patients with a severe form, primarily related to cervical instability and compression, often do not live beyond the second or third decade of life. A minor fall or extension of the neck can result in serious cord injury and subsequent quadriparesis or sudden death.
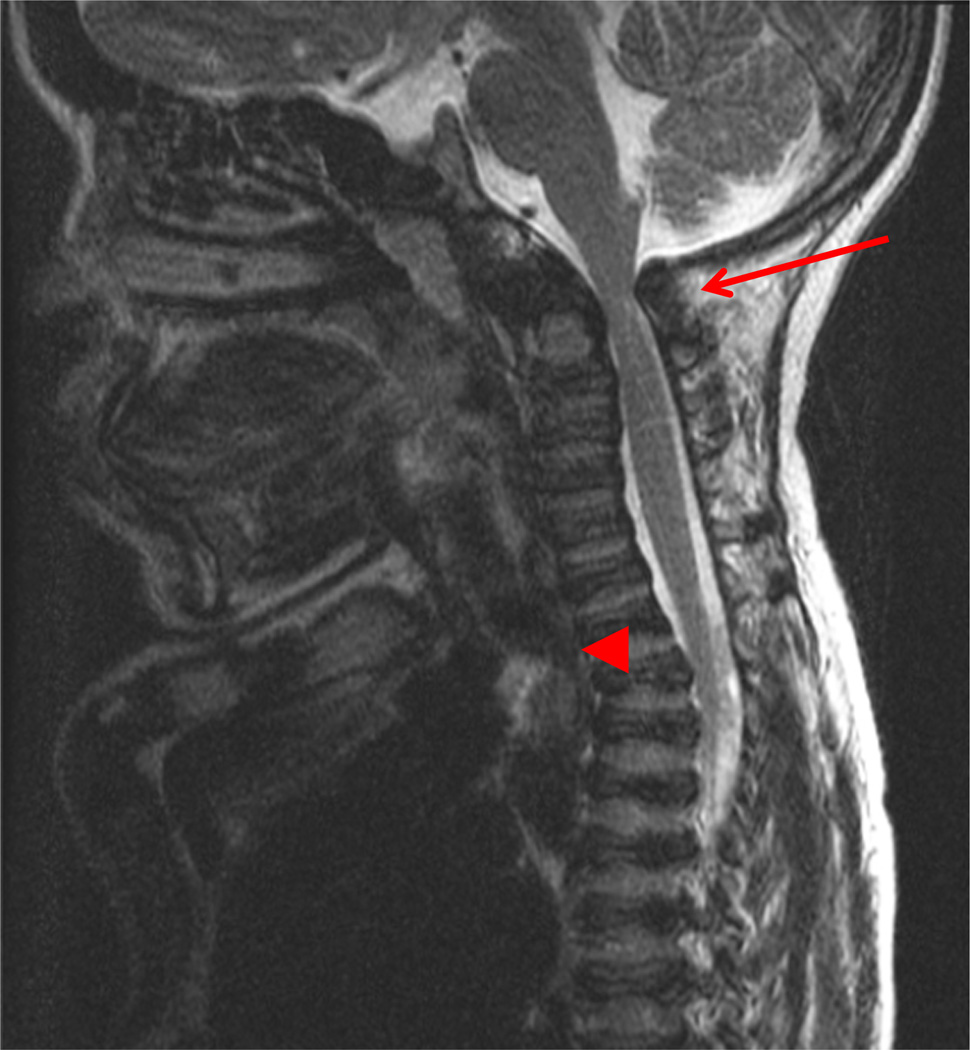
MRI of the cervical spine in a 13-year-old patient shows hypoplasia of odontoid process, atlantoaxial subluxation, and spinal cord compression (Fig. 3). A baseline study of the upper cervical region is recommended no later than two years or at diagnosis using flexion/extension X-ray films. If severe pain or pain associated with weakness of strength or tremors (or clonus) in the arms or legs occur, the patient should have studies of the neck to evaluate for the slippage (subluxation) of the neck vertebrae and compression of the spinal cord.
Fig. 3.
MRI of cervical spine in a 13 years patient. The arrow shows C1-C2 spinal cord compression and the arrow head specifies tracheal obstruction. A baseline study of the upper cervical anatomy is recommended no later than 2 years or at diagnosis using flexion/extension X-ray films. If severe pain or pain associated with weakness of strength or tremors (or clonus) in the arms or legs occur, the patient should have studies of the neck to evaluate for the slippage (subluxation) of the neck vertebrae and compression of the spinal cord.
X-rays and MRI will be taken with the head bent forward (flexion) and with the neck back (extension) and will be monitored annually to check the situation.
2.3.2. Upper limb
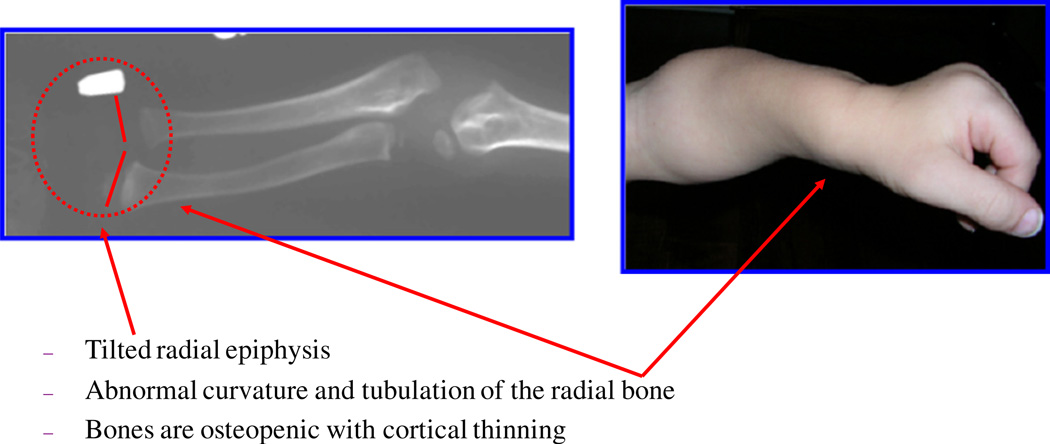
The epiphyseal involvement characteristic of MPS IVA is exemplified by the tapered irregular distal radius and ulna. The bones are osteopenic with cortical thinning. Upper extremities show the irregular epiphyses and widened metaphyses even at an early stage (Fig. 4). Cortical thinning and mild widening of the diaphysis of the humerus are visible. With aging, the bone deformity is in progress with tilting of the radial epiphysis towards the ulna. The humerus usually appears shortened later.
Fig. 4.
Characteristic bone deformity in the upper extremity (adapted from Educational CD for Morquio and permitted by Carol Ann Foundation). The epiphyseal involvement characteristic of MPS IVA is exemplified by the tapered irregular distal radius and ulna. The bones are osteopenic with cortical thinning. Upper extremities in a child aged 2 years 3 months (left panel). Note the irregular epiphyses and widened metaphyses. Cortical thinning and mild widening of the diaphysis of the humerus are visible. With age, the bone deformity progresses, e.g. with tilting of the radial epiphysis towards the ulna (10 years old; right panel). The humerus usually appears shortened later.
The tapering of the proximal portion of metacarpals 2 through 5 as well as the small irregular carpal bones appear (Fig. 5). The joints may become hyperlaxity by the age of 2 years.
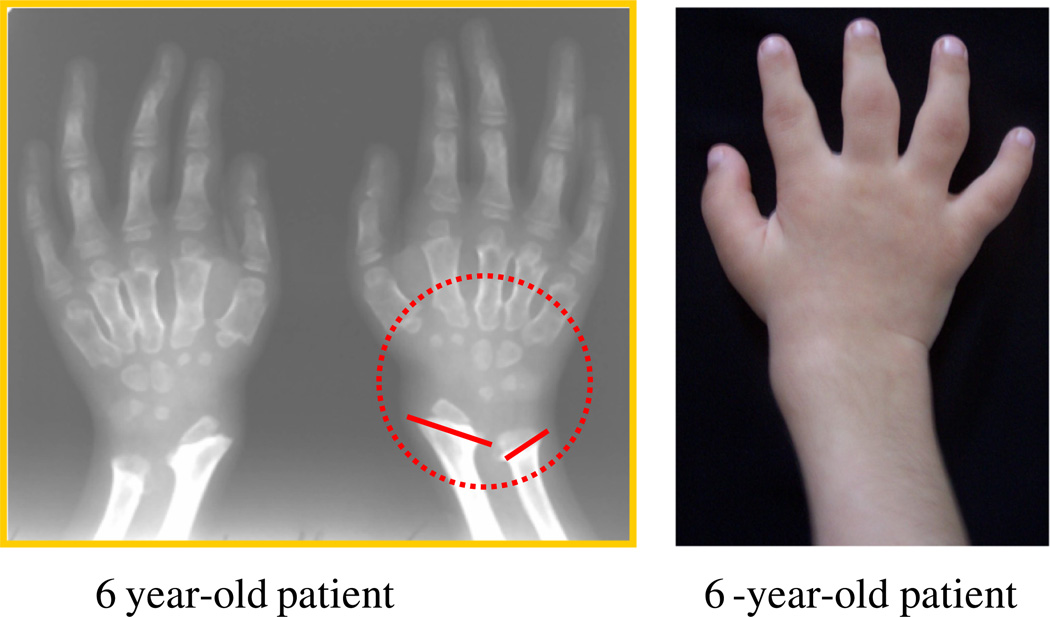
Fig. 5.
Hand deformity in MPS IVA (adapted from Educational CD for Morquio and permitted by Carol Ann Foundation). The radiograph in a 6-year-old patient shows the tapering of the proximal portion of metacarpals 2 through 5 and small irregular carpal bones. The joints may become hyperlaxity by the age of 2 years. The hands with age take on a characteristic with tilting of the radial epiphysis towards the ulna, resulting from a combination of metaphyseal deformities, hypoplasia of the bones, and degradation of connective tissues near the joint secondary to GAG accumulation.
2.3.3. Lower extremities
Multiple abnormalities are present in the pelvis, including spondyloepiphyseal dysplastic femoral heads and oblique acetabular roof with coxa valgus deformity and flared iliac wings (Fig. 6). The detailed lower extremities are described in orthopedics surgery section.
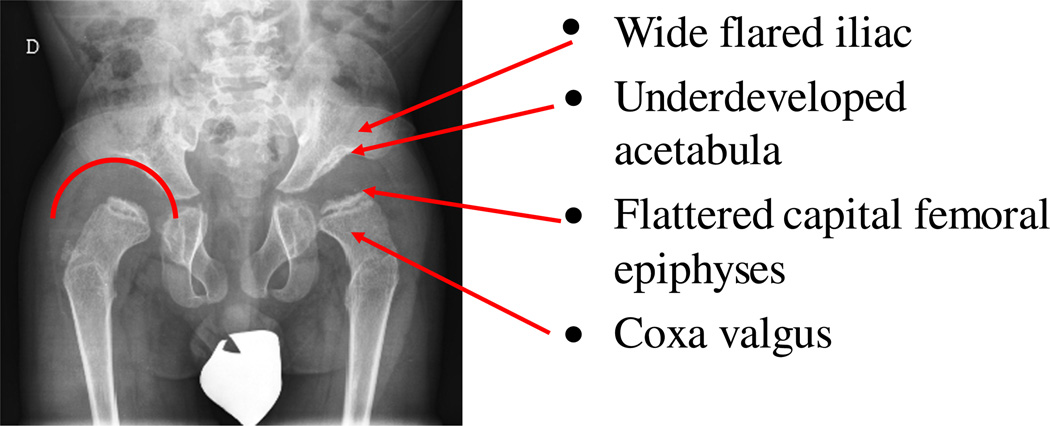
Fig. 6.
Hip joint deformity. The radiograph of a 6-year-old patient shows bone abnormality in lower extremities. Multiple abnormalities are present in the pelvis, including spondyloepiphyseal dysplastic femoral heads and oblique acetabular roof with coxa valgus deformity and flared iliac wings.
2.4. Biochemical diagnosis and enzyme activity
2.4.1. Analysis of urinary and blood KS
Once clinical suspicion of MPS IVA has been established, biochemical analysis and enzyme activity can be used to reach a diagnosis. Screening can be performed either by testing for abnormal elevation of urinary GAG levels and presence of excess KS or by measuring enzyme activity from a dried blood spot (DBS) [83]. The urinary excretion of GAGs, when normalized to creatinine, was high in infants and young children, decreases with age, plateaus in the second decade of life, and remained constant through adulthood [84–88]. It is important to evaluate both quantitative and qualitative analysis to avoid false negative results as KS can be present without elevating the total amount of GAG above the upper limits for unaffected individuals [40,85–87]. The quantitative analysis of total urinary GAG is usually performed by spectrophotometric analysis using dimethylmethylene blue (DMB) [89]. Although most MPS IVA patients do excrete KS, in many of them total urinary GAG levels are not sufficiently higher than those found in unaffected individuals, resulting false negative diagnoses for urinary analysis. False negative results are found for almost 15% of all MPS patients and even more for MPS IV patients [40,85,87].
In 2007, Oguma et al. developed a LC-MS/MS method that is highly specific and sensitive to measure KS without the false negatives, followed by other groups [30,34,35,39,90–94]. In healthy individuals, blood KS levels rise progressively during the first four years of life and remain elevated until 12 years of age. KS levels decline rapidly over the next three years and levels continue to decline until the age of 20 years when they reach a stable low level [34,39,90]. The decrease of KS levels after 13 years of age is consistent with the fact that the growth rate in healthy children declines after 13 years old. In patients with MPS IVA, the level of blood KS is the highest between 5 and 10 years of age while the level of urine KS peaked between ages 0 and 5 [34,39,90]. Blood and urine KS levels were higher in MPS IVA patients than those in age-matched controls under 20 years of age. The level of urine KS remained higher in MPS IVA patients than in controls even after 20 years of age, but the levels of blood KS in adult MPS IVA patients were indistinguishable from those in unaffected individuals [34,39,90]. The levels of blood and urine KS correlate with clinical severity during early and progressive stages of the disease, and therefore, they are a good prognostic biomarker. Blood KS directly reflects growth, turnover, disruption and/or repair of cartilage, where KS is mainly synthesized. The advantage of measurement of KS in DBS is feasible transport of samples for screening purposes. Blood KS could be more important to assess the therapeutic effect in bone lesions and their vicinity and association with clinical improvement. GALNS removes the sulfate at C(6) in Gal of the di-sulfated KS, Gal(6S)β1 → 4GlcNAc(6S), producing mono-sulfated KS, Galβ1 → 4GlcNAc(6S). Our previous results showed that the proportion of di-sulfated KS in total KS in the patients with MPS IVA was higher than that in the control subjects [39]. Thus, the ratio of di-sulfated KS to total KS in the patients with MPS IVA in blood and urine is higher than that in the control subjects [35,39].
Overall, determination of KS concentrations provides a potential biomarker to screen for high risk of MPS IVA patients and to measure pharmacodynamic effects of therapy [38].
2.4.2. Analysis of C6S
GALNS plays an important role to hydrolyze the sulfate group of C6S, converting it into CS. C6S is distributed mainly in growth plates, aorta, and cornea. Levels of C6S in blood and urine were significantly elevated in MPS IVA patients compared with age-matched controls and declined with age in both MPS IVA patients and control subjects. There is no rapid, accurate, quantitative method to measure C6S. In 2014, Shimada et al. developed measurement of C6S by using LC-MS/MS [95]. C6S levels were assayed in the blood from control subjects and patients with MPS IVA aged from 0 to 58 years old. Levels of C6S in blood decreased with age and were significantly elevated in patients with MPS IVA compared with age-matched controls. The difference of C6S in the patients and controls were more pronounced than the increased levels of KS in patients with MPS IVA. C6S and KS together provided enhanced discrimination of patients from controls than either C6S or KS levels alone. Thus, C6S could be a useful biomarker for MPS IVA [95].
2.4.3. Analysis of enzyme activity
Another important aspect of biochemical analysis is to demonstrate deficiency in GALNS activity in MPS IVA patients. Fibroblasts and leukocytes samples may be used for enzyme activities. In order to facilitate prenatal diagnosis, dissected chorionic villi, cultured chorionic villus cells, and amniocytes, are very useful to diagnose prenatal MPS [96, 97]. Additionally, protocols for evaluating GALNS activity in a DBS have recently been proposed as screening methods [98,99]. Due to the temperature sensitive nature of GALNS in a DBS [98], cards should be stored at 4 °C after drying and shipped promptly; the longer the period of time between collection and analysis, the higher the risk of a false positive result. For this reason, low levels of enzyme activity in DBS cannot be used alone for diagnosis. Positive results from DBS need to be confirmed by enzyme activity analysis in fibroblasts or leukocytes. More data are needed to evaluate GALNS stability in a DBS, since DBS samples are more likely to be exposed to environmental extremes during shipping than leukocytes. If GALNS activities appear normal, β-galactosidase, the enzyme deficient in MPS IVB, should be tested as MPS IVA and B can present with very similar symptoms and can both cause elevated urinary KS [100].
3. Glycosaminoglycans: KS and C6S
3.1. Biological roles of KS
Lack of GALNS causes accumulation of KS in lysosomes of cartilage cells that leads to the progressive skeletal dysplasia. Undegraded GAGs are released into the circulation and thus are an important biomarker for screening of MPS IVA. Blood KS is directly related to turnover of proteoglycans in cartilage, the primary tissue where KS is synthesized, due to tissue remodeling during growth and disruption and/or repair of cartilage. Urinary KS is filtered in the kidney, and consequently only selected smaller molecules are excreted into urine and consequently urinary KS does not measure all forms of undigested KS. The proportion of di-sulfated KS to total KS in the patients with MPS IVA in blood and urine is higher than that in the control subjects [35,39]. As cartilage is the primary tissue containing KS, replacement enzyme needs to penetrate this tissue to enable effective degradation of polymeric GAGs containing KS in MPS IVA patients. Replacement enzyme in the circulation will not effectively convert di-sulfated KS to mono-sulfated KS in blood since infused enzyme is not penetrated into bone and is inactive at neutral pH in blood, so that a reduction of the proportion of di-sulfated KS in total KS in blood will not be changed with ERT [33,35,39,40,56,101,102].
3.2. Biological roles of C6S
C6S is distributed mainly in the growth plates, aorta, and cornea; however, the physiological function of C6S is not entirely understood. Shimada et al. developed a method to measure C6S using LC-MS/MS [95]. C6S levels were assayed in the blood from control subjects and patients with MPS IVA aged from 0 to 58 years old. Levels of C6S in the blood decreased with age and were significantly elevated in patients with MPS IVA, compared with age-matched controls. C6S accumulates in the heart valves and aorta in patients with MPS IVA [65]. Foam cells/macrophages in the aorta and heart valves contain C6S rather than KS. While accumulation of KS appears to be the primary driver for the bone dysplasia the role that C6S accumulation plays in MPS IVA is not yet clear. A more detailed analysis of tissue distribution pattern of C6S may reveal pathogenic roles of this GAG in MPS IVA.
4. Animal models of mucopolysaccharidosis IVA
Three mouse models of MPS IVA have been established: knock-out (KO) by gene deletion, knock-in (KI) with an active site point mutation, p.C76S, and tolerant mice expressing inactive human GALNS (humanoid mouse) [27–29]. These model mice have been used for in vivo and in vitro studies designed to develop new therapies for MPS IVA [24,26,30, 103]. First, homozygous GALNS KO mouse were generated by disrupting exon 2 of GALNS. This mouse model has no detectable GALNS enzyme activity in multiple tissues and shows elevated levels of urinary GAGs [28]. The KO mouse also shows excess accumulation of GAGs in multiple organs such as liver, spleen, kidney, brain, and bone marrow. In the liver and spleen, storage materials are seen in Kupffer cells and sinusoidal lining cells within 2 months after birth. At 12 months, there is a vacuolar change in the visceral epithelial cells of glomeruli and cells at the base of heart valves but there is no vacuolar change in parenchymal cells such as hepatocytes and renal tubular epithelial cells. In the brain, lysosomal storage exists in the hippocampal and neocortical neurons and meningeal cells. Immunohistochemistry revealed accumulation of KS and C6S in the cytoplasm of corneal epithelial cells of this knock-out mouse. The recent study has demonstrated that KO mice with MPS IVA on the congenic C57BL background have marked elevation of blood KS (unpublished) and that clinical phenotype with high KS is under investigation.
Secondly, an MPS IVA mouse model was generated that was tolerant to human GALNS to prevent immune response during enzyme replacement therapy (ERT) [27]. In early preclinical trials of ERT for other LSDs, it was reported that a long-term administration of enzyme impaired therapeutic effectiveness due to a humoral immune response [104, 105]. We created a construct vector containing both an active site mutation (C76S) in exon 2 of the mouse gene and a transgene that contained a cDNA for inactive human GALNS in intron 1. This vector was inserted into murine GALNS by targeted mutagenesis. This tolerant mouse had no GALNS activity and GAGs accumulated in multiple tissues such as visceral organs, cornea, brain, bone, cartilage and bone marrow. At 3 months, lysosomal storage material is seen within not only reticuloendothelial Kupffer cells, and cells of the sinusoidal lining of the spleen but also hepatocytes. There is also marked lysosomal distention and increased vacuolation of osteoblasts, osteocytes and chondrocytes, and disorganization of layers in cartilage. Radiographic analysis reveals that the MPS IVA tolerant mouse has normal spine, leg and ribs compared with wild-type mouse. However, the mouse shows an abnormal calcaneus that is angled upward. Micro-CT analysis of knee bones showed no significant differences in bone mineral density between MPS IVA tolerant and wild type mice (MPS IVA: 500.73 ± 22.25 mg HA/cm3, WT: 496.24 ± 38.75 mg HA/cm3).
Serum KS levels in the MPS IVA tolerant mouse on the congenic background of C57BL are not significantly changed compared with wild-type mouse [101]. These findings suggest that the MPS IVA tolerant mouse has no severe bone dysplasia because of limited accumulation of GAGs in chondrocytes and that KS is not released from chondrocytes, resulting normal level of KS in blood [101].
Knock-in mouse was made by replacing the Cys76 with Ser in the endogenous murine Galns by targeted mutagenesis. Homozygous Galns(tm(C76S)slu) mice had no detectable GALNS enzyme activity [29]. At age of 2–4 months, lysosomal storage was present primarily within reticuloendothelial cells such as Kupffer cells and spleen sinusoidal lining cells. Vacuolar change was present in glomerular visceral epithelial cells and was not present in hepatocytes or renal tubular cells. In the brain, hippocampal and neocortical neurons and meningeal cells showed lysosomal storage. Radiographs revealed no change in the skeletal bones of mice up to 12 months old. Thus, the Galns(tm(C76S)slu) mice had visceral storage of GAGs in organs but lacked the skeletal features of human MPS IVA. We conclude that the milder phenotype is characteristic of isolated GALNS deficiency.
Overall, none of these MPS IVA model mice have the same phenotype seen in MPS IVA patients, even though abundant storage materials do accumulate in multiple tissues including cartilage, heart valves, cornea, and visceral organs. One major reason is that rodents, including mice, synthesize far less KS compared to human (10–100 folds lower in blood KS level). More sophisticated MPS IVA mouse models that more closely mimic the human disease are needed.
5. Therapy and management
5.1. Enzyme replacement therapy
5.1.1. Preclinical trial
Recombinant GALNS for ERT has been produced in CHO cells with specific activities of 170,000 [24,106] to 120,000 nmol h−1 mg−1 [107]. As an alternative, recombinant GALNS have also been produced in E. coli [10,108–111] and in the yeast P. pastoris [112–114]. In all cases, the recombinant enzymes show highest activity at pH 5.0 and are taken-up by cultured fibroblast, chondrocytes, or HEK293 cells in a dose-dependent manner via the mannose-6-phosphate receptor (CHO-produced enzyme) [26,107,115] or mannose receptor (yeast-produced enzyme) [116]. Enzyme produced in E. coli does not have N-glycosylation, so that the enzyme can only be taken up into cells by nonspecific endocytosis [109,117].
After a single intravenous dose of 250 units/g of CHO produced enzyme into MPS IVA knock-out mice, recombinant GALNS showed a biphasic clearance with a rapid initial t1/2 between 2.4 and 3.3 min and a slower second t1/2 between 13.8 and 21 min, with no detectable enzyme in blood in 30 to 60 min after infusion [24,26,106,115]. The enzyme was delivered mainly in liver and spleen and was detected in bone marrow to less extent. The enzyme was not detectable in any tissue 48 h post-infusion [106,115]. Weekly intravenous infusions of 250, 600, or 1000 U/g during a 12-week period to a MPS IVA mouse model showed a complete clearance of GAG storage in liver, spleen, and sinus lining cells in bone marrow [24,106]. By contrast, the growth plate showed little improvement in column structure and GAG storage [26,115].
In another group, after five administrations of fluorescently tagged enzyme every two days into wild-type mice, low levels of tagged enzyme could be detected in the growth plate and articular cartilage of wild-type mice, indicating that it is very hard to penetrate the bone by a native enzyme infused [107].
To improve the delivery of the enzyme to bone, a bone-targeting drug delivery system was constructed by adding an N-terminal hexaglutamate residue to GALNS [30]. The bone-tagged enzyme showed a longer blood circulation time than that of the native enzyme. The bone-tagged enzyme was also detected 48 h post-infusion in femur, spine, and bone marrow; while native enzyme was not detected in these tissues. Adult and newborn Morquio A mice treated with the bone-tagged enzyme showed a complete clearance of the storage in Kupffer cells, in the liver and sinus-lining cells in bone marrow [30]. After 24 weekly infusions of the targeting enzyme, a substantial effect on storage and cellular organization of the growth plate was detected in adult mice. Newborn mice treated for 8 weeks, showed a partial reduction of storage material and a partial restoration of cell organization in the growth plate. Similar results were observed in heart valves, with better clearance of storage in newborn treated animals. Thus, the bone-targeted enzyme showed an improvement in the pathology of chondrocytes and heart valves in comparison with the results observed by using the native enzyme [30] (Fig. 7).
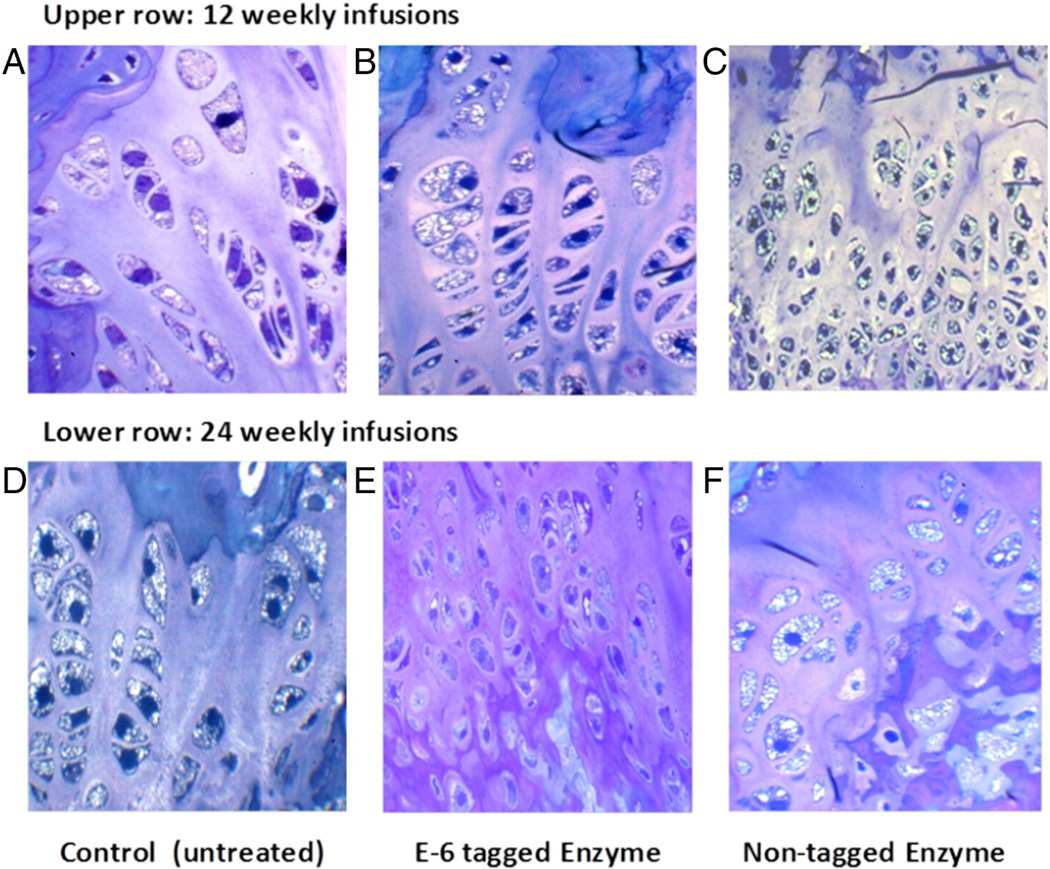
Fig. 7.
ERT on Morquio A mice at 8 weeks old treated for 12 (upper) and 24 (lower) weeks with 250 U/g of E6-GALNS. Light microscopy of growth plates in femur from untreated, E6 GALNS-treated, or non-tagged (native) enzyme. An untreated mouse shows marked storage material in chondrocytes (A and D). Vacuolated chondrocytes are obvious. Chondrocytes showed a slight response after 12 and 24 weeks of treatment with native enzyme (C and F). The cartilage cells treated with bone-targeting enzymes have substantial reduction of storage material after 24 weeks of treatment (E). ERT, enzyme-replacement therapy; GALNS, N-acetylgalactosamine-6-sulfate sulfatase.
Long-term treatment (14 weeks) of newborn Morquio A mice with native recombinant GALNS allowed complete clearance of lysosomal storage in the liver, spleen, and bone marrow; while in heart a slight reduction in the storage was observed. In the growth plate, ligaments, articular cartilage, and articular disc, the storage materials (vacuoles) were reduced but still remained to a variable extent although cellular organization was improved [118].
5.1.2. Clinical trial
In 2014, ERT for Morquio A disease was approved in EMA and FDA using a recombinant enzyme produced in CHO cells (elosulfase alfa) [119]. The phase 1/2 trial included 20 patients in an open-label, dose escalation trial; while the phase 3 trial was a 24-weeks, randomized, double-blind, placebo-controlled trial with 176 patients (aged between 5 and 57 years old) in two dosing regimens: 2 mg/kg/dose weekly (2 mg/kg QW; n = 58) and 2 mg/kg/dose every other week (2 mg/kg QOW; n = 59), compared with placebo (PBO) (n = 59) [55,120]. Overall, treatment with 2 mg/kg elosulfase alfa weekly resulted in a modest improvement in the 6-min walk test (6MWT) while there was not improvement in the 3-min stair climb test (3MSCT). Patients treated with ERT showed positive changes in maximal voluntary ventilation, MPS-Health Assessment Questionnaire (MPS-HAQ), and height/growth rate, although these changes were not statistically significant. All treated patients developed anti-elosulfase alfa antibodies, and most of them developed neutralizing antibodies.
5.1.2.1. Six-minute walk test (6MWT)
The primary clinical endpoint was defined as the change in distance walked in the 6MWT. At baseline, all enrolled patients walked >30 m but <325 m in 6 min. Patients in the 2 mg/kg QW treatment group showed a significant change, 22.5 m (95% CI 4.0, 40.9; P = 0.017) in the 6MWT, compared to placebo at week 24, while patients in the 2 mg/kg QOW treatment group performed similarly to those in the placebo group [55]. Several exploratory analyses were conducted by the FDA Advisory Committee to better assess the clinical meaningfulness of this result [121,122]. Patients who walked shorter distances at baseline (6MWT ≤ 200 m) had a greater improvement in walking distance than those who walked >200 m at baseline. Patients who walked ≤200 m at baseline showed a mean change in 6MWT from baseline to week 24 of 53 ± 67 m on 2 mg/kg QW treatment vs. 13 ± 39 m on placebo; while patients who walked >200 m at baseline showed a mean change in 6MWT from baseline to week 24 of 25 ± 49 m on 2 mg/kg QW treatment vs. 14 ± 57 m on placebo [55,123]. Based on long-term data from an extension study, patients who continued to receive 2 mg/kg QW for another 48 weeks (a total of 72-weeks treatment), did not show further improvement on 6MWT beyond what was demonstrated during the first 24-week placebo-controlled trial [122]. There was no comparable placebo group in this extension trial [124]. It is noteworthy that patients, who received placebo during the initial 24-week controlled trial and were subsequently randomized to receive 2 mg/kg QW in the extension study, did not show any improvement in 6MWT compared to baseline [93,121,122].
The 6MWT was originally developed to measure the sub maximal level of functional capacity in adult patients with moderate to severe heart or lung diseases, as a predictor of morbidity and mortality in these patients [122]. The 6MWT has been adopted to indicate clinical improvement in several LSDs prior to U.S. marketing approval, including Pompe disease, MPS I, MPS II, and MPS VI. However, clinical features and prognoses vary between different diseases. The 6MWT is influenced by at least three organ systems affected in MPS IVA (i.e. musculoskeletal, respiratory, and cardiovascular) and is also affected by motivation or pschological effect, training, and water supply. Therefore, it may not be good measure of overall efficacy of treatment, and it is a less specific measure of the effect on the skeletal function affected by additional factors. In this sense, other measures of short and long-term treatment benefits need to be developed in future clinical trials involving patients with MPS IVA [122].
5.1.2.2. 3-Minute stair climb test (3MSCT)
The secondary clinical end-point, the change in the 3MSCT, did not show any significant improvement for treated patients. Neither 2 mg/kg QW nor 2 mg/kg QOW treatment group demonstrated a significant improvement in the 3MSCT, compared to placebo at week 24. The mean difference in the stair climb rate between the two treatment groups and the placebo group was only 1.1 stairs per min [123]. A long-term study with 173 MPS IVA patients for 120 weeks, receiving 2 mg/kg every week at the end of study, showed significant improvement, 5.5 (1.9) stair/min and 6.7 (2.0) stair/min in ITT and MPP groups respectively, in the 3MSCT in QW-QW cohort, compared with baseline [125].
5.1.2.3. Other clinical effects
Patients treated with ERT showed positive changes in maximal voluntary ventilation and MPS-Health Assessment Questionnaire (MPS-HAQ) [55,120] although no significant improvement was observed in normalized standing height (0.1 [95% CI 0.0, 0.3] and growth rate z-scores (0.4 [95% CI −0.1, 0.9]) [120]. Furthermore, no significant improvement was observed in cervical and lumber spines, corneal clouding, lower extremity, bone length, and echocardiogram and audiometry alterations [120].
Cardio-pulmonary exercise test (CPET), evaluated in 15 patients after 25 weekly infusions of elosulfase alfa showed a positive change in exercise capacity with treatment (i.e. increase in exercise duration, peak workload, and O2 pulse, which were statistically significant from baseline). There was also a reduction in the oxygen uptake during work (VO2/watt that was not statically significant), suggesting that patients were performing work at a reduced oxygen cost [126]. Muscle strength testing showed numerical improvement in knee extension (11%) while smaller improvements were observed in elbow (7.1%) and knee flexion (2.9%), which were not significant from baseline [126]. Furthermore, there was also a numerical improvement (decrease, from −74.4 to −30.6 that was not significant) in the score of the Adolescent Pediatric Pain Tool [126]. A study of oxidative stress parameters and pro-inflammatory cytokine in 8 months weekly treated Morquio A patients showed that pro-inflammatory and pro-oxidant states occur in MPS IVA patients even under ERT [127]. However, since baseline measurements were not reported in this study, it is not possible to determine the impact of ERT on oxidative stress biomarkers.
5.1 2.4. Urinary KS
In a phase 3 trial, urinary KS levels in the 2 mg/kg QW treatment group decreased by 41% from the baseline, and in the extension clinical trial, the urinary KS levels continued to decline through the 72 weeks. However, changes in urinary KS levels did not correlate with changes in 6MWT [55,120]. MPS IVA patients that had received weekly ERT for about 8 months still presented significantly higher levels of urinary GAGs (2 ± 0.02 µg/mg Cr) when compared to healthy controls (1.57 ± 0.07 µg/mg Cr) [127]. Patients in the 2 mg/kg QOW treatment group had a 30% reduction in urinary KS level from the baseline, without any clinical improvement [123]. Since most clinical features of MPS IVA are due to skeletal dysplasia caused by malfunction of vacuolated chondrocytes and successive abnormal ECM formation, it is critical to determine whether a measured biomarker correlates with improvement of these clinical outcomes. To date urinary KS is a useful to differentiate MPS IVA from other forms of MPS and to demonstrate pharmacodynamic effects of ERT treatment, but it does not appear to be a valuable predictor of skeletal improvement during ERT for MPS IVA [122].
5.1.2.5. Safety
Of the 235 patients, 175 (74%) patients had an adverse reaction. The most common adverse reactions occurring in ≥10% of patients were pyrexia (26%), vomiting (22%), headache (20%), nausea (18%), abdominal pain (14%), and fatigue (12%). Twenty-five (10.6%) patients presented a serious adverse effect, in which the most serious adverse reactions were acute reactions associated with infusions, such as anaphylaxis or hypersensitivity reactions. The signs and symptoms of anaphylactic events observed in treated patients included dyspnea, bronchospasm, cough, hypoxia, hypotension, flushing, angioedema of the throat, urticaria, and gastrointestinal symptoms in conjunction with urticaria. Anaphylaxis occurred as early as 30 min from the time of infusion and up to 3 h after infusion. Anaphylaxis has also occurred as late into treatment as the 47th infusion. It should be noted that all patients in the phase 3 trial received premedication with antihistamines. Hypersensitivity reactions were reported in 64 (27%) patients. Most commonly reported hypersensitivity reactions included angioedema, urticaria, peripheral edema, facial edema, wheezing, flushing, cough, dyspnea, and rash. One patient discontinued treatment due to anaphylaxis after the tenth infusion of elosulfase alfa, and another patient discontinued treatment after the 45th infusion due to recurrent severe hypersensitivity reactions and anaphylaxis [55,120]. In addition, 1345 total infusions were administered over a period of 6 months to 2.0 mg/kg/week group. Fourteen patients were interrupted in infusion and 3 were discontinued and they also required medical intervention [55].
5.1.2.6. Immunogenicity
Development of anti-elosulfase alfa antibodies has been reported for all patients receiving the ERT [128]. QW patients were positive for anti-elosulfase alfa antibodies faster (week 4) than QOW patients (week 16), and then antibody levels were stable until the end of the study at week 24, at that time titers were similar for both treatment groups. The majority of the patients (87 and 80% for QW and QOW, respectively) were positive for antibodies capable of preventing the enzyme from binding to the mannose-6-phosphate receptor (neutralizing antibodies, NAb) [128]. However, there was no correlation between severity of hypersensitivity adverse reactions and anti-elosulfase alfa antibodies or NAb. No association was found between Nab positivity and decrease in urinary KS levels [128].
5.1.2.7. Treatment of young patients (<5 years old)
Fifteen Morquio A patients between 9 months and 4.9 years old were enrolled in a study treating at 2 mg/kg/week for 52 weeks [129]. Overall, elosulfase alfa treatment was well-tolerated, with safety issues similar to those reported for older patients. At least one adverse effect was observed in all treated patients, with 73% reporting at least one drug-related adverse effect, and all patients reported at least one infusion-associated reaction. However, most adverse effects and infusion-associated reactions were mild to moderate, and no life-threatening adverse effects or deaths were observed [129]. As observed in the clinical trial with patients >5 years old, anti-elosulfase alfa antibodies were detected in all treated patients. Urinary KS levels were reduced by 30% after 2 weeks, and 43% after 52 weeks [129]. A mean percentage increase of 13.8% in weight was observed from baseline to week 52. The mean height z-score at baseline was −2.0 (±1.5) for the 12 treated patients ≥2 years of age compared to −2.2 (± 1.3) and for 24 untreated subjects (2–5 years old) obtained from the Morquio A Clinical Assessment Program (MorCAP). After 52 weeks, height z-scores in treated group and MorCAP group changed to −2.2 (±1.7) and −3.0 (±1.2), respectively [129], indicating that growth of treated patients was less affected than that of untreated patients. In patients >2 years old, the mean normalized growth rate z-score was −0.8 (±0.8) at baseline and −0.3 (± 0.5; N = 12) at week 52, which, together with data from the MorCAP study, suggest that elosulfase alfa improves growth rates in Morquio A patients. However, a longer study is necessary to determine the long-term impact of ERT [129]. Nevertheless, this study has shown a favorable benefit/risk profile for elosulfase alfa for Morquio A patients younger than 5 years old, decreasing urinary KS and having a positive impact on growth [129]. We have evaluated the effect of ERT on activity of daily living (ADL) and surgical intervention in patients with MPS IVA [130]. ADL scores for ERT patients under 10 years of age (2.5 years follow-up on average) were similar to those of age-matched controls, but declined in older patients. To date, there is no report to prove the effect of ERT on bone and cartilage lesions in patients with MPS IVA. Chondrocytes and ECM derived from MPS IVA patients with ERT for 6–30 months showed no decrease of vacuoles in bone pathology [56,122,131]. These pathological findings are consistent with the fact that frequency of need for orthopedic surgical interventions has not been reduced by ERT (average duration of ERT: 2.5 ± 1.0 years) [129]. Early ERT treatment at 21 months old did not improve the bone outcome in a severe MPS IVA patient after the 30 months-long treatment [132]. In the UK, a specific Managed Access Agreement for elosulfase alfa was formulated in December 2015, in which a drug is made available for a limited period of time (e.g. for 5 years) often at a discounted price, to allow further evidence to be gathered on its effectiveness while ensuring that patients receive access to the drug [133]. In Canada, Canadian Drug Expert Committee followed the UK agreement, suggesting a substantial reduction in price [134]. Through this study, it can be monitored how well the medicine works in practice before future funding decisions are taken. In The Netherlands, it was initially reimbursed, but reimbursement was stopped when the authorities decided that the data provided were limited to a suboptimal short term outcome in a heterogeneous population, showing a small effect that was not clinically relevant as several other EU countries (Sweden, Belgium, and Spain) made the same decision [135].
This is agreeable with the statement of Australia’s PBAC, which recently rejected to reimburse elosulfase alfa [136]. The statements by these authorities emphasize importance of cost/benefit to assess the ERT ($250,000 to $500,000 per year per patient). Careful long-term observations will be required to determine whether limited enzyme delivery to bone and cartilage is sufficient to improve outcomes for these patients. The response to ERT is likely to depend on the age of the patient when treatment is initiated and the severity of the clinical condition. It is notable that some patients respond to ERT while others do not respond to ERT although that the mechanism remains unclear [61, 130,132]. The reduction of urine KS seen in treated MPS IVA patients does not predict any improvement in bone pathology and chondrocyte function [131].
Urinary KS results from small KS fragments (not large fragments) that filter through the kidneys from the circulation. Or the infused enzyme is delivered to the kidneys and digests KS present in kidneys. Therefore, urinary KS does not reflect blood KS coming directly from the bone and cartilage unless blood KS is reduced to the same extent like urinary KS. Thus, it is important to measure blood KS directly derived from the bone as a biomarker of improvement in true clinical endpoints including bone pathology or any other skeletal signs and symptoms. A limitation of both blood and urinary KS levels is that these biomarkers are only of value in younger patients before closure or destruction of growth plates since synthesis of KS decreases markedly and levels of KS in MPS IVA patients are normalized or subnormal naturally [33,51,56,122].
There may be a clinical effect of ERT by reducing the pro-inflammatory factors that are induced when excessive KS results in an abnormal structure of extracellular matrix, leading to relieving arthritis and pain in joints, enabling an increase in physical activity, as described in other types of MPS [137]. ERT with native enzyme could also improve hearing, reduce recurrent infection, and reduce airway narrowing (if the storage materials are released from the airway). Thus, while ERT may benefit some MPS IVA patients by arresting some aspects of disease progression, the fundamental problems associated with advanced skeletal deformity and laxity of joints are unlikely to be solved by current ERT.
The limitations of ERT for MPS IVA disease open new challenges and opportunities to researchers to improve it and deliver a better product to the patient population.
5.2. Hematopoietic stem cell therapy (HSCT)
The potential advantage of HSCT for MPS is deemed to be that the marrow-derived donor macrophages provide a consistent secreting source of enzyme that can gain access to various storage tissues. HSCT has been successfully achieved for other MPS diseases including MPS I, II, and VI [138,139,174–176] but there are some adverse effects [140]. The patients need immunosuppression and steroid, following transplantation to protect against graft-versus-host disease (GVHD). HSCT does not reverse advanced skeletal deformities, in spite of improving growth development [141].
The clinical outcome of HSCT relies upon the following factors; 1) the age and the clinical condition of the recipients when undergoing HSCT, 2) prognosis of the recipient, 3) donor type, and 4) preparative regimen. In patients with MPS I, II, and VI, HSCT results in reversal of visceral organ involvement and improvement of heart function and hearing. However, the pre-existing skeletal abnormality remains, and corneal clouding is not diminished.
The primary concern of HSCT is immunological reaction; in early cases approximately 20% of patients died of HSCT-related adverse effects shortly after treatment. New international HSCT guidelines for MPS in 2005 have improved outcomes [142]. The survival and graft outcomes of MPS patients receiving HSCT were evaluated based on conditioning regimens including busulfan. A non-carrier matched sibling donor, matched unrelated cord blood, or matched unrelated donor were regarded as preferred donors. Study of MPS patients receiving HSCT at median age 13.5 months (range, 3 to 44 months) showed high overall survival (95.2%) and event-free survival (90.3%) with only low toxicity. Since 1999 at Tokai University in Japan, the survival rate after HSCT was comparable to this report.
Overall, HSCT is a therapeutic option for selected cases of MPS IVA with careful pre-transplantation counseling and clinical evaluation at the well-trained facility.
HSCT for MPS IVA has been approved prior to ERT. We have experienced 5 cases with MPS IVA who underwent HSCT; none of patients died of HSCT procedure although one patient had a rejection of engraftment [53,130,143]. Other groups also reported HSCT for MPS IVA previously [144,145].
We have described the detailed outcomes of the MPS IVA patient, a 15-year-old male, treated by allogeneic bone marrow transplantation (BMT) [9,43,53]. Eleven years after BMT, GALNS activity was maintained to the level of the enzyme activity of the donor in the lymphocytes of the recipient. This patient showed 1) recovery of ambulation with osteotomies, 2) release of narrow airway and disappearance of a shortage of breath, 3) diminished snoring, and 5) increase of bone mineral density. However, restriction of physical activity and hyperlaxity of joints remained in this patient. We have reported on a total of four cases of MPS IVA patients treated with HSCT (age at HSCT: 4–15 years, mean 10.5 years) and followed them for least 10 years post-treatment (range 11–28 years; mean 19 years of post-treatment) [143]. Current age ranged between 25 and 36 years of age (mean 29.5 years). All 4 cases had a successful full engraftment of allogeneic BMT without serious GVHD. Transplanted bone marrow was derived from HLA-identical siblings (three cases) or HLA-identical unrelated donor (one case). GALNS activity in lymphocytes was maintained at the same level as the donors for at least 10 years in all patients. Except for one case that had osteotomies in both legs one year after BMT, they have not yet had any orthopedic surgical interventions. All cases have remained ambulatory, and three of them can walk over 400 m in 6MWT. ADL in patients with HSCT was much better than untreated patients. The patient, who underwent HSCT at four years of age, showed the best ADL score in all untreated and ERT patients investigated over 20 years of age. This patient could walk without limitation, squat easily, move joint easily, and work independently as a dentist assistant [143; video 1 in 143].
Overall, the long-term study of HSCT has indicated therapeutic effect in amelioration of advance of the disease in respiratory function, ADL, and biochemical findings, suggesting that HSCT is a therapeutic option as a permanent treatment.
If HSCT is executed for MPS IVA patients at an earlier stage, skeletal deformities, restrictive and obstructive airway, and abnormal growth development could be prevented or improved. In 2016, the report on HSCT for MPS IVA has also demonstrated that one year after HSCT, a significant improvement in ligamentous laxity and joint hypermobility, hepatosplenomegaly, upper-airway obstruction, and recurrent otitis media and a smaller improvement in height and thoracic deformity [146]. Spinal cord compression syndrome remained stable after transplantation.
Although regimens for HSCT have been improved substantially over recent years, the process still bears some risk of mortality from infection, GVHD, and additional complications. For these reasons, HSCT should be applied to selected cases with careful pre-transplantation counseling, clinical assessment, and systemic longitudinal monitoring of the outcome. The cost/risk/benefit of HSCT should be conisdered; the cost of HSCT is $100,000 to 250,000 for one time treatment.
5.3. Gene therapy
The first study in development of gene therapy for Morquio A was conducted in vitro using a γ-retrovirus vector, which resulted in increased GALNS activity and reduced GAGs in transduced normal and Morquio A human lymphoblastoid B cells, human keratinocytes, murine myoblasts (C2C12), and rabbit synoviocytes (HIG-82) [147]. Subsequently, AAV vectors were selected for Morquio A gene therapy studies, divided into three phases. In the first phase, the effect of the promoter region on GALNS expression was evaluated in vitro, showing that eukaryotic promoters (elongation factor 1a or α1-antitrypsin) induced similar expression levels to those observed with cytomegalovirus (CMV) promoter [31,103,148,149], which might avoid the gene silencing [150] associated with the use of CMV promoter. Co-expression of GALNS with SUMF1, the enzyme required to convert the active-site cysteine to formylglycine [151], resulted in up to 4-fold increase in enzyme activity, depending on the evaluated cell (i.e. fibroblast or chondrocyte) and favored the secretion of the enzyme [103]. In a second phase study, AAV vectors were evaluated in vivo using a Morquio A mouse model after a single intravenous administration [31]. Twelve-weeks after infusion of AAV-GALNS vector, the enzyme activity in plasma was restored to 8.5% of wild-type levels, while the co-administration with AAV-SUMF1 vector increased GALNS activity to 19% of wild-type levels. Enzyme activity was also increased in the peripheral tissues by up to 22% of wild-type levels. Notably, co-administration of AAV-GALNS and AAV-SUMF1 vectors resulted in 33% of wild-type levels in bone tissue. Thus, SUMF1 co-expression enabled delivery of therapeutic levels of the enzyme to the most severely affected tissue in a Morquio A mouse [31]. Finally, in a third phase, we took advantage of the benefits of AAV retargeting by modification of the viral capsid [152]. The AAV vector was modified by insertion of a short acidic amino acid peptide within the viral capsid, to confer affinity of the virus for hydroxyapatite (HA), the major constituent of bone matrix. This increase in affinity for HA resulted in higher vector genome copies in bone, which led to a significant increase in gene expression in bone cells and enzyme activity levels reached 42% of those observed in wild-type animals [153,154]. Recently, a new set of AAV vectors carrying both GALNS and SUMF1 genes were evaluated using internal ribosome entry site (IRES), which allowed higher enzyme activity than that observed with GALNS and SUMF1 genes be transduced with separated vectors [155]. Finally, GALNS transduction mediated by the lentiviral vectors in HEK293 cells and Morquio A skin fibroblasts showed enzyme activity levels 100-fold higher than those observed by using AAV vectors, as well as the normalization of β-hexosaminidase and β-galactosidase activity [155], which has been reported as secondary biomarkers for Morquio A [28,156].
5.4. Management of airway
Progressive tracheal obstruction is commonly seen in patients with MPS, leading to life-threatening complications. The critical aspects of airway management include the ability to provide positive pressure ventilation via a face mask and the ability to intubate the trachea safely. While upper airway obstruction and history of snoring are widely recognized, it is not until recently that the critical narrowing and buckling of trachea was well described. Some degrees of respiratory involvement are common to all forms of MPS IVA and patients often may become progressively difficult to manage during their anesthesia care. Difficult airway is very common especially as patients become older and the causative features are lack of cervical spine mobility, very short neck, limited mouth opening, a narrowed trachea, thickened vocal cords, redundant tissue in the upper airway, and an enlarged tongue (Fig. 8). Clinically, patients with obstructive airway may exhibit loud snoring, daytime hypersomnolence, sleep apnea, and alveolar hypoventilation. The pathophysiology of MPS IVA in the area of airway demonstrates the progressive issues and the presence of vicious cycles. The respiratory problems in MPS IVA comprise both restrictive and obstructive pattern. The restrictive defect is due to thoracic cage deformity and subsequent small lung, and the obstructive defect is from tracheobronchial abnormalities, large tongue, adenoidal, tonsillar, and vocal cord hypertrophy [157]. Moreover, MPS IVA patients have small nasal passages by thickened mucous membranes as a result of GAG deposits contributing to the overall obstructive pathology.
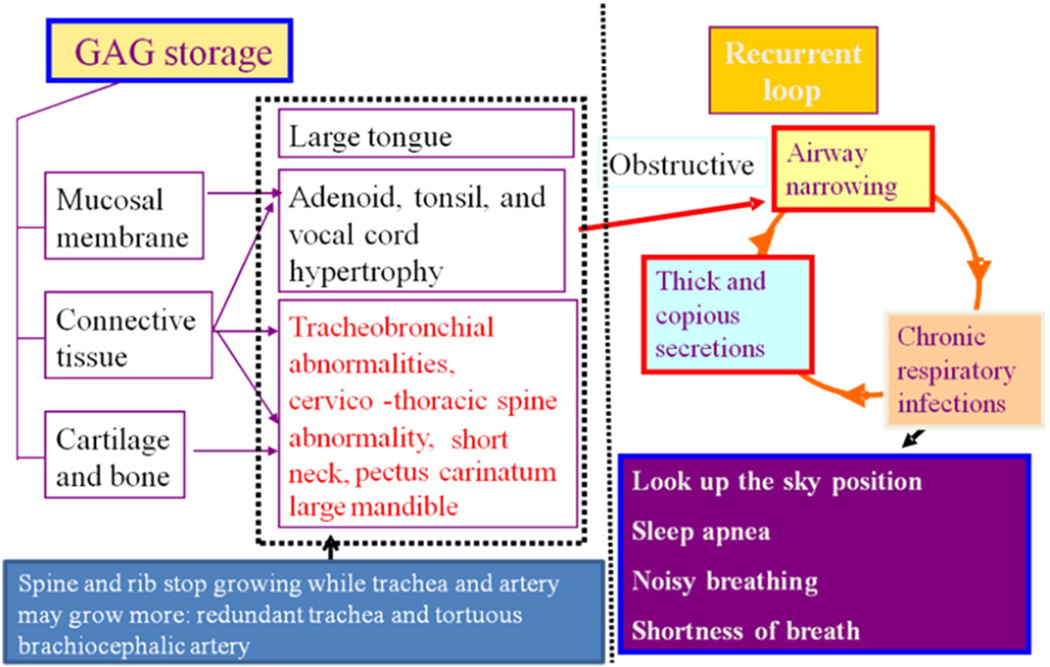
Fig. 8.
Pathophysiology of difficult airway in Morquio A patients. Both restrictive and obstructive respiratory pathology are common in Morquio A patients. The restrictive defect is due to thoracic cage deformity, and the obstructive defect is from tracheobronchial abnormalities, large tongue and mandible, adenoidal, tonsillar, and vocal cord hypertrophy by accumulation of storage materials. Imbalance of growth between trachea, cervical spine, and brachiocephalic artery causes tracheal obstruction. Moreover, Morquio A patients have small nasal passages caused by thickened mucous membranes and thick and copious secretions. Chronic upper respiratory tract infection further decrease the already diminished airway lumen (adapted from Educational CD for Morquio and permitted by Carol Ann Foundation).
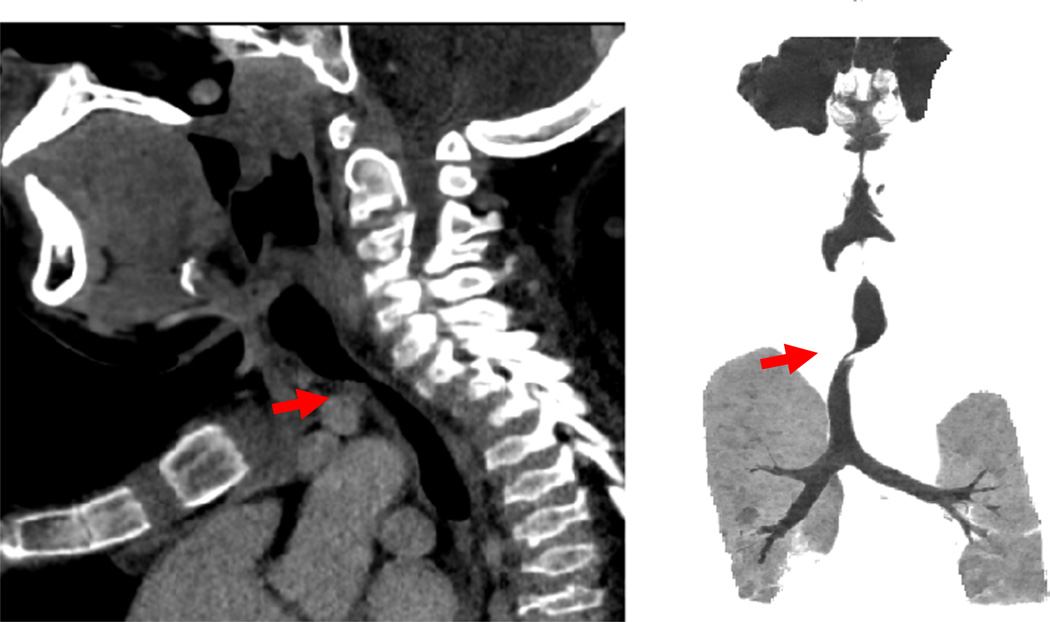
Patients with severe tracheal obstruction in MPS IVA are at risk of dying of sleep apnea and related complications. Tracheal obstruction also leads to life-threatening complications during anesthesia as a result of the difficulty in managing the upper airway due to factors inherent to the MPS IVA, compounded by the difficulty in intubating and extubating the trachea. Sagittal MRI images of the cervical spine of MPS IVA patients (12 ± 8.14 years) showed that 67.9% patients had at least 25% tracheal narrowing and that narrowing worsened with age (all 8 patients over 15 years had >50% narrowing). Around 30% of the patients were categorized as severe (>75%) tracheal narrowing [61]. Evidence of such tracheal narrowing was evident as early as at two years of age. The etiology of tracheal impingement by the innominate artery in MPS IVA appears to be due to a combination of the narrow thoracic inlet crowding the structures and the disproportionate growth of trachea in relationship to the chest cavity leading to tracheal tortuosity (Fig. 8). Tracheal narrowing increases with age in MPS IVA patients (Fig. 9). Greater attention to the trachea is needed with the goal of establishing a timely treatment protocol to reduce the mortality rate.
Fig. 9.
Tracheal obstruction. CT in a 13-year-old patient shows a severe tracheal obstruction. Tracheal narrowing, often due to impression from the crossing bracheocephalic (innominate) artery, increases with age. Note the position of the brachiocephalic artery anterior to the trachea (arrows). Cervico-thoracic spine moves forward while a severe pectus carinatum compresses backward.
Treatment for tracheal and airway obstruction is complicated. Sleep apnea will be diminished either with continuous airway pressure therapy (C-PAP) or adenoidectomy and tonsillectomy. Although tracheostomy can be used to rescue an otherwise inaccessible airway, performing a tracheostomy in a patient with MPS IVA is challenging due to an extremely short neck, fixed cervical vertebrae with inability to hyperex-tend the neck, and a torturous and redundant trachea. Airway obstruction associated with a narrow thoracic inlet and vascular compression requires an alternative approach.
Recently, we have experienced the first case of a 16-year-old MPS IVA patient, whose near-fatal tracheal obstruction was relieved by timely surgical intervention where the innominate artery was relocated and in addition, trachea was resected at the stenotic site and anastomosed trachea vascular reconstruction with dramatic resolution of his respiratory symptoms [60]. Successively, additional three patients, who had a severe tracheal obstruction with respiratory distress, underwent successful tracheal reconstruction surgery, and all of these three patients have shown improvement in their symptoms and are waiting longer term follow up [130]. In addition to the possibility of resolving the airway stenosis by tracheal and vascular reconstruction, this type of procedure permits parallel alignment of the trachea and the innominate artery, which could allow the safe placement of a trachestomy if necessary, while eliminating the potential risk of a tracheo-innominate fistula.
5.5. Orthopedic surgery
While medical therapies including ERT have been, and continue to be, developed for the treatment of Morquio syndrome, these therapies however have provided only modest benefit for skeletal deformities, necessitating monitoring and often times surgical intervention [9]. Anesthesia in individuals with MPS should be regarded with extreme respect. The difficulties of anesthesia in this population include significant upper airway obstruction, tracheomalacia and tracheal stenosis [158]. Most affected children have odontoid hypoplasia, with subluxation, thus preventing usual head positioning required for visualization of the vocal cords at intubation. In older children, there is more difficulty with airway management.
Preoperative evaluation by the anesthesia team, as well as evaluation by cardiology, pulmonology, and otolaryngology is strongly recommended [159]. Placement of a tracheostomy should be considered very carefully although it is not recommended since a new tracheal reconstructive surgery is now available. The reservation of a postoperative critical care bed is also recommended. Neuromonitoring for spinal cord injury is recommended for all surgeries (including non-spinal surgery) longer than 45 min [58,160].
5.5.1. Cervical spine
Prophylactic treatment of atlantoaxial instability is no longer recommended [161]. Close clinical and imaging evaluation should dictate the need for surgical stabilization of the occipital-cervical junction. An absolute minimum space available for the cord (SAC) has not established. While published minimums for adults suggest treatment for SAC < 14 mm on plain radiograph, it is not uncommon for Morquio patients to have narrowing below 10 mm on MRI, but still show reasonable CSF flow. Often the amount of instability in MPS can be subtle, with only several millimeters of physiologic motion. When combined with stenosis however, small amounts of instability can become problematic. In “classic” Morquio however, overt atlantoaxial instability is the preeminent finding [162,163]. Decompression with fusion, often from occiput to C2, is recommended for these patients. Atlantoaxial instability should be surgically addressed when there are signs and symptoms of myelopathy (unsteady gait, upper and lower extremity weakness, dysesthesias, urinary retention), significant instability (>8 mm) or cord signal change on T2-weighted MRI. As instability is part and parcel of the disease in these patients, decompression with fusion is recommended [164–166]. Instrumentation can be challenging as the spinal anatomy is usually hypoplastic. Halo vest support post-operatively is often required.
5.5.2. Gibbus deformity (thoracolumbar kyphosis)
Severe focal kyphotic deformities at the thoracolumbar junction are less commonly seen in Morquio syndrome than other forms of MPS [167,168]. The rate of progression is variable and, therefore, needs to be followed. Surgical stabilization is recommended for continued progression or myelopathy. Progressive curves may be observed up to 70° as they tend to sit below the level of the conus and, therefore, do not represent an immediate threat to the neural elements. Anterior and posterior fusion or three column correction (i.e., vertebral column resection), is recommended, particularly for advanced curves. Instrumentation is usually possible with small stature instrumentation. Postoperative bracing for three months, due to the hypoplastic elements is also recommended. Kyphotic deformities may be accompanied by scoliosis, a necessitating extension of the fusion to include these deformities. The role of delay tactics such as bracing, casting and growth-friendly surgical techniques have not been studied in MPS, but these should be considered part of the armamentarium required to care for these complex patients.
5.5.3. Hip dysplasia and osteonecrosis
Acetabular dysplasia and progressive subluxation of the hip is essentially universal in Morquio syndrome and does not resolve despite the presence of a significant cartilaginous analogue on MRI and arthrogram [47]. Surgical treatment should be considered to treat the global acetabular deficiency and to prevent progressive subluxation and debilitating arthrosis. Surgical treatment of hip dysplasia is controversial, due to associated femoral head osteonecrosis, but may be appropriate with painful subluxation. Literature suggests that excellent radiographic outcomes can be achieved with surgical reconstruction of the hip. Most authors recommend treatment of both acetabular and femoral sided pathology [47,169]. San Diego (Dega) innominate osteotomy or shelf arthroplasty for the pelvic side with a femoral varus osteotomy have been recommended. Frank dislocation is uncommon, so capsulorraphy is not typically required. Patients with MPS IVA are prone to resubluxation, and so augmentation with an acetabular shelf procedure has also been recommended. Total hip arthroplasty is advocated in young adult patients with MPS IVA who have pain limiting hip disease, beyond traditional reconstructive capabilities [170].
5.5.4. Lower extremity valgus
Patients with MPS commonly develop genu valgum severe enough to require treatment. Genu valgum appears to be responsive to growth guidance techniques [47,171]. Two-hole growth modulation plates are recommended, particularly in smaller children as they allow more reliable fixation in the small cartilaginous epiphyses. Osteotomy of the proximal tibia or distal femur may be required in patients with limited growth potential. Total knee arthroplasty has been reported for patients with severe degenerative arthritis. In these cases, significant lateral release and allograft augmentation may be required [172,173].
Ankle valgus (tibiotalar joint) may contribute the valgus deformity of the lower extremities. Screw hemiepiphysiodesis through the medial malleolus provides a low profile, effective treatment to augment overall extremity malalignment. Pes valgus (flat foot) responds well to custom orthotics. Surgical intervention is rarely needed, but may be considered if the pain is not responsive to orthotics treatment.
5.6. Future therapies
Conventional ERT and HSCT are currently available in clinical practice for patients with Morquio A. These therapies show some improvement in symptoms and ADL; but they have a limited effect on established bone and cartilage lesions. If patients are treated earlier with ERT or HSCT it is likely that fewer bone and cartilage lesions will arise but it is not yet known whether these lesions can be prevented completely by these therapies. Bone-targeted therapy is expected to improve cartilage and bone lesion in patients with Morquio A. The targeting of GALNS to bone via a hexaglutamate sequence as described above is a promising approach to improve the impact of ERT on bone lesions in Morquio A [30].
Like HSCT, gene therapy is expected to be a one-time permanent treatment as enzyme activity would be maintained continuously.
6. Conclusion
Conventional ERT and HSCT are currently available in clinical practice for patients with MPS IVA. These therapies have a partial improvement in symptoms and ADL; however, ERT and HSCT have a limited effect on established bone and cartilage lesions. While our studies indicate that HSCT can prevent and slow the advancing of skeletal dysplasia in patients with MPS IVA [53,143] more HSCT cases for MPS IVA are needed to compare it with ERT and to determine best transplant processes and treatment benefits and consequences. Gene therapy is expected a one-time permanent treatment and enzyme activity would be maintained continuously. Type of vector, route of administration and adverse effects remains unanswered. Surgical intervention is often required to improve quality of life in patients with MPS IVA, and currently it is important to use surgical intervention in combination with ERT or HSCT. A new surgical intervention for tracheal obstruction will be the current best option to improve severe narrowing trachea leading to reduction of high mortality rate and marked improvement of ADL. We need to accumulate more surgical cases to establish the best operative procedure. A well-trained team and facility are required to deal with respiratory failure risk.
International Morquio A investigational team
We have established a basic and clinical research team for Morquio A to develop novel non-invasive assessment methods and therapeutic approaches, leading to accurate and practical evaluations of prognosis, therapeutic efficacy, and clinical severity. This multi-disciplinary team comprises experts in the fields of orthopedics, cardiothoracic surgery, anesthesiology, genetics, hematology and transplantation, pharmacokinetics, biochemistry, radiology, molecular biology, pulmonology, and hearing function. The team has over 50 publications related to Morquio A over the last five years in basic and clinical research, diagnosis, bio-markers, and treatments including orthopedic and tracheal surgery, ERT, HSCT, and gene therapy. Importantly, the Japanese team members have direct experience in both ERT and HSCT for multiple forms of MPS, including Morquio A. The experts at Gifu University alone have over 100 publications related to Morquio A since 1971. Thus, this team is the most internationally recognized group of scientists that have taken Morquio A research from the bench to the clinic.
Acknowledgments
This work was supported by grants from the Austrian MPS Society and International Morquio Organization (Carol Ann Foundation). This work was also supported by Japanese MPS Family Society. Y.S. and K.E.O. were supported by the Ministry of Health, Labor, and Welfare of Japan. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant number P30GM114736. S.T. was supported by the National Institutes of Health grant R01HD065767. The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Abbreviations
- MPS
mucopolysaccharidosis
- GAG
glycosaminoglycans
- C6S
chondroitin-6-sulfate
- KS
keratin sulfate
- ECM
extracellular matrix
- GALNS
N-acetylgalactosamine 6-sulfate sulfatase
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- LSD
lysosomal storage disorder
- DBS
dried blood spot
- DMB
dimethylmethylene blue
- ERT
enzyme replacement therapy
- 6MWT
6 min walk test
- 3MSCT
3 min stair climb test
- ADL
activity of daily living
- HSCT
Hematopoietic Stem Cell Therapy
- BMT
bone marrow transplantation
- GVHD
graft-versus-host disease
- AAV
adenoassociated viral vector
Footnotes
Conflict of interest
All the authors contributed to the Review Article and had no conflict of interest with any other party.
Shaukat Khan, Carlos J. Alméciga-Díaz, Kazuki Sawamoto, William G. Mackenzie, Mary C. Theroux, Christian Pizarro, Robert W. Mason, Tadao Orii, and Shunji Tomatsu declare that they have no conflict of interests.
Contributions to the project
Shaukat Khan is the primary author for this review article and an expert in molecular biology and chemistry. He has over 10 year of experience in MPS field from basic science to gene therapy. He has contributed to the concept and planning of the article, collection of previous articles and data, and reporting of the work described.
Carlos J. Alméciga-Díaz is the primary author for this review article and expert in pharmacology, ERT, and gene therapy. He has contributed to the planning, data analysis, and reporting of the work described.
Kazuki Sawamoto is an expert in pharmacokinetics. He has 10 years of research experience in model mouse to translational research and drug development. He has contributed to the planning, data analysis, and reporting of the work described.
William G. Mackenzie is an orthopedic surgeon and has over 30 years of experience in Morquio surgery. He has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Mary C. Theroux is an experienced anesthesiologist for Morquio patients over 20 years and has contributed to the concept of the manuscript, planning of the article, and reporting of the work described.
Christian Pizarro is a pediatric cardiothoracic surgeon who has first performed tracheal reconstructive surgery in Morquio A syndrome and has contributed to the planning of the article, treating Morquio A patients who had a tracheal obstruction, and reporting of the work described.
Robert W. Mason has contributed to data analysis, and reporting of the work described.
Tadao Orii is a medical doctor with 50 years of clinical and research experiences in Morquio A. He published over 150 articles and chapter books in this field. He has contributed to the planning, data analysis, and reporting of the work described.
Shunji Tomatsu is a Principal Investigator and has 30 years of clinical and research experience in Morquio A, publishing over 100 articles in this field. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the review.
References
- 1.Morquio L. Sur une forme de dystrophic osseuse familiale. Arch. Med. Infant. 1929;32:129–135. [Google Scholar]
- 2.JF B. Chondro-osteo-dystrophy. Roentgenographic and clinical features of a child with dislocation of vertebrae. Am. J. Surg. 1929;7:404–410. [PubMed] [Google Scholar]
- 3.Pedrini V, Lennzi L, Zambotti V. Isolation and identification of keratosulphate in urine of patients affected by Morquio-Ullrich disease. Proc. Soc. Exp. Biol. Med. 1962;110:847–849. doi: 10.3181/00379727-110-27668. [DOI] [PubMed] [Google Scholar]
- 4.McKusick VA, Kaplan D, Wise D, Hanley WB, Suddarth SB, Sevick ME, Maumanee AE. The genetic mucopolysaccharidoses. Medicine. 1965;44:445–483. doi: 10.1097/00005792-196511000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Orii T, Minami R, Chiba T, Yamaguchi M, Tsugawa S, Nakao T, Horino K, Sakuma K. Study on Morquio syndrome bone metabolism (in Japanese) 1971;5:72–78. [Google Scholar]
- 6.Orii T, Kiman T, Sukegawa K, Kanemura T, Hattori S, Taga T, Hirota K. Late onset Nacetylgalactosamine-6-sulfate sulfatase deficiency in two brothers. Connect. Tissue Res. 1981;13:169–175. [Google Scholar]
- 7.Sukegawa K, Orii T. Residual activity in fibroblasts from two brothers with the late-onset form of N-acetylgalactosamine-6-sulphate sulphatase deficiency. J. Inherit. Metab. Dis. 1982;5:231–232. doi: 10.1007/BF02179150. [DOI] [PubMed] [Google Scholar]
- 8.Hecht JT, Scott CI, Jr, Smith TK, Williams JC. Mild manifestations of the Morquio syndrome. Am. J. Med. Genet. 1984;18:369–371. doi: 10.1002/ajmg.1320180222. [DOI] [PubMed] [Google Scholar]
- 9.Tomatsu S, Montano AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T. Mucopolysaccharidosis type IVA (morquio a disease): clinical review and current treatment. Curr. Pharm. Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 10.Hendriksz CJ, Harmatz P, Beck M, Jones S, Wood T, Lachman R, Gravance CG, Orii T, Tomatsu S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matalon R, Arbogast B, Justice P, Brandt IK, Dorfman A. Morquio’s syndrome: deficiency of a chondroitin sulfate N-acetylhexosamine sulfate sulfatase. Biochem. Biophys. Res. Commun. 1974;61:759–765. doi: 10.1016/0006-291x(74)91022-5. [DOI] [PubMed] [Google Scholar]
- 12.Dorfman A, Arbogast B, Matalon R. The enzymic defects in Morquio and Maroteaux-Lamy syndrome. Adv. Exp. Med. Biol. 1976;68:261–276. doi: 10.1007/978-1-4684-7735-1_18. [DOI] [PubMed] [Google Scholar]
- 13.Singh J, Di Ferrante N, Niebes P, Tavella D. N-acetylgalactosamine-6-sulfate sulfa-tase in man. Absence of the enzyme in Morquio disease. J. Clin. Invest. 1976;57:1036–1040. doi: 10.1172/JCI108345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Ferrante N, Ginsberg LC, Donnelly PV, Di Ferrante DT, Caskey CT. Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses. Science. 1978;199:79–81. doi: 10.1126/science.199.4324.79. [DOI] [PubMed] [Google Scholar]
- 15.Glossl J, Kresse H. A sensitive procedure for the diagnosis of N-acetyl-galactos-amine-6-sulfate sulfatase deficiency in classical Morquio’s disease. Clin. Chim. Acta. 1978;88:111–119. doi: 10.1016/0009-8981(78)90157-2. [DOI] [PubMed] [Google Scholar]
- 16.Cozma C, Eichler S, Wittmann G, Flores Bonet A, Kramp GJ, Giese AK, Rolfs A. Diagnosis of morquio syndrome in dried blood spots based on a new MRM-MS assay. PLoS One. 2015;10:e0131228. doi: 10.1371/journal.pone.0131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielicki J, Hopwood JJ. Human liver N-acetylgalactosamine 6-sulphatase. Purification and characterization. Biochem. J. 1991;279(Pt 2):515–520. doi: 10.1042/bj2790515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masue M, Sukegawa K, Orii T, Hashimoto T. N-acetylgalactosamine-6-sulfate sul-fatase in human placenta: purification and characteristics. J. Biochem. 1991;110:965–970. doi: 10.1093/oxfordjournals.jbchem.a123697. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima Y, Tomatsu S, Hori T, Fukuda S, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IVA: molecular cloning of the human N-acetylgalactosamine-6-sulfatase gene (GALNS) and analysis of the 5′-flanking region. Genomics. 1994;20:99–104. doi: 10.1006/geno.1994.1132. [DOI] [PubMed] [Google Scholar]
- 20.Tomatsu S, Fukuda S, Masue M, Sukegawa K, Fukao T, Yamagishi A, Hori T, Iwata H, Ogawa T, Nakashima Y, et al. Morquio disease: isolation, characterization and expression of full-length cDNA for human N-acetylgalactosamine-6-sulfate sulfatase. Biochem. Biophys. Res. Commun. 1991;181:677–683. doi: 10.1016/0006-291x(91)91244-7. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda S, Tomatsu S, Masue M, Sukegawa K, Iwata H, Ogawa T, Nakashima Y, Hori T, Yamagishi A, Hanyu Y, et al. Mucopolysaccharidosis type IVA. N-acetylgalactosamine-6-sulfate sulfatase exonic point mutations in classical Morquio and mild cases. J. Clin. Invest. 1992;90:1049–1053. doi: 10.1172/JCI115919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi GM, Yamagishi A, Yamada N, Kato Z, Isogai K, Sukegawa K, et al. Mucopolysaccharidosis type IVA: identification of six novel mutations among non-Japanese patients. Hum. Mol. Genet. 1995;4:741–743. doi: 10.1093/hmg/4.4.741. [DOI] [PubMed] [Google Scholar]
- 23.Montano AM, Yamagishi A, Tomatsu S, Fukuda S, Copeland NG, Orii KE, Isogai K, Yamada N, Kato ZI, Jenkins NA, Gilbert DJ, Sukegawa K, Orii T, Kondo N. The mouse N-acetylgalactosamine-6-sulfate sulfatase (Galns) gene: cDNA isolation, genomic characterization, chromosomal assignment and analysis of the 5′-flanking region. Biochim. Biophys. Acta. 2000;1500:323–334. doi: 10.1016/s0925-4439(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 24.Tomatsu S, Montano AM, Gutierrez M, Grubb JH, Oikawa H, Dung VC, Ohashi A, Nishioka T, Yamada M, Yamada M, Tosaka Y, Trandafirescu GG, Orii T. Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase. Mol. Genet. Metab. 2007;91:69–78. doi: 10.1016/j.ymgme.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Sukegawa K, Nakamura H, Kato Z, Tomatsu S, Montano AM, Fukao T, Toietta G, Tortora P, Orii T, Kondo N. Biochemical and structural analysis of missense mutations in N-acetylgalactosamine-6-sulfate sulfatase causing mucopolysaccharidosis IVA phenotypes. Hum. Mol. Genet. 2000;9:1283–1290. doi: 10.1093/hmg/9.9.1283. [DOI] [PubMed] [Google Scholar]
- 26.Tomatsu S, Montano AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, Dung VC, Nishioka T, Orii T, Sly WS. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 27.Tomatsu S, Gutierrez M, Nishioka T, Yamada M, Yamada M, Tosaka Y, Grubb JH, Montano AM, Vieira MB, Trandafirescu GG, Pena OM, Yamaguchi S, Orii KO, Orii T, Noguchi A, Laybauer L. Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum. Mol. Genet. 2005;14:3321–3335. doi: 10.1093/hmg/ddi364. [DOI] [PubMed] [Google Scholar]
- 28.Tomatsu S, Orii KO, Vogler C, Nakayama J, Levy B, Grubb JH, Gutierrez MA, Shim S, Yamaguchi S, Nishioka T, Montano AM, Noguchi A, Orii T, Kondo N, Sly WS. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns−/−) produced by targeted disruption of the gene defective in Morquio A disease. Hum. Mol. Genet. 2003;12:3349–3358. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 29.Tomatsu S, Vogler C, Montano AM, Gutierrez M, Oikawa H, Dung VC, Orii T, Noguchi A, Sly WS. Murine model (Galns(tm(C76S)slu)) of MPS IVA with missense mutation at the active site cysteine conserved among sulfatase proteins. Mol. Genet. Metab. 2007;91:251–258. doi: 10.1016/j.ymgme.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Tomatsu S, Montano AM, Dung VC, Ohashi A, Oikawa H, Oguma T, Orii T, Barrera L, Sly WS. Enhancement of drug delivery: enzyme-replacement therapy for murine Morquio A syndrome. Mol. Ther. 2010;18:1094–1102. doi: 10.1038/mt.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeciga-Diaz CJ, Rueda-Paramo MA, Espejo AJ, Echeverri OY, Montano A, Tomatsu S, Barrera LA. Effect of elongation factor 1alpha promoter and SUMF1 over in vitro expression of N-acetylgalactosamine-6-sulfate sulfatase. Mol. Biol. Rep. 2009;36:1863–1870. doi: 10.1007/s11033-008-9392-3. [DOI] [PubMed] [Google Scholar]
- 32.Tomatsu S, Almeciga-Diaz CJ, Barbosa H, Montano AM, Barrera LA, Shimada T, Yasuda E, Mackenzie WG, Mason RW, Suzuki Y, Orii KE, Orii T. Therapies of mucopolysaccharidosis IVA (Morquio A syndrome) Expert Opin. Orphan Drugs. 2013;1:805–818. doi: 10.1517/21678707.2013.846853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomatsu S, Yasuda E, Patel P, Ruhnke K, Shimada T, Mackenzie WG, Mason R, Thacker MM, Theroux M, Montano AM, Almeciga-Diaz CJ, Barrera LA, Chinen Y, Sly WS, Rowan D, Suzuki Y, Orii T. Morquio A syndrome: diagnosis and current and future therapies. Pediatr. Endocrinol. Rev. 2014;12(Suppl. 1):141–151. [PMC free article] [PubMed] [Google Scholar]
- 34.Hintze JP, Tomatsu S, Fujii T, Montano AM, Yamaguchi S, Suzuki Y, Fukushi M, Ishimaru T, Orii T. Comparison of liquid chromatography-tandem mass spectrom-etry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark. Insights. 2011;6:69–78. doi: 10.4137/BMI.S7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimada T, Tomatsu S, Mason RW, Yasuda E, Mackenzie WG, Hossain J, Shibata Y, Montano AM, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T. Di-sulfated keratan sulfate as a novel biomarker for mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thonar EJ, Pachman LM, Lenz ME, Hayford J, Lynch P, Kuettner KE. Age related changes in the concentration of serum keratan sulphate in children journal of clinical chemistry and clinical biochemistry. Z. Klin. Chem. Klin. Biochem. 1988;26:57–63. doi: 10.1515/cclm.1988.26.2.57. [DOI] [PubMed] [Google Scholar]
- 37.Tomatsu S, Dieter T, Schwartz IV, Sarmient P, Giugliani R, Barrera LA, Guelbert N, Kremer R, Repetto GM, Gutierrez MA, Nishioka T, Serrato OP, Montano AM, Yamaguchi S, Noguchi A. Identification of a common mutation in mucopolysaccharidosis IVA: correlation among genotype, phenotype, and keratan sulfate. J. Hum. Genet. 2004;49:490–494. doi: 10.1007/s10038-004-0178-8. [DOI] [PubMed] [Google Scholar]
- 38.Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, Kida K, Shibata Y, Futatsumori H, Montano AM, Mason RW, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Kida K, Kubota M, Barrera L, Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J. Inherit. Metab. Dis. 2010;33(Suppl. 3):S35–S42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 40.Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr. Res. 2004;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 41.Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 42.Montano AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am. J. Med. Genet. A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 43.Tomatsu S, Montaño A, Oikawa H, Giugliani R, Harmatz P, Smith M, Suzuki Y, Orii T. Impairment of body growth in mucopolysaccharidoses, Impairment of Body Growth in Mucopolysaccharidoses. Springer New York Dordrecht Heidelberg London: Springer Science + Businessmedia, LLC; 2012. [Google Scholar]
- 44.Baratela WA, Bober MB, Thacker MM, Belthur MV, Oto M, Rogers KJ, Mackenzie WG. Cervicothoracic myelopathy in children with Morquio syndrome A: a report of 4 cases. J. Pediatr. Orthop. 2014;34:223–228. doi: 10.1097/BPO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 45.Dede O, Thacker MM, Rogers KJ, Oto M, Belthur MV, Baratela W, Mackenzie WG. Upper cervical fusion in children with Morquio syndrome: intermediate to long-term results. J. Bone Joint Surg. Am. 2013;95:1228–1234. doi: 10.2106/JBJS.J.01135. [DOI] [PubMed] [Google Scholar]
- 46.Dhawale AA, Church C, Henley J, Holmes L, Jr, Thacker MM, Mackenzie WG, Miller F. Gait pattern and lower extremity alignment in children with Morquio syndrome. J. Pediatr. Orthop. B. 2013;22:59–62. doi: 10.1097/BPB.0b013e32835a0e6d. [DOI] [PubMed] [Google Scholar]
- 47.Dhawale AA, Thacker MM, Belthur MV, Rogers K, Bober MB, Mackenzie WG. The lower extremity in Morquio syndrome. J. Pediatr. Orthop. 2012;32:534–540. doi: 10.1097/BPO.0b013e318259fe57. [DOI] [PubMed] [Google Scholar]
- 48.Sitoula P, Mackenzie WG, Shah SA, Thacker M, Ditro C, Holmes L, Jr, Campbell JW, Rogers KJ. Occipitocervical fusion in skeletal dysplasia: a new surgical technique. Spine. 2014;39:E912–E918. doi: 10.1097/BRS.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 49.Solanki GA, Martin KW, Theroux MC, Lampe C, White KK, Shediac R, Lampe CG, Beck M, Mackenzie WG, Hendriksz CJ, Harmatz PR. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): presentation, diagnosis and management. J. Inherit. Metab. Dis. 2013;36:339–355. doi: 10.1007/s10545-013-9586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomatsu S, Almeciga-Diaz CJ, Montano AM, Yabe H, Tanaka A, Dung VC, Giugliani R, Kubaski F, Mason RW, Yasuda E, Sawamoto K, Mackenzie W, Suzuki Y, Orii KE, Barrera LA, Sly WS, Orii T. Therapies for the bone in mucopolysaccharidoses. Mol. Genet. Metab. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomatsu S, Mackenzie WG, Theroux MC, Mason RW, Thacker MM, Shaffer TH, Montano AM, Rowan D, Sly W, Almeciga-Diaz CJ, Barrera LA, Chinen Y, Yasuda E, Ruhnke K, Suzuki Y, Orii T. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res. Rep. Endocr. Disord. 2012;2012:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White KK, Jester A, Bache CE, Harmatz PR, Shediac R, Thacker MM, Mackenzie WG. Orthopedic management of the extremities in patients with Morquio A syndrome. J. Child. Orthop. 2014;8:295–304. doi: 10.1007/s11832-014-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol. Genet. Metab. Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendriksz CJ, Berger KI, Giugliani R, Harmatz P, Kampmann C, Mackenzie WG, Raiman J, Villarreal MS, Savarirayan R. International guidelines for the management and treatment of Morquio A syndrome. Am. J. Med. Genet. A. 2015;167A:11–25. doi: 10.1002/ajmg.a.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, Lin SP, Mengel E, Scarpa M, Valayannopoulos V, Giugliani R, Investigators S, Slasor P, Lounsbury D, Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomatsu S, Sawamoto K, Almeciga-Diaz CJ, Shimada T, Bober MB, Chinen Y, Yabe H, Montano AM, Giugliani R, Kubaski F, Yasuda E, Rodriguez-Lopez A, Espejo-Mojica AJ, Sanchez OF, Mason RW, Barrera LA, Mackenzie WG, Orii T. Impact of enzyme replacement therapy and hemato-poietic stem cell transplantation in patients with Morquio A syndrome. Drug Des. Devel. Ther. 2015;9:1937–1953. doi: 10.2147/DDDT.S68562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomatsu S, Sawamoto K, Shimada T, Bober MB, Kubaski F, Yasuda Y, Mason RW, Khan S, Almeciga-Diaz CJ, Barrera LA, Mackenzie WG, Orii T. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations. Expert Opin. Orphan Drugs. 2015;3:1279–1290. doi: 10.1517/21678707.2015.1086640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drummond JC, Krane EJ, Tomatsu S, Theroux MC, Lee RR. Paraplegia after epi-dural-general anesthesia in a Morquio patient with moderate thoracic spinal stenosis. Can. J. Anaesth. 2015;62:45–49. doi: 10.1007/s12630-014-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and periopera-tive complications of children with Morquio syndrome. Paediatr. Anaesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 60.Pizarro C, Davies RR, Theroux M, Spurrier EA, Averill LW, Tomatsu S. Surgical reconstruction for severe tracheal obstruction in Morquio A syndrome. Ann. Thorac. Surg. 2016;102:e329–e331. doi: 10.1016/j.athoracsur.2016.02.113. [DOI] [PubMed] [Google Scholar]
- 61.Tomatsu S, Averill LW, Sawamoto K, Mackenzie WG, Bober MB, Pizarro C, Goff CJ, Xie L, Orii T, Theroux M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol. Genet. Metab. 2016;117:150–156. doi: 10.1016/j.ymgme.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neufeld E, Muenzer J. The metabolic and molecular bases of inherited disease, in. In: Scriver CBASWVD, editor. The Mucopolysaccharidoses. New York: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- 63.Patel P, Suzuki Y, Maeda M, Yasuda E, Shimada T, Orii KE, Orii T, Tomatsu S. Growth charts for patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014;1:5–18. doi: 10.1016/j.ymgmr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomatsu S, Montaño A, Oikawa H, Giugliani R, Harmatz P, Smith M, Suzuki Y, Orii T. Impairment of body growth in mucopolysaccharidoses. 2012;1:2091–2117. [Google Scholar]
- 65.Yasuda E, Fushimi K, Suzuki Y, Shimizu K, Takami T, Zustin J, Patel P, Ruhnke K, Shimada T, Boyce B, Kokas T, Barone C, Theroux M, Mackenzie W, Nagel B, Ryerse JS, Orii KE, Iida H, Orii T, Tomatsu S. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez ME, Mackenzie WG, Ditro C, Miller TL, Chidekel A, Shaffer TH. Skeletal dysplasias: evaluation with impulse oscillometry and thoracoabdominal motion analysis. Pediatr. Pulmonol. 2010;45:679–686. doi: 10.1002/ppul.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pshezhetsky AV, Potier M. Association of N-acetylgalactosamine-6-sulfate sulfa-tase with the multienzyme lysosomal complex of beta-galactosidase, cathepsin A, and neuraminidase. Possible implication for intralysosomal catabolism of keratan sulfate. J. Biol. Chem. 1996;271:28359–28365. doi: 10.1074/jbc.271.45.28359. [DOI] [PubMed] [Google Scholar]
- 68.Morrone A, Caciotti A, Atwood R, Davidson K, Du C, Francis-Lyon P, Harmatz P, Mealiffe M, Mooney S, Oron TR, Ryles A, Zawadzki KA, Miller N. Morquio A syndrome-associated mutations: a review of alterations in the GALNS gene and a new locus-specific database. Hum. Mutat. 2014;35:1271–1279. doi: 10.1002/humu.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomatsu S, Montano AM, Nishioka T, Gutierrez MA, Pena OM, Tranda Firescu GG, Lopez P, Yamaguchi S, Noguchi A, Orii T. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum. Mutat. 2005;26:500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- 70. http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GALNS 2016.
- 71.Bunge S, Kleijer WJ, Tylki-Szymanska A, Steglich C, Beck M, Tomatsu S, Fukuda S, Poorthuis BJ, Czartoryska B, Orii T, Gal A. Identification of 31 novel mutations in the N-acetylgalactosamine-6-sulfatase gene reveals excessive allelic heterogeneity among patients with Morquio A syndrome. Hum. Mutat. 1997;10:223–232. doi: 10.1002/(SICI)1098-1004(1997)10:3<223::AID-HUMU8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 72.Hori T, Tomatsu S, Nakashima Y, Uchiyama A, Fukuda S, Sukegawa K, Shimozawa N, Suzuki Y, Kondo N, Horiuchi T, et al. Mucopolysaccharidosis type IVA: common double deletion in the N-acetylgalactosamine-6-sulfatase gene (GALNS) Genomics. 1995;26:535–542. doi: 10.1016/0888-7543(95)80172-i. [DOI] [PubMed] [Google Scholar]
- 73.Kato Z, Fukuda S, Tomatsu S, Vega H, Yasunaga T, Yamagishi A, Yamada N, Valencia A, Barrera LA, Sukegawa K, Orii T, Kondo N. A novel common missense mutation G301C in the N-acetylgalactosamine-6-sulfate sulfatase gene in mucopolysaccharidosis IVA. Hum. Genet. 1997;101:97–101. doi: 10.1007/s004390050594. [DOI] [PubMed] [Google Scholar]
- 74.Ogawa T, Tomatsu S, Fukuda S, Yamagishi A, Rezvi GM, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Oru T. Mucopolysaccharidosis IVA: screening and identification of mutations of the N-acetylgalactosamine-6-sulfate sulfatase gene. Hum. Mol. Genet. 1995;4:341–349. doi: 10.1093/hmg/4.3.341. [DOI] [PubMed] [Google Scholar]
- 75.Tomatsu S, Filocamo M, Orii KO, Sly WS, Gutierrez MA, Nishioka T, Serrato OP, Di Natale P, Montano AM, Yamaguchi S, Kondo N, Orii T, Noguchi A. Mucopolysaccharidosis IVA (Morquio A): identification of novel common mutations in the N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene in Italian patients. Hum. Mutat. 2004;24:187–188. doi: 10.1002/humu.9265. [DOI] [PubMed] [Google Scholar]
- 76.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Ferreira P, Di Natale P, Tortora P, Fujimoto A, Kato Z, Yamada N, Isogai K, Yamagishi A, Sukegawa K, Suzuki Y, Shimozawa N, Kondo N, Sly WS, Orii T. Fourteen novel mucopolysaccharidosis IVA producing mutations in GALNS gene. Hum. Mutat. 1997;10:368–375. doi: 10.1002/(SICI)1098-1004(1997)10:5<368::AID-HUMU6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 77.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi GM, Yamagishi A, Yamada N, Kato Z, Isogai K, Sukegawa K, et al. Mucopolysaccharidosis IVA: identification of a common missense mutation I113F in the N-acetylgalactosamine-6-sulfate sulfa-tase gene. Am. J. Hum. Genet. 1995;57:556–563. [PMC free article] [PubMed] [Google Scholar]
- 78.Tomatsu S, Fukuda S, Yamagishi A, Cooper A, Wraith JF, Hori T, Kato Z, Yamada N, Isogai K, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IVA: four new exonic mutations in patients with N-acetylgalactosamine-6-sulfate sulfatase deficiency. Am. J. Hum. Genet. 1996;58:950–962. [PMC free article] [PubMed] [Google Scholar]
- 79.Tomatsu S, Montano AM, Lopez P, Trandafirescu G, Gutierrez MA, Oikawa H, Nishioka T, Vieira MB, Orii T, Noguchi A. Determinant factors of spectrum of mis-sense variants in mucopolysaccharidosis IVA gene. Mol. Genet. Metab. 2006;89:139–149. doi: 10.1016/j.ymgme.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Yamada N, Fukuda S, Tomatsu S, Muller V, Hopwood JJ, Nelson J, Kato Z, Yamagishi A, Sukegawa K, Kondo N, Orii T. Molecular heterogeneity in mucopolysaccharidosis IVA in Australia and Northern Ireland: nine novel mutations including T312S, a common allele that confers a mild phenotype. Hum. Mutat. 1998;11:202–208. doi: 10.1002/(SICI)1098-1004(1998)11:3<202::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 81.Tamarozzi ER, Torrieri E, Semighini EP, Giuliatti S. In silico analysis of mutations occurring in the protein N-acetylgalactosamine-6-sulfatase (GALNS) and causing mucopolysaccharidosis IVA. Genet. Mol. Res. 2014;13:10025–10034. doi: 10.4238/2014.November.28.7. [DOI] [PubMed] [Google Scholar]
- 82.Ohashi A, Montano AM, Colon JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–769. doi: 10.1111/j.1651-2227.2009.01134.x. (910-762) [DOI] [PubMed] [Google Scholar]
- 83.Wood TC, Harvey K, Beck M, Burin MG, Chien YH, Church HJ, D’Almeida V, van Diggelen OP, Fietz M, Giugliani R, Harmatz P, Hawley SM, Hwu WL, Ketteridge D, Lukacs Z, Miller N, Pasquali M, Schenone A, Thompson JN, Tylee K, Yu C, Hendriksz CJ. Diagnosing mucopolysaccharidosis IVA. J. Inherit. Metab. Dis. 2013;36:293–307. doi: 10.1007/s10545-013-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin. Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- 85.Gray G, Claridge P, Jenkinson L, Green A. Quantitation of urinary glycosaminoglycans using dimethylene blue as a screening technique for the diagnosis of mucopolysaccharidoses: an evaluation. Ann. Clin. Biochem. 2007;44:360–363. doi: 10.1258/000456307780945688. [DOI] [PubMed] [Google Scholar]
- 86.Piraud M, Boyer S, Mathieu M, Maire I. Diagnosis of mucopolysaccharidoses in a clinically selected population by urinary glycosaminoglycan analysis: a study of 2,000 urine samples. Clin. Chim. Acta. 1993;221:171–181. doi: 10.1016/0009-8981(93)90031-x. [DOI] [PubMed] [Google Scholar]
- 87.Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminogly-can in urine samples collected on a paper matrix. Clin. Chem. 1989;35:2074–2081. [PubMed] [Google Scholar]
- 88.Wood T, Bodamer OA, Burin MG, D’Almeida V, Fietz M, Giugliani R, Hawley SM, Hendriksz CJ, Hwu WL, Ketteridge D, Lukacs Z, Mendelsohn NJ, Miller N, Pasquali M, Schenone A, Schoonderwoerd K, Winchester B, Harmatz P. Expert recommendations for the laboratory diagnosis of MPS VI. Mol. Genet. Metab. 2012;106:73–82. doi: 10.1016/j.ymgme.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 89.Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycos-aminoglycan excretion. Clin. Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- 90.Martell LA, Cunico RL, Ohh J, Fulkerson W, Furneaux R, Foehr ED. Validation of an LC-MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis. 2011;3:1855–1866. doi: 10.4155/bio.11.172. [DOI] [PubMed] [Google Scholar]
- 91.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed. Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 92.Tomatsu S, Shimada T, Mason RW, Kelly J, LaMarr WA, Yasuda E, Shibata Y, Futatsumori H, Montano AM, Yamaguchi S, Suzuki Y, Orii T. Assay for glycosamino-glycans by tandem mass spectrometry and its applications. J. Anal. Bioanal. Tech. 2014;2014:006. doi: 10.4172/2155-9872.S2-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomatsu S, Shimada T, Mason RW, Montano AM, Kelly J, LaMarr WA, Kubaski F, Giugliani R, Guha A, Yasuda E, Mackenzie W, Yamaguchi S, Suzuki Y, Orii T. Establishment of glycosaminoglycan assays for mucopolysaccharidoses. Metabolites. 2014;4:655–679. doi: 10.3390/metabo4030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomatsu S, Shimada T, Patel P, Mason R, Mikami T, Kitagawa H, Akama T, Montano AM, Orii T. Chondroitin and keratan sulfate. Nova Sci. 2015 [Google Scholar]
- 95.Shimada T, Tomatsu S, Yasuda E, Mason RW, Mackenzie WG, Shibata Y, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii K, Orii T. Chondroitin 6-sulfate as a novel biomarker for mucopolysaccharidosis IVA and VII. JIMD Rep. 2014;16:15–24. doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kleijer WJ, Geilen GC, Garritsen V, Huijmans JG, Los FJ, Voznyi YV, van Diggelen OP. First-trimester diagnosis of Morquio disease type. Prenat. Diagn. 2000;20:183–185. [PubMed] [Google Scholar]
- 97.Zhao H, Van Diggelen OP, Thoomes R, Huijmans J, Young E, Mazurczak T, Kleijer WJ. Prenatal diagnosis of Morquio disease type A using a simple fluorometric enzyme assay. Prenat. Diagn. 1990;10:85–91. doi: 10.1002/pd.1970100204. [DOI] [PubMed] [Google Scholar]
- 98.Camelier MV, Burin MG, De Mari J, Vieira TA, Marasca G, Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio syndrome type A) in dried blood samples. Clin. Chim. Acta. 2011;412:1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Duffey TA, Khaliq T, Scott CR, Turecek F, Gelb MH. Design and synthesis of substrates for newborn screening of Maroteaux-Lamy and Morquio A syndromes. Bioorg. Med. Chem. Lett. 2010;20:5994–5996. doi: 10.1016/j.bmcl.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKusick V, Neufeld E. The Metabolic Basis of Inherited Disease. New York: McGraw-Hill; 1983. [Google Scholar]
- 101.Rowan DJ, Tomatsu S, Grubb JH, Montano AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J. Inherit. Metab. Dis. 2013;36:235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomatsu S, Okamura K, Maeda H, Taketani T, Castrillon SV, Gutierrez MA, Nishioka T, Fachel AA, Orii KO, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Haskins M, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Okuyama T, Tanaka A, Noguchi A. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 103.Almeciga-Diaz CJ, Montano AM, Tomatsu S, Barrera LA. Adeno-associated virus gene transfer in Morquio A disease - effect of promoters and sulfatase-modifying factor 1. FEBS J. 2010;277:3608–3619. doi: 10.1111/j.1742-4658.2010.07769.x. [DOI] [PubMed] [Google Scholar]
- 104.Kakkis ED, McEntee MF, Schmidtchen A, Neufeld EF, Ward DA, Gompf RE, Kania S, Bedolla C, Chien SL, Shull RM. Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. Biochem. Mol. Med. 1996;58:156–167. doi: 10.1006/bmme.1996.0044. [DOI] [PubMed] [Google Scholar]
- 105.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 106.Tomatsu S, Montaño A, Gutiérrez M, Grubb J, Oikawa H, Dung V, Ohashi A, Nishioka T, Yamada N, Tosaka Y, Trandafirescu G, Orii T. Characterization and phar-macokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfa-tase. Mol. Genet. Metab. 2007;91:69–78. doi: 10.1016/j.ymgme.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 107.Dvorak-Ewell M, Wendt D, Hague C, Christianson T, Koppaka V, Crippen D, Kakkis E, Vellard M. Enzyme replacement in a human model of mucopolysaccharidosis IVA in vitro and its biodistribution in the cartilage of wild type mice. PLoS One. 2010;5:e12194. doi: 10.1371/journal.pone.0012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hernandez A, Velasquez O, Leonardi F, Soto C, Rodriguez A, Lizaraso L, Mosquera A, Bohorquez J, Coronado A, Espejo A, Sierra R, Sanchez OF, Almeciga-Diaz CJ, Barrera LA. Effect of culture conditions and signal peptide on production of human recombinant N-acetylgalactosamine-6-sulfate sulfatase in Escherichia coli BL21. Microb. Biotechnol. 2013;23:689–698. doi: 10.4014/jmb.1211.11044. [DOI] [PubMed] [Google Scholar]
- 109.Mosquera A, Rodríguez A, Vargas C, Leonardi F, Espejo A, Sánchez O, Alméciga-Díaz C, Barrera L. Characterization of a recombinant N-acetylgalactosamine-6-sulfate sulfatase produced in E. coli for enzyme replacement therapy of Morquio A disease. Process Biochem. 2012;47:2097–2102. [Google Scholar]
- 110.Mosquera A, Rodríguez A, Vargas C, Leonardi F, Espejo A, Sánchez O, Alméciga-Díaz C, L B. Characterization of a recombinant N-acetylgalactosamine-6-sulfate sulfatase produced in E. coli for enzyme replacement therapy of Morquio A disease. Process Biochem. 2012;47:2097–2102. [Google Scholar]
- 111.Rodriguez A, Espejo AJ, Hernandez A, Velasquez OL, Lizaraso LM, Cordoba HA, Sanchez OF, Almeciga-Diaz CJ, Barrera LA. Enzyme replacement therapy for Morquio A: an active recombinant N-acetylgalactosamine-6-sul-fate sulfatase produced in Escherichia coli BL21. J. Ind. Microbiol. Biotechnol. 2010;37:1193–1201. doi: 10.1007/s10295-010-0766-x. [DOI] [PubMed] [Google Scholar]
- 112.Alméciga-Díaz C, Rodríguez-López A, Sánchez J, Moreno J, Diaz D, Beltran LM, Espejo AJ, Ruiz FO, Barrera Pontificia LA. Production of an active recombinant human N-acetylgalactosamine-6-sulfate sulfatase enzyme in Pichia pastoris. Mol. Genet. Metab. 2014;111:S19. [Google Scholar]
- 113.Rodriguez A, Moreno J, Sánchez J, Díaz D, Beltran L, Espejo A, Alméciga-Díaz C, Barrera L. Production of recombinant human N-acetylgalactosamine-6-sulfate sulfa-tase enzyme in Pichia pastoris . Mol. Genet. Metab. 2013;108:S79–S80. [Google Scholar]
- 114.Rodriguez-Lopez A, Almeciga-Diaz CJ, Sanchez J, Moreno J, Beltran L, Diaz D, Pardo A, Ramirez AM, Espejo-Mojica AJ, Pimentel L, Barrera LA. Recombinant human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) produced in the methylotrophic yeast Pichia pastoris . Sci. Rep. 2016;6:29329. doi: 10.1038/srep29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomatsu S, Montaño A, Ohashi A, Oikawa H, Oguma T, Dung V, Nishioka T, Orii T, Sly W. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 116.Almeciga CJ, Espejo-Mojica A, Rodriguez-López A, Losada C, Sanchez J, Ramírez AM, Pimentel N, Beltran LM, Diaz D, Barrera LA. Cell uptake evaluation of human recombinant lysosomal enzymes produced in Pichia pastoris . Mol. Genet. Metab. 2016;117:S17–S18. [Google Scholar]
- 117.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J. Clin. Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomatsu S, Montano AM, Oikawa H, Dung VC, Hashimoto A, Oguma T, Gutierrez ML, Takahashi T, Shimada T, Orii T, Sly WS. Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions? Mol. Genet. Metab. 2015;114:195–202. doi: 10.1016/j.ymgme.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sanford M, Lo JH. Elosulfase alfa: first global approval. Drugs. 2014;74:713–718. doi: 10.1007/s40265-014-0210-z. [DOI] [PubMed] [Google Scholar]
- 120.Hendriksz CJ, Giugliani R, Harmatz P, Mengel E, Guffon N, Valayannopoulos V, Parini R, Hughes D, Pastores GM, Lau HA, Al-Sayed MD, Raiman J, Investigators S, Yang K, Mealiffe M, Haller C. Multi-domain impact of elosufase alfa in Morquio A syndrome in the pivotal phase III trial. Mol. Genet. Metab. 2015;114:178–185. doi: 10.1016/j.ymgme.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 121.FDA Advisory Committee Briefing Document, Elosulfase alfa for mucopolysaccharidosis type IVA. 2014 http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm375126.pdf.
- 122.Tomatsu S, Sawamoto K, Shimada T, Bober MB, Kubaski F, Yasuda E, Mason RW, Khan S, Almeciga-Diaz CJ, Barrera LA, Mackenzie WG, Orii T. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations. Expert Opin. Orphan Drugs. 2015;3:1279–1290. doi: 10.1517/21678707.2015.1086640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, Lin SP, Mengel E, Scarpa M, Valayannopoulos V, Giugliani R, STRIVE Investigators. Slasor P, Lounsbury D, Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harmatz PR, Mengel KE, Giugliani R, Valayannopoulos V, Lin SP, Parini R, Guffon N, Burton BK, Hendriksz CJ, Mitchell JJ, Martins AM, Jones SA, Guelbert N, Vellodi A, Wijburg FA, Yang K, Slasor P, Decker C. Longitudinal analysis of endurance and respiratory function from a natural history study of Morquio A syndrome. Mol. Genet. Metab. 2015;114:186–194. doi: 10.1016/j.ymgme.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 125.Hendriksz CJ, Parini R, AlSayed MD, Raiman J, Giugliani R, Solano Villarreal ML, Mitchell JJ, Burton BK, Guelbert N, Stewart F, Hughes DA, Berger KI, Slasor P, Matousek R, Jurecki E, Shaywitz AJ, Harmatz PR. Long-term endurance and safety of elosulfase alfa enzyme replacement therapy in patients with Morquio A syndrome. Mol. Genet. Metab. 2016;119:131–143. doi: 10.1016/j.ymgme.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 126.Burton BK, Berger KI, Lewis GD, Tarnopolsky M, Treadwell M, Mitchell JJ, Muschol N, Jones SA, Sutton VR, Pastores GM, Lau H, Sparkes R, Genter F, Shaywitz AJ, Harmatz P. Safety and physiological effects of two different doses of elosulfase alfa in patients with morquio a syndrome: a randomized, double-blind, pilot study. Am. J. Med. Genet. A. 2015;167A:2272–2281. doi: 10.1002/ajmg.a.37172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Donida B, Marchetti DP, Biancini GB, Deon M, Manini PR, da Rosa HT, Moura DJ, Saffi J, Bender F, Burin MG, Coitinho AS, Giugliani R, Vargas CR. Oxidative stress and inflammation in mucopolysaccharidosis type IVA patients treated with enzyme replacement therapy. Biochim. Biophys. Acta. 2015;1852:1012–1019. doi: 10.1016/j.bbadis.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 128.Schweighardt B, Tompkins T, Lau K, Jesaitis L, Qi Y, Musson DG, Farmer P, Haller C, Shaywitz AJ, Yang K, O’Neill CA. Immunogenicity of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome: results from MOR-004, a phase III trial. Clin. Ther. 2015;37:1012–1021. doi: 10.1016/j.clinthera.2014.11.005. (e1016) [DOI] [PubMed] [Google Scholar]
- 129.Jones SA, Bialer M, Parini R, Martin K, Wang H, Yang K, Shaywitz AJ, Harmatz P. Safety and clinical activity of elosulfase alfa in pediatric patients with Morquio A syndrome (mucopolysaccharidosis IVA) less than 5 y. Pediatr. Res. 2015;78:717–722. doi: 10.1038/pr.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yasuda E, Suzuki Y, Shimada T, Sawamoto K, Mackenzie WG, Theroux MC, Pizarro C, Xie L, Miller F, Rahman T, Kecskemethy HH, Nagao K, Morlet T, Shaffer TH, Chinen Y, Yabe H, Tanaka A, Shintaku H, Orii KE, Orii KO, Mason RW, Montano AM, Fukao T, Orii T, Tomatsu S. Activity of daily living for Morquio A syndrome. Mol. Genet. Metab. 2016;118:111–122. doi: 10.1016/j.ymgme.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sawamoto K, Suzuki Y, Mackenzie WG, Theroux MC, Pizarro C, Yabe H, Orii KE, Mason RW, Orii T, Tomatsu S. Current therapies for Morquio A syndrome and their clinical outcomes. Expert Opin. Orphan Drugs. 2016;4 doi: 10.1080/21678707.2016.1214572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Do Cao J, Wiedemann A, Quinaux T, Battaglia-Hsu SF, Mainard L, Froissart R, Bonnemains C, Ragot S, Leheup B, Journeau P, Feillet F. 30 months follow-up of an early enzyme replacement therapy in a severe Morquio A patient: about one case. Mol. Genet. Metab. Rep. 2016;9:42–45. doi: 10.1016/j.ymgmr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. https://www.nice.org.uk/guidance/hst2/resources/managed-access-agreement-december-2015-22389358692015.
- 134. https://www.cadth.ca/sites/default/files/cdr/complete/SR0456_complete_Vimizim_May-26_16.pdf 2016.
- 135. https://www.zorginstituutnederland.nl/binaries/content/documents/zinl-www/documenten/publicaties/geneesmiddelbeoordelingen/2016/1603-elosulfase-alfa-vimizim-bij-de-behandeling-van-morquio-a-syndroom/elosulfase+alfa+28Vimizim29+bij+de+behandeling+van+Morquio+A+syn-droom.pdf2016.
- 136. https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2016-03/files/elosulfase-alfa-psd-march-2016.pdf 2016.
- 137.Simonaro CM, D’Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, Tanaka T, Otomo T, Ohashi T, Ishige-Wada M, Yabe H, Ohura T, Suzuki N, Kato K, Adachi S, Kobayashi R, Mugishima H, Kato S. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 139.Tolar J, Grewal SS, Bjoraker KJ, Whitley CB, Shapiro EG, Charnas L, Orchard PJ. Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome. Bone Marrow Transplant. 2008;41:531–535. doi: 10.1038/sj.bmt.1705934. [DOI] [PubMed] [Google Scholar]
- 140.Turbeville S, Nicely H, Rizzo JD, Pedersen TL, Orchard PJ, Horwitz ME, Horwitz EM, Veys P, Bonfim C, Al-Seraihy A. Clinical outcomes following hematopoi-etic stem cell transplantation for the treatment of mucopolysaccharidosis VI. Mol. Genet. Metab. 2011;102:111–115. doi: 10.1016/j.ymgme.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khanna G, Van Heest AE, Agel J, Bjoraker K, Grewal S, Abel S, Krivit W, Peters C, Orchard PJ. Analysis of factors affecting development of carpal tunnel syndrome in patients with Hurler syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;39:331–334. doi: 10.1038/sj.bmt.1705586. [DOI] [PubMed] [Google Scholar]
- 142.Aldenhoven M, Jones SA, Bonney D, Borrill RE, Coussons M, Mercer J, Bierings MB, Versluys B, van Hasselt PM, Wijburg FA, van der Ploeg AT, Wynn RF, Boelens JJ. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol. Blood Marrow Transplant. 2015;21:1106–1109. doi: 10.1016/j.bbmt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 143.Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, Shintaku H, Suzuki Y, Orii T, Tomatsu S. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol. Genet. Metab. 2016;117:84–94. doi: 10.1016/j.ymgme.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson FL, Mentzer WC, Kalinyak KA, Sullivan KM, Abboud MR. Bone marrow transplantation for sickle cell disease. The United States experience. Am. J. Pediatr. Hematol. Oncol. 1994;16:22–26. [PubMed] [Google Scholar]
- 145.Ramsay SL, Meikle PJ, Hopwood JJ. Determination of monosaccharides and disaccharides in mucopolysaccharidoses patients by electrospray ionisation mass spectrometry. Mol. Genet. Metab. 2003;78:193–204. doi: 10.1016/s1096-7192(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 146.Wang J, Luan Z, Jiang H, Fang J, Qin M, Lee V, Chen J. Allogeneic hematopoietic stem cell transplantation in thirty-four pediatric cases of mucopolysaccharidosis-a ten-year report from the China children transplant group. Biol. Blood Marrow Transplant. 2016;22:2104–2108. doi: 10.1016/j.bbmt.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 147.Toietta G, Severini GM, Traversari C, Tomatsu S, Sukegawa K, Fukuda S, Kondo N, Tortora P, Bordignon C. Various cells retrovirally transduced with N-acetylgalactosoamine-6-sulfate sulfatase correct Morquio skin fibroblasts in vitro. Hum. Gene Ther. 2001;12:2007–2016. doi: 10.1089/104303401753204571. [DOI] [PubMed] [Google Scholar]
- 148.Gutierrez MA, Garcia-Vallejo F, Tomatsu S, Ceron F, Almeciga-Diaz CJ, Dominguez MC, Barrera LA. Construction of an adenoassociated, viral derived, expression vector to correct the genetic defect in Morquio A disease. Biomedica. 2008;28:448–459. [PubMed] [Google Scholar]
- 149.Handley CJ, Samiric T, Ilic MZ. Structure, metabolism, and tissue roles of chon-droitin sulfate proteoglycans. Adv. Pharmacol. 2006;53:219–232. doi: 10.1016/S1054-3589(05)53010-2. [DOI] [PubMed] [Google Scholar]
- 150.Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr. Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 151.Cosma MP, Pepe S, Annunziata I, Newbold RF, Grompe M, Parenti G, Ballabio A. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113:445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 152.Alméciga-Díaz C, Cuaspa R, Barrera L. Gene delivery systems: tailoring vectors to reach specific tissues. In: Yuan X, editor. Non-viral Gene Therapy. Croatia: Intech, Rijeka; 2011. pp. 51–76. [Google Scholar]
- 153.Alméciga Díaz CJ, Montaño AM, Tomatsu S, Barrera AL. Contribución Colombiana al Conocimiento de la Enfermedad de Morquio. Medicina. 2012;34:221–241. [Google Scholar]
- 154.Tomatsu S, Montaño A, Alméciga-Diaz C, Barrera L. Delivery of Therapeutic Agents to the Bone. USA: Saint Louis University; 2010. [Google Scholar]
- 155.Alméciga-Díaz C, Herrera J, Barbosa H, Barrera L. New viral vectors for Morquio syndrome type A gene therapy. Mol. Genet. Metab. 2013:S19. [Google Scholar]
- 156.Salazar DA, Rodriguez-Lopez A, Herreno A, Barbosa H, Herrera J, Ardila A, Barreto GE, Gonzalez J, Almeciga-Diaz CJ. Systems biology study of mucopolysaccharidosis using a human metabolic reconstruction network. Mol. Genet. Metab. 2016;117:129–139. doi: 10.1016/j.ymgme.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 157.Shinhar SY, Zablocki H, Madgy DN. Airway management in mucopolysaccharide storage disorders. Arch. Otolaryngol. Head Neck Surg. 2004;130:233–237. doi: 10.1001/archotol.130.2.233. [DOI] [PubMed] [Google Scholar]
- 158.Belani KG, Krivit W, Carpenter BL, Braunlin E, Buckley JJ, Liao JC, Floyd T, Leonard AS, Summers CG, Levine S, et al. Children with mucopolysaccharidosis: perioperative care, morbidity, mortality, and new findings. J. Pediatr. Surg. 1993;28:403–408. doi: 10.1016/0022-3468(93)90240-l. (discussion 408–410) [DOI] [PubMed] [Google Scholar]
- 159.Walker RW, Dearlove OR. Anaesthesia for children with mucopolysaccharidoses. Anaesth. Intensive Care. 1997;25:197–198. [PubMed] [Google Scholar]
- 160.Pruszcznski B, Mackenzie W, Rogers K, Ahmadian A, White K. Spinal cord injury following extremity surgery in children with thoracic kyphosis: case series. Clin. Orthop. Relat. Res. 2016 doi: 10.1007/s11999-015-4437-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.White KK, Steinman S, Mubarak SJ. Cervical stenosis and spastic quadriparesis in morquio disease (MPS IV). A case report with twenty-six-year follow-up. J. Bone Joint Surg. Am. 2009;91:438–442. doi: 10.2106/JBJS.H.00148. [DOI] [PubMed] [Google Scholar]
- 162.Ashraf J, Crockard HA, Ransford AO, Stevens JM. Transoral decompression and posterior stabilisation in Morquio’s disease. Arch. Dis. Child. 1991;66:1318–1321. doi: 10.1136/adc.66.11.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lipson SJ. Dysplasia of the odontoid process in Morquio’s syndrome causing quadriparesis. J. Bone Joint Surg. Am. 1977;59:340–344. [PubMed] [Google Scholar]
- 164.Ain MC, Chaichana KL, Schkrohowsky JG. Retrospective study of cervical ar-throdesis in patients with various types of skeletal dysplasia. Spine. 2006;31:E169–E174. doi: 10.1097/01.brs.0000202758.61848.61. [DOI] [PubMed] [Google Scholar]
- 165.Ransford AO, Crockard HA, Stevens JM, Modaghegh S. Occipito-atlanto-axial fusion in Morquio-Brailsford syndrome. A ten-year experience. J. Bone Joint Surg. 1996;78:307–313. [PubMed] [Google Scholar]
- 166.Stevens JM, Kendall BE, Crockard HA, Ransford A. The odontoid process in Morquio-Brailsford’s disease. The effects of occipitocervical fusion. J. Bone Joint Surg. 1991;73:851–858. doi: 10.1302/0301-620X.73B5.1910048. [DOI] [PubMed] [Google Scholar]
- 167.Irfan I SR, Oxborrow N, et al. Thoracolumbar kyphosis in mucopolysaccharidosis I (Hurler Syndrome) Scoliosis Research Society; San Antonio, Texas, Texas. Proceedings 44th Annual Meeting & Course.2009. [Google Scholar]
- 168.Tandon V, Williamson JB, Cowie RA, Wraith JE. Spinal problems in mucopolysaccharidosis I (Hurler syndrome) J. Bone Joint Surg. 1996;78:938–944. doi: 10.1302/0301-620x78b6.1279. [DOI] [PubMed] [Google Scholar]
- 169.White KK, Sousa T. Mucopolysaccharide disorders in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2013;21:12–22. doi: 10.5435/JAAOS-21-01-12. [DOI] [PubMed] [Google Scholar]
- 170.Lewis JR, Gibson PH. Bilateral hip replacement in three patients with lysosomal storage disease: mucopolysaccharidosis type IV and mucolipidosis type III. J. Bone Joint Surg. 2010;92:289–292. doi: 10.1302/0301-620X.92B2.23104. [DOI] [PubMed] [Google Scholar]
- 171.Odunusi E, Peters C, Krivit W, Ogilvie J. Genu valgum deformity in Hurler syndrome after hematopoietic stem cell transplantation: correction by surgical intervention. J. Pediatr. Orthop. 1999;19:270–274. doi: 10.1097/00004694-199903000-00026. [DOI] [PubMed] [Google Scholar]
- 172.Atinga M, Hamer AJ. Total knee replacements in a patient with the Morquio syndrome. J. Bone Joint Surg. 2008;90:1631–1633. doi: 10.1302/0301-620X.90B12.20641. [DOI] [PubMed] [Google Scholar]
- 173.de Waal Malefijt MC, van Kampen A, van Gemund JJ. Total knee arthroplasty in patients with inherited dwarfism—a report of five knee replacements in two patients with Morquio’s disease type A and one with spondylo-epiphyseal dysplasia. Arch. Orthop. Trauma Surg. 2000;120:179–182. doi: 10.1007/s004020050039. [DOI] [PubMed] [Google Scholar]
- 174.Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, James DC, Lucas CF, Rogers TR, Benson PF, Tansley LR, Patrick AD, Mossman J, Young EP. Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet. 1981;2:709–712. doi: 10.1016/s0140-6736(81)91046-1. [DOI] [PubMed] [Google Scholar]
- 175.Krivit W, Pierpont ME, Ayaz K, Tsai M, Ramsay NK, Kersey JH, Weisdorf S, Sibley R, Snover D, McGovern MM, et al. Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI), Biochemical and clinical status 24 months after transplantation. N. Engl. J. Med. 1984;311:1606–1611. doi: 10.1056/NEJM198412203112504. [DOI] [PubMed] [Google Scholar]
- 176.Hugh-Jones K, Hobbs J, Chambers D, White S, Byrom N, Williamson S, Barrett J, Henry K, Patrick D. Bone marrow transplantation in Mucopolysaccharidoses, Molecular Basis of Lysosomal Storage Disorders. Academic Press; 1984. pp. 411–428. [Google Scholar]