Abstract
The sympathetic nervous system has been identified as a major contributor to the pathophysiology of chronic heart failure (CHF) and other diseases such as hypertension and diabetes, both in experimental animal models and patients. The kidneys have a dense afferent sensory innervation positioning it to be the origin of multimodal input to the central nervous system. Afferent renal nerve (ARN) signals are centrally integrated, and their activation results in a general increase in sympathetic tone, which is directed toward the kidneys as well as other peripheral organs innervated by the sympathetic nerves. In the central nervous system, stimulation of ARN increases the neuronal discharge frequency and neuronal activity in the paraventricular nucleus (PVN) of the hypothalamus. The activity of the neurons in the PVN is attenuated during iontophoretic application of glutamate receptor blocker, AP5. An enhanced afferent renal input to the PVN may be critically involved in dictating sympathoexcitation in CHF. Furthermore, renal denervation abrogates the enhanced neuronal activity within the PVN in rats with CHF, thereby possibly contributing to the reduction in sympathetic tone. Renal denervation also restores the decreased endogenous levels of neuronal nitric oxide synthase (nNOS) in the PVN of rats with CHF. Overall, these data demonstrate that sensory information originating in the kidney excites pre-autonomic sympathetic neurons within the PVN and this “renal-PVN afferent pathway” may contribute to elevated sympathetic nerve activity in hyper-sympathetic disease conditions such as CHF and hypertension.
Keywords: sympathetic activity, cardiovascular, paraventricular nucleus, afferent renal nerves
Introduction
The paraventricular nucleus (PVN) of the hypothalamus is an important site that integrates and responds to a variety of neural and humoral signals regulating sympathetic drive and extracellular fluid volume status (Coote, 2005; Patel, 2000). The kidneys have a dense afferent sensory and efferent sympathetic innervation and are positioned to be the origin as well as target of sympathetic nervous system activation (Booth et al., 2015; Johns et al., 2011; Kopp, 2015). It has been demonstrated that the discharge frequency of putative vasopressinergic magnocellular neurosecretory neurons (MNCs) in the PVN are increased during stimulation of afferent renal nerve (ARN) as well as during the activation of specific renal receptors (Ciriello, 1998). ARN stimulation has been shown to increase neurons containing Fos-like immunoreactivity positive neurons in the PVN, indicating that the PVN neurons are activated by ARN stimulation (Solano-Flores et al., 1997).
The PVN includes neuroendocrine-related functional neurons that project to the median eminence, posterior pituitary and pre-autonomic neurons that send long descending projections to the brain stem and spinal cord regions that are important in dictating autonomic outflow (Armstrong et al., 1980; Swanson et al., 1980a). There are a number of PVN neurons that project to the rostral ventrolateral medulla (RVLM), which have been shown to correlate with activation of renal sympathetic nerve activity (RSNA) (Chen et al., 2010). Recently, we have shown that ARN stimulation activates RVLM projecting PVN neurons (Xu et al., 2015). Stimulation of ARN also increases sympathetic activity and arterial pressure (Patel et al., 1986; Patel et al., 2016; Xu et al., 2015). Afferent information from the kidney may play an important role in the coordination of neural and humeral activation, concerned with body fluid balance and the regulation of arterial blood pressure in normal and disease conditions such as chronic heart failure (CHF) and hypertension (Caverson et al., 1988; Ciriello et al., 1987; Day et al., 1987; Kopp, 2015; Patel et al., 2016; Solano-Flores et al., 1997).
This review highlights and describes the studies that examine activation of renal sensory afferent contribution to the sympathetic outflow, particularly the activation of the PVN by ARN stimulation, ultimately leading to the activation of sympathetic nervous system. Furthermore, an enhanced afferent renal input to the PVN is shown to be intimately involved in processes leading to sympathoexcitation in the CHF condition (Patel et al., 2016; Xu et al., 2012 ).
PVN and Sympathetic Outflow
Of the five major central nervous system sites that directly control sympathetic outflow (Strack et al., 1989), PVN is the most rostral and only site located in the hypothalamus. This fact, combined with the known role for PVN in fluid balance and vasopressin release, makes the PVN a prime candidate site within the forebrain, responsible for mediating sympathetic outflow. The role of PVN in cardiovascular reflexes is twofold: 1) the MNCs are responsible for the humoral component of the regulation of fluid balance (Swanson et al., 1980b); while 2) the parvocellular neurons of PVN (pPVN) are involved in the mediation of the neural component of cardiovascular reflexes by influencing RSNA (Haselton et al., 1994; Lovick et al., 1993; Patel et al., 1988). Specifically, we have demonstrated that lesion the pPVN with kainic acid altered the renal sympathoinhibition produced in response to acute volume expansion (Haselton et al., 1994). These observations suggest that the PVN plays an essential role in the mediation of RSNA under both resting and reflex conditions (Haselton et al., 1994; Lovick et al., 1993; Patel et al., 1988). Stimulation of PVN has been shown to elicit an increased discharge from several sympathetic nerves, including: renal (Kannan et al., 1989), adrenal (Katafuchi et al., 1988), and splanchnic (Lu et al., 1991). Stimulation of PVN elevates serum norepinephrine via a neural mechanism (Martin et al., 1992). Activation of the PVN is thought to produce an increase in overall sympathetic outflow. On the contrary, a few studies have also shown sympathoinhibition originating from the PVN (Coote, 2005; Zhang et al., 1997).
In the rat CHF model, induced by coronary ligation, norepinephrine is increased in several forebrain and brain stem cell groups, including the PVN (Sole et al., 1982). In the same model of CHF, we have observed a significantly increased hexokinase activity [an index of neuronal activity (Krukoff, 1993)] in the pPVN and MNCs portions of the PVN of rats with CHF compared to sham operated controls (Patel et al., 1993). We also have shown that there is increased FosB [fos family gene, indicating chronic neuronal activation (Dampney et al., 2003)] staining in the PVN of rats with CHF (Zheng et al., 2012), consistent with increased Fra-like (fos family gene, indicating chronic neuronal activation also) staining reported by the others (Kang et al., 2006; Vahid-Ansari et al., 1998). Further, by direct electrophysiological recording, we have demonstrated an increased firing of RVLM projecting PVN neurons in rats with CHF (Xu et al., 2012 ; Zhang et al., 2002b). RVLM projecting PVN neurons are more active under basal conditions and are endogenously driven by an enhanced glutamatergic mechanism in the CHF condition (Li et al., 2003). The responses of RVLM projecting PVN neurons to baroreflex challenge are attenuated, whereas the responses to hypertonic osmotic stimulation are enhanced in rats with CHF (Xu et al., 2012 ). So far, there is mounting evidence to support the idea that the increase in activation of the PVN neurons that drives the sympathoexcitation in CHF is a result of the imbalance between the inhibitory, nitric oxide (NO) and GABA mechanisms, and the excitatory glutamatergic and angiotensinergic mechanisms (Li et al., 2003; Patel et al., 2012; Zhang et al., 2001; Zhang et al., 2002a; Zheng et al., 2009). In addition, it has been reported that increased circulating cytokines cause the induction of cyclooxygenase-2 expression in the microvasculature of the PVN, resulting in enhanced proinflammatory cytokines in the PVN, resulting in sympathoexcitation in CHF (Kang et al., 2011; Kang et al., 2009; Kang et al., 2006; Yu et al., 2013; Yu et al., 2016).
Renal Sensory Receptor Afferents
The kidneys are distinctly innervated with sensory afferents (Kopp, 2015). The majority of the sensory nerves are located in the renal pelvic area with the greatest density in the pelvic wall (Marfurt et al., 1991). Afferent signals from the kidneys are transmitted by 2 modalities of receptors, mechanoreceptors and chemoreceptors (Recordati et al., 1978). These receptors transmit information to the central nervous system via the ARN.
Mechanoreceptors are found within the renal parenchyma and in the wall of the renal pelvis (Niijima, 1975). These receptors respond to increases in intra-renal pressure and are stimulated by renal vein occlusion/compression and physical compression of the hilus of the kidney (Kostreva et al., 1981; Ueda et al., 1967). Stimulation of renal mechanoreceptors leads to an increase in ipsilateral renal afferent activity and a decrease in ipsilateral and contralateral efferent RSNA (Kopp et al., 1985; Ueda et al., 1967). The main responses to renal mechanoreceptor activation are abolished by spinal cord transection at the level of T6, indicating that the mechanoreceptor reno-renal reflex is dependent on central integration (Kopp et al., 1985).
The second class of renal sensory receptors is the chemoreceptors: R1 and R2 receptors, which are activated by the chemical environment of intra-renal tissue and renal pelvis (Recordati et al., 1978). R1 receptors are activated by renal ischemia, arterial and venous occlusion and systemic asphyxia (Recordati et al., 1978). R1 receptor activation is associated with an increase in efferent RSNA, which persists after spinal cord transection at the level of T6 (Recordati et al., 1982). R2 receptors are activated by backflow of concentrated urine, hypertonic NaCl and KCl (Recordati et al., 1978). Activation of R2 receptors results in an increase in efferent RSNA and is invariably accompanied by small increases in blood pressure and heart rate (Recordati et al., 1982). The R2 receptor response remains after spinal cord transection at the level of T6 and is enhanced by transection at the level of C3, indicating that the chemoreceptor reflex integrated at a spinal level (Recordati et al., 1982).
There are numerous studies suggesting a supra-spinal integration of the afferent renal signals (Ciriello et al., 1983; Solano-Flores et al., 1997). Within the spinal cord, the ARN project to the ipsilateral dorsal horn in laminae I, III-V (Ciriello et al., 1983), where they synapse with interneurons projecting to various sites within the central nervous system including the PVN, associated with cardiovascular regulation and sympathetic outflow. There is also evidence for a monosynaptic projection of the ARN to areas within the brain stem directly (Wyss et al., 1984).
Activation of the PVN by Afferent Renal Nerve Stimulation
A number of studies utilizing anterograde tract tracing of fluorescent dyes (Wyss et al., 1984), horseradish peroxidase transport (Ciriello et al., 1983; Kuo et al., 1983), or pseudorabies virus injected into the kidneys (Weiss et al., 2001) as well as electrophysiological evidence (Calaresu et al., 1981b; Ciriello et al., 1980; Xu et al., 2015) indicate that the renal afferent information is transmitted to various sites within the spinal cord and brain stem. ARN relay information to the central nervous system associated with cardiovascular regulation, including nucleus tractus solitaries (NTS), RVLM, preoptic area, subfornical organ (SFO), lateral hypothalamus and the PVN. In the hypothalamus, stimulation of ARN affected the activity of 197 of the 407 units, the majority of the units were excited but 8% were found to be inhibited (Calaresu et al., 1981b). Renal afferent nerve signals can elicit both inhibitory reno-renal reflexes as well as long looped supra-medullary reno-excitatory responses (Ciriello et al., 1987; Saeki et al., 1988).
Renal afferent nerve fibers are mainly unmyelinated (primarily C-fibers) with a small population of faster conducting, A-delta, myelinated fibers (Knuepfer et al., 1987; Simon et al., 1984). We have found that the mean onset latency of response in RVLM projecting PVN neurons to ARN stimulation was comparable to those reported in cats (Caverson et al., 1983) and rats (Day et al., 1985) with a fairly wide range of values (Xu et al., 2015). The onset latency of RVLM projecting PVN neurons to low frequency ARN stimulation was longer compared with high frequency ARN stimulation. One possible explanation for these observations is the differences in conduction velocities of the different afferent fibers. The renal afferent A fibers are elicited by stimulation with trains of pulses at low voltage and high frequency, and the renal afferent C fibers are stimulated by trains of pulses at high voltage and low frequency (Calaresu et al., 1980; Felder, 1986). It is thought that mechanoreceptor information is carried by the myelinated large fibers and the chemoreceptor information is transmitted by the unmyelinated small fibers (Calaresu et al., 1980; Ciriello, 1998; Simon et al., 1984).
The precise pathway by which ARN information is relayed to the PVN neurons is not fully clear. A possible pathway may involve the NTS and the RVLM, since neurons in these areas alter firing rates in response to ARN stimulation (Calaresu et al., 1981a; Felder, 1986). By stimulating myelinated renal afferent fibers, investigators have shown that there are direct projections from the kidney to the most medial segment of the fasciculatus gracilis and the caudal half of the NTS (Simon et al., 1984). The fluorescent tracer studies examining the connection between the kidneys and posterior medulla show that some of the renal afferents directly project to the medulla but not to higher regions of the brain. Approximately 8% of the total amount of renal afferents are typically shown to have direct projections to the medulla (Wyss et al., 1984).
In cats, electrical stimulation of ARN affects activity of medullary neurons in the lateral tegmental field, paramedical reticular nucleus and dorsal vagal complex, and hypothalamic neurons in the lateral preoptic area, lateral hypothalamic area, and the PVN (Calaresu et al., 1981b). These data provide the electrophysiological evidence for a neural pathway originating in the kidney and projecting to hypothalamic structures implicated in central cardiovascular regulation. Our recent studies have shown that there is a neural connection from the PVN to the RVLM that is activated by stimulation of ARN (Xu et al., 2015). Increased Fos-labelled neurons were found in the PVN and the brain stem after ARN stimulation, suggesting ARN information originating in kidneys is conveyed to a number of central areas known to be involved in the regulation of body fluid balance and arterial pressure (Solano-Flores et al., 1997).
Calaresu and Ciriello confirmed projections to the hypothalamus in the rat by determining the effect of RDN on hypothalamic catecholamine concentrations (Calaresu et al., 1981a). Four days after RDN, a decreased epinephrine concentration has been reported in the PVN. The finding of catecholamine changes in the PVN after RDN is interesting because brain stem catecholaminergic pathways are thought to provide direct inputs to MNCs in the PVN. This suggests that ARN may alter the activity of MNCs in the PVN via ascending brain stem catecholamine pathways. Caverson et al have demonstrated that neurons in the PVN that project directly to the neurohypophysis increase their rate of discharge during stimulation of ARN (Caverson et al., 1987). Administration of the vasopressin antagonist abolished the rise in arterial pressure that had a long onset latency and outlasted the duration of ARN stimulation (Caverson et al., 1987). These findings demonstrate that afferent information from renal receptors contributes to a reflex pathway by which the kidney may alter the release of vasopressin from the neurohypophysis to influence circulatory and body fluid homeostasis.
Renal afferent nerve signals are centrally integrated and their activation results in an increase in sympathetic tone, which is not only directed toward the kidneys, but also toward other organs (Grisk et al., 2004; Malpas et al., 2006; Schlaich et al., 2009a). The ARN pressor response, which was locked in time with stimulus duration, was shown to be due to the activation of the sympathetic nervous system (Caverson et al., 1987). Renal afferent signals are also involved in spinal feedback loops, termed reno-renal reflexes, whereby afferent activity from one kidney can modulate ipsilateral and contralateral efferent renal nerve activity to regulate diuresis and natriuresis to balance overall renal function between the two kidneys (Kopp, 2015). Kopp et al. showed that the inhibitory mechanoreceptor reno-renal reflex was blunted in CHF, due to desensitization of renal mechanoreceptors by high circulating angiotensin II (Kopp et al., 2003) and activation of endothelin A receptors (Kopp et al., 2010). Blunting of the inhibitory reno-renal reflex may be a mechanism by which sodium is retained and efferent sympathetic drive to non-renal vascular beds is stimulated in CHF.
PVN and Vasopressin
Anatomically the PVN includes vasopressin-producing MNCs which project to neurohypophysis. Day and Ciriello have demonstrated that putative vasopressin MNCs in the hypothalamus increase their rate of discharge during electrical stimulation of ARN (Day et al., 1987). In contrast, ARN stimulation has no effect on the rate of discharge of putative oxytocin neurons. Putative vasopressin neurosecretory neurons have also been shown to increase their rate of discharge during intra-renal infusion of bradykinin and capsaicin, whereas renal vein occlusion and increased systemic arterial pressure after transection of sinoaortic and cardiopulmonary afferent fibers did not alter the discharge rates of these cells. Vasopressin neurosecretory neurons selectively receive an excitatory input from chemoreceptors within the kidney, but are unresponsive to activation of renal vascular mechanoreceptors. These findings also suggest that afferent information originating from renal receptors and baroreceptors converge on hypothalamic neurons and contribute to neural mechanisms controlling the release of vasopressin from the neurohypophysis.
In the hypothalamus, vasopressin neurosecretory neurons selectively receive an excitatory input from chemoreceptors within the kidney, but are unresponsive to activation of renal vascular mechanoreceptors (Ciriello et al., 2002). These findings suggest that afferent information originating from renal receptors and baroreceptors converge on hypothalamic neurons and contribute to neural mechanisms controlling the release of vasopressin from the neurohypophysis. We have previous found that ARN stimulation activates RVLM projecting PVN neurons (Xu et al., 2015). In the same study, we also observed that part of the non-antidromically identified neurons responded to the stimulation of the ARN. It is possible that this population of PVN neurons represents the projection to the neurohypophysis to influence vasopressin release observed previously (Caverson et al., 1987).
Chronically elevated plasma vasopressin levels have been reported both in animal models and human patients with CHF, being an important factor contributing to altered fluid/electrolyte balance, as well as detrimental myocardial effects (Goldsmith et al., 1983; Riegger et al., 1985). Studies in animal models of CHF support elevated vasopressin neuronal activity in the PVN (Potapenko et al., 2011). Postsynaptic properties of GABAergic and glutamatergic synaptic function contribute to enhanced MNCs activity in the CHF rats (Potapenko et al., 2011; Stern et al., 2013). In the PVN, vasopressin can also act in a diffusible manner to stimulate the activity of neighboring pre-autonomic neurons, leading to an increased RSNA (Son et al., 2013).
Activation of the Afferent Renal Nerve Produces Exaggerated Sympathetic Outflow during Chronic Heart Failure
The sympathetic nervous system has been identified as a major contributor to the complex pathophysiology of CHF both in experimental models and patients (Grassi, 2009; Parati et al., 2012). There are very few studies that have examined the role of the ARN in CHF. CHF is associated with a number of symptoms that would be expected to stimulate renal afferent activity, such as increased venous congestion and decreased renal blood flow (Ciriello, 1998; Recordati et al., 1978). Direct electrical stimulation of the ARN in animals has been shown to produce sympathoexcitation in various vascular beds and an increase in arterial pressure (Caverson et al., 1988; Chinushi et al., 2013; Spelman et al., 1991). It is possible that a pathological positive feedback signal/s, which remains to be identified, from the level of the kidney may exist, whereby causing an increase in overall sympathetic tone. Ablation of the renal sympathetic nerves remains an attractive therapeutic approach in hypertension and CHF (Krum et al., 2009; Lambert et al., 2012; Laurent et al., 2012; Sobotka et al., 2012).
It is unknown whether the excitatory renal-chemoreflex is enhanced in CHF, potentially in parallel with the enhanced arterial chemoreflex response already observed in CHF. In this regard, it is of interest to note that such inhibitory reno-renal reflexes are reported to be blunted in rats with CHF (Kopp, 2015; Kopp et al., 2003). These data are consistent with the concept that this reduced inhibitory input may be overwhelmed by excitatory input generated within the kidneys of rats during the CHF condition, since RDN is able to abrogate the increase in global sympathetic activation. Therefore, the kidney is not only a target of sympathetic outflow, but also a source of signals that have the potential to directly modulate overall sympathetic outflow in disease conditions such as CHF (Kopp, 2015).
Evidence for excitatory reflexes originating in diseased kidneys is derived from studies in rats with chronic renal failure and patients with renal failure (Kopp, 2015). There is also considerable strong evidence that the diseased kidneys exert an excitatory effect on sympathetic nerve activity in various pathological conditions involving renal injury, including hypertension, CHF, chronic renal failure, diabetes, and obesity (Giamouzis et al., 2011; Henegar et al., 2014; Kopp, 2015; Linz et al., 2015; Ott et al., 2014; Schlaich et al., 2009b). It has been proposed that renal inflammation is prevalent in many of these pathological conditions and may contribute to the increased sympathetic outflow via activation of ARN (Kopp, 2015).
In CHF rats induced by left coronary ligation surgery, there was a two-fold increase in FosB-positive cells in the PVN compared to sham-operated rats. Extracellular recordings in the PVN have also shown that RVLM projecting PVN neurons are more active in rats with CHF at 4 weeks. (Xu et al., 2012 ), suggesting that perhaps there is a potential for tonic ongoing activation by the ARN during CHF condition, which may contribute to the state of activation of the PVN which would then translate into an increased overall sympathoexcitation under basal conditions. It should be noted that there are multiple triggers in the CHF condition that have the potential for increased activation of the ARN activity, including reduced perfusion pressure, increased venous pressure, increased inflammation, increased oxidative stress to name a few.
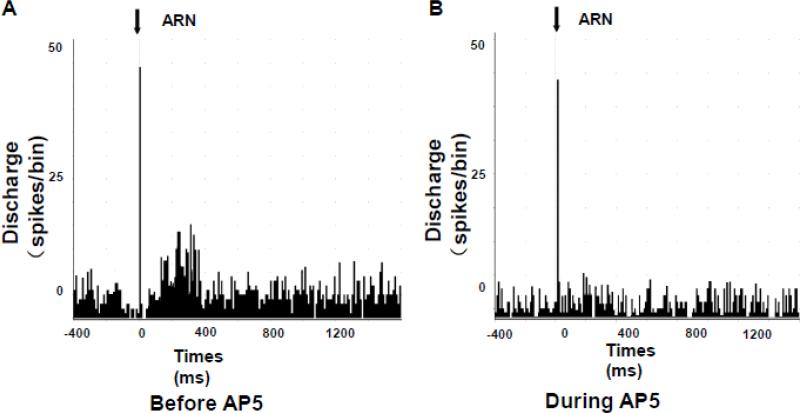
Our previous studies have shown that the PVN is activated in rats with CHF in conjunction with enhanced glutamatergic tone and blunted NO mechanism within the PVN (Li et al., 2003; Patel et al., 2012; Zhang et al., 2001; Zheng et al., 2011). NR1 receptor mRNA expression and protein in the PVN are significantly increased in CHF, which may contribute to the elevated sympathoexcitation during CHF (Li et al., 2003). In our recent study, NMDA activated all PVN neurons that were excited by inputs from ARN, and these responses were attenuated during iontophoretic application of the glutamate receptor blocker, AP5 (Figure 1) (Xu et al., 2015). Thus, it is possible that the upregulation of NMDA receptor and the subsequent increase in glutamate activity within the PVN may be contributing to the altered compensatory responses in disease states such as CHF.
Figure 1.
Peristimulus histogram of spike occurrence triggered by electrical stimulation of the ARN with 50 sweeps. A: before iontophoretic application of NMDA receptor antagonist AP5 and B: during iontophoretic application of AP5. [From (Xu et al., 2015)]
Renal Denervation Abrogates Activation of the PVN during Chronic Heart Failure
The kidneys communicate with integral structures in the central nervous system via the renal sensory afferent nerves (Grisk et al., 2004). Intra-renal stimuli or pathology, such as ischemia or hypoxia, results in an increase in ARN activity (Ye et al., 1998; Ye et al., 2002). Renal sensory afferent nerve activity directly influences sympathetic outflow to the kidneys and other organs such as the heart and peripheral blood vessels, which are also modulated by the PVN (DiBona, 2003). Thus, RDN is likely to be valuable in the treatment of several clinical conditions such as hypertension and CHF characterized by increased sympathetic outflow overall, and particularly RSNA to the kidney (Kline, 1987; Patel et al., 2016; Patel et al., 1996).
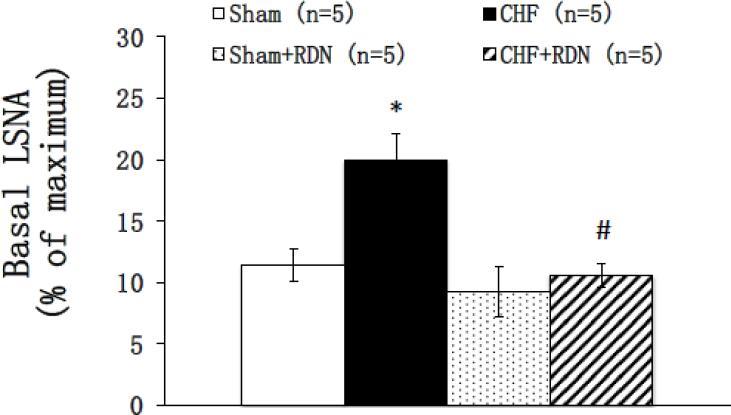
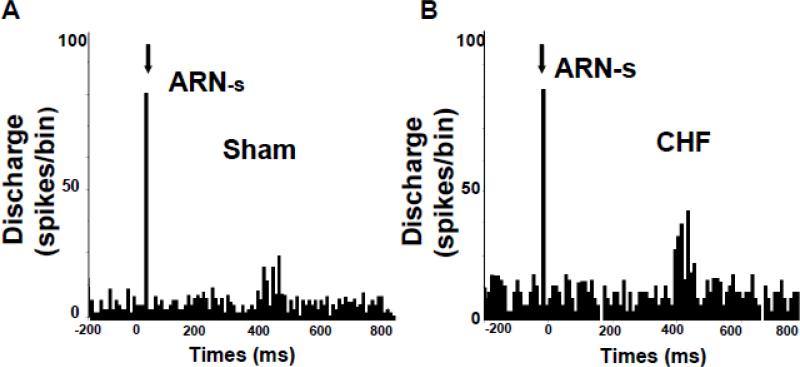
Renal denervation in most experimental forms of hypertension as well as drug resistant hypertensive patient has been shown to reduce arterial pressure and sympathetic activity (DiBona et al., 1997; Kline, 1987; Krum et al., 2009). Since exaggerated sympathoexcitation is characteristic of CHF, the efficacy of RDN to reduce sympathoexcitation has been explored in both ischemia-induced and pacing heart models of CHF (Hu et al., 2012; Nozawa et al., 2002; Schiller et al., 2013) and patients with CHF (Bohm et al., 2014; Schiller et al., 2015). Recently, we have shown that bilateral RDN was sufficient to reduce global sympathetic outflow, as evident by a reduction in urinary excretion of norepinephrine as well as basal level of lumbar sympathetic nerve activity in the CHF rats (Figure 2) (Patel et al., 2016). One plausible interpretation of these data is that there may be an enhanced tonic level of ARN activity during the CHF condition (Figure 3), which may contribute to the state of activation of the PVN resulting in an increased overall sympathoexcitation. Reducing norepinephrine is very important, as CHF patients with lower levels of plasma norepinephrine have better prognosis (Cohn et al., 1984). These results suggest that RDN may alter the activity of neurons in central cardiovascular regulatory sites, such as the PVN, thereby contributing to the reduction in sympathetic tone.
Figure 2.
Basal lumbar sympathetic nerve activity (LSNA) in four groups of rats: sham, sham+RDN, CHF and CHF+RDN. Data are presented as mean ± SE. CHF: chronic heart failure; RDN: renal denervation. *P < 0.05 vs. sham; #P < 0.05 vs. without RDN.
Figure 3.
Peristimulus histogram of spike occurrence triggered by electric stimulation of the afferent renal nerve with 100 sweeps, bin = 0.02 s. ARN-s: afferent renal nerve stimulation. Segments of original recordings of changes in discharge after afferent renal nerve (ARN) stimulation in sham (A) and CHF (B) rats. [From (Patel et al., 2016)]
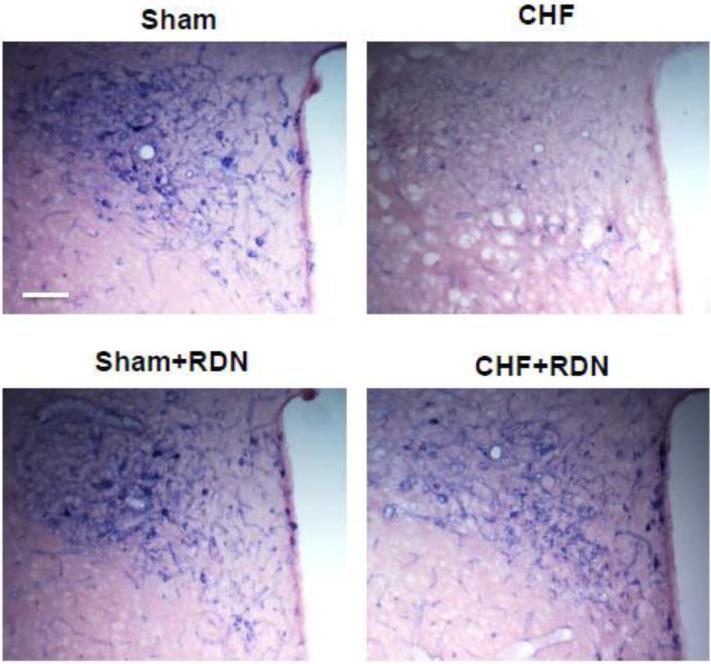
Particularly, we have found RDN restored the endogenous neuronal NO synthase (nNOS) in the PVN that had been decreased in rats with CHF (Figure 4) (Patel et al., 2016). Previously, we have shown nNOS mediated sympathoinhibition from the PVN was blunted in CHF (Zhang et al., 2001). RDN normalized the blunted lumbar sympathetic nerve activity response to inhibition of endogenous NOS within the PVN observed in CHF rats (Patel et al., 2016). We proposed that a possible mechanism for the therapeutic effects of RDN during CHF might be through an NO-dependent mechanism within the PVN. This is consistent with previous observations showing NO synthesizing cells in the PVN may affect renal autonomic pathways by interacting with the renal sensory information (Weiss et al., 2001).
Figure 4.
A. The effect of renal denervation (RDN) on NADPH-diaphorase in the paraventricular nucleus (PVN) of rats with CHF. Representative pictures of PVN with NOS positive staining in four groups of rats, sham, CHF, sham+RDN and CHF+RDN. Bar = 100 μm.
Summary
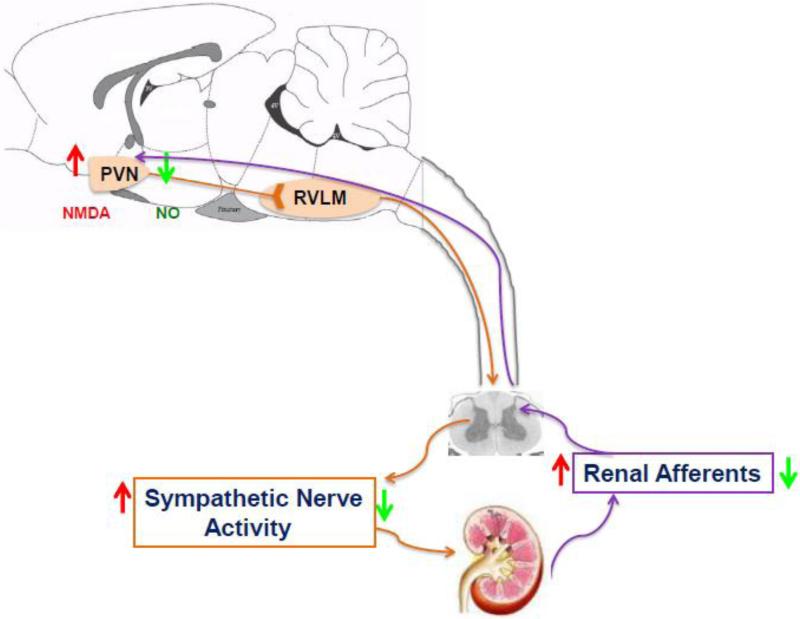
In summary, sensory information originating in the kidney excites pre-autonomic neurons within the PVN know to be involved in the regulation of sympathetic outflow, suggests that this “renal-PVN afferent pathway” may contribute to overall sympathetic tone (Figure 5). It is thus conceivable that an enhanced/altered afferent renal input to the PVN in disease conditions such as CHF and hypertension may be critically involved in producing elevated sympathetic nerve activity commonly observed in these disease states.
Figure 5.
Schematic diagram illustrating that sensory information originating in the kidney is transmitted by renal afferent nerves to the dorsal column of the spinal cord which results in exciting pre-autonomic neurons in the PVN via multiple synaptic pathway. The pre-autonomic neurons in the PVN project to the RVLM as well as the intermediary column of the spinal cord, where pre-sympathetic neurons terminating in the kidney reside. These pre-autonomic neurons are activated by NMDA and inhibited by NO. This long loop pathway involving the PVN suggests that this renal-PVN pathway may contribute to the elevated sympathetic nerve activity in disease conditions such as CHF. Renal denervation reduces afferent renal nerve signaling, neuronal activity in the PVN and sympathetic nerve activity in the CHF. RVLM, rostral ventrolateral medulla; NMDA, N-methyl-d-aspartate; NO, nitric oxide. Orange arrows indicate efferent pathway affecting the kidney. Purple arrows indicate afferent pathway to the PVN. Red arrows: indicate changes during CHF; green arrows: indicate changes after renal denervation.
Non-standard Abbreviations and Acronyms
- PVN
paraventricular nucleus
- MNCs
magnocellular neurosecretory neurons
- ARN
afferent renal nerves
- RVLM
rostral ventrolateral medulla
- RSNA
renal sympathetic nerve activity
- CHF
chronic heart failure
- pPVN
parvocellular neurons of the PVN
- NO
nitric oxide
- NTS
nucleus tractus solitaries
- SFO
subfornical organ
- RDN
Renal denervation
- nNOS
neuronal nitric oxide synthase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Bohm M, Ewen S, Kindermann I, Linz D, Ukena C, Mahfoud F. Renal denervation and heart failure. Eur J Heart Fail. 2014;16:608–613. doi: 10.1002/ejhf.83. [DOI] [PubMed] [Google Scholar]
- Booth LC, May CN, Yao ST. The role of the renal afferent and efferent nerve fibers in heart failure. Front Physiol. 2015;6:270. doi: 10.3389/fphys.2015.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaresu FR, Ciriello J. Projections to the hypothalamus from buffer nerves and nucleus tractus solitarius in the cat. Am J Physiol. 1980;239:R130–136. doi: 10.1152/ajpregu.1980.239.1.R130. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Ciriello J. Altered concentration of catecholamines in the hypothalamus of the rat after renal denervation. Can J Physiol Pharmacol. 1981a;59:1274–1277. doi: 10.1139/y81-199. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst. 1981b;3:311–320. doi: 10.1016/0165-1838(81)90072-2. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J. Effect of stimulation of afferent renal nerves on plasma levels of vasopressin. Am J Physiol. 1987;252:R801–807. doi: 10.1152/ajpregu.1987.252.4.R801. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol. 1988;254:R531–543. doi: 10.1152/ajpregu.1988.254.3.R531. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J, Calaresu FR. Cardiovascular afferent inputs to neurons in the ventrolateral medulla projecting directly the central autonomic area of the thoracic cord in the cat. Brain Res. 1983;274:354–358. doi: 10.1016/0006-8993(83)90718-7. [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol. 2010;103:4–15. doi: 10.1152/jn.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinushi M, Izumi D, Iijima K, Suzuki K, Furushima H, Saitoh O, Furuta Y, Aizawa Y, Iwafuchi M. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61:450–456. doi: 10.1161/HYPERTENSIONAHA.111.00095. [DOI] [PubMed] [Google Scholar]
- Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1745–R1754. doi: 10.1152/ajpregu.1998.275.6.R1745. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Hypothalamic projections of renal afferent nerves in the cat. Can J Physiol Pharmacol. 1980;58:574–576. doi: 10.1139/y80-095. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–285. doi: 10.1016/0165-1838(83)90110-8. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Caverson MM. Central organization of afferent renal nerve pathways. Clinical and experimental hypertension. Part A, Theory and practice. 1987;9(Suppl 1):33–46. doi: 10.3109/10641968709160162. [DOI] [PubMed] [Google Scholar]
- Ciriello J, de Oliveira CV. Renal afferents and hypertension. Curr Hypertens Rep. 2002;4:136–142. doi: 10.1007/s11906-002-0038-x. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. New Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Day TA, Ciriello J. Afferent renal nerve stimulation excites supraoptic vasopressin neurons. Am J Physiol. 1985;249:R368–371. doi: 10.1152/ajpregu.1985.249.3.R368. [DOI] [PubMed] [Google Scholar]
- Day TA, Ciriello J. Effects of renal receptor activation on neurosecretory vasopressin cells. Am J Physiol. 1987;253:R234–241. doi: 10.1152/ajpregu.1987.253.2.R234. [DOI] [PubMed] [Google Scholar]
- DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621–624. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- Felder RB. Excitatory and inhibitory interactions among renal a cardiovascular afferent nerves in dorsomedial medulla. Am J Physiol. 1986;250:R580–588. doi: 10.1152/ajpregu.1986.250.4.R580. [DOI] [PubMed] [Google Scholar]
- Giamouzis G, Butler J, Triposkiadis F. Renal function in advanced heart failure. Congest Heart Fail. 2011;17:180–188. doi: 10.1111/j.1751-7133.2011.00240.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith SR, Francis GS, Cowley AW, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;106:1385–1390. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- Grisk O, Rettig R. Interactions between the sympathetic nervous system and the kidneys in arterial hypertension. Cardiovasc Res. 2004;61:238–246. doi: 10.1016/j.cardiores.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst. 1994;50:1–11. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–1292. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ji M, Niu C, Aini A, Zhou Q, Zhang L, Jiang T, Yan Y, Hou Y. Effects of renal sympathetic denervation on post-myocardial infarction cardiac remodeling in rats. PLoS One. 2012;7:e45986. doi: 10.1371/journal.pone.0045986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–767. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- Kang YM, Gao F, Li HH, Cardinale JP, Elks C, Zang WJ, Yu XJ, Xu YY, Qi J, Yang Q, Francis J. NF-kappaB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. 2011;106:1087–1097. doi: 10.1007/s00395-011-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83:737–746. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Katafuchi TY, Oomura Y, Kurosawa M. Effects of chemical stimulation of paraventricular nucleus on adrenal and renal nerve activity in rats. Neurosci Lett. 1988;86:195–200. doi: 10.1016/0304-3940(88)90570-8. [DOI] [PubMed] [Google Scholar]
- Kline RL. Renal nerves and experimental hypertension: evidence and controversy1. Can J Physiol Pharmacol. 1987;65:1540–1547. doi: 10.1139/y87-243. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM, Schramm LP. The conduction velocities and spinal projections of single renal afferent fibers in the rat. Brain Res. 1987;435:167–173. doi: 10.1016/0006-8993(87)91598-8. [DOI] [PubMed] [Google Scholar]
- Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Jones SY. Activation of endothelin A receptors contributes to impaired responsiveness of renal mechanosensory nerves in congestive heart failure. Can J Physiol Pharmacol. 2010;88:622–629. doi: 10.1139/y10-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: role of endogenous angiotensin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R116–124. doi: 10.1152/ajpregu.00336.2002. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Smith LA, DiBona GF. Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am J Physiol. 1985;249:F507–517. doi: 10.1152/ajprenal.1985.249.4.F507. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Seagard JL, Castaner A, Kampine JP. Reflex effects of renal afferents on the heart and kidney. Am J Physiol. 1981;241:R286–292. doi: 10.1152/ajpregu.1981.241.5.R286. [DOI] [PubMed] [Google Scholar]
- Krukoff TL. Expression of c-fos in studies of central autonomic and sensory systems. Mol Neurobio. 1993;7:247–263. doi: 10.1007/BF02769178. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich MP, Whitbourn R, Sobotka P, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler MD. Catheter-based renal sympathetic denervation for resistant hypertension. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Kuo DC, Nadelhaft I, Hisamitsu T, de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:162–174. doi: 10.1002/cne.902160205. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Hering D, Esler MD, Marusic P, Lambert EA, Tanamas SK, Shaw J, Krum H, Dixon JB, Barton DA, Schlaich MP. Health-related quality of life after renal denervation in patients with treatment-resistant hypertension. Hypertension. 2012;60:1479–1484. doi: 10.1161/HYPERTENSIONAHA.112.200865. [DOI] [PubMed] [Google Scholar]
- Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet. 2012;380:591–600. doi: 10.1016/S0140-6736(12)60825-3. [DOI] [PubMed] [Google Scholar]
- Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- Linz D, Hohl M, Schutze J, Mahfoud F, Speer T, Linz B, Hubschle T, Juretschke HP, Dechend R, Geisel J, Rutten H, Bohm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: the role of renal sympathetic innervation. Am J Hypertens. 2015;28:256–265. doi: 10.1093/ajh/hpu123. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Malpas S, Mahoney MT. Renal vasodilatation in respone to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Auton Nerv Syst. 1993;43:247–256. doi: 10.1016/0165-1838(93)90331-n. [DOI] [PubMed] [Google Scholar]
- Lu XZ, Sun XY, Yao T. Inhibition of renal nerve activity induced by chemical stimulation of the paraventricular nucleus: mediation of the vasopressinergic spinally-projecting pathway. Chin J Physiol Sci. 1991;7:215–221. [Google Scholar]
- Malpas SC, Ramchandra R, Guild SJ, McBryde F, Barrett CJ. Renal sympathetic nerve activity in the development of hypertension. Curr Hypertens Rep. 2006;8:242–248. doi: 10.1007/s11906-006-0057-0. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol. 1991;311:389–404. doi: 10.1002/cne.903110309. [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- Niijima A. Observation on the localization of mechanoreceptors in the kidney and afferent nerve fibres in the renal nerves in the rabbit. J Physiol. 1975;245:81–90. doi: 10.1113/jphysiol.1975.sp010836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, Inoue H. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels. 2002;16:51–56. doi: 10.1007/s380-002-8317-8. [DOI] [PubMed] [Google Scholar]
- Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, Ewen S, Ukena C, Uder M, Bohm M, Schmieder RE. Improvement of albuminuria after renal denervation. Inter J Cardiol. 2014;173:311–315. doi: 10.1016/j.ijcard.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Patel KP. Role of paraventrivular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- Patel KP, Knuepfer M. Effect of afferent renal nerve stimulation on blood pressure and heart rate and noradrenergic activity in conscious rats. J Auton Nerv Syst. 1986;17:121–130. doi: 10.1016/0165-1838(86)90087-1. [DOI] [PubMed] [Google Scholar]
- Patel KP, Schmid PG. Role of the paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst. 1988;22:211–219. doi: 10.1016/0165-1838(88)90109-9. [DOI] [PubMed] [Google Scholar]
- Patel KP, Xu B, Liu X, Sharma NM, Zheng H. Renal denervation improves exaggerated sympathoexcitation in rats with heart failure: a role for neuronal nitric oxide synthase in the paraventricular nucleus. Hypertension. 2016;68:175–184. doi: 10.1161/HYPERTENSIONAHA.115.06794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KP, Zhang PL, Carmines PK. Neural influences on renal responses to acute volume expansion in rats with heart failure. Am J Physiol. 1996;271:H1441–H1448. doi: 10.1152/ajpheart.1996.271.4.H1441. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R923–R928. doi: 10.1152/ajpregu.1993.265.4.R923. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H527–537. doi: 10.1152/ajpheart.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol. 2011;106:1545–1557. doi: 10.1152/jn.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recordati G, Genovesi S, Cerati D. Renorenal reflexes in the rat elicited upon stimulation of renal chemoreceptors. J Auton Nerv Syst. 1982;6:127–142. doi: 10.1016/0165-1838(82)90046-7. [DOI] [PubMed] [Google Scholar]
- Recordati GM, Moss NG, Waselkov L. Renal chemoreceptors in the rat. Circ Res. 1978;43:534–543. doi: 10.1161/01.res.43.4.534. [DOI] [PubMed] [Google Scholar]
- Riegger GA, Liebau G, Bauer E, Kochsiek K. Vasopressin and renin in high output heart failure of rats: hemodynamic effects of elevated plasma hormone levels. J Cardiovasc Pharmacol. 1985;7:1–5. doi: 10.1097/00005344-198501000-00001. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Terui N, Kumada M. Participation of ventrolateral medullary neurons in the renal-sympathetic reflex in rabbits. Jpn J Physiol. 1988;38:267–281. doi: 10.2170/jjphysiol.38.267. [DOI] [PubMed] [Google Scholar]
- Schiller AM, Haack KK, Pellegrino PR, Curry PL, Zucker IH. Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am J Physiol Regul Integr Comp Physiol. 2013;305:R886–892. doi: 10.1152/ajpregu.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller AM, Pellegrino PR, Zucker IH. The renal nerves in chronic heart failure: efferent and afferent mechanisms. Front Physiol. 2015;6:224. doi: 10.3389/fphys.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009a;54:1195–1201. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009b;20:933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- Simon OR, Schramm LP. The spinal course and medullary termination of myelinated renal afferents in the rat. Brain Res. 1984;290:239–247. doi: 10.1016/0006-8993(84)90941-7. [DOI] [PubMed] [Google Scholar]
- Sobotka PA, Krum H, Bohm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep. 2012;14:285–292. doi: 10.1007/s11886-012-0258-x. [DOI] [PubMed] [Google Scholar]
- Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res. 1997;753:102–119. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- Sole MJ, Hussain MN, Versteeg DHG, Ronald de Kloet ER, Adams D, Lixfeld W. The identification of specific brain nuclei in which catecholamine turnover is increased by left ventricular receptors during acute myocardial ischemia in the rat. Brain Res. 1982;235:315–325. doi: 10.1016/0006-8993(82)91010-1. [DOI] [PubMed] [Google Scholar]
- Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman FA, Oberg PA. Continuous measurement of renal cortical blood flow and renal arterial blood flow during stimulation of the renal nerve. Med Biol Eng Comput. 1991;29:121–128. doi: 10.1007/BF02447096. [DOI] [PubMed] [Google Scholar]
- Stern JE, Potapenko ES. Enhanced NMDA receptor-mediated intracellular calcium signaling in magnocellular neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R414–422. doi: 10.1152/ajpregu.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of the projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980a;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980b;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Ueda H, Nishimura H, Yasuda H. Experimental vascular lesions elicited by collagenase in rats. Jpn Heart J. 1967;8:378–387. doi: 10.1536/ihj.8.378. [DOI] [PubMed] [Google Scholar]
- Vahid-Ansari F, Leenen FHH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol. 1998;275:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- Weiss ML, Chowdhury SI, Patel KP, Kenney MJ, Huang J. Neural circuitry of the kidney: NO-containing neurons. Brain Res. 2001;919:269–282. doi: 10.1016/s0006-8993(01)03030-x. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Donovan MK. A direct projection from the kidney to the brainstem. Brain Res. 1984;298:130–134. doi: 10.1016/0006-8993(84)91154-5. [DOI] [PubMed] [Google Scholar]
- Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–1111. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zheng H, Patel KP. Enhanced Activation of RVLM Projecting PVN Neurons in Rats with Chronic Heart Failure. Am J Physiol Heart Circ Physiol. 2012;302:H1700–1711. doi: 10.1152/ajpheart.00722.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens. 1998;11:723–728. doi: 10.1016/s0895-7061(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Ye S, Zhong H, Yanamadala V, Campese VM. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am J Hypertens. 2002;15:717–724. doi: 10.1016/s0895-7061(02)02959-x. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Suo YP, Qi J, Yang Q, Li HH, Zhang DM, Yi QY, Zhang J, Zhu GQ, Zhu Z, Kang YM. Interaction between AT1 receptor and NF-kappaB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol. 2013;13:381–390. doi: 10.1007/s12012-013-9219-x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;310:H732–739. doi: 10.1152/ajpheart.00703.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002a;282:R1006–1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensinaldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002b;283:H423–433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–973. doi: 10.1161/HYPERTENSIONAHA.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Sharma NM, Liu X, Patel KP. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2012;303:R387–394. doi: 10.1152/ajpregu.00046.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]