Abstract
The homeostasis of peripheral B cell compartment requires lifelong B lymphopoiesis from hematopoietic stem cells (HSC). As a result, the B cell repertoire is susceptible to disruptions of hematopoiesis. Increasing evidence, primarily from rodent models, shows that the aryl hydrocarbon receptor (AHR) regulates hematopoiesis. To study the effects of persistent AHR activation on human B cell development, a potent AHR agonist and known environmental contaminant, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was utilized. An in vitro B cell development model system was established by co-culturing human cord blood-derived HSCs with irradiated human primary bone marrow stromal cells. Using this in vitro model, we found that TCDD significantly suppressed the total number of hematopoietic stem and progenitor cells (HSPC) in a concentration-dependent manner. Cell death analysis demonstrated that the decrease in cell number was not due to cytotoxicity by TCDD. In addition, TCDD markedly decreased CD34 expression on HSPCs. Structure-activity relationship studies using dioxin congeners demonstrated a correlation between the relative AHR binding affinity and the magnitude of decrease in the number of HSPCs and CD34 expression, suggesting that AHR mediates the observed TCDD-elicited changes in HSPCs. Moreover, a significant reduction in lineage committed B cell derived from HSCs was observed in the presence of TCDD, indicating impairment of human B cell development. Similar effects of TCDD were observed regardless of the use of stromal cells in cultures indicating a direct effect of TCDD on HSCs. Collectively, we demonstrate that AHR activation by TCDD on human HSCs impairs early stages of human B lymphopoiesis.
Keywords: human B lymphopoiesis; hematopoietic stem cell; 2,3,7,8-tetrachlorodibenzo-p-dioxin; aryl hydrocarbon receptor
1. Introduction
The generation of B cells from self-renewing hematopoietic stem cells (HSC) involves successive rounds of lineage restrictions (Nutt and Kee 2007). Cytokine receptor signaling and a lineage-specific transcription factor network progressively direct this developmental process. The interplay between transcription factor Ikaros and PU.1 during hematopoiesis leads to lymphoid lineage restriction and give rise to common lymphoid progenitors (CLP) (Somasundaram et al. 2015). CLPs have lost myeloid capacity but maintain the potential of lymphoid lineage differentiation. At this stage, cells start expressing the α chain of interleukin-7 receptor (IL7R), which is under the regulation of PU.1 (DeKoter et al. 2002). Signaling through the IL7R on CLPs activates the expression of early B cell factor 1 (EBF1) (Dias et al. 2005), which in turn up-regulates PAX5 (Roessler et al. 2007). The elevated expression of EBF1 and PAX5 eventually leads to B cell lineage commitment and generation of CD19 expressing pre-B cells (Cobaleda et al. 2007).
Halogenated aromatic hydrocarbons (HAH) are a group of structurally-related, ubiquitous environmental contaminants. Due to the chemical stability and lipophilicity of these compounds, HAHs are persistent in the environment and tend to bioaccumulate in the food chain and hence representing the primary route of human exposure (Poland and Knutson 1982). Within HAHs, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic member (Poland and Knutson 1982; Whitlock 1990). TCDD exhibits a broad spectrum of toxicity in mammals including wasting syndrome, hepatotoxicity, endocrine disruption, reproductive and developmental toxicity, carcinogenesis and immunotoxicity (Peterson et al. 1993; Poland and Knutson 1982). Most of the toxic effects of TCDD are primarily mediated by the aryl hydrocarbon receptor (AHR) (Hankinson 1995; Schmidt and Bradfield 1996). AHR is a ligand-activated transcription factor belonging to Per-ARNT-Sim (PAS) protein superfamily. As other PAS proteins, the AHR is believed to act as a sensor of endogenous and exogenous chemicals to trigger cellular responses mainly by regulating expression of target genes (Denison et al. 2011).
Among the spectrum of toxicities elicited by TCDD, immune toxicity, specifically suppression of B cell function, represents a highly sensitive sequela of TCDD exposure across animal species (Holsapple et al. 1991; Kerkvliet 2002; Sulentic and Kaminski 2011). Epidemiological studies suggest an association between exposure to TCDD and B cell disorders, most notably decreased humoral immune competence and increased incidence of B cell-derived cancers (Baccarelli et al. 2002; Becher et al. 1996; Floret et al. 2003; Kogevinas et al. 1997; Kramarova et al. 1998; Viel et al. 2008). In addition, several studies utilizing in vitro models reveal that TCDD suppresses human B cell activation and immunoglobulin M (IgM) antibody production (Lu et al. 2011; Lu et al. 2010; Wood and Holsapple 1993). These studies demonstrate that TCDD affects the function of already established mature B cells; however, it is presently unclear whether TCDD also affects human B cell developmental process.
The vulnerability of hematopoietic stem and progenitor cells to TCDD during B cell development has been previously shown in mice, as evidenced by a decrease in the number of B cell progenitors (Thurmond and Gasiewicz 2000). Subsequent studies revealed that in mice TCDD skewed the differentiation of HSC by increasing the number of myeloid progenitors and decreasing lymphoid progenitors, which give rise to B cells (Singh et al. 2009). Concordantly, HSCs in Ahr-null mice exhibited higher proliferative activity compared to heterozygote controls (Singh et al. 2011). To date, the effects of TCDD on human B cell development have not been investigated. Interestingly, it has been reported that treatment of human HSC with an AHR antagonist produced retention of the stem cell marker, CD34, and markedly promoted the expansion of human HSCs (Boitano et al. 2010), suggesting that the AHR plays a role in regulating human HSC development. The objective of the present study was to investigate the effects of TCDD, a high affinity AHR agonist, on human HSC to B cell lineage commitment.
2. Materials and Methods
2.1 Chemicals
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 1-chlorodibenzo-p-dioxin (MCDD), 2,3,7-trichlorodibenzo-p-dioxin (TriCDD), and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin (HxCDD) were purchased from AccuStandard (New Haven, CT). DMSO was purchased from Sigma Aldrich (St. Louis, MO).
2.2 Cells
Primary human marrow stromal cells (HMSC) were obtained from Cell Applications (San Diego, CA). Fresh human CD34+ hematopoietic stem cells (HSC) isolated from cord blood of mixed donors were purchased from All Cells (Emeryville, CA).
2.3 Cultures
Two different in vitro culture systems were utilized for human B lymphopoiesis in this study. The first culture system was a co-culture system previously described by Parrish et al. (Parrish et al. 2009) in which HMSCs were used as feeder cells to support lymphopoiesis of HSCs. HMSCs were cultured in marrow stromal cell growth medium (Cell Applications, Inc) for less than 8 rounds of cell division. Then, 24 hr prior to co-culture, HMSCs were sub-lethally irradiated (2000 rad) and seeded (1×104cells/well) in 96-well tissue culture plate. Fresh human CD34+ HSCs (1×104cells/well) were co-cultured with irradiated HMSCs in complete RPMI media (RPMI-1640 medium (Life Technologies) supplemented with 5% human AB serum (serum from human blood type AB donors) (Valley Biomedical), 100 U/ml of penicillin (Life Technologies), 100 μg/ml of streptomycin (Life Technologies), and 50 μM 2-mercaptoethanol). In addition, the cultures were supplemented with IL-3 (1ng/ml) (week 1 only), Flt3 ligand (1ng/ml), IL-7 (5ng/ml) and stem cell factor (25ng/ml) (Miltenyi Biotec). At indicated time points, the non-adherent hematopoietic stem and progenitor cells (HSPC) were harvested by gentle resuspension without disrupting the monolayer of HMSCs.
The second culture system was stromal cell-free as described previously (Ichii et al. 2010). Briefly, fresh cord blood CD34+ HSCs (1×104cells/well) were cultured in complete RPMI media supplemented with cytokines as described in co-culture system. In addition, conditioned media, which was supernatant of one week HMSC culture, was filtered and added into stromal cell-free culture (20% v/v) to support B lymphopoiesis (Ichii et al. 2010).
In all cases, cells were treated with TCDD (1, 10 or 30 nM) or vehicle (VH, 0.02% DMSO) on day 0 prior to addition of cytokines. For both culture systems, half of the media was replaced weekly with fresh media containing supplements as described above without addition of any additional TCDD or VH.
2.4 Flow cytometric analysis
Antibodies used for flow cytometry included Alexa Fluor 488 anti-human CD34 (clone: 581), Pacific Blue anti-human CD45 (clone: HI30), APC anti-human CD127 (IL7Rα) (clone: A019D5), and PE/Cy7 anti-human CD19 (clone: HIB19) from Biolegend (San Diego, CA), PE anti-human CD127 (IL7Rα) (clone: hIL-7R-M21) from BD Bioscience (San Jose, CA). At the indicated time points, cells were harvested and washed using 1X Hank’s Balanced Salt Solution (HBSS, pH 7.4, Invitrogen). Viable cells were identified using Live/Dead Fixable Aqua Dead Cell Stain (Invitrogen) prior to cell surface and intracellular staining. Cell surface Fc receptors were blocked by incubating cells with human AB serum (Valley Biomedical). For cell surface staining, cells were incubated with antibodies in FACS buffer (1X HBSS containing 1% BSA and 0.1% sodium azide, pH 7.4–7.6) for 30 min and then fixed using Cytofix fixation buffer (BD Biosciences) for 10 min. For intracellular staining, fixed cells were permeabilized by incubating in Perm/Wash Buffer (BD Biosciences) for 20 min and incubated with antibodies for 30 min. To assess cell death, cells were harvested and stained with PE Annexin V and 7-aminoactinomycin D (7-AAD) using Apoptosis Detection Kit (BD Pharmingen) per manufacturer’s instructions. In all cases, flow cytometric analyses were performed on a FACS Canto II cell analyzer (BD Biosciences) and data were analyzed using FlowJo or Kaluza software. For cell marker expression analysis, the gating strategy was to first gate on singlets and viable cells, and then gated on lymphocytes. For cell death analysis, the level of Annexin V and 7-AAD was analyzed based on singlets gate.
2.5 Real-time quantitative PCR
Total RNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA) and was reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The expression level of target gene was assessed by TaqMan Gene Expression Assays: CYP1B1 (Hs02382916_s1). Real-time qPCR was performed on ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The relative steady-state mRNA level for target gene was calculated by normalizing to the 18S ribosomal RNA and fold changes were calculated by ΔΔCT method (Livak and Schmittgen 2001).
2.6 Statistics
Statistical analyses were performed using GraphPad Prism 5.00 (Graphpad Software, San Diego, CA). Data were graphed as mean ± SEM. Statistical comparisons were performed using t-test, one-way ANOVA with Dunnett’s multiple comparison posttest or two way ANOVA with Bonferroni posttest depending on the experimental design. Data presented as fold-change were transformed using logarithmic transformation prior to statistic analysis.
3. Results
3.1 An in vitro co-culture system supporting human B lymphopoiesis
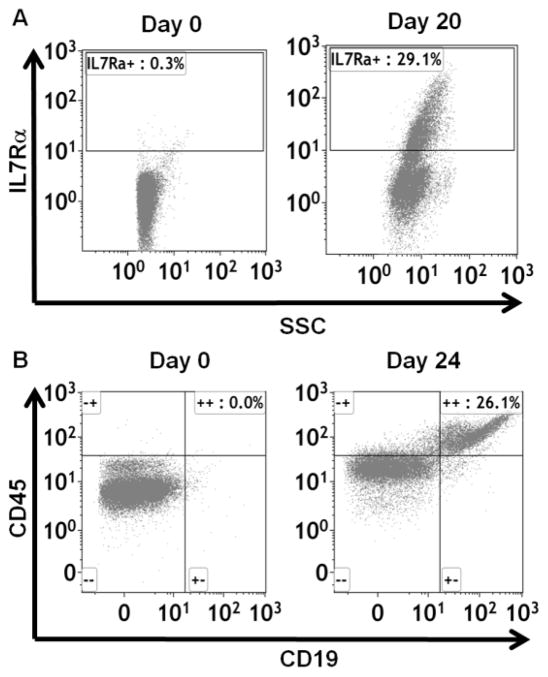
An in vitro culture system was established to investigate the effects of TCDD on human B lymphopoiesis. Specifically, fresh human cord blood CD34+ HSCs were co-cultured with primary human marrow stromal cells (HMSC) and supplemented with cytokines. During the 3–5 week culture period, cell surface markers demarcating discrete stages of B cell development were measured to monitor the progression of B lymphopoiesis. The expression of interleukin-7 receptor α chain (IL7Rα), which identified lymphoid lineage restriction (Nutt and Kee 2007), increased from 0.3% on day 0 to 29.1% on day 20 (Fig. 1A), indicating the generation of common lymphoid progenitors. By day 24, 26.1% of cells expressed CD19, a hallmark of B cell lineage commitment and demonstrating that this in vitro co-culture system supports human B cell development from HSCs (Fig. 1B).
Figure 1. Establishment of an in vitro model system of human B cell development.
Human HSCs were co-cultured with HMSCs in RPMI media supplemented with cytokines. The HSC-derived hematopoietic stem and progenitor cells (HSPCs) were harvested by gentle resuspension from co-cultures. The expression of cell surface IL7Rα (A) and CD19 (B) were analysed by flow cytometry. Data are representative of two independent experiments with similar results.
3.2 TCDD decreased the total number of hematopoietic stem and progenitor cells
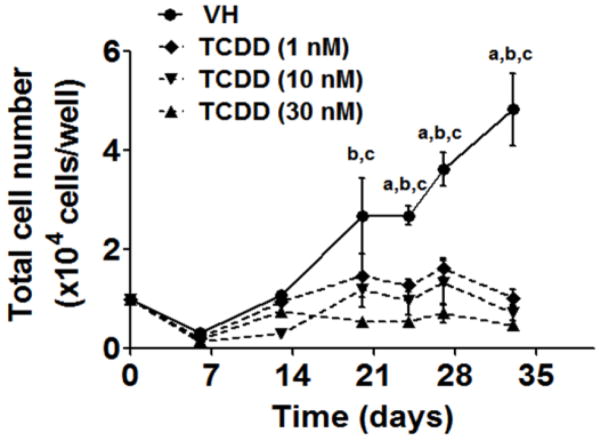
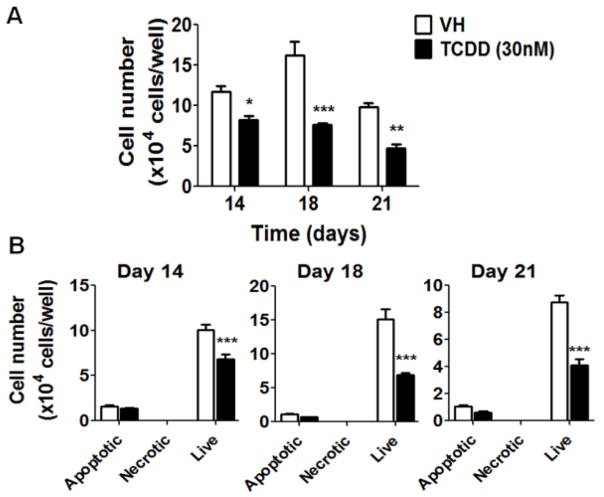
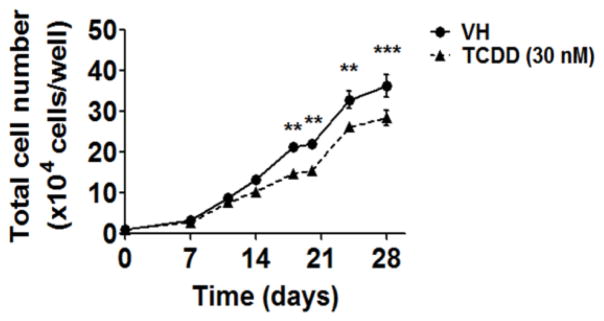
To explore the effects of TCDD on early stages of human HSC to B cell development, HSCs were treated with vehicle (VH, 0.02% DMSO) or TCDD (1, 10 or 30 nM) on day 0 and co-cultured with HMSCs for 5 weeks. The effects of TCDD on B cell development were first assessed by enumerating the total number of HSC-derived hematopoietic stem and progenitor cells (HSPCs). The growth curves for HSPCs show that TCDD treatment significantly decreased the total number of HSPCs at each time point (Fig. 2). To determine whether the decrease in cell number was due to TCDD-induced cell death, Annexin V and 7-AAD staining assay was performed. Consistently, a significant decrease in total cell number was observed with TCDD treatment on days 14, 18 and 21 (Fig. 3A). While the number of cells undergoing apoptosis (Annexin V+ 7-AAD+/−) or necrosis (Annexin V− 7-AAD+) was unchanged by TCDD treatment, a significant decrease in the number of live cells (Annexin V− 7-AAD−) was observed in the presence of TCDD (Fig. 3B). Collectively these findings suggest that the decrease in the total number of HSPCs in the presence of TCDD is not due to necrotic or apoptotic cell death.
Figure 2. TCDD decreased the total cell number in co-culture.
Human HSCs (104 cells/well) were treated with vehicle (VH) (0.02% DMSO) or TCDD (1, 10 or 30 nM) on day 0 and co-cultured with HMSCs. The HSC-derived HSPCs were harvested by gentle resuspension from co-culture and enumerated using a hemocytometer. Data are presented as the mean ± SE of triplicate measurements. Statistic analysis was performed using two way ANOVA with Bonferroni posttest. The symbols a, b and c designate the significant differeces (p<0.05) between VH group and the three TCDD treatment groups respectively. Data are representative of two independent experiments.
Figure 3. TCDD does not affect cell viability.
Human HSCs were treated with vehicle (VH) (0.02% DMSO) or TCDD (30 nM) on day 0 and co-cultured with HMSCs. A) The HSC-derived HSPCs were harvested by gentle resuspension from co-culture and enumerated using a hemocytometer. B) Cell death analysis was performed by Annexin V and 7-AAD staining. The numbers of live (Annexin V− 7-AAD−), apoptotic (Annexin V+ 7-AAD+/−) and necrotic (Annexin V− 7-AAD+) HSPCs from VH or TCDD treated groups were enumerated on day 14, 18 and 21. Data are presented as mean ± SE of triplicate measurements. * p <0.05, **p<0.01, ***p <0.001, compared to the VH by two way ANOVA with Bonferroni posttest. Data are representative of four independent experiments.
3.3 TCDD diminished CD34 expression on HSPCs
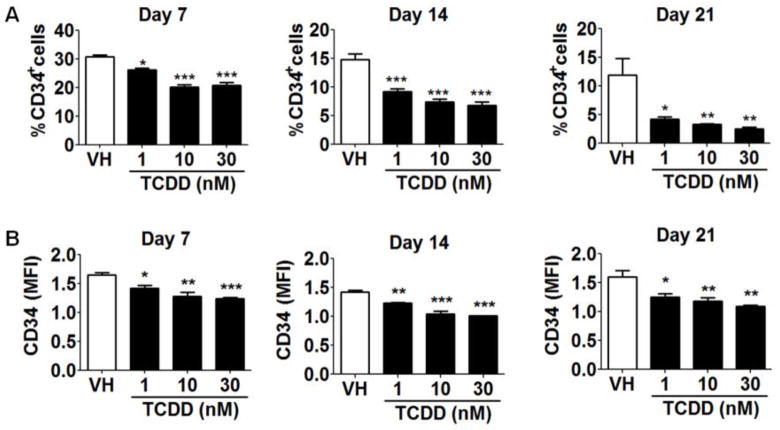
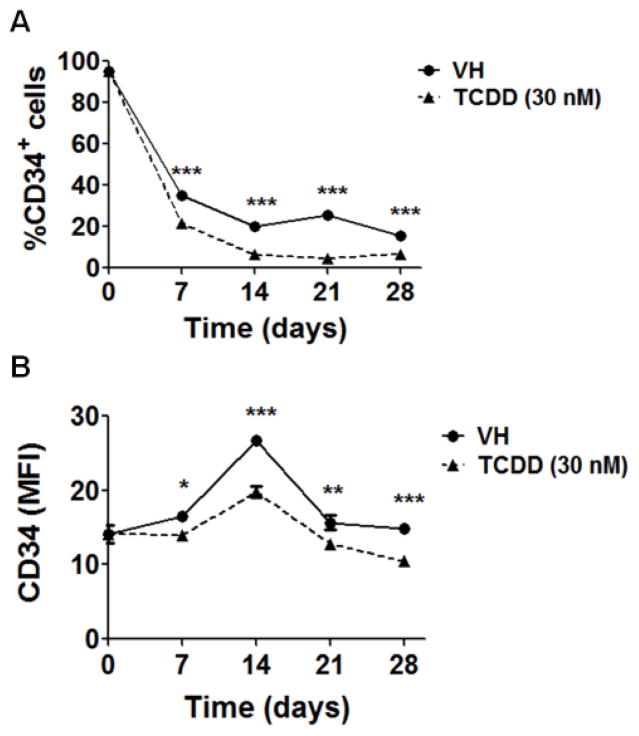
To further investigate impairment of B cell development by TCDD, the expression of CD34 on HSPCs was examined. CD34 has been used widely as a stem cell marker for HSCs as it is absent from most mature hematopoietic lineages. In addition, the expression of CD34 by HSCs has been identified as an indicator of stem cell activation status. Quiescent HSCs, largely in G0 phase of cell cycle, are CD34− whereas cytokine-activated HSCs in G0/G1 phase are CD34+ (Roberts and Metcalf 1995; Tajima et al. 2000). Using the HSC-stromal cell co-culture, TCDD significantly decreased the expression of CD34 on HSPCs (Fig. 4) as evidenced by both the percentage of CD34+ cells (Fig. 4A) as well as by the average level of CD34 surface expression on individual CD34+ cells (represented by mean fluorescence intensity - MFI) (Fig. 4B). In light of the aforementioned association between CD34 expression and HSC activation, decreased CD34 on HSPCs in the presence of TCDD suggests either suppression of HSC activation or that HSCs are being driven more rapidly toward a differentiated state. The former is more likely since TCDD treatment led to a reduction in the total cell number in culture (Fig. 2).
Figure 4. TCDD reduced the percentage of CD34+ cells and the average expression level of CD34 on CD34+ cells.
HSCs (CD34+) were treated with vehicle (VH) (0.02% DMSO) or TCDD (1, 10 or 30 nM) on day 0 and co-cultured with HMSCs. The expression of CD34 on HSC-derived HSPCs was measured on day 7, 14 and 21 using flow cytometry. A) The percentage of CD34+ cells in VH or TCDD treatment groups. B) The average expression level of CD34 on CD34+ HSPCs is represented by MFI. Data are mean ± SE of triplicate measurements. * p <0.05, **p <0.01, ***p <0.001, compared to VH by one way ANOVA with Dunnett’s multiple comparison test. Data are representative of three independent experiments.
3.4 Structure-activity-relationship studies to assess involvement of AHR in impairment of early B lymphopoiesis by TCDD
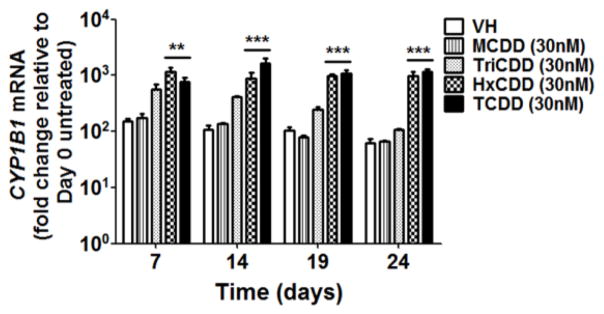
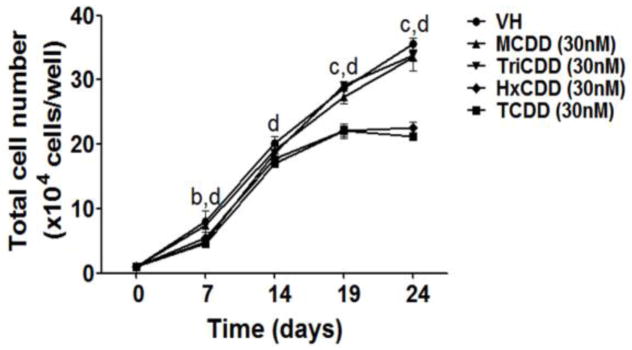
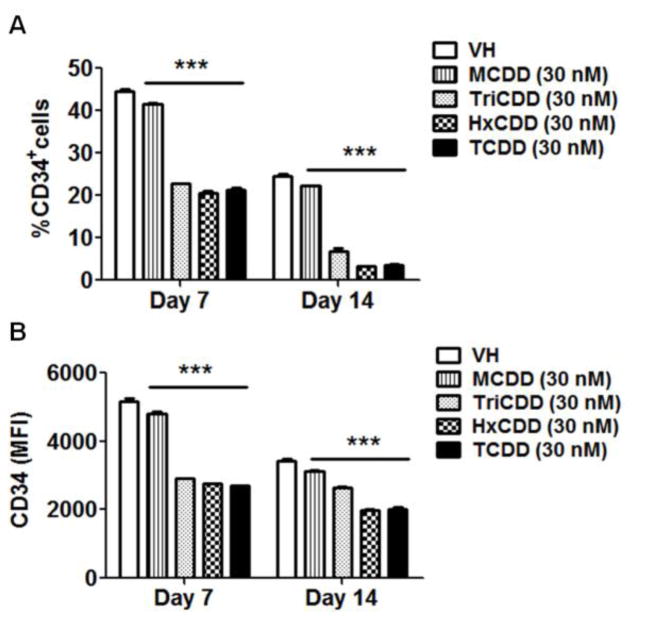
It is widely established that most of the effects produced by TCDD are mediated by the AHR (Hankinson 1995; Schmidt and Bradfield 1996). To explore the involvement of the AHR in TCDD-mediated impairment of B cell development, structure-activity-relationship studies were conducted using four polychlorinated dibenzo-p-dioxin (PCDD) congeners: 1-chlorodibenzo-p-dioxin (MCDD), 2,3,7-trichlorodibenzo-p-dioxin (TriCDD), 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin (HxCDD) and TCDD. First we measured the induction of CYP1B1 expression by PCDD congeners, a AHR-mediated response. The rank order for CYP1B1 induction was: MCDD < TriCDD < HxCDD < TCDD (Fig. 5), which correlated with AHR binding affinity for the respective congeners (Poland et al. 1976; Sulentic et al. 2000). Consistently, the magnitude of reduction in the total cell number (Fig. 6), down-regulation of CD34 expression, and reduction in the percentage of CD34+ cells (Fig. 7) all correlated with the congener’s AHR binding affinity, suggesting AHR involvement.
Figure 5. Effect of selected PCDD congeners on CYP1B1 induction.
HSCs were treated with vehicle (VH) (0.02% DMSO) or PCDD congeners (30 nM) on day 0 and co-cultured with HMSCs. The mRNA level of CYP1B1 in HSC-derived HSPCs was measured by RT-qPCR. The fold change was calculated relative to day 0 untreated cells. Data are presented as mean ± SE of triplicate measurements. **p<0.01, ***p <0.001, compared to the VH by two way ANOVA with Bonferroni posttest.
Figure 6. Effect of selected PCDD congeners on cell number.
HSCs (104 cells/well) were treated with vehicle (VH) (0.02% DMSO) or PCDD congeners (30 nM) on day 0 and co-cultured with HMSCs. The HSC-derived HSPCs were harvested by gentle resuspension from co-culture and enumerated using a hemocytometer. Data are presented as mean ± SE of triplicate measurements. The symbols a, b, c and d designate significant differeces (p<0.05) between the VH group and four PCDD congeners treatment groups (MCDD, TriCDD, HxCDD and TCDD) respectively. Statistical analysis was performed using a two way ANOVA with Bonferroni posttest. Data are representative of two independent experiments.
Figure 7. Effect of selected PCDD congeners on CD34 expression.
HSCs (CD34+) were treated with vehicle (VH) (0.02% DMSO) or PCDD congeners (30 nM) on day 0 and co-cultured with HMSCs. The expression of CD34 on HSC-derived HSPCs was measured on day 7 and 14 by flow cytometry. A) The percentage of CD34+ HSPCs. B) The average expression level of CD34 on CD34+ HSPCs, represented by MFI. Data are presented as mean ± SE of triplicate measurements. ***p <0.001, compared to VH by two way ANOVA with Bonferroni posttest. Data are representative of two independent experiments.
3.5 TCDD diminished B cell lineage commitment by HSC
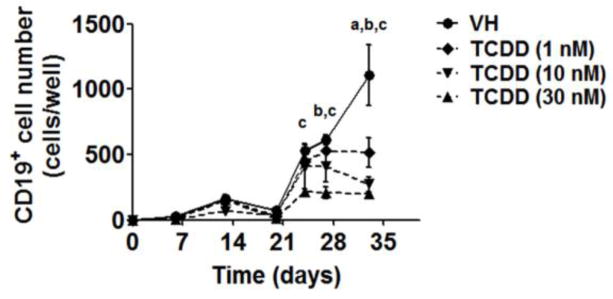
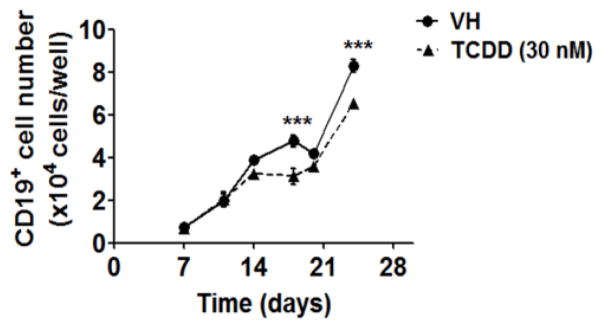
To investigate the effects of TCDD on human B cell lineage commitment, we assessed the capacity of HSCs to give rise to pre-B cells in the absence and presence of TCDD (1, 10 and 30 nM). The generation of pre-B cells from HSCs was identified based on the expression of CD19, a hallmark of B cell lineage commitment, using flow cytometry. During the culture period, pre-B cells started emerging after the third week and rapidly increased in number thereafter. In contrast, the generation of pre-B cells was significantly reduced in the presence of TCDD (Fig. 8), suggesting impairment of B cell lineage commitment.
Figure 8. TCDD decreased the number of lineage committed B cells in co-culture.
HSCs (104 cells/well) were treated with vehicle (VH) (0.02% DMSO) or TCDD (1, 10 or 30 nM) on day 0 and were co-cultured with HMSCs. The CD19+ lineage committed B cells were quantified by flow cytometry. Data are presented as mean ± SE of triplicate measurements. The symbols a, b and c designate significant differeces (p<0.05) between VH group and three TCDD treatment groups respectively. Statistic analysis was performed using a two way ANOVA with Bonferroni posttest. Data are representative of two independent experiments.
3.6 The effects of TCDD on HSCs in the absence of stromal cells
A previous study demonstrated that the AHR agonist, 7,12-Dimethylbenz[a]anthracene (DMBA), altered stromal cell cytokine production (Jensen et al. 2003). In addition, DMBA metabolites generated by stromal cells triggered bone marrow B cell apoptosis (Teague et al. 2010). Although TCDD is not readily metabolized and does not require metabolism to suppress B cell function, studies with DMBA raised the question whether TCDD-elicited impairment of early human B cell development was dependent on stromal cells. To investigate the direct effects of TCDD on HSCs in the absence of stromal cells, we adopted and modified a previously described stromal cell-free culture system (Ichii et al. 2010). As observed in HSC/HMSC co-cultures, the total number of HSPCs in the stromal cell-free cultures was significantly reduced by TCDD treatment (Fig. 9). In addition, TCDD treatment also decreased the percentage of CD34+ cells as well as the magnitude of CD34 expression (represented by MFI) on CD34+ cells in stromal cell-free cultures (Fig. 10). Moreover, the number of CD19+ lineage committed B cells was also diminished by TCDD treatment (Fig. 11). Comparing between HSC/HMSC co-cultures and stromal cell-free cultures, similar effects of TCDD on B cell development were observed, including the decrease in total cell number, decline in CD34 expression and reduction in lineage committed B cell production. This similarity, in spite of the difference in magnitude, suggests a direct effect by TCDD on HSCs, which is sufficient to disrupt early B cell development.
Figure 9. TCDD decreased the total cell number in stromal cell-free cultures.
HSCs (104 cells/well) were treated with vehicle (VH) (0.02% DMSO) or TCDD (30 nM) on day 0 and cultured in stromal cell-free cultures (RPMI media supplemented with cytokines and 20% v/v of conditioned media). The HSC-derived HSPCs were harvested and enumerated using a hemocytometer. Data are presented as mean ± SE of triplicate measurements. **p <0.01, ***p <0.001, compared to the VH by a two way ANOVA with Bonferroni posttest. Data are representative of three independent experiments.
Figure 10. TCDD reduced CD34 expression in stromal cell-free cultures.
HSCs (104 cells/well) were treated with vehicle (VH) (0.02% DMSO) or TCDD (30 nM) on day 0 and cultured in stromal cell-free cultures (RPMI media with supplemented with cytokines and 20% v/v of conditioned media). The expression of CD34 on HSC-derived HSPCs was measured by flow cytometry. A) The percentage of CD34+ HSPCs. B) The average expression level of CD34 on CD34+ HSPCs, represented by MFI. Data are presented as mean ± SE of triplicate measurements. * p <0.05, **p <0.01, ***p <0.001, compared to the VH by a two way ANOVA with Bonferroni posttest. Data are representative of three independent experiments.
Figure 11. TCDD decreased the total number of lineage committed B cells in stromal cell-free culture.
HSCs (104 cells/well) were treated with vehicle (VH) (0.02% DMSO) or TCDD (30 nM) on day 0 and cultured in stromal cell-free cultures (RPMI media supplemented with cytokines and 20% v/v of conditioned media). The CD19+ lineage committed B cells were identified by flow cytometry. Data were presented as mean ± SE of triplicate measurements. ***p <0.001, compared to the VH by two way ANOVA with Bonferroni posttest.
4. Discussion
Here we show that TCDD impaired human B cell development from HSCs in light of the following observations: (i) The number of HSC-derived HSPCs was decreased in the presence of TCDD, which was not due to cell death but rather a decrease in cell proliferation. This finding is consistent with the observation that HSPCs from Ahr-null mice exhibited an increased rate of proliferation (Singh et al. 2011); (ii) TCDD decreased the expression of CD34, an indicator of activation status of HSCs; (iii) The generation of lineage committed B cells from human HSCs was diminished by TCDD. This observation is consistent with previous reports in mice showing decreased B cell progenitors in response to TCDD administration (Singh et al. 2009), and Ahr-null mice exhibited an increase in the number of common lymphoid progenitors (Singh et al. 2011). We also demonstrated AHR involvement by structure-activity relationship experiments. Last, using parallel cultures with or without stromal cells suggest a direct effect of TCDD on HSCs. Overall, to our knowledge these are the first studies demonstrating that AHR activation by TCDD impairs human HSC to B cell lineage commitment.
Increasing evidence suggests a physiological/developmental role for the AHR in variety of biological responses, including hematopoiesis (Fernandez-Salguero et al. 1995; Schmidt et al. 1996). Enhanced proliferation of HSCs in Ahr−/− mice (Singh et al. 2011) as well as increased expansion of human HSCs in the presence of AHR antagonist (Boitano et al. 2010) suggests the existence of endogenous AHR ligands that influence hematopoiesis. The search for endogenous AHR ligands has identified a growing list of compounds, including indigoids, equilenin, tryptophan metabolites, arachidonic acid metabolites and heme metabolites (Nguyen and Bradfield 2008). It is believed that the endogenous AHR activation is controlled via an autoregulatory feedback pathway such that endogenous ligands activate AHR, which in turn upregulate the expression of cytochrome P450 enzymes that degrade endogenous ligands (Chiaro et al. 2007). Here we explored the effects of persistent AHR activation on hematopoiesis using TCDD, a stable high affinity ligand and environmental contaminant. Although the in vitro concentrations of TCDD used in this study are higher than the levels to which the general public is typically exposed, the goal of the present investigation was to utilize TCDD as a mechanistic probe. With that said, 1 nM TCDD is comparable with serum levels observed in exposed individuals after the Sevoso, Italy accident in 1976 (Needham et al. 1999). It is important to emphasize that in the current study, HSCs were treated with TCDD only on day 0 with half of the media then being replaced weekly without further addition of TCDD. Hence the concentration of TCDD at the end of the 28-day culture period was reduced over time.
To mimic the microenvironment of human B lymphopoiesis in vitro, human feeder cells (primary human marrow stromal cells) and human recombinant cytokines were used to facilitate HSC to B cell development. The present in vitro findings showing that TCDD impaired human B lymphopoiesis are consistent with in vivo animal studies (Singh et al. 2009; Thurmond and Gasiewicz 2000), suggesting mechanistic parallels across species. In addition, our studies suggest that TCDD directly affects the HSCs rather than mediating its effects indirectly through actions on stromal cells. It is noteworthy that the magnitude of TCDD-mediated impairment (i.e. decrease of total HSPCs (Fig. 9), CD34 expression (Fig. 10) and pro-B cell number (Fig. 11)) was modestly diminished in the absence of HMSCs, which could be attributable to several factors. One possibility is that TCDD also mediates some effects on the HMSCs, which further impairs B cell development. Although we cannot exclude this possibility, it is unlikely since the HMSCs undergo significant irradiation prior to co-culture with HSCs and are functionally compromised as evidenced by a complete absence of proliferative capability (data not shown). A second possibility is that under stromal cell-free culture conditions, HSCs are subjected to supraoptimal concentrations of growth factors present in HMSC conditioned media, which attenuate some of the inhibitory effects produced on HSCs by TCDD. The latter explanation is supported by our observation that the same number of HSCs generated more HSPCs in stromal cell-free cultures than in co-cultures (Fig. 2 and 9, vehicle groups). Although the effects of TCDD in stromal cell-free culture are modest, they are consistent with the effects in HSC/stromal cell co-cultures, suggesting direct effects of TCDD on HSCs. In addition, given that CD19+ pre-B cells undergo significant proliferation during B lymphopoiesis, the modest disturbance by TCDD will be magnified over time.
One of the most consistent and profound effects by TCDD in the present study was on the decrease of CD34 expression by HSCs. CD34 has been identified as a hematopoietic stem cell marker which has been used clinically in HSC transplantation for more than 20 years. As CD34 expression is restricted on hematopoietic stem and progenitor cells and is absent from terminally differentiated hematopoietic lineages, one possible interpretation is that TCDD drives HSC more rapidly toward a differentiated state. This abnormal acceleration of development might disrupt the sequential progression required for B cell development and result in reduced production of lineage committed B cells. On the other hand, studies have shown that the expression of CD34 associates with the activation status of HSCs (Roberts and Metcalf 1995; Tajima et al. 2000). Specifically, quiescent HSCs express low levels of CD34, whereas activated and proliferating HSCs express CD34 at a relatively high level. Therefore, decreased CD34 expression could reflect the transition of activated HSCs into a quiescent stage by TCDD, hence, accounting for suppressed HSC proliferation. Concordantly, a previous study has shown that Ahr-null mice had more activated HSCs (in the G1 and S phase of cell cycle) compared to WT mice (Singh et al. 2011), supporting the possibility that TCDD activates AHR in HSCs which leads to reduced cell activation and proliferation. This possibility is also supported by our observation that TCDD reduced the total number of HSPCs via mechanism not involving cell death.
Historically, humoral immunity has been identified as a very sensitive target of TCDD and dioxin-like compound exposure as evidenced by suppression of antibody response across virtually all animal species evaluated (Holsapple et al. 1991; Sulentic and Kaminski 2011). Epidemiological studies have also shown an association between TCDD exposure and decreased humoral immunity. A cohort study in Dutch children found that high perinatal exposure to dioxin-like compounds was correlated with low vaccine titters and a high incidence of chicken pox and otitis (ten Tusscher et al. 2003). Likewise, a case-control study in Sevoso, Italy, identified a significant association between decreased plasma immunoglobulin G (IgG) levels and increasing TCDD plasma concentration (Baccarelli et al. 2002). Although these dioxin-associated effects on humoral immunity could have arisen from direct effects by dioxin on mature B cells, impairment of B cell developmental processes by TCDD, as suggested by our study, may represent an additional contributing factor responsible for compromised humoral immunity. Because mature B cells have a relatively short half-life (3–8 weeks), the peripheral B cell repertoire requires life-long replenishment by B lymphopoiesis from HSCs. Therefore, disruptions of B lymphopoiesis by TCDD could have significant impact on humoral immunity.
Collectively, our study shows that TCDD, by activating AHR, impairs the early stages of human hematopoiesis and eventually results in a decrease in B cell lineage commitment. As growing evidence suggests AHR is involved in many aspects of hematopoiesis, future investigations focusing on AHR-mediated alterations of gene expression will shed light on the molecular mechanism and putative target genes by which TCDD inhibits human B cell development.
Acknowledgments
The authors would like to thank Ms. Kimberly Hambleton for assistance with submission of this manuscript.
Funding
This work was supported by NIH ES002520 and Michigan State University Intramural Support.
Abbreviations
- HSC
hematopoietic stem cell
- AHR
aryl hydrocarbon receptor
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- HSPC
hematopoietic stem and progenitor cell
- CLP
common lymphoid progenitor
- IL7R
interleukin-7 receptor
- IL7Rα
interleukin-7 receptor α chain
- EBF1
early B cell factor 1
- HAH
halogenated aromatic hydrocarbon
- HMSC
human marrow stromal cell
- PCDD
polychlorinated dibenzo-p-dioxin
- MCDD
1-chlorodibenzo-p-dioxin
- TriCDD
2,3,7-trichlorodibenzo-p-dioxin
- HxCDD
1,2,3,4,7,8-hexachlorodibenzo-p-dioxin
- DMBA
7,12-Dimethylbenz[a]anthracene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baccarelli A, Mocarelli P, Patterson DG, Jr, Bonzini M, Pesatori AC, Caporaso N, Landi MT. Immunologic effects of dioxin: new results from Seveso and comparison with other studies. Environmental health perspectives. 2002;110:1169–1173. doi: 10.1289/ehp.021101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher H, Flesch-Janys D, Kauppinen T, Kogevinas M, Steindorf K, Manz A, Wahrendorf J. Cancer mortality in German male workers exposed to phenoxy herbicides and dioxins. Cancer causes & control: CCC. 1996;7:312–321. doi: 10.1007/BF00052936. [DOI] [PubMed] [Google Scholar]
- Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Molecular pharmacology. 2007;72:1369–1379. doi: 10.1124/mol.107.038968. [DOI] [PubMed] [Google Scholar]
- Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nature immunology. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- DeKoter RP, Lee HJ, Singh H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 2002;16:297–309. doi: 10.1016/s1074-7613(02)00269-8. [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological sciences: an official journal of the Society of Toxicology. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Floret N, Mauny F, Challier B, Arveux P, Cahn JY, Viel JF. Dioxin emissions from a solid waste incinerator and risk of non-Hodgkin lymphoma. Epidemiology. 2003;14:392–398. doi: 10.1097/01.ede.0000072107.90304.01. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annual review of pharmacology and toxicology. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Morris DL, Wood SC, Snyder NK. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: possible mechanisms. Annual review of pharmacology and toxicology. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- Ichii M, Oritani K, Yokota T, Schultz DC, Holter JL, Kanakura Y, Kincade PW. Stromal cell-free conditions favorable for human B lymphopoiesis in culture. Journal of immunological methods. 2010;359:47–55. doi: 10.1016/j.jim.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BA, Leeman RJ, Schlezinger JJ, Sherr DH. Aryl hydrocarbon receptor (AhR) agonists suppress interleukin-6 expression by bone marrow stromal cells: an immunotoxicology study. Environmental health: a global access science source. 2003;2:16. doi: 10.1186/1476-069X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. International Immunopharmacology. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Kogevinas M, Becher H, Benn T, Bertazzi PA, Boffetta P, Bueno-de-Mesquita HB, Coggon D, Colin D, Flesch-Janys D, Fingerhut M, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Saracci R. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. American journal of epidemiology. 1997;145:1061–1075. doi: 10.1093/oxfordjournals.aje.a009069. [DOI] [PubMed] [Google Scholar]
- Kramarova E, Kogevinas M, Anh CT, Cau HD, Dai LC, Stellman SD, Parkin DM. Exposure to Agent Orange and occurrence of soft-tissue sarcomas or non-Hodgkin lymphomas: an ongoing study in Vietnam. Environmental health perspectives. 1998;106(Suppl 2):671–678. doi: 10.1289/ehp.106-1533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu H, Crawford RB, Kaplan BL, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated disruption of the CD40 ligand-induced activation of primary human B cells. Toxicology and applied pharmacology. 2011;255:251–260. doi: 10.1016/j.taap.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Crawford RB, Suarez-Martinez JE, Kaplan BL, Kaminski NE. Induction of the aryl hydrocarbon receptor-responsive genes and modulation of the immunoglobulin M response by 2,3,7,8-tetrachlorodibenzo-p-dioxin in primary human B cells. Toxicological sciences: an official journal of the Society of Toxicology. 2010;118:86–97. doi: 10.1093/toxsci/kfq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, Gerthoux PM, Patterson DG, Jr, Brambilla P, Smith SJ, Sampson EJ, Mocarelli P. Exposure assessment: serum levels of TCDD in Seveso, Italy. Environmental research. 1999;80:S200–S206. doi: 10.1006/enrs.1998.3928. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The Search for Endogenous Activators of the Aryl Hydrocarbon Receptor. Chemical research in toxicology. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, Sahakian E, Kagoda M, Huang G, Hao QL, Sevilla Y, Barsky LW, Zielinska E, Price MA, Wall NR, Dovat S, Payne KJ. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Critical reviews in toxicology. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. The Journal of biological chemistry. 1976;251:4936–4946. [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annual review of pharmacology and toxicology. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Metcalf D. Noncycling state of peripheral blood progenitor cells mobilized by granulocyte colony-stimulating factor and other cytokines. Blood. 1995;86:1600–1605. [PubMed] [Google Scholar]
- Roessler S, Gyory I, Imhof S, Spivakov M, Williams RR, Busslinger M, Fisher AG, Grosschedl R. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol Cell Biol. 2007;27:579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annual review of cell and developmental biology. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KP, Garrett RW, Casado FL, Gasiewicz TA. Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem cells and development. 2011;20:769–784. doi: 10.1089/scd.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30:11–19. doi: 10.1093/carcin/bgn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram R, Prasad MA, Ungerback J, Sigvardsson M. Transcription factor networks in B-cell differentiation link development to acute lymphoid leukemia. Blood. 2015;126:144–152. doi: 10.1182/blood-2014-12-575688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Putative link between transcriptional regulation of IgM expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and the aryl hydrocarbon receptor/dioxin-responsive enhancer signaling pathway. J Pharmacol Exp Ther. 2000;295:705–716. [PubMed] [Google Scholar]
- Sulentic CE, Kaminski NE. The long winding road toward understanding the molecular mechanisms for B-cell suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicological sciences: an official journal of the Society of Toxicology. 2011;120(Suppl 1):S171–191. doi: 10.1093/toxsci/kfq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F, Sato T, Laver JH, Ogawa M. CD34 expression by murine hematopoietic stem cells mobilized by granulocyte colony-stimulating factor. Blood. 2000;96:1989–1993. [PubMed] [Google Scholar]
- Teague JE, Ryu HY, Kirber M, Sherr DH, Schlezinger JJ. Proximal events in 7,12-dimethylbenz[a]anthracene-induced, stromal cell-dependent bone marrow B cell apoptosis: stromal cell-B cell communication and apoptosis signaling. J Immunol. 2010;185:3369–3378. doi: 10.4049/jimmunol.0902541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Tusscher GW, Steerenberg PA, van Loveren H, Vos JG, von dem Borne AE, Westra M, van der Slikke JW, Olie K, Pluim HJ, Koppe JG. Persistent hematologic and immunologic disturbances in 8-year-old Dutch children associated with perinatal dioxin exposure. Environmental health perspectives. 2003;111:1519–1523. doi: 10.1289/ehp.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond TS, Gasiewicz TA. A single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin produces a time- and dose-dependent alteration in the murine bone marrow B-lymphocyte maturation profile. Toxicological sciences: an official journal of the Society of Toxicology. 2000;58:88–95. doi: 10.1093/toxsci/58.1.88. [DOI] [PubMed] [Google Scholar]
- Viel JF, Daniau C, Goria S, Fabre P, de Crouy-Chanel P, Sauleau EA, Empereur-Bissonnet P. Risk for non Hodgkin’s lymphoma in the vicinity of French municipal solid waste incinerators. Environmental health: a global access science source. 2008;7:51. doi: 10.1186/1476-069X-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP. Genetic and Molecular Aspects of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin Action. Annual review of pharmacology and toxicology. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Wood SC, Holsapple MP. Direct suppression of superantigen-induced IgM secretion in human lymphocytes by 2,3,7,8-TCDD. Toxicology and applied pharmacology. 1993;122:308–313. doi: 10.1006/taap.1993.1200. [DOI] [PubMed] [Google Scholar]