Abstract
Calcium silicate-based cements have superior sealing ability, bioactivity, and marginal adaptation, which make them suitable for different dental treatment applications. However, they exhibit some drawbacks such as long setting time and poor handling characteristics. To overcome these limitations calcium silicates are engineered with various constituents to improve specific characteristics of the base material, and are the focus of this review. An electronic search of the PubMed, MEDLINE, and EMBASE via OVID databases using appropriate terms and keywords related to the use, application, and properties of calcium silicate-based cements was conducted. Two independent reviewers obtained and analyzed the full texts of the selected articles. Although the effects of various constituents and additives to the base Portland cement-like materials have been investigated, there is no one particular ingredient that stands out as being most important. Applying nanotechnology and new synthesis methods for powders most positively affected the cement properties.
Keywords: Calcium silicate-based cements, Dicalcium silicate, Mineral trioxide aggregate, Nanotechnology
INTRODUCTION
Recent advances have generated wide interest in the regenerative dentistry particularly Endodontics to restore or supplement the function of the maxillofacial system during disease. Among inorganic biomaterials, which recently have received great attention in regenerative medicine1–3), calcium silicate-based cements (mineral trioxide aggregate (MTA); MTA like materials) are cements or root canal sealers that are prepared based on a composition of calcium and silicate. MTA is prepared by mechanical mixing of Portland cement, bismuth oxide, and gypsum powders4). Since Portland cement is the major constituent of MTA, there are also studies regarding differences between MTA and Portland cement5–8). There are four major components in Portland cement: tricalcium silicate [(CaO)3•SiO2; C3S], dicalcium silicate [(CaO)2•SiO2; C2S], tricalcium aluminate [(CaO)3•Al2O3; C3A], and tetracalcium aluminoferrite [(CaO)4•Al2O3•Fe2O3; C4AF]. According to a study by Dammaschke et al.6), the gypsum content of MTA is approximately half that of Portland cement, which indicates that a prolonged maximum setting time is required for MTA. Moreover, there were also fewer aluminum species found, and this also results in longer setting times. In another study Portland cement was significantly more soluble, achieved lower microhardness values, and was less radiopaque than MTA9).
MTA has some drawbacks such as long setting time10), poor handling characteristics11), and costs12). The promising results obtained from MTA research have encouraged further investigation of materials with similar favorable properties that are less expensive, as well as have fewer drawbacks when compared with the original MTA. These investigators claimed that their new materials had a similar composition to MTA with some modifications that may improve some of the clinical drawbacks of MTA. Some of the new formulations and their components are presented in Table 112–15). In addition to formulation of the calcium silicate base materials, some other parameters also affect the final product properties including setting reaction and particle size. Thus, this review aimed to discuss the role of all the constituents in calcium silicate-based cement formulation, along with the parameters of their reactions and physical properties.
Table 1.
| Name of Ingredients (%) | Portland cement |
Bioden- tine |
MTA | Angelous MTA |
White MTA |
Nano WMTA |
Biaoaggr- egate |
Grey MTA |
Grey Angelous MTA |
|---|---|---|---|---|---|---|---|---|---|
| Calcium silicate oxides (Ca3SiO5 and Ca2SiO4) | 94.9 | 80.1 | 75.6 | 74.5 | 34.1 | 65 | 65 | 30.3 | 30.1 |
| Magnesium phosphate (Mg3(PO4)2) | *** | *** | *** | *** | 0.9 | *** | *** | 2.3 | *** |
| (Bi2O3) | *** | *** | 21.6 | 14.0 | 56.7 | 17 | *** | 58.8 | 38.8 |
| Calcium carbonate (CaCO3) | *** | 14.9 | *** | *** | 0.9 | *** | *** | *** | 3.9 |
| Calcium phosphate (Ca3(PO4)2) | *** | *** | *** | *** | 1.6 | *** | 6 | 1.0 | *** |
| Calcium silicate (Ca2SiO4) | *** | *** | *** | *** | 1.7 | *** | *** | 1.0 | |
| Calcium magnesium aluminum (Ca2MgO. 2AlFeO. 6SiO.2O5) | *** | *** | *** | *** | *** | *** | *** | 2.9 | 4.2 |
| Barium zinc phosphate (BaZn2(PO4)2) | *** | *** | *** | *** | *** | *** | *** | *** | 3.4 |
| Tantalum Pentoxide (Ta2O5) | *** | *** | *** | *** | *** | *** | 25 | *** | *** |
| Silicon Oxide (SiO2) | *** | *** | *** | 0.5 | *** | *** | 4 | *** | *** |
| Zirconium Oxide (ZrO2) | *** | 5.0 | *** | *** | *** | *** | *** | *** | *** |
| Tricalcium Aluminate (Ca3Al2O6) | 0.8 | *** | *** | 2.0 | *** | 4 | *** | *** | *** |
| Calcium oxide (CaO) | *** | *** | *** | 8.0 | *** | *** | *** | *** | *** |
| Strontium carbonate (SrCO3) | *** | *** | *** | *** | *** | 3 | *** | *** | *** |
| Gypsum (CaSO4•2H2O) | *** | *** | *** | *** | *** | 5 | *** | *** | *** |
MTA cement has similar hydration mechanisms as Portland cement16). The surface of the particles is the first part that undergoes hydration. The hydration process continues by mixing more powder and liquid, followed by ion release from the calcium silicate surfaces. Camilleri believed that after mixing tricalcium silicate and dicalcium silicate with water they contribute to calcium hydrate's formation17), whereas Dammaschke et al.6) reported that tricalcium aluminates hydrogenation is the main cause for formation of calcium hydrate. However, characteristics of the mixture depends on the ratio of powder to liquid, environmental humidity and pH, compaction pressure, mixing technique, material's thickness, temperature, type of media, and the time interval between mixing and evaluation6, 9, 18–37).
Calcium silicates based materials are engineered and additives are included to improve a specific characteristic of the base material. For example, if tricalcium silicate (C3S) and dicalcium silicate (C2S) mixtures employed as the main components instead of conventional mineral trioxide aggregate (MTA)36), there would be some drawbacks including long setting time, intrinsic radiopacity, and alterations in handling characteristics. Thus, additives are included with the base material to give the desirable properties of the final product. However, a concern always remains that the additives may adversely affect biocompatibility, physical properties, and clinical applications of the base material.
Smaller particle size and increased surface area of white mineral trioxide aggregate (WMTA) compared with Portland cement12,38–40) play important roles in its physical and chemical properties, partly because of better and more rapid hydration38). Initial and final setting time of WMTA were reported to be more than 40 min and 3 h, respectively26,41), which is not desirable when WMTA is used as a root-end filling material42). A number of studies evaluated MTA's particle size and shape, which attribute to its handling ability demonstrating finer particles for WMTA compared to two types of Portland cement6,28,43–53). It is also reported that WMTA's good mechanical and biocompatibility properties are due to the uniform distribution of particles and their surface morphology6). The gray mineral trioxide aggregate (GMTA) particle size reported to range from 1–10 μm28), whereas Camilleri49) reported WMTA's particle size to be less than 1 to 30 μm. As particle size decreases the amount of surface contact that is ready to get mixed with the liquid increases contributing to faster setting time, greater early strength and improvement in handling. MTA particles as small as 1.5 μm have been reported and in comparison with some dentinal tubules diameter they are finer51). This might be a significant feature while the sealing ability of MTA are important in hydration action51).
A nano modified version of MTA has been patented in the USA and claimed to set faster with acceptable resistance to acidic environments by adding a small amount of strontium and reducing particle size (US Patent Application No. 13/211.880). Saghiri et al.40,54) analyzed the physicochemical properties of a Nano WMTA (NWMTA) and compared it with WMTA. In their study surface and biocompatibility area, setting time and microhardness were evaluated. These studies indicated significant differences in surface area, setting time and surface hardness for both cements. Despite the lack of significant differences in chemical composition of WMTA and NWMTA, the initial setting time of NWMTA was approximately 6 min. The difference in initial setting time might be attributed to the total surface area of NWMTA that was greater than WMTA specimens. Thus, NWMTA may react more rapidly with water and prevent washout of the cement plug before final setting. NWMTA had significantly greater microhardness and lower porosity than WMTA at different pH54). Moreover, a smaller particle size also leads to a smoother surface, which causes less irritation when in direct contact with living tissues55). This review was performed to determine the impact of various constituents of MTA, as a calcium silicate based cement. The main aspects perused in this review include: 1) Determining the exact role of each major component including tricalcium silicate and dicalcium silicate. 2) What physical and/or mechanical properties of MTA are attributed to these major components. 3) What are the common additive constituents to basic elements of MTA?
MATERIALS AND METHODS
The review purpose
The present review was conducted to evaluate the role of calcium silicate's constituents on its properties. Specifically, the potential effects of tricalcium silicate, dicalcium silicate, tricalcium aluminate, tertacalcium alumino ferrate, strontium salts, calcium sulfate, bismuth oxide, zirconium oxide, tantalum pentoxide, calcium chloride, methylcellulose, barium sulfate and their role in hydration of MTA were reviewed.
Inclusion and exclusion criteria
The inclusion criteria considered all articles including review studies, in-vitro and in-vivo studies, and case reports in peer reviewed journals published in English up to September 2015 that evaluated the role of tricalcium silicate, dicalcium silicate, tricalcium aluminate, tertacalcium alumino ferrate, strontium salts, calcium sulfate, bismuth oxide, zirconium oxide, tantalum pentoxide, calcium chloride, methylcellulose, barium sulfate in calcium silicate based materials. The properties of each component are briefly discussed.
Search methodology and strategy
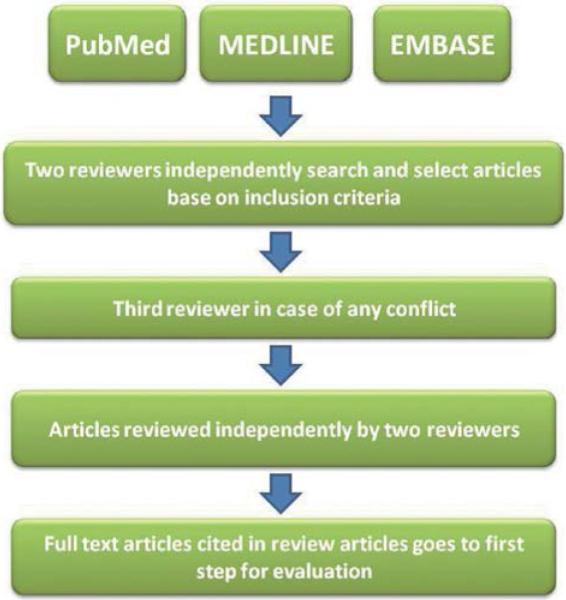
An electronic search was conducted of the PubMed, MEDLINE, and EMBASE via OVID databases using appropriate terms and keywords related to the use, application, and properties of calcium silicate-based cements. A hand search also was conducted of issues cited by the reviewed articles. The following keywords were used to identify a list of potential papers: mineral trioxide aggregate composition, tricalcium silicate, dicalcium silicate, tricalcium aluminate, tertacalcium alumino ferrate, strontium salts, calcium sulfate, bismuth oxide, zirconium oxide, tantalum pentoxide, calcium chloride, methylcellulose, barium sulfate and their role in hydration of MTA. Two independent reviewers obtained and analyzed the full texts of the selected articles. The relevant full text articles and the reference lists of the related articles were evaluated to supplement the search as well. The assessment of the eligibility and finding related data were performed by two reviewers independently. A third reviewer was selected for further discussion and final agreement on any conflict met in the mentioned processes (Fig. 1). Of 1666 articles, 96 articles met the inclusion criteria and this review summarizes the role of calcium silicate-based cements constituents and outlines why each component was added to the mixture.
Fig. 1.
Process used to select and review articles.
RESULTS AND DISCUSSION
Tricalcium silicate
Tricalcium silicate has been used on its own or with additives as bone cement56–59), as a die material for the plastic-forming process by extrusion when admixed with cellulose based polymers60), as a posterior restorative material61), and as a root fi material62). The tricalcium silicate has been postulated to be able to replace the cement component in the MTA due to similar composition and bioactivity of the material63,64), advantageously shortened setting time compared with MTA64), and being more bioactive with respect to time65).
Tricalcium silicate cement possesses good injectability, good bioactivity and moderate in vitro degradability, thus ultimately the body may be able to replace the implanted cement by natural tissue56). The addition of up to 30% calcium carbonate, calcium sulfate, and calcium chloride resulted in improvement in physical properties of tricalcium silicate cement57,59,62), as well as enhancing the bioactivity and degradability of the resultant composite material57). Tricalcium silicate cement is presently used as the main constituent of a number of proprietary brands namely Biodentine (Active Biosilicate TechnologyTM, Septodont, Saint-Maur-des-Fossés Cedex, France) and Bioaggregate (Verio Dental, Vancouver, Canada). Its hydration greatly influences the setting and development of early strength. However, dicalcium silicate contributes to late strength, due to its lower reactivity (Fig. 2). The main product of the reaction of tricalcium silicate with water is calcium silicate hydrate, a nearly amorphous material, which primarily contributes to the strength and volume stability of cement-based materials66,67). Numerous studies have been conducted to understand various steps and mechanisms in the hydration of tricalcium silicate67,68). More detailed review on kinetic mechanisms of TRICALCIUM SILICATE could be find elsewhere69). Briefly, tricalcium silicate is more reactive because of its higher calcium content, and the presence of an oxide ion in the lattice. During clinker grinding, first step of partial dissolution of tricalcium silicate involves hydration of superficial oxide ions that leads to a hydroxylated tricalcium silicate surface.
Fig. 2.
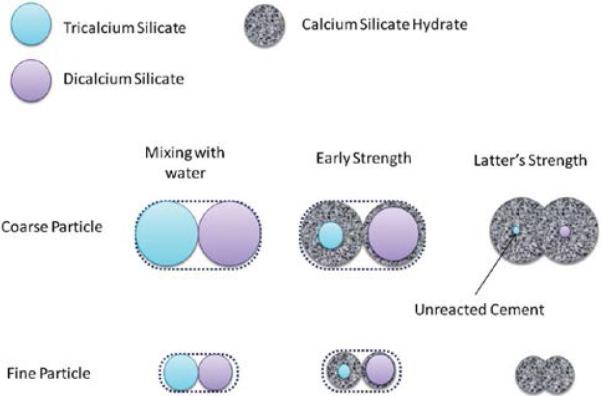
Hydration sequence and the effect of particle size on hydration of C3S and C2S.
C3S hydration greatly influences the setting and development of early strength. On the other hand, C2S contributes to late strength due to its lower reactivity.
The tricalcium silicate in Portland cement reacts with water through a dissolution-precipitation process to form calcium silicate hydrate gel and calcium hydrate67). This reaction is typical of calcium silicates and has been reported both for industrial Portland cement67), and for MTA49,50). Both hydrated cements were composed of un-reacted cement particle surrounded by a rim of hydration product (Fig. 2). The calcium silicate hydrate of the hydrated tricalcium silicate cement had a ratio of Si/Ca of approximately 0.40 as opposed to the higher Si/Ca ratio of the Portland cement63). The lower Si/Ca ratio was due to a greater abundance of calcium in tricalcium silicate, which can result in a greater deposition of hydroxyapatite when the material is in contact with a physiological solution63). The ettringite and monosulphate phases were absent from the tricalcium silicate as the aluminate phase was not present in the un-reacted powder63). Tricalcium silicate reacts with water (roughly) according to the reaction:
Tricalcium silicate can be manufactured by sol-gel70), or spark plasma sintering methods using pure raw materials71). Calcium oxide and silicon oxide are used as raw materials to avoid aluminum which has been linked to Parkinson's and Alzheimer's disease72). Furthermore, tricalcium silicate ceramics sintered by spark plasma sintering have higher density and superior mechanical properties compared with those fabricated by pressureless sintering process71).
Dicalcium silicate
There are five polymorphs of dicalcium silicate, designated α, α'H, α'L, β, and γ73). Dicalcium silicate hydrates much more slowly than tricalcium silicate and is responsible for the latter's strength. The impure form of dicalcium silicate is referred to as belite. Belite is stabilized by foreign ions in solid solution with respect to γ-dicalcium silicate. The γ-dicalcium silicate (fabricated by hydrothermal synthesis) is not hydrated in synthetic body fluid solution and exhibits a slower formation of carbonate-containing hydroxyapatite on the surface when compared with β-dicalcium silicate (fabricated by sol gel)74). Generally, there is a higher content of foreign ions taken into solid solution than with alite. Cations, such as Al3+, Fe3+, Mg2+ and K+, and anions, such SiO42− and PO43−, stabilize dicalcium silicate at high temperatures. No link was found among the impurity content, dislocation density, and reactivity of different kinds of dicalcium silicate75).
Calcium silicate hydrate is the principal phase that forms during the hydration process. The cement's pH values change from an initial pH 11 to a high pH 1364). It is hypothesized that the newly developed β-dicalcium silicate cement might possess the in vitro bioactivity and biocompatibility since its components are similar to that of MTA and SiO2-CaO-based bioactive glass. The cement could quickly form bone-like apatite spherulites after immersion in a simulated body fluid at 1 h.
Dicalcium silicate releases silicon ions, which have important roles in skeletal development and repair76). Dicalcium silicate possesses excellent bioactivity when used as a coating material for titanium alloy substrates77,78). The in vitro and in vivo bioactivities and biocompatibilities of such implants increase when they are coated with α-tricalcium phosphate (αTCP) doped with dicalcium silicate79).
Dicalcium silicate could be fabricated by hydrothermal synthesis74), plasma spary (on titanum coatings), and sol-gel methods64,74). In sol gel method reagent grade tetraethyl orthosilicate and calcium nitrate are used as precursors for SiO2 and CaO, respectively. Nitric acid is used as the catalyst and ethanol as the solvent. The molar ratio of calcium nitrate to tetraethyl orthosilicate was 3:2. Detailed description of the fabrication of the powder has been given in an earlier article64). Dicalcium silicate also can be fabricated by hydrothermal synthesis74).
Dicalcium silicate cement exhibits high apatite-forming activity and low degradation in acidic environments when used as a root-end filling material80). Regarding its cytotoxicity, dicalcium silicate cement is significantly superior to the traditional root-end filler, MTA81). Dicalcium silicate cement is also a model system for drug release74). Dicalcium silicate cement has adequate biological properties and can be used as a root-end filling and pulp capping material, and exhibits good bioactivity and biocompatibility in in vitro studies74,82–86).
Tricalcium aluminate
Tricalcium aluminate is known to have the fastest hydration rate amongst the main components of Portland cement (Portland cement). Thus, it accelerates the hydration process and improves the short-term compressive strength of tricalcium silicate/tricalcium aluminates composites when compared with that of pure tricalcium silicate67,87). The reactivity of tricalcium aluminate with water is decreased by the incorporation of sodium, but evidence suggests that its early reactivity is increased75). Tricalcium aluminates can be manufactured by sol-gel88), solid state reaction89), combustion synthesis90,91), and polymeric precursor processes92).
Calcium aluminoferrite has little effect on the physical properties and hydration of calcium silicate cements, but precipitation of an insoluble layer of hydrated iron oxide upon the calcium aluminoferrite crystal surface, forms a barrier to further the hardening without any contribution to the strength.
In single-phase, tricalcium silicate has several undesirable shortcomings including setting time (3–4 h), similar to MTA, and low mechanical strength at the early stage59). The effect of tricalcium aluminates on physical and ex vivo biological properties of the tricalcium silicate/tricalcium aluminate mixtures derived from MTA was investigated by adding tricalcium aluminate into tricalcium silicate93). The addition of tricalcium aluminate to tricalcium silicate accelerated the hydration process, reduced setting time and improved the compressive strength. Tricalcium aluminate both in regular and nano form enhanced the osseous reaction of the implanted material may be due to its high calcium content40). The order of main constitutes of Portland cement and MTA base materials hydration rate during the first few days are: C3A>C3S>C2S.
Strontium salts
Incorporation of a small amount of strontium into bone cements can create or increase bioactivity and bioconductivity properties94,95). Strontium fluoride, strontium oxide or hydroxides are used in fast setting MTA formulations96).
The ingredient strontium carbonate improves osteopromotive properties, and bio-activeness of the dental cement, while preventing agglomeration or clustering of the nanoparticle constituents. The difference between the constituent elements of NWMTA and WMTA was related to the presence of strontium with its uniform distribution on the surface12,54). Micrographs from the back scattered electron mode illustrated hydrated products, better interlocking solid and better nucleation of calcium silicate hydrate needle in NWMTA than that observed in WMTA. However, other compounds were not significantly different54).
Strontium shares the same physiological pathway as calcium in the human body and can be deposited in the bone mineral structure97). Strontium carbonate has a solubility close to that of calcium carbonate, making strontium carbonate resorbable. Strontium was found to exert beneficial effects on the osteoblastic activity98), and enhancing cell viability and differentiation99). The main limitation with using strontium appears to be the high cost.
Calcium sulfate
Calcium sulfate hemihydrate and dehydrate are widely recognized as safe and bioactive implant materials and have been successfully used in bone substitution100–102). The hemihydrate form of calcium sulfate reacts with water and creates a resorbable phase. The reaction results in the growth of interlocking needle-like crystals that form the set cement. Besides serving as a bone filler, calcium sulfate has also been used as a drug delivery material100,103).
Calcium sulfate, typically in the form of dehydrate (gypsum), may be incorporated as a mean of controlling the rate of setting reaction104). Comparatively, Portland cement contains approximately double the amount of calcium sulfate as that found in MTA105,106). ProRoot MTA containing calcium sulfate has a much longer setting time (3–4 h) than MTA Angelus (about 15 min) without calcium sulfate because the calcium sulfate, as a setting retarder, preferentially reacts with tricalcium aluminate to produce a layer on the cement surface and delays the hydration process107).
Radiopacifier
Calcium silicate cements have intrinsic radiopacity values ranging from 0.86–2.02 mm aluminium (Al)26,108,109), while higher than the 3 mm Al is recommended by the International Standards for dental root canal sealing materials (ISO 6876 Section 7.8 2002). Radiopacifying agents need to be added to make the cement distinguishable from the surrounding anatomical structures110,111). The first radiopacifier used in MTA was bismuth oxide, while alternative radiopacifiers with a high relative molecular mass have been suggested including gold powder, silver/tin alloy112), barium sulfate112–114), iodoform113,114), zirconium oxide113,114), zinc oxide112,114), and calcium tungstate114).
Bismuth oxide
Bismuth oxide size affects physical properties of the hydrated MTA like cements52). Strong linear correlations were observed between relative porosity, dry and strut densities and bismuth oxide content. Bismuth oxide drastically affected the strength of Portland cement, varying the compressive strength from 82.1 to 28.7 MPa as the bismuth concentration increased from 0 to 40% by weight20). In addition, other studies have failed to observe undesirable effects associated with the addition of bismuth oxide to Portland cement in terms of biocompatibility108,115,116), compressive strength109), cytotoxicity and genotoxicity117).
Radiopacifiers such as gold and silver/tin alloys added to Portland cement were considered suitable substitutes for bismuth oxide118), as their chemical characteristics and physical properties were similar to those of ProRoot MTA. Other radiopacifying agents, such as calcium tungstate and zirconium oxide, have also been considered as potential radiopacifiers in combination with Portland cement114).
Zirconium oxide
Investigation of the replacement of bismuth oxide with zirconium oxide in MTA, and evaluation of the radiopacity and physical properties of varying zirconium oxide levels (0 to 50%) were performed. The materials' microstructures, radiopacity, strength, setting time, water uptake, solubility, sorption and porosity of the specimens were evaluated119). These studies indicated that Portland cement with replaced 30% zirconium oxide resulted in optimum combination of properties. This material exhibited radiopacity, compressive strength, setting time, water uptake, solubility and sorption comparable to ProRoot MTA. Both microscopy and the evaluation of porosity from the solubility and sorption experiments indicated a degree of porosity consisting mainly of capillary pores and entrapped air voids119). The same group investigated the hydration characteristics of 30% zirconium oxide in Portland cement and reported that it acted as an inert filler and did not react with the hydration by-products of Portland cement120). Zirconium oxide is used as radiopacifier with glass ionomer cements121), and Biodentine (Septodont). Similar efficacies in the clinical setting were observed when the clinical, radiographic, and histologic responses of the pulp-dentin complex after direct capping with the Biodentine and MTA in human teeth were investigated122).
Tantalum pentoxide
Bismuth can induce discoloration when used as a radiopacifying agent. In order to avoid discoloration, tantalum pentoxide or zirconia of non-discoloration induced radiocifying material were used123). Zirconia is used in other BioAggregate family products to increase the radiopacity. However, tantalum pentoxide is used as a radiopacifying agent in BioAggregate for more biocompatible properties.
Barium sulfate
Barium sulfate is a compound characterized by an extremely low solubility and is clinically used as a radio-contrast agent for X-ray imaging and other diagnostic procedures124). The addition of barium sulfate to glass ionomer cement at low concentrations reduced working and initial setting times, but further addition delayed the setting reaction of glass ionomer cements. However, both compressive strength and surface hardness decreased with increasing concentrations of the radiopacifier125). The effects of barium sulfate on the physiochemical properties of Portland cement are yet to be investigated.
Barium sulfate is used as a radiopacifi in composite resins and novel endodontic cements (e.g., Epiphany; Pentron, Wallingford, CT, USA), and has the advantage of its white color. Cathers et al.126) have reported barium sulfate to be nondegradable, with possibly toxic effects. Smith et al.127) found that barium ions are not biocompatible, and severe foreign body reactions occurred in tissues contacting this material. Up to 20% barium sulfate mixed with Portland cement showed poor radiopacity, thus greater amounts of radiopaque agent should be added to meet the ISO recommendations. This may negatively affect the already poor biocompatibility of the material113).
Methylcellulose
The addition of methylcellulose to these cements improves the tensile strength properties, while degrading the compressive properties and thermal stability. The larger the methylcellulose content, the greater is the effect128). Higher concentrations of methylcellulose have the capacity to entrap air and retain higher amounts of H2O, altering Portland cement strength and retarding the setting reaction. Both of these effects are a function of concentration129).
The methylcellulose anti-washout admixture binds water molecules within the cement, accomplishing two things. First, the addition of methylcellulose to cement increases the cohesiveness and plasticity of the material, making it easier to handle. Second, using an admix of methylcellulose should increase the washout resistance, a benefit when placed in a contaminated site. This rate of washout resistance increases as the amount of anti-washout admixture is increased129).
Calcium chloride
Calcium chloride (CaCl2) is one of the most effective accelerators of hydration and setting in tricalcium silicate and Portland cement pastes130). The accelerative power of CaCl2 may come, at least in part, from its ability to flocculate hydrophilic colloids, such as calcium silicate hydrate, facilitating diffusion of ions and water through the initial calcium silicate hydrate layer due to an increased mean pore diameter, and thus allowing a higher rate of hydration during the early diffusion-controlled period131). The accelerative power of this salt increases with increasing concentration, with a practical dosage being 1–2% by weight of cement131,132). A percentage greater than 2% CaCl2 adversely affects the cement by increasing the risk of drying shrinkage and reducing ultimate strength133).
MTA materials and Portland cement have been mixed with CaCl2134–136), and with methylcellulose137). Ber et al.137) illustrated that the combination of methylcellulose and CaCl2 significantly reduced the setting time of the Portland cement. Most importantly, the 1% methylcellulose/CaCl2 combination had a significantly shorter setting time while the methylcellulose had a tendency to retard the setting reaction of Portland cement. In a study by Kogan et al.27), the setting time of MTA was found to decrease to 20–25 min if sodium hypochlorite (NaOCl) gel, K-Y jelly, and 5% CaCl2 were used as additives, but the compressive strength was also found to be much lower than MTA mixed with water.
Researchers examining the effects of salts on Portland cement and C3S hydration have arrived at the following sequences of cations and anions, ranked in order of their effectiveness as accelerators138–140):
CONCLUSIONS
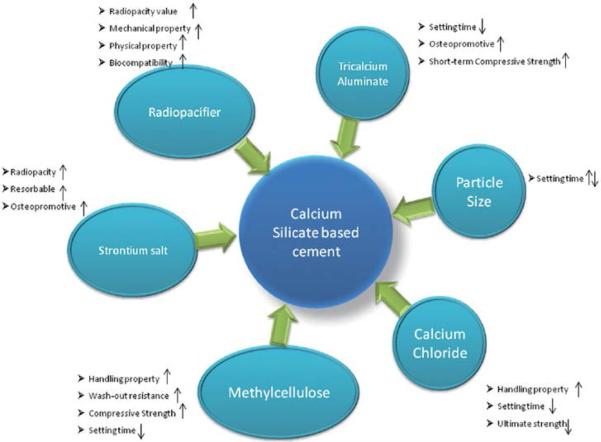
According to the studied overviewed, the role of major and minor constituents of calcium silicate-based cements the following conclusions can be drawn (Fig. 3):
Tricalcium silicate and dicalcium silicate are the main constituents of the calcium silicate based cements that are believed to be promising biomaterials with applications in several aspects of endodontic surgery. Tricalcium silicate hydration greatly influences the setting and development of early strength. While dicalcium silicate hydrates much more slowly and is responsible for the latter's strength. In addition to formulation of the calcium silicate-based materials, some other parameters including setting reaction, liquid to powder mixing ratio, and particle's morphology should be considered when engineering an ideal material. Further studies are necessary to determine the influence of its constituents on the final properties and shortcomings of the base material.
Although the effects of various constituents and additives on Portland cement-like materials have been investigated, there is no one superior material that impacts on the ideal properties of these materials. The literature review indicates that a large volume of research is focused on developing the main drawbacks of MTA, the handling characteristics, degradability, and setting time10,11,27,40,42,134–136,141–146). However, the concern would always remain that additives may adversely affect biocompatibility, physical properties, and clinical applications of MTA.
Applying nanotechnology and new synthesizing methods for powders positively affected the cement properties. Moreover, there are still many chemical components that could be used to improve the base formulation of the currently used materials. Realistic and detailed studies of the mechanism of each ingredient action in the mixture will enable advances in the properties of these materials.
Fig. 3.
Components added to calcium silicate-based cements to improve their intrinsic properties.
The effect of each component is briefly presented here.
ACKNOWLEDGMENTS
This publication is dedicated to the memory of Dr. Hajar Afsar Lajevardi, a legendry Iranian Pediatrician (1955–2015) who passed away during the writing of this article. We will never forget Dr. H Afsar Lajevardi's kindness and support. She was the only clinician-scientist with a particular focus on infectious diseases of children in Iran. The authors also acknowledge the unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, and EY022883. In addition, we thank Drs. Ali Mohammad Saghiri and Mona Momeni Moghadam for their assistance with the manuscript. Also, none of the supporters have had any role in direction of this project.
Footnotes
CONFLICT OF INTEREST
Mohammad Ali Saghiri and Mehrdad Lotfi hold a US patent for Nano Cement.
REFERENCES
- 1).Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis—Part I: N, Fe, Se, P, Au, and Ca. J Crit Rev Oncol Hematol. 2015;96:129–142. doi: 10.1016/j.critrevonc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 2).Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis—Part II: Cr, Si, Zn, Cu, and S. J Crit Rev Oncol Hematol. 2015;96:143–155. doi: 10.1016/j.critrevonc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 3).Saghiri MA, Orangi J, Asatourian A, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis part III:(Ti, Li, Ce, As, Hg, Va, Nb and Pb) J Crit Rev Oncol Hematol. 2016;98:290–301. doi: 10.1016/j.critrevonc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ma J, Chen W, Zhang L, Tucker B, Zhu G, Sasaki H, Hao L, Wang L, Ci H, Jiang H, Stashenko P, Li YP. RNA interference-mediated silencing of Atp6i prevents both periapical bone erosion and inflammation in the mouse model of endodontic disease. Infect Immun. 2013;81:1021–1030. doi: 10.1128/IAI.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Khan S, Kaleem M, Fareed MA, Habib A, Iqbal K, Aslam A, Ud Din S. Chemical and morphological characteristics of mineral trioxide aggregate and Portland cements. Dent Mater J. 2016;35:112–117. doi: 10.4012/dmj.2015-117. [DOI] [PubMed] [Google Scholar]
- 6).Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21:731–738. doi: 10.1016/j.dental.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 7).Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. J Braz Dent. 2000;11:3–9. [PubMed] [Google Scholar]
- 8).Lin JC, Lu JX, Zeng Q, Zhao W, Li WQ, Ling JQ. Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. J Formos Med Assoc. 2016;115:523–530. doi: 10.1016/j.jfma.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 9).Danesh G, Dammaschke T, Gerth HU, Zandbiglari T, Schäfer E. A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J. 2006;39:213–219. doi: 10.1111/j.1365-2591.2006.01076.x. [DOI] [PubMed] [Google Scholar]
- 10).Saoud TM, Zaazou A, Nabil A, Moussa S, Aly HM, Okazaki K, Rosenberg PA, Lin LM. Histological observations of pulpal replacement tissue in immature dog teeth after revascularization of infected pulps. Dent Traumatol. 2015;31:243–249. doi: 10.1111/edt.12169. [DOI] [PubMed] [Google Scholar]
- 11).Chng HK, Islam I, Yap AUJ, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31:665–668. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 12).Saghiri MA, Lotfi M, Aghili H. Dental Cement Composition. US Patent 20,120,012,030. 2012
- 13).Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:809–815. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 14).Park JW, Hong SH, Kim JH, Lee SJ, Shin SJ. X-Ray diffraction analysis of white ProRoot MTA and Diadent BioAggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:155–158. doi: 10.1016/j.tripleo.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 15).Guven Y, Tuna EB, Dincol ME, Aktoren O. X-ray diffraction analysis of MTA-Plus, MTA-Angelus and DiaRoot BioAggregate. Eur J Dent. 2014;8:211–215. doi: 10.4103/2278-344X.130603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Hsieh SC, Teng NC, Lin YC, Lee PY, Ji DY, Chen CC, Ke ES, Lee SY, Yang JC. A novel accelerator for improving the handling properties of dental filling materials. J Endod. 2009;35:1292–1295. doi: 10.1016/j.joen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 17).Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41:408–417. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 18).Aminoshariae A, Hartwell GR, Moon PC. Placement of mineral trioxide aggregate using two different techniques. J Endod. 2003;29:679–682. doi: 10.1097/00004770-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 19).Chogle S, Mickel AK, Chan DM, Huffaker K, Jones JJ. Intracanal assessment of mineral trioxide aggregate setting and sealing properties. Gen Dent. 2007;55:306–311. [PubMed] [Google Scholar]
- 20).Coomaraswamy KS, Lumley PJ, Hofmann MP. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement–based (MTA-like) system. J Endod. 2007;33:295–298. doi: 10.1016/j.joen.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 21).Faraco IM, Junior, Holland R. Histomorphological response of dogs' dental pulp capped with white mineral trioxide aggregate. J Braz Dent. 2004;15:104–108. doi: 10.1590/s0103-64402004000200004. [DOI] [PubMed] [Google Scholar]
- 22).Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003;29:814–817. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 23).Gancedo-Caravia L, Garcia-Barbero E. Influence of humidity and setting time on the push-out strength of mineral trioxide aggregate obturations. J Endod. 2006;32:894–896. doi: 10.1016/j.joen.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24).Hachmeister DR, Schindler WG, Walker WA, III, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28:386–390. doi: 10.1097/00004770-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 25).Holt DM, Watts JD, Beeson TJ, Kirkpatrick TC, Rutledge RE. The anti-microbial effect against (enterococcus faecalis) and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. J Endod. 2007;33:844–847. doi: 10.1016/j.joen.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 26).Islam I, Kheng Chng H, Jin Yap AU. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod. 2006;32:193–197. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 27).Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32:569–572. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 28).Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787–793. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 29).Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, Mohammadi MM, Dummer PM. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J. 2008;41:108–116. doi: 10.1111/j.1365-2591.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 30).Nekoofar MH, Adusei G, Sheykhrezae MS, Hayes SJ, Bryant ST, Dummer PM. The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2007;40:453–461. doi: 10.1111/j.1365-2591.2007.01236.x. [DOI] [PubMed] [Google Scholar]
- 31).Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V, Ranjkesh B. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod. 2008;34:1226–1229. doi: 10.1016/j.joen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 32).Sluyk SR, Moon PC, Hartwell GR. Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod. 1998;24:768–771. doi: 10.1016/S0099-2399(98)80171-4. [DOI] [PubMed] [Google Scholar]
- 33).Smith JB, Loushine RJ, Weller RN, Rueggeberg FA, Whitford GM, Pashley DH, Tay FR. Metrologic evaluation of the surface of white MTA after the use of two endodontic irrigants. J Endod. 2007;33:463–467. doi: 10.1016/j.joen.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 34).Storm B, Eichmiller FC, Tordik PA, Goodell GG. Setting expansion of gray and white mineral trioxide aggregate and Portland cement. J Endod. 2008;34:80–82. doi: 10.1016/j.joen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 35).Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21:603–608. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 36).Torabinejad M, Watson T, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–595. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 37).Walker MP, Diliberto A, Lee C. Effect of setting conditions on mineral trioxide aggregate flexural strength. J Endod. 2006;32:334–336. doi: 10.1016/j.joen.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 38).Tennis PD, Jennings HM. A model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cement Concrete Res. 2000;30:855–863. [Google Scholar]
- 39).Komabayashi T, Spångberg LS. Particle size and shape analysis of MTA finer fractions using Portland cement. J Endod. 2008;34:709–711. doi: 10.1016/j.joen.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 40).Saghiri MA, Orangi J, Tanideh N, Janghorban K, Sheibani N. Effect of Endodontic Cement on bone mineral density using serial dual-energy X-ray absorptiometry. J Endod. 2014;40:648–651. doi: 10.1016/j.joen.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Torabinejad M, Hong C, McDonald F, Pitt Ford T. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 42).Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review —part II: leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 43).Saghiri MA, Asatourian A, Garcia-Godoy F, Gutmann JL, Sheibani N. The impact of thermocycling process on the dislodgement force of different endodontic cements. BioMed Res Int. 2013;2013:317185. doi: 10.1155/2013/317185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Saghiri MA, Garcia-Godoy F, Asatourian A, Lotfi M, Banava S, Khezri-Boukani K. Effect of pH on compressive strength of some modification of mineral trioxide aggregate. J Med Oral Patol Oral Cir Bucal. 2013;18:714–720. doi: 10.4317/medoral.18922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Saghiri MA, Garcia-Godoy F, Gutmann JL, Lotfi M, Asatourian A, Ahmadi H. Push-out bond strength of a nano-modified mineral trioxide aggregate. Dent Traumatol. 2013;29:323–327. doi: 10.1111/j.1600-9657.2012.01176.x. [DOI] [PubMed] [Google Scholar]
- 46).Saghiri MA, Nazari A, Garcia-Godoy F, Asatourian A, Malekzadeh M, Elyasi M. Mechanical response of dental cements as determined by nanoindentation and scanning electron microscopy. J Microsc Microanal. 2013;19:1458–1464. doi: 10.1017/S1431927613013457. [DOI] [PubMed] [Google Scholar]
- 47).Saghiri MA, Garcia-Godoy F, Gutmann JL, Lotfi M, Asatourian A. Effects of various mixing techniques on physical properties of white mineral trioxide aggregate. Dent Traumatol. 2014;30:240–245. doi: 10.1111/edt.12067. [DOI] [PubMed] [Google Scholar]
- 48).Asgary S, Parirokh M, Eghbal MJ, Stowe S, Brink F. A qualitative X-ray analysis of white and grey mineral trioxide aggregate using compositional imaging. J Mater Sci Mater Med. 2006;17:187–191. doi: 10.1007/s10856-006-6823-3. [DOI] [PubMed] [Google Scholar]
- 49).Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40:462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 50).Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TRP. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 51).Komabayashi T, Spångberg LS. Comparative analysis of the particle size and shape of commercially available mineral trioxide aggregates and Portland cement: a study with a flow particle image analyzer. J Endod. 2008;34:94–98. doi: 10.1016/j.joen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 52).Saghiri MA, Gutmann JL, Orangi J, Asatourian A, Sheibani N. Radiopacifier particle size impacts the physical properties of tricalcium silicate–based cements. J Endod. 2015;41:225–230. doi: 10.1016/j.joen.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Saghiri MA, Asatourian A, Orangi J, Lotfi M, Soukup JW, Garcia-Godoy F, Sheibani N. Effect of particle size on calcium release and elevation of pH of endodontic cements. Dent Traumatol. 2015;31:196–201. doi: 10.1111/edt.12160. [DOI] [PubMed] [Google Scholar]
- 54).Saghiri MA, Asgar K, Lotfi M, Garcia-Godoy F. Nanomodification of mineral trioxide aggregate for enhanced physiochemical properties. Int Endod J. 2012;45:979–988. doi: 10.1111/j.1365-2591.2012.02056.x. [DOI] [PubMed] [Google Scholar]
- 55).Spangberg L, Langeland K. Biologic effects of dental materials: 1. Toxicity of root canal filling materials on HeLa cells in vitro. Oral Surg Oral Med Oral Pathol. 1973;35:402–414. doi: 10.1016/0030-4220(73)90078-9. [DOI] [PubMed] [Google Scholar]
- 56).Huan Z, Chang J. Study on physicochemical properties and in vitro bioactivity of tricalcium silicate–calcium carbonate composite bone cement. J Mater Sci Mater Med. 2008;19:2913–2918. doi: 10.1007/s10856-008-3423-4. [DOI] [PubMed] [Google Scholar]
- 57).Zhao W, Chang J, Zhai W. Self-setting properties and in vitro bioactivity of Ca3SiO5/CaSO4 1/2H2o composite cement. J Biomed Mater Res A. 2008;85:336–344. doi: 10.1002/jbm.a.31523. [DOI] [PubMed] [Google Scholar]
- 58).Huan Z, Chang J. Novel tricalcium silicate/monocalcium phosphate monohydrate composite bone cement. J Biomed Mater Res B Appl Biomater. 2007;82:352–359. doi: 10.1002/jbm.b.30740. [DOI] [PubMed] [Google Scholar]
- 59).Zhao W, Wang J, Zhai W, Wang Z, Chang J. The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials. 2005;26:6113–6121. doi: 10.1016/j.biomaterials.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 60).Ridi F, Fratini E, Mannelli F, Baglioni P. Hydration process of cement in the presence of a cellulosic additive. A calorimetric investigation. J Phys Chem B. 2005;109:14727–14734. doi: 10.1021/jp050237n. [DOI] [PubMed] [Google Scholar]
- 61).Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a Ca 3 SiO 5-based posterior restorative material. Dent Mater. 2008;24:1486–1494. doi: 10.1016/j.dental.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 62).Wang X, Sun H, Chang J. Characterization of Ca 3 SiO 5/CaCl 2 composite cement for dental application. Dent Mater. 2008;24:74–82. doi: 10.1016/j.dental.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 63).Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater. 2011;27:836–844. doi: 10.1016/j.dental.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 64).Chen CC, Ho CC, Chen CHD, Ding SJ. Physicochemical properties of calcium silicate cements for endodontic treatment. J Endod. 2009;35:1288–1291. doi: 10.1016/j.joen.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 65).Formosa LM, Mallia B, Bull T, Camilleri J. The microstructure and surface morphology of radiopaque tricalcium silicate cement exposed to different curing conditions. Dent Mater. 2012;28:584–595. doi: 10.1016/j.dental.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 66).Greenberg S, Chang T. The hydration of tricalcium silicate. J Phys Chem. 1965;69:553–561. [Google Scholar]
- 67).Taylor HF. Cement chemistry. Thomas; Telford: 1997. [Google Scholar]
- 68).Ramachandran VS. Concrete admixtures handbook: properties, science and technology. Cambridge University Press; 1996. [Google Scholar]
- 69).Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ. Mechanisms of cement hydration. Cement Concrete Res. 2011;41:1208–1223. [Google Scholar]
- 70).Ding SJ, Shie MY, Wang CY. Novel fast-setting calcium silicate bone cements with high bioactivity and enhanced osteogenesis in vitro. J Mater Chem. 2009;19:1183–1190. [Google Scholar]
- 71).Zhong H, Wang L, He L, Jiang W, Zhai W, Lin K, Chen L, Chang J. Fabrication and characterization of tricalcium silicate bioceramics with high mechanical properties by spark plasma sintering. J Int App Ceram Tech. 2011;8:501–510. [Google Scholar]
- 72).Forbes WF, Gentleman JF. Risk factors, causality, and policy initiatives: the case of aluminum and mental impairment. Exp Gerontol. 1998;33:141–154. doi: 10.1016/s0531-5565(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 73).Odler I. In: Constituents and composition. Cements SI, editor. Taylor & Francis; Oxford: 2000. [Google Scholar]
- 74).Gou Z, Chang J, Zhai W, Wang J. Study on the self-setting property and the in vitro bioactivity of β-Ca2SiO4. J Biomed Mater Res B Appl Biomater. 2005;73:244–251. doi: 10.1002/jbm.b.30203. [DOI] [PubMed] [Google Scholar]
- 75).Bye GC. Portland cement: composition, production and properties. Thomas; Telford: 1999. [Google Scholar]
- 76).De Aza PN, García-Bernal D, Cragnolini F, Velasquez P, Meseguer-Olmo L. The effects of Ca2SiO4–Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. J Mater Sci Eng C Mater Biol Appl. 2013;33:4009–4020. doi: 10.1016/j.msec.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 77).Liu X, Xie Y, Ding C, Chu PK. Early apatite deposition and osteoblast growth on plasma-sprayed dicalcium silicate coating. J Biomed Mater Res A. 2005;74:356–365. doi: 10.1002/jbm.a.30273. [DOI] [PubMed] [Google Scholar]
- 78).Liu X, Tao S, Ding C. Bioactivity of plasma sprayed dicalcium silicate coatings. Biomaterials. 2002;23:963–968. doi: 10.1016/s0142-9612(01)00210-1. [DOI] [PubMed] [Google Scholar]
- 79).Velasquez P, Luklinska ZB, Meseguer-Olmo L, Mate-Sanchez de Val JE, Delgado-Ruiz RA, Calvo-Guirado JL, Ramirez-Fernandez MP, de Aza PN. αTCP ceramic doped with dicalcium silicate for bone regeneration applications prepared by powder metallurgy method: in vitro and in vivo studies. J Biomed Mater Res A. 2013;101:1943–1954. doi: 10.1002/jbm.a.34495. [DOI] [PubMed] [Google Scholar]
- 80).Chiang TY, Ding SJ. Physicochemical properties of radiopaque dicalcium silicate cement as a root-end filling material in an acidic environment. Int Endod J. 2013;46:234–241. doi: 10.1111/j.1365-2591.2012.02112.x. [DOI] [PubMed] [Google Scholar]
- 81).Chiang TY, Ding SJ. Comparative physicochemical and biocompatible properties of radiopaque dicalcium silicate cement and mineral trioxide aggregate. J Endod. 2010;36:1683–1687. doi: 10.1016/j.joen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 82).Liangjiao C, Ping Z, Ruoyu L, Yanli Z, Ting S, Yanjun L, Longquan S. Potential proinflammatory and osteogenic effects of dicalcium silicate particles in vitro. J Mech Behav Biomed Mater. 2015;44:10–22. doi: 10.1016/j.jmbbm.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 83).Wu BC, Wei CK, Hsueh NS, Ding SJ. Comparative cell attachment, cytotoxicity and antibacterial activity of radiopaque dicalcium silicate cement and white-coloured mineral trioxide aggregate. Int Endod J. 2015;48:268–276. doi: 10.1111/iej.12310. [DOI] [PubMed] [Google Scholar]
- 84).Chen CC, Ho CC, David Chen CH, Wang WC, Ding SJ. In vitro bioactivity and biocompatibility of dicalcium silicate cements for endodontic use. J Endod. 2009;35:1554–1557. doi: 10.1016/j.joen.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 85).Chen CC, Shie MY, Ding SJ. Human dental pulp cell responses to new calcium silicate-based endodontic materials. Int Endod J. 2011;44:836–842. doi: 10.1111/j.1365-2591.2011.01890.x. [DOI] [PubMed] [Google Scholar]
- 86).Gandolfi MG, Ciapetti G, Taddei P, Perut F, Tinti A, Cardoso MV, Van Meerbeek B, Prati C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent Mater. 2010;26:974–992. doi: 10.1016/j.dental.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 87).Black L, Breen C, Yarwood J, Deng CS, Phipps J, Maitland G. Hydration of tricalcium aluminate (C 3 A) in the presence and absence of gypsum —studied by Raman spectroscopy and X-ray diffraction. J Mater Chem. 2006;16:1263–1272. [Google Scholar]
- 88).Voicu G, Ghiţulică CD, Andronescu E. Modified Pechini synthesis of tricalcium aluminate powder. Mater Charact. 2012;73:89–95. [Google Scholar]
- 89).Mukhopadhyay S, Dutta S. Comparison of solid state and sol–gel derived calcium aluminate coated graphite and characterization of prepared refractory composite. Ceram Int. 2012;38:4997–5006. [Google Scholar]
- 90).Ianoş R, Lazău I, Păcurariu C, Barvinschi P. Fuel mixture approach for solution combustion synthesis of Ca3Al2O6 powders. Cement Concrete Res. 2009;39:566–572. [Google Scholar]
- 91).Fumo D, Morelli M, Segadaes A. Combustion synthesis of calcium aluminates. Mater Res Bull. 1996;31:1243–1255. [Google Scholar]
- 92).Gaki A, Chrysafi R, Perraki T, Kakali G. Synthesis of calcium aluminates through the polymeric precursor route. Chem Ind Chem Eng Q. 2006;12:137–140. [Google Scholar]
- 93).Liu WN, Chang J, Zhu YQ, Zhang M. Effect of tricalcium aluminate on the properties of tricalcium silicate–tricalcium aluminate mixtures: setting time, mechanical strength and biocompatibility. Int Endod J. 2011;44:41–50. doi: 10.1111/j.1365-2591.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 94).Yang F, Yang D, Tu J, Zheng Q, Cai L, Wang L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. J Stem Cells. 2011;29:981–991. doi: 10.1002/stem.646. [DOI] [PubMed] [Google Scholar]
- 95).Peng S, Liu XS, Wang T, Li Z, Zhou G, Luk KD, Guo XE, Lu WW. In vivo anabolic effect of strontium on trabecular bone was associated with increased osteoblastogenesis of bone marrow stromal cells. J Orthop Res. 2010;28:1208–1214. doi: 10.1002/jor.21127. [DOI] [PubMed] [Google Scholar]
- 96).Lovschall H, Kjaergaard P, Thomsen JS. Mineral trioxide aggregate (MTA) composition and use. US Patent 8,722,100. 2014
- 97).Blake GM, Zivanovic MA, Mcewan AJ. Sr-89 therapy strontium kineticls in metastatic bone disease. J Nucl Med. 1986;27:1030–1035. doi: 10.1007/BF00254749. [DOI] [PubMed] [Google Scholar]
- 98).Saint-Jean SJ, Camire C, Nevsten P, Hansen S, Ginebra M. Study of the reactivity and in vitro bioactivity of Sr-substituted α-TCP cements. J Mater Sci Mater Med. 2005;16:993–1001. doi: 10.1007/s10856-005-4754-z. [DOI] [PubMed] [Google Scholar]
- 99).Kim HW, Koh YH, Kong YM, Kang JG, Kim HE, et al. Strontium substituted calcium phosphate biphasic ceramics obtained by a powder precipitation method. J Mater Sci Mater Med. 2004;15:1129–1134. doi: 10.1023/B:JMSM.0000046395.76435.60. [DOI] [PubMed] [Google Scholar]
- 100).Kokubo T, editor. Bioceramics and their clinical applications. Woodhead Publishing Ltd.; Abington, Cambridge, MA, USA: 2008. p. 784. [Google Scholar]
- 101).Lazáry Á , Balla B, Kósa JP, Bácsi K, Nagy Z, Takács I, Vargab PP, Speera G, Lakatosa P. Effect of gypsum on proliferation and differentiation of MC3T3-E1 mouse osteoblastic cells. Biomaterials. 2007;28:393–399. doi: 10.1016/j.biomaterials.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 102).Fernández E, Vlad MD, Gel MM, López J, Torres R, Cauich JV, Bohnerc M. Modulation of porosity in apatitic cements by the use of α-tricalcium phosphate—calcium sulphate dihydrate mixtures. Biomaterials. 2005;26:3395–3404. doi: 10.1016/j.biomaterials.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 103).Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26:2677–2684. doi: 10.1016/j.biomaterials.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 104).Al-Daafas A, Al-Nazhan S. Histological evaluation of contaminated furcal perforation in dogs' teeth repaired by MTA with or without internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e92–e99. doi: 10.1016/j.tripleo.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 105).Darvell BW, Wu RC. “MTA”—An Hydraulic Silicate Cement: Review update and setting reaction. Dent Mater. 2011;27:407–422. doi: 10.1016/j.dental.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 106).Gandolfi MG, Van Landuyt K, Taddei P, Modena E, Van Meerbeek B, Prati C. Environmental scanning electron microscopy connected with energy dispersive x-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time. J Endod. 2010;36:851–857. doi: 10.1016/j.joen.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 107).Fleming GJ, Farooq AA, Barralet JE. Influence of powder/liquid mixing ratio on the performance of a restorative glass-ionomer dental cement. Biomaterials. 2003;24:4173–4179. doi: 10.1016/s0142-9612(03)00301-6. [DOI] [PubMed] [Google Scholar]
- 108).Kim EC, Lee BC, Chang HS, Lee W, Hong CU, Min KS. Evaluation of the radiopacity and cytotoxicity of Portland cements containing bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e54–e57. doi: 10.1016/j.tripleo.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 109).Saliba E, Abbassi-Ghadi S, Vowles R, Camilleri J, Hooper S. Evaluation of the strength and radiopacity of Portland cement with varying additions of bismuth oxide. Int Endod J. 2009;42:322–328. doi: 10.1111/j.1365-2591.2008.01512.x. [DOI] [PubMed] [Google Scholar]
- 110).Beyer-Olsen EM, Orstavik D. Radiopacity of root canal sealers. Oral Surg Oral Med Oral Pathol. 1981;51:320–328. doi: 10.1016/0030-4220(81)90062-1. [DOI] [PubMed] [Google Scholar]
- 111).Ruch JV, Lesot H, Peterkova R, Peterka M. Evolving rodent dentition. J Bioessays. 1997;19:1041. doi: 10.1002/bies.950191115. [DOI] [PubMed] [Google Scholar]
- 112).Camilleri J, Gandolfi M. Evaluation of the radiopacity of calcium silicate cements containing different radiopacifiers. Int Endod J. 2010;43:21–30. doi: 10.1111/j.1365-2591.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 113).Bortoluzzi EA, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Duarte MAH. Radiographic effect of different radiopacifiers on a potential retrograde filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:628–632. doi: 10.1016/j.tripleo.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 114).Hungaro Duarte MA, Minotti PG, Rodrigues CT, Zapata RO, Bramante CM, Vivan RR, Gomes de Moraes I, Bombarda de Andrade F. Effect of different radiopacifying agents on the physicochemical properties of white Portland cement and white mineral trioxide aggregate. J Endod. 2012;38:394–397. doi: 10.1016/j.joen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 115).Coutinho-Filho T, De-Deus G, Klein L, Manera G, Peixoto C, Gurgel-Filho ED. Radiopacity and histological assessment of Portland cement plus bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e69–77. doi: 10.1016/j.tripleo.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 116).Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, Son HH, Oh WM. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e96–102. doi: 10.1016/j.tripleo.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 117).Zeferino E, Bueno C, Oyama L, Ribeiro D. Ex vivo assessment of genotoxicity and cytotoxicity in murine fibroblasts exposed to white MTA or white Portland cement with 15% bismuth oxide. Int Endod J. 2010;43:843–848. doi: 10.1111/j.1365-2591.2010.01747.x. [DOI] [PubMed] [Google Scholar]
- 118).Camilleri J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int Endod J. 2010;43:231–240. doi: 10.1111/j.1365-2591.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 119).Cutajar A, Mallia B, Abela S, Camilleri J. Replacement of radiopacifier in mineral trioxide aggregate; characterization and determination of physical properties. Dent Mater. 2011;27:879–891. doi: 10.1016/j.dental.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 120).Camilleri J, Cutajar A, Mallia B. Hydration characteristics of zirconium oxide replaced Portland cement for use as a root-end filling material. Dent Mater. 2011;27:845–854. doi: 10.1016/j.dental.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 121).McCabe JF, Walls WG. Applied Dental Materials. 8th edn Blackwell Science Ltd; Oxford, UK: 1998. pp. 10–11. [Google Scholar]
- 122).Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–747. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 123).Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012;45:942–949. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 124).Ott DJ, Gelfand DW. Gastrointestinal contrast agents: indications, uses, and risks. JAMA. 1983;249:2380–2384. [PubMed] [Google Scholar]
- 125).Prentice LH, Tyas MJ, Burrow MF. The effect of ytterbium fluoride and barium sulphate nanoparticles on the reactivity and strength of a glass-ionomer cement. Dent Mater. 2006;22:746–751. doi: 10.1016/j.dental.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 126).Cathers SJ, Kaminski EJ, Osetek EM. The cellular response to Hydron within the rat peritoneal cavity. J Endod. 1984;10:173–181. doi: 10.1016/S0099-2399(84)80079-5. [DOI] [PubMed] [Google Scholar]
- 127).Smith JW, Leeb I, Torney DL. A comparison of calcium hydroxide and barium hydroxide as agents for inducing apical closure. J Endod. 1984;10:64–70. doi: 10.1016/S0099-2399(84)80039-4. [DOI] [PubMed] [Google Scholar]
- 128).Xuli F, Chung D. Effect of methylcellulose admixture on the mechanical properties of cement. Cement Concrete Res. 1996;26:535–538. [Google Scholar]
- 129).Nawy EG. Concrete construction engineering handbook. CRC press; 2008. [Google Scholar]
- 130).Ramachandran VS, Paroli RM, Beaudoin JJ, Delgado AH. Handbook of thermal analysis of construction materials: William Andrew. 2002. [Google Scholar]
- 131).Juenger M, Monteiro P, Gartner E, Denbeaux G. A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration. Cement Concrete Res. 2005;35:19–25. [Google Scholar]
- 132).Gartner E, Young J, Damidot D, Jawed I. Hydration of Portland cement. In: Bensted J, Barnes P, editors. Structure and performance of cements. Vol. 13. 2002. pp. 978–0. [Google Scholar]
- 133).Kosmatka SH, Panarese WC. Design and Control of Concrete Mixtures. 13th ed Portland Cement Association; 1988. [Google Scholar]
- 134).Bortoluzzi EA, Broon NJ, Bramante CM, Garcia RB, de Moraes IG, Bernardineli N. Sealing ability of MTA and radiopaque Portland cement with or without calcium chloride for root-end filling. J Endod. 2006;32:897–900. doi: 10.1016/j.joen.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 135).Antunes Bortoluzzi E, Juárez Broon N, Antonio Hungaro Duarte M, de Oliveira Demarchi AC, Monteiro Bramante C. The use of a setting accelerator and its effect on pH and calcium ion release of mineral trioxide aggregate and white Portland cement. J Endod. 2006;32:1194–1197. doi: 10.1016/j.joen.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 136).Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J Endod. 2007;33:1235–1238. doi: 10.1016/j.joen.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 137).Ber BS, Hatton JF, Stewart GP. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J Endod. 2007;33:1231–1234. doi: 10.1016/j.joen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 138).Kantro DL. Tricalcium silicate hydration in the presence of various salts. ASTM Journal of Testing and Evaluation. 1975;3:312–321. [Google Scholar]
- 139).Double D, Hewlett P, Sing K, Raffle J. New developments in understanding the chemistry of cement hydration [and discussion] Philos Trans R Soc Lond A. 1983;310:53–66. [Google Scholar]
- 140).Wilding C, Walter A, Double D. A classification of inorganic and organic admixtures by conduction calorimetry. Cement Concrete Res. 1984;14:185–194. [Google Scholar]
- 141).Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009;35:550–554. doi: 10.1016/j.joen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 142).Hong ST, Bae KS, Baek SH, Kum KY, Lee W. Microleakage of accelerated mineral trioxide aggregate and Portland cement in an in vitro apexification model. J Endod. 2008;34:56–58. doi: 10.1016/j.joen.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 143).Jafarnia B, Jiang J, He J, Wang Y-H, Safavi KE, Zhu Q. Evaluation of cytotoxicity of MTA employing various additives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:739–744. doi: 10.1016/j.tripleo.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 144).McNamara RP, Henry MA, Schindler WG, Hargreaves KM. Biocompatibility of accelerated mineral trioxide aggregate in a rat model. J Endod. 2010;36:1851–1855. doi: 10.1016/j.joen.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 145).Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review —part I: chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 146).Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review —part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]