Abstract
With the aims of sharing information about the technical aspects of immunohistochemistry (IHC) and making it possible to make a suitable choice of antibody for histopathological examination, this technical report describes the results of a questionnaire administered during the period of 2014 to 2015 to members of the Conference on Experimental Animal Histopathology. It also describes the immunological properties of primary antibodies (clone, supplier, catalog number, species reactivity, etc.) and the IHC staining conditions (fixing solution, fixing time, embedding, antigen retrieval method, antibody dilution, incubation time, incubation temperature, positive control tissue, secondary antibody information, etc.) for a total number of 733 primary antibodies (425 kinds of primary antibody).
Keywords: antibody, immunohistochemistry, toxicological pathology
Immunohistochemistry (IHC) is a biochemical method that applies to any use of an antibody-based method to identify a specific antigen in order to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue1. IHC is widely utilized for diagnostic interpretation and understanding of pathogenesis and has become a routine tool for toxicological pathology. However, the variable staining conditions of IHC, such as those relating to the antibody clone/supplier, fixation, antigen retrieval, antigen-antibody reactions, positive controls, antibody dilution, and incubation time, raise many challenges for pathologists. A questionnaire about IHC was administered during the period of 2014 to 2015 to members of the Conference on Experimental Animal Histopathology (CEAH), which is composed of 93 research institutes involved in experimental animal pathological research in Japan and Korea, such as pharmaceutical companies, chemical companies, universities, public research institutes and contract research organizations. A total of 733 primary antibodies (425 kinds of primary antibody) were available from 47 research institutes according to the responses to the questionnaire. With the aims of sharing information about the technical aspects of IHC and making it possible to make a suitable choice of antibody for histopathological examination, the present technical report describes the IHC questionnaire results. In addition, the IHC histological photographs of some primary antibodies are provided in the figures to clarify the antigen localization and staining condition in the tissues.
The immunological properties of primary antibodies (clone, supplier, catalog number, species reactivity, etc.) and IHC staining conditions (fixing solution, fixing time, embedding, antigen retrieval method, antibody dilution, incubation time, incubation temperature, positive control tissue, secondary antibody information, etc.) are shown in Tables 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and Supplementary Tables 1–15: online only, and IHC histological photographs are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235..
Table 1.
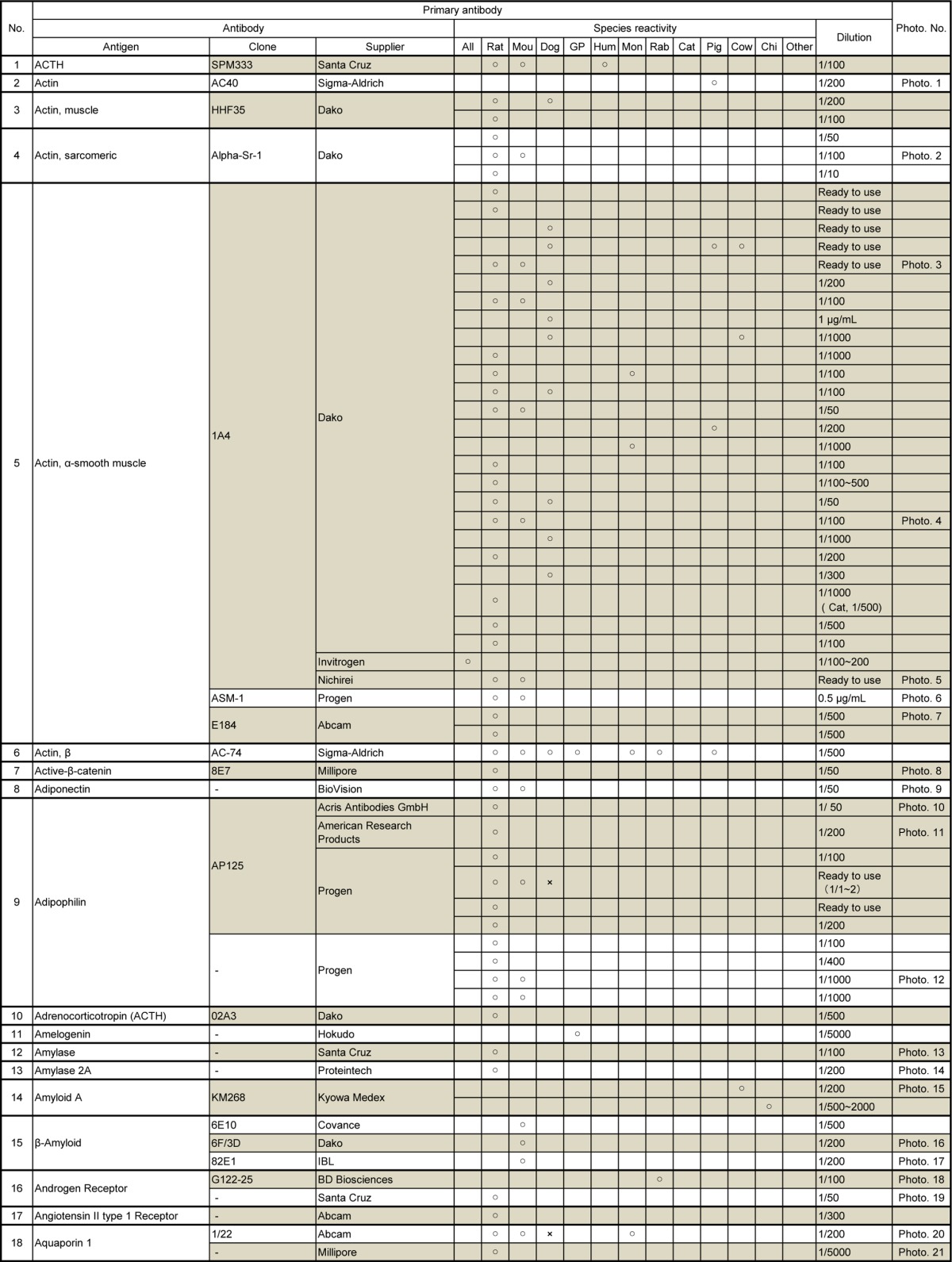
Table 2.
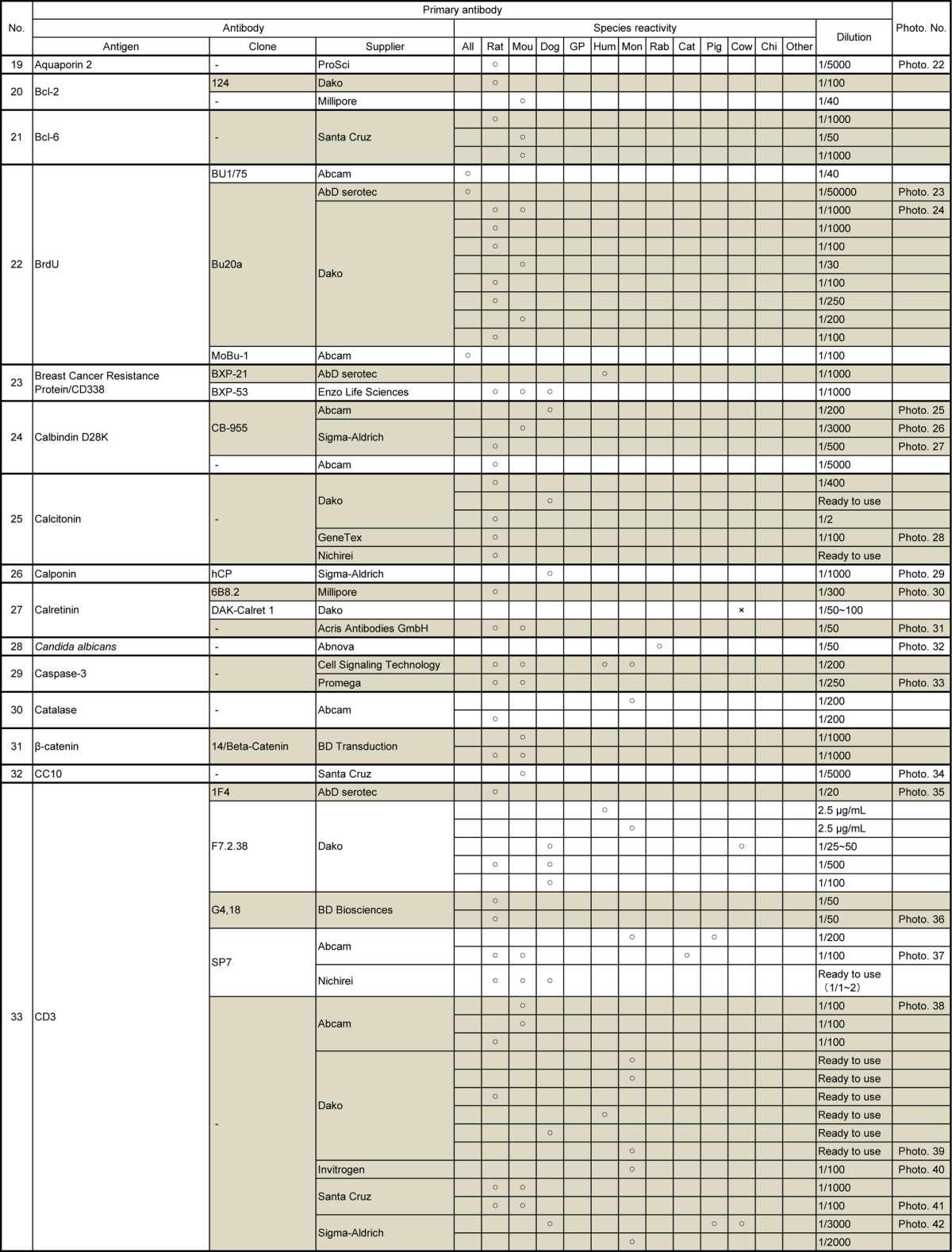
Table 3.
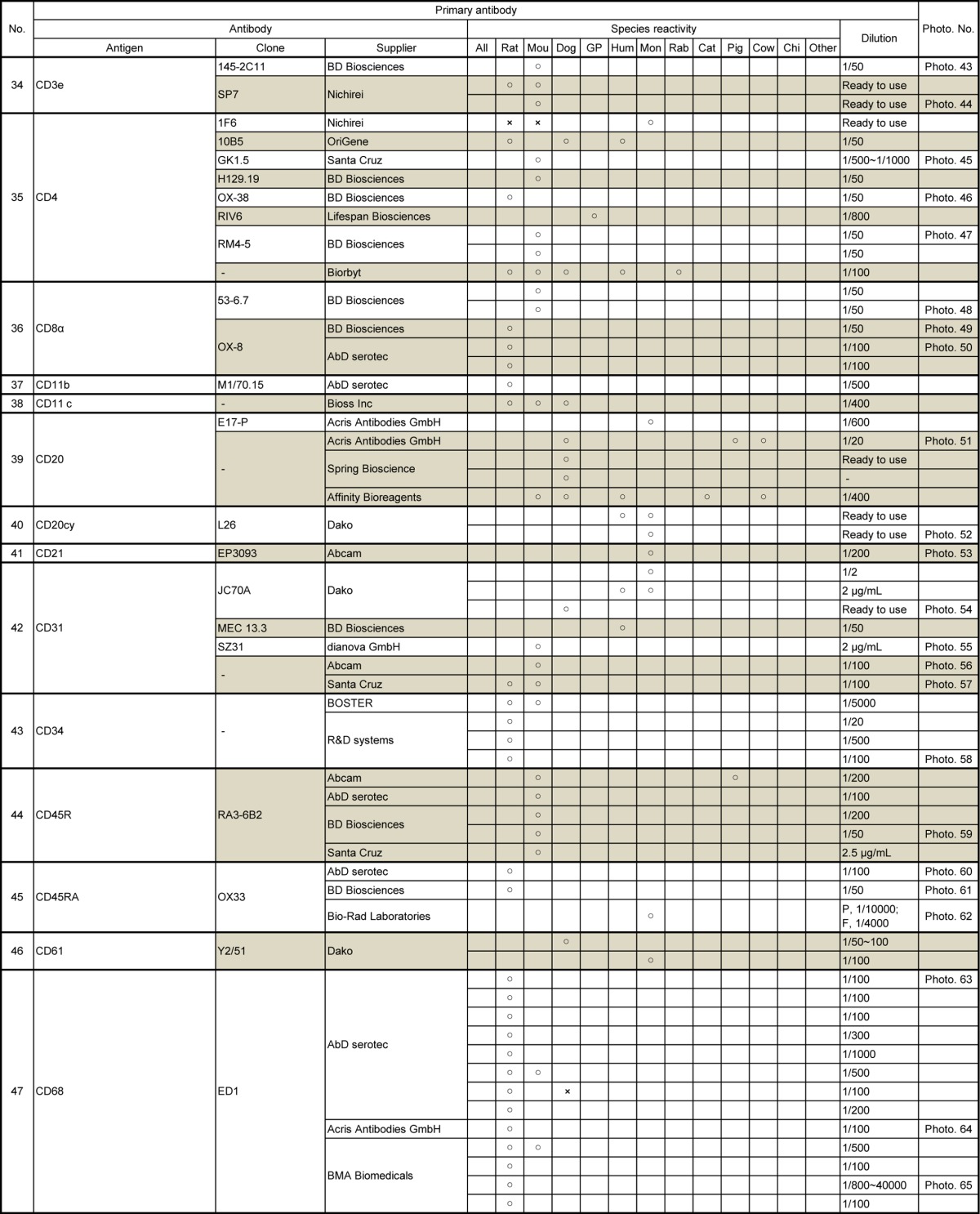
Table 4.
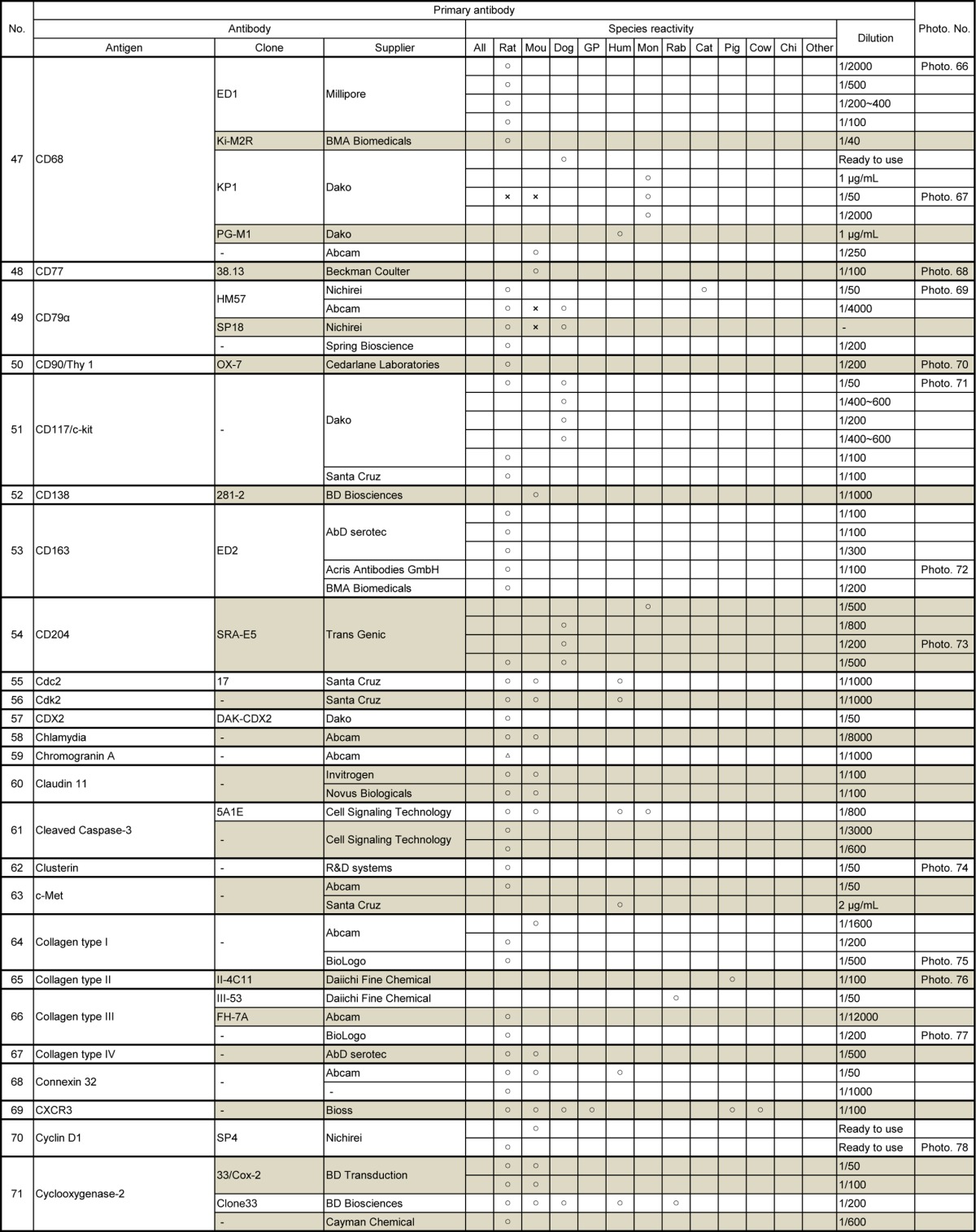
Table 5.
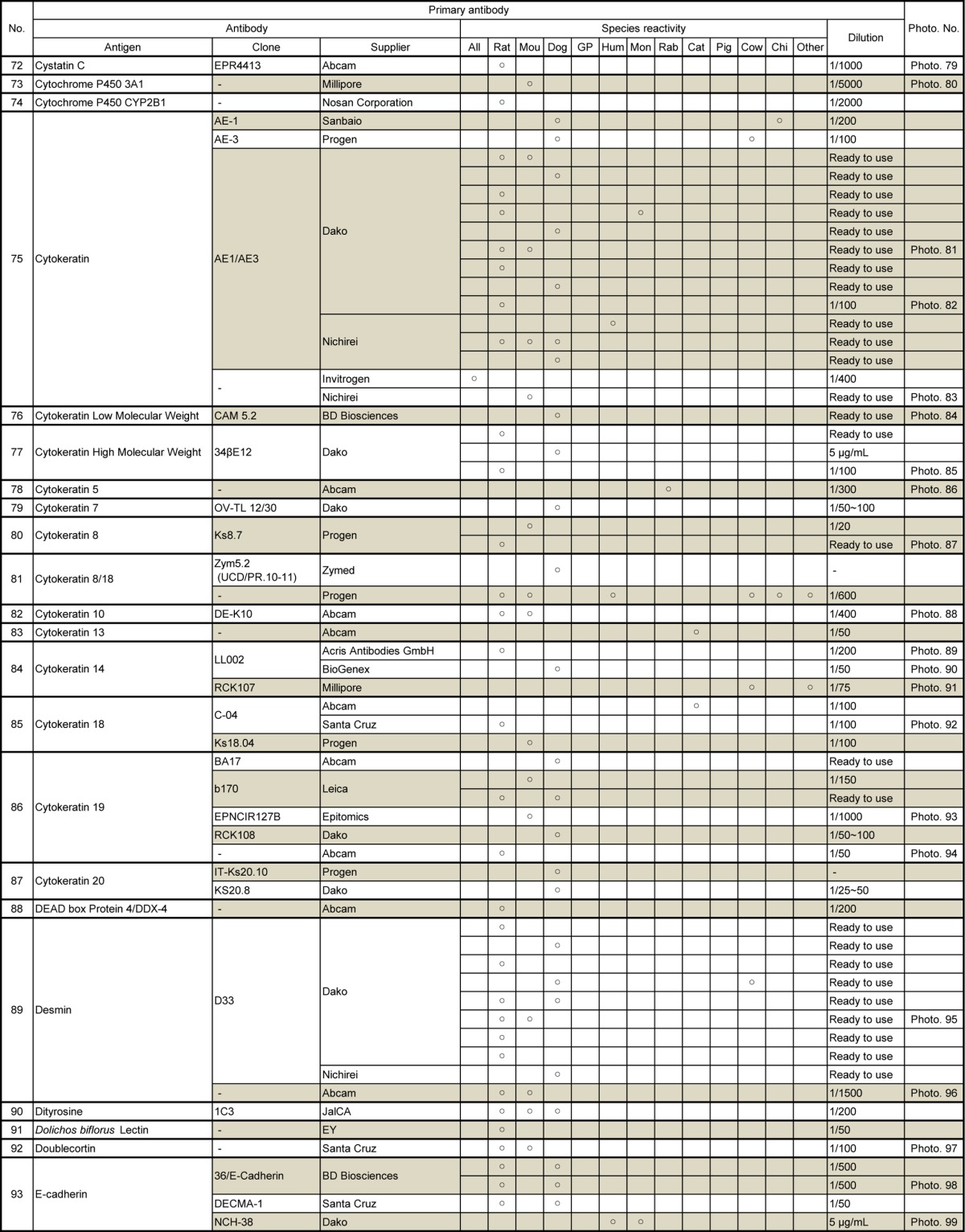
Table 6.
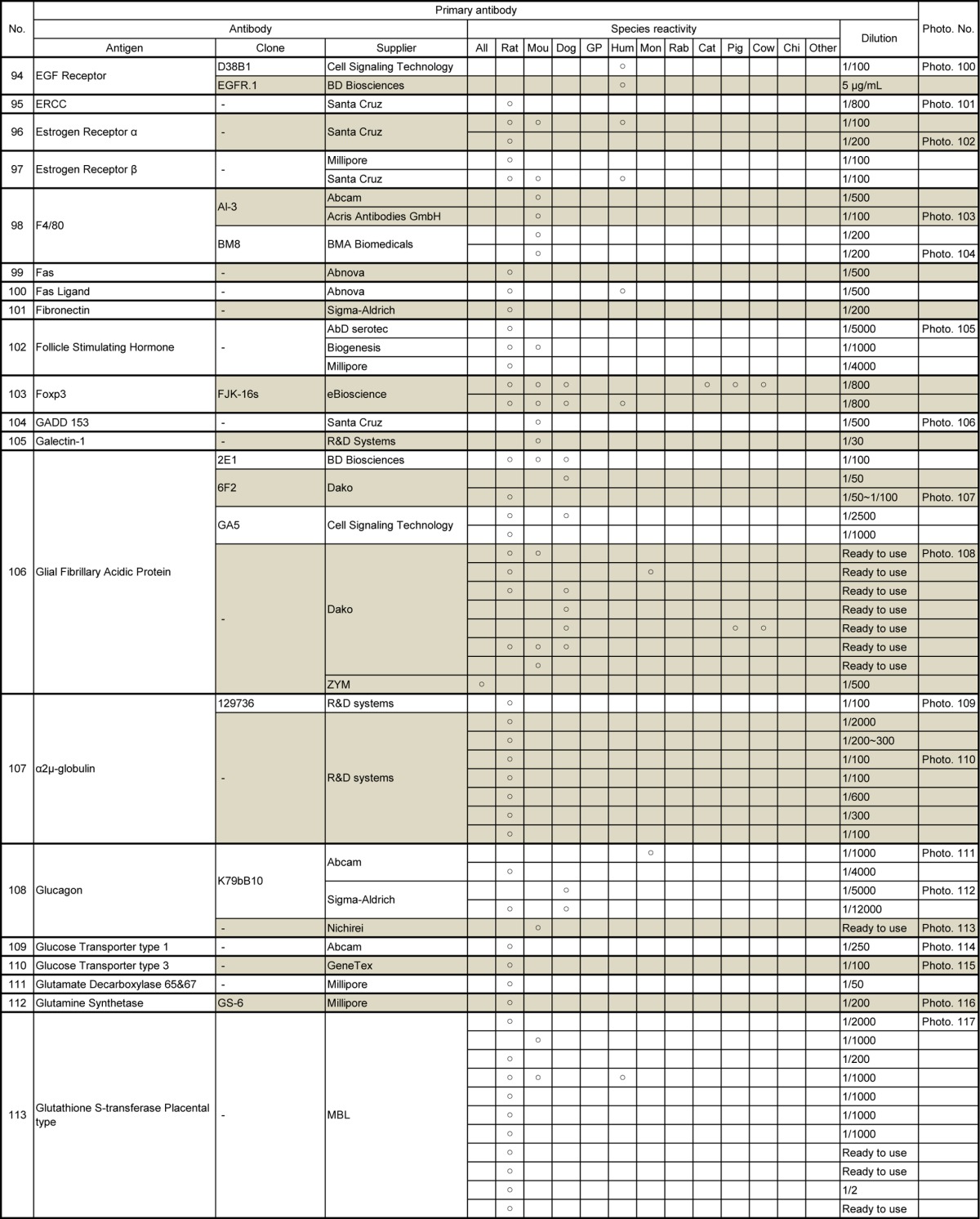
Table 7.
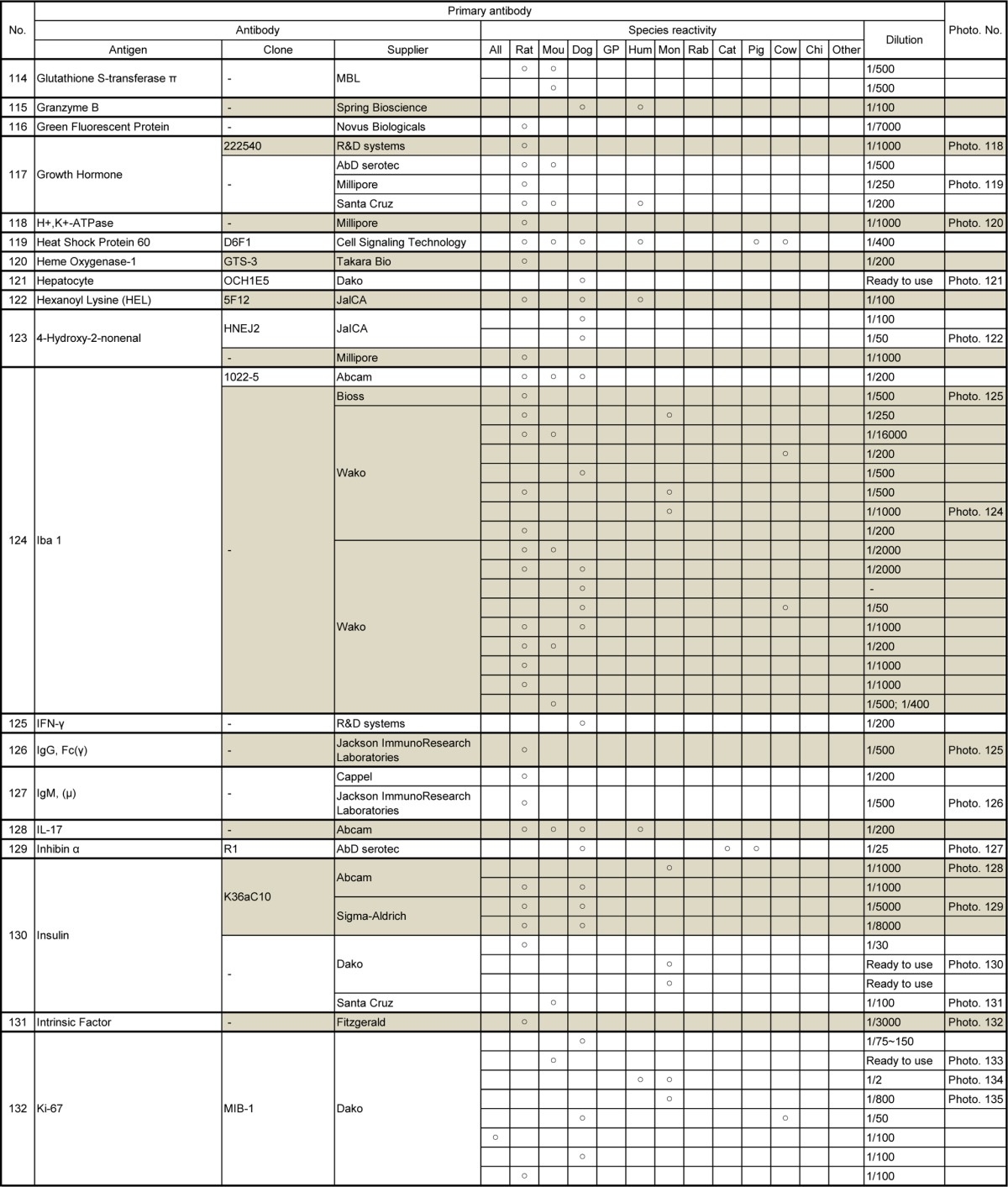
Table 8.
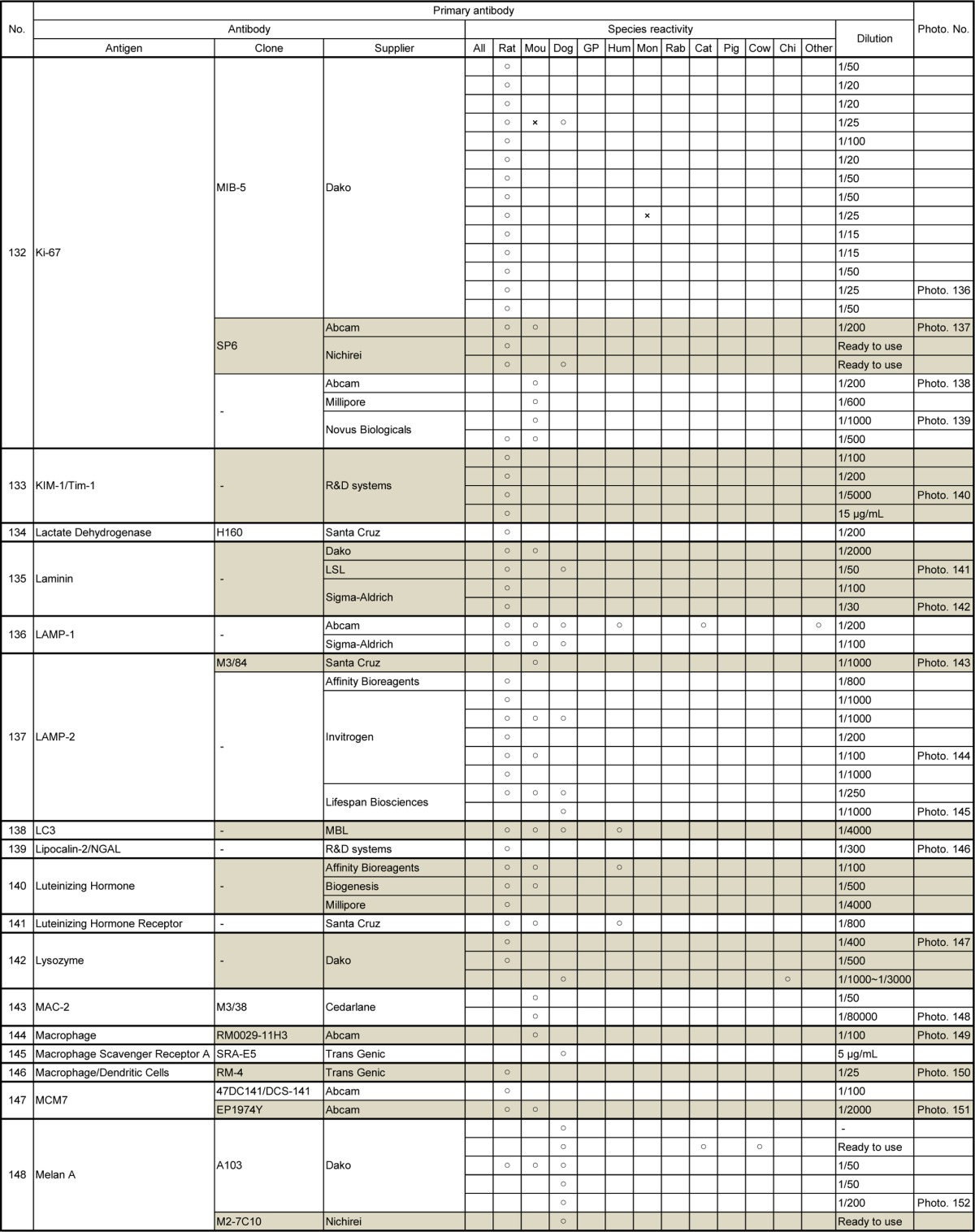
Table 9.
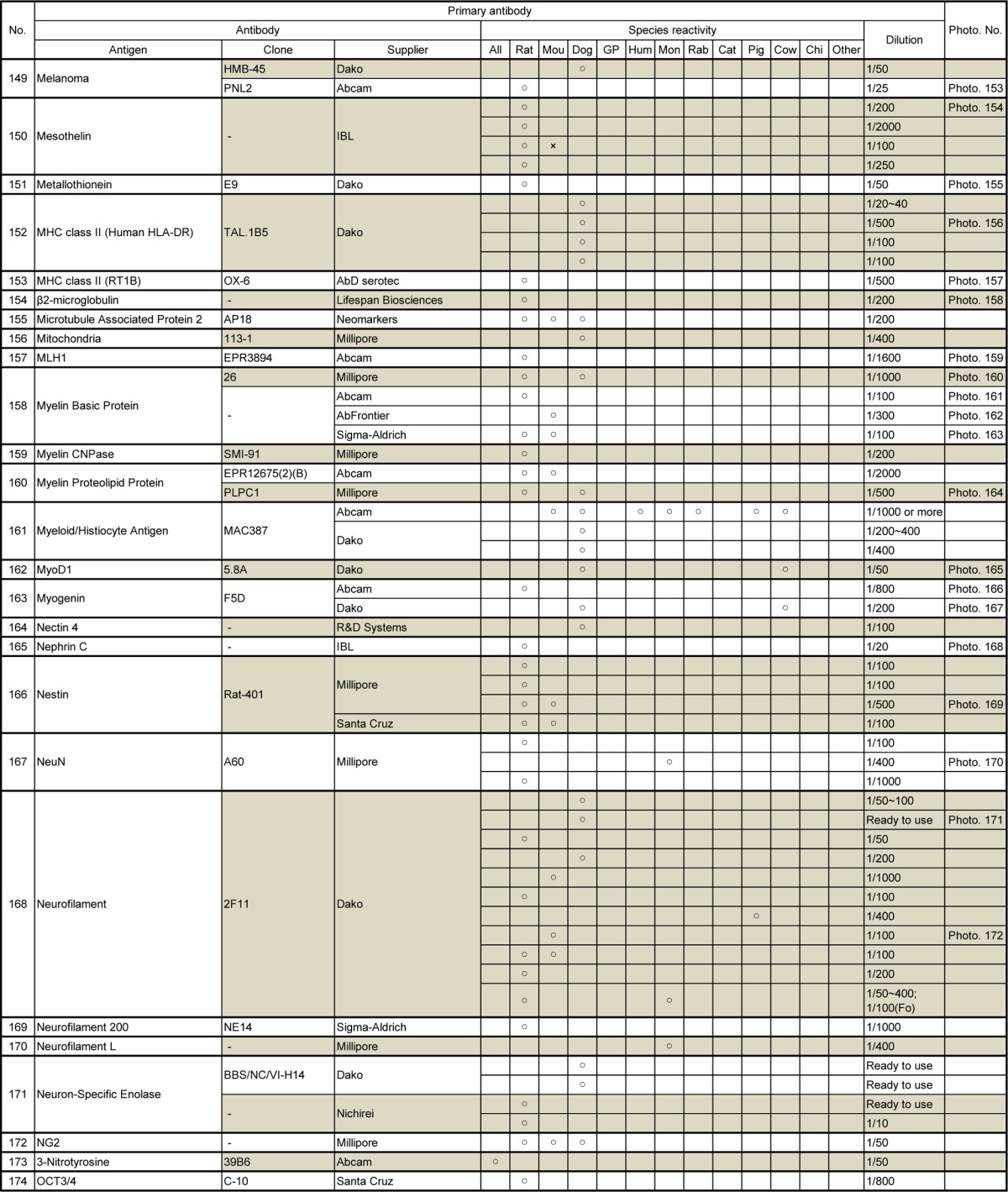
Table 10.
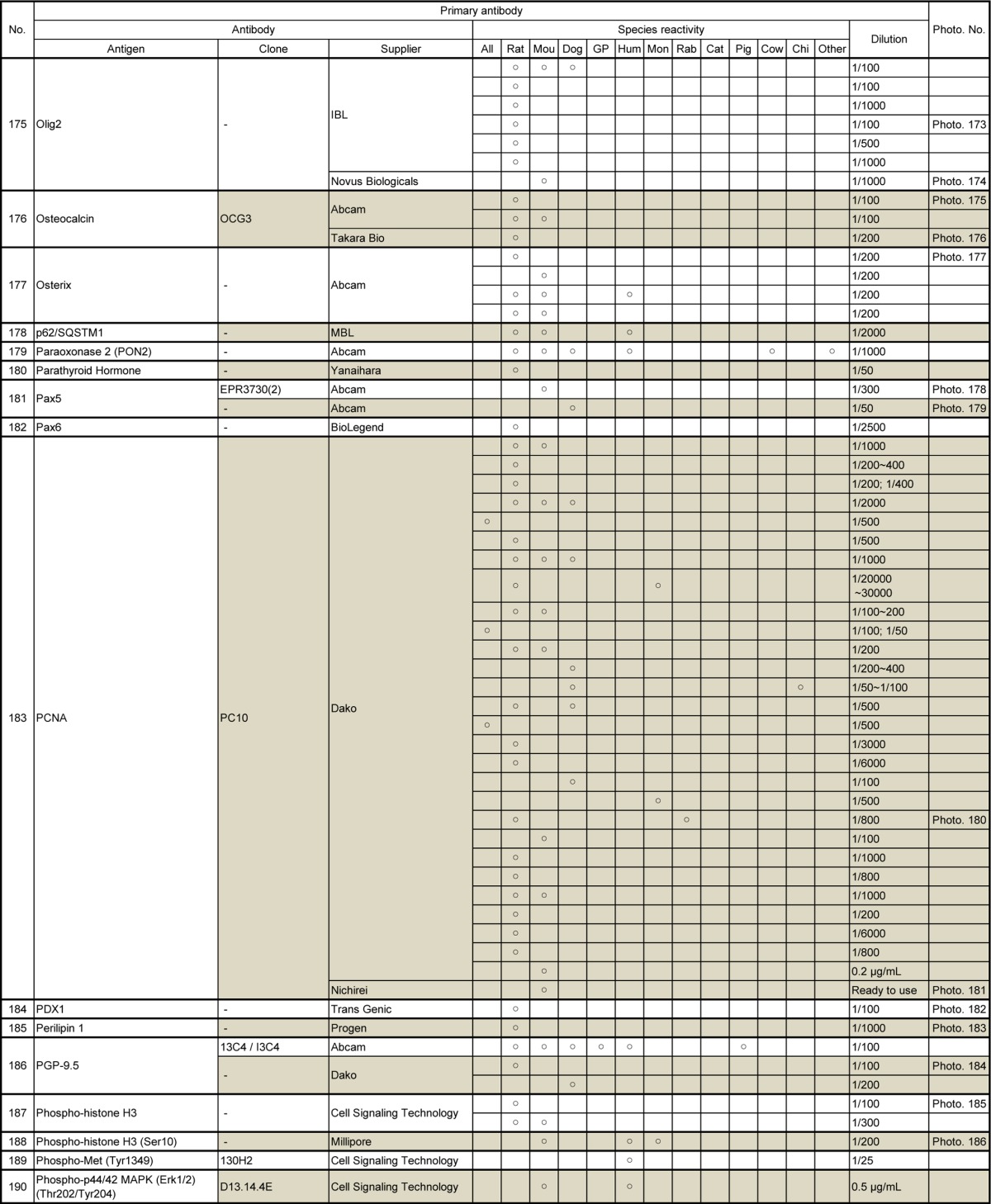
Table 11.
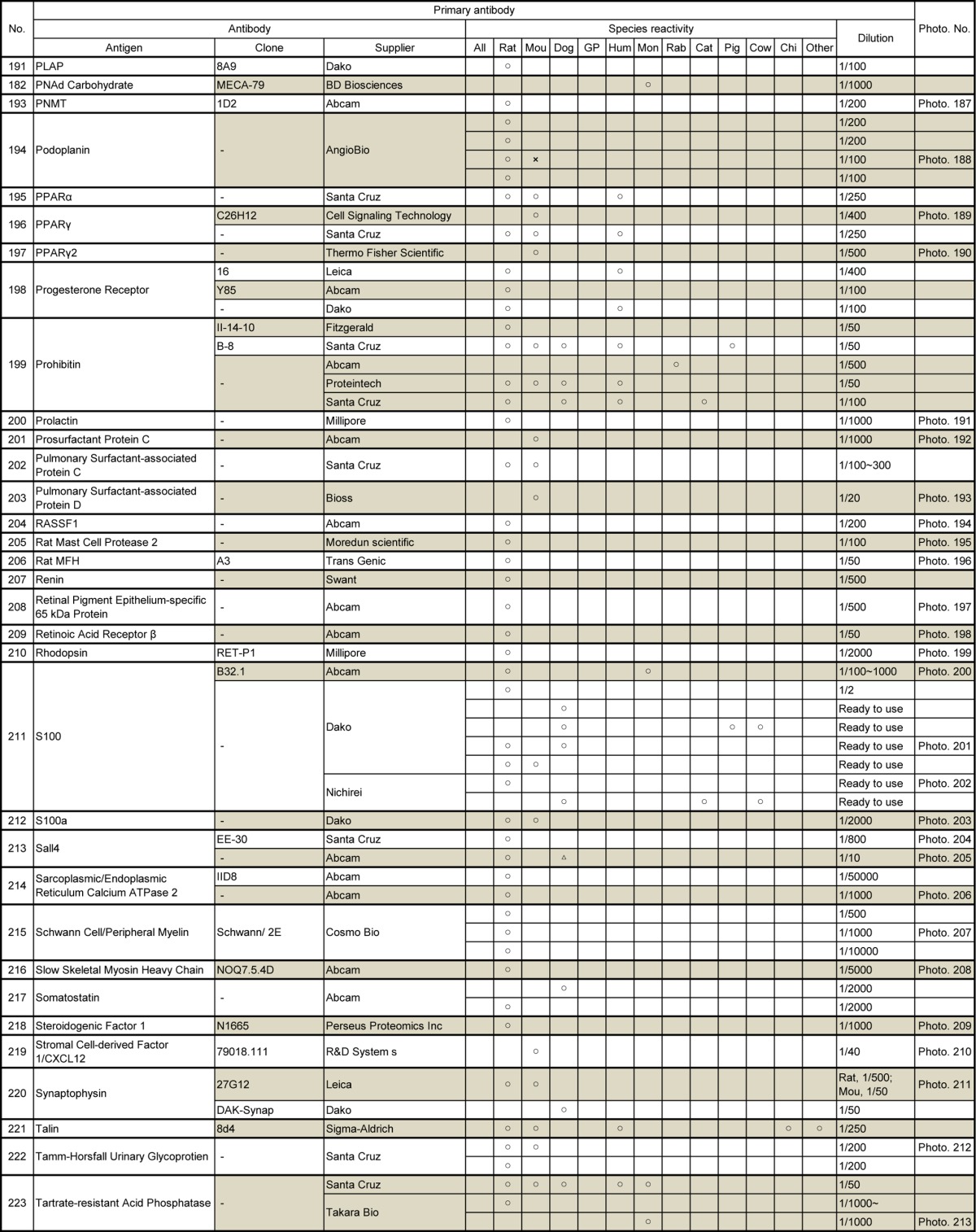
Table 12.
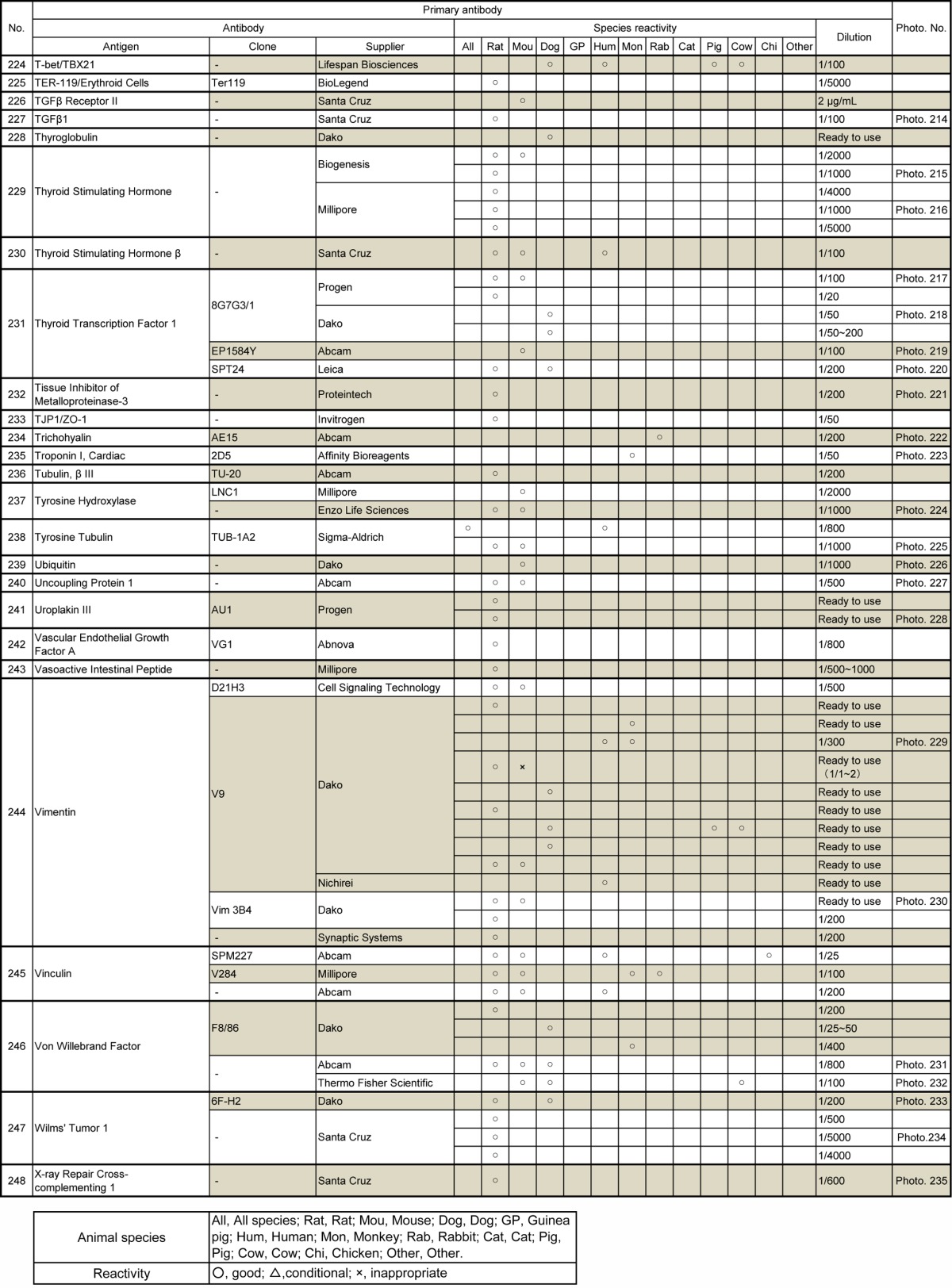
Fig. 1.
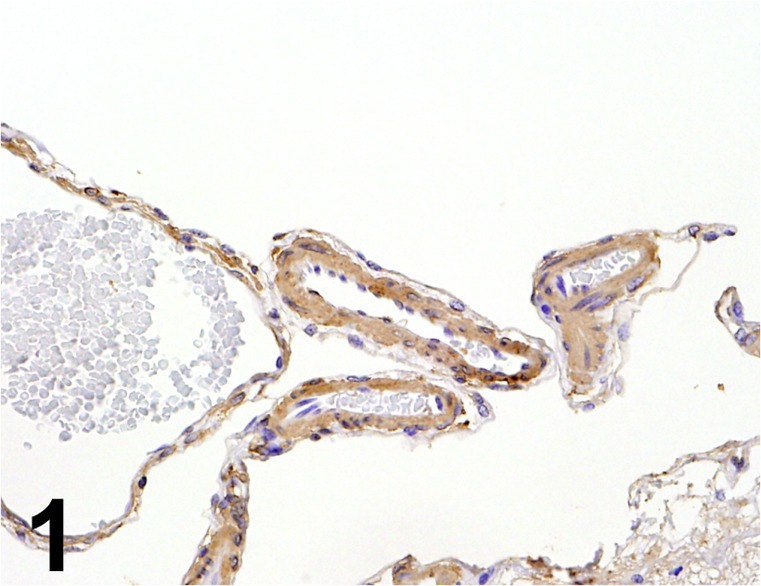
Actin/ AC40/ Sigma/ A4700, Brain (Arachnoid)/ Minipig.
Fig. 2.

Actin, sarcomeric/ Alpha-Sr-1/ Dako/ M0874, Muscle/ Rat.
Fig. 3.
Actin, α-smooth muscle/ 1A4 / Dako/ IR611, Small intestine/ Rat.
Fig. 4.
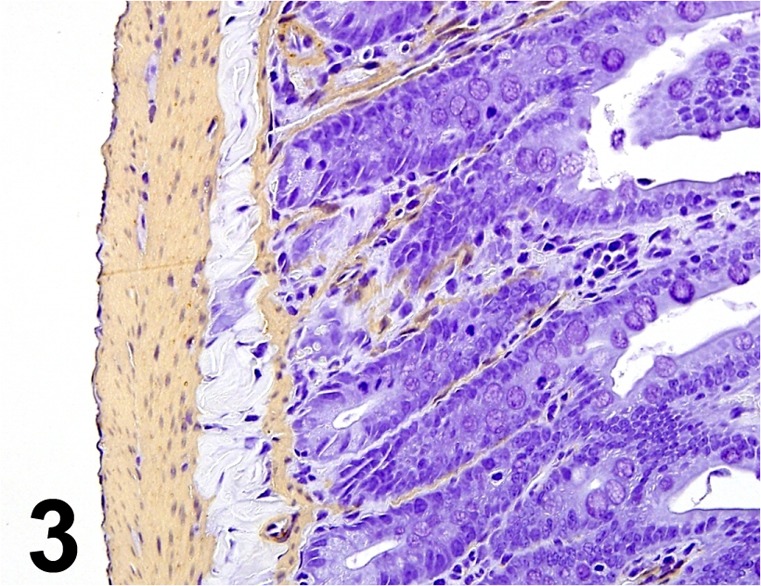
Actin, α-smooth muscle/ 1A4/ Dako/ M0851, Jejunum/ Rat.
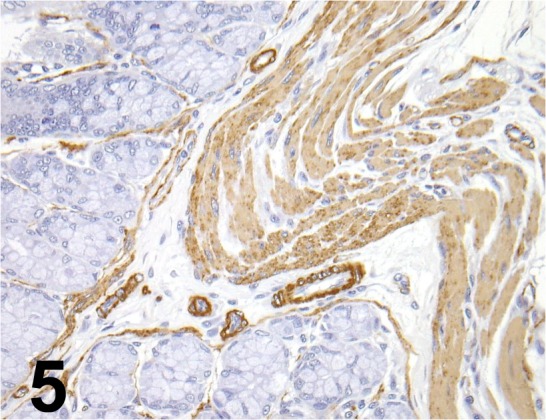
Fig. 5.
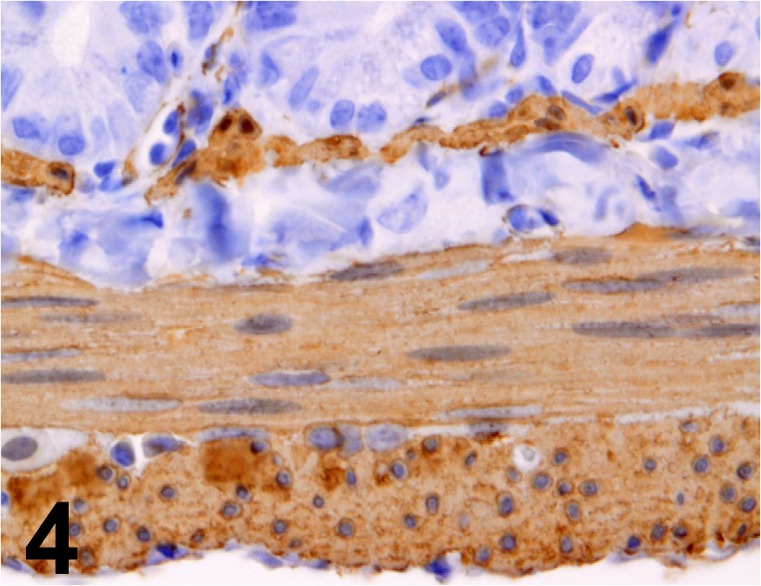
Actin, α-smooth muscle/ 1A4/ Nichirei/ 412021, Colon/ Rat.
Fig. 6.
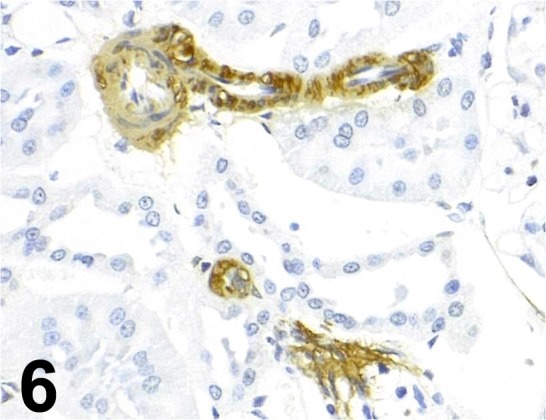
Actin, α-smooth muscle/ ASM-1/ Progen/ 61001, Kidney/ Rat.
Fig. 7.
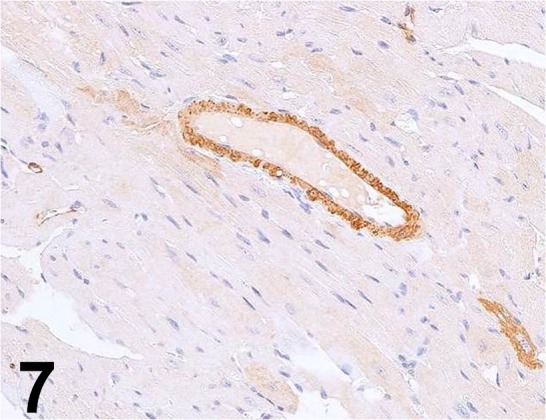
Actin, α-smooth muscle/ E184/ Abcam/ ab32575, Heart/ Rat.
Fig. 8.
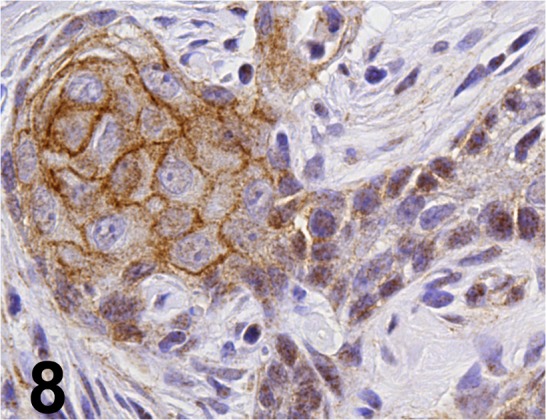
Active-β-catenin/ 8E7/ Millipore/ 05-665, Stomach/ Rat.
Fig. 9.
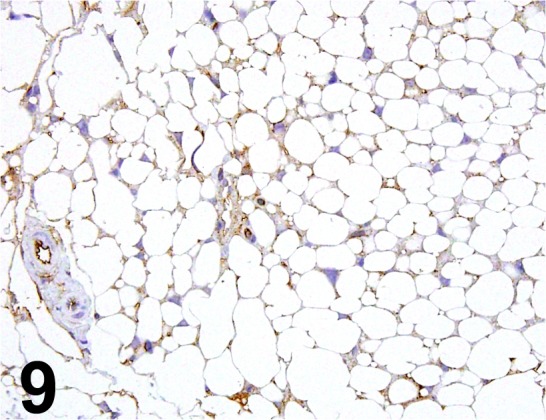
Adiponectin/ - / BioVision/ 5902-50, Adipose tissue/Mouse.
Fig. 10.
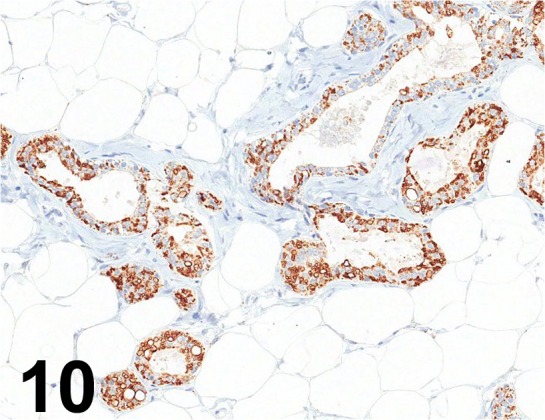
Adipophilin/ AP125/ Acris Antibodies GmbH/ BM5051, Mammary gland/ Rat.
Fig. 11.
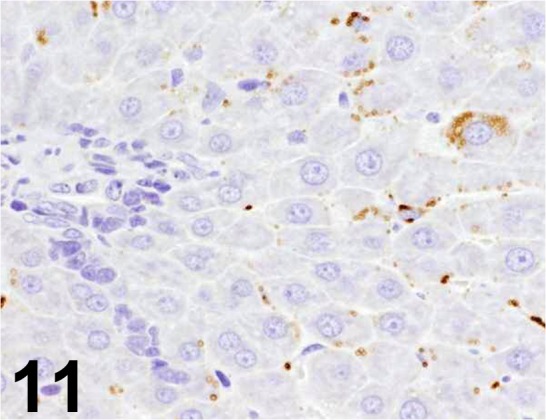
Adipophilin/ AP125/ American Research Products/ 03-651102, Liver/ Rat.
Fig. 12.
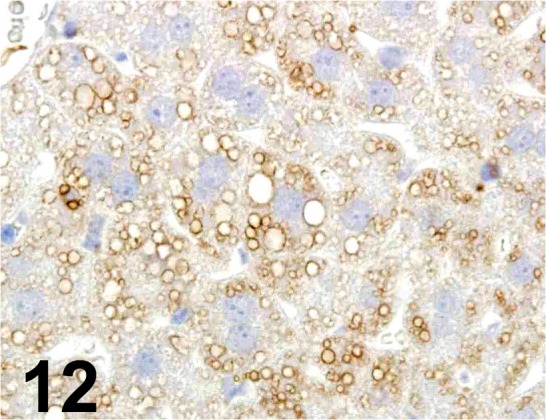
Adipophilin/ - / Progen/ GP40, Liver/ Mouse.
Fig. 13.
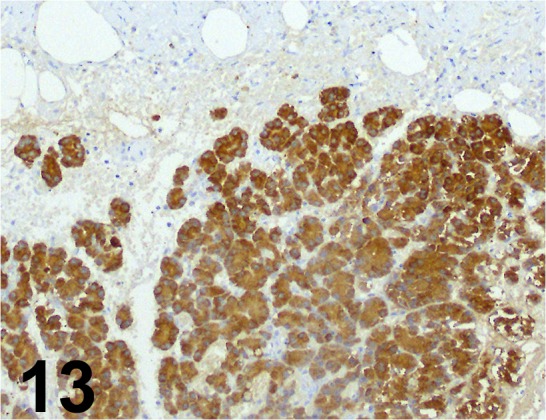
Amylase/ - / Santa Cruz/ sc-12821, Pancreas/ Rat.
Fig. 14.
Amylase 2A/ - / Proteintech/ 15845-1-AP, Pancreas/ Rat.
Fig. 15.
Amyloid A/ KM268/ Kyowa Medex/ HM01, Kidney/ Cow.
Fig. 16.
β-Amyloid/ 6F/ 3D/ Dako/ M0872, Cerebrum/ Mouse.
Fig. 17.
β-Amyloid/ 82E1/ IBL/ 10326, Brain/ Mouse.
Fig. 18.
Androgen Receptor/ G122-25/ BD Biosciences/ 554224, Genital gland/ Rabbit.
Fig. 19.
Androgen Receptor/ - / Santa Cruz/ sc-816, Prostatic carcinoma/ Rat.
Fig. 20.
Aquaporin 1/ 1/22/ Abcam/ ab9566, Kidney/ Monkey.
Fig. 21.
Aquaporin 1/ - / Millipore/ AB2219, Kidney/ Rat.
Fig. 22.
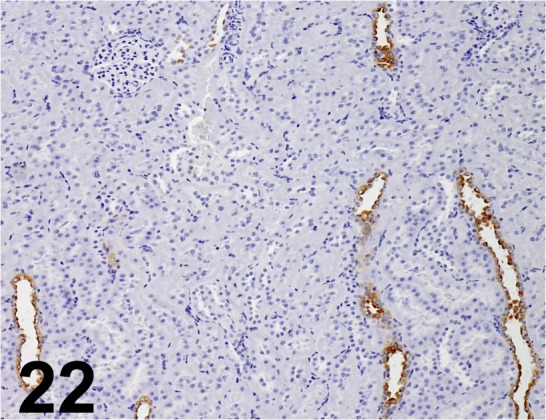
Aquaporin 2/ - / ProSci/ 50-225, Kidney/ Rat.
Fig. 23.
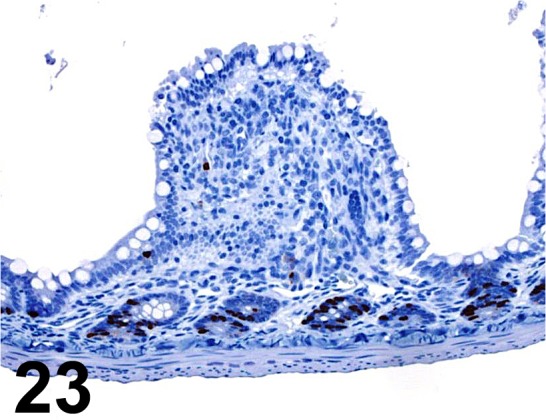
BrdU/ Bu20a/ AbD serotec/ MCA2483, Small intestine/ Rat.
Fig. 24.
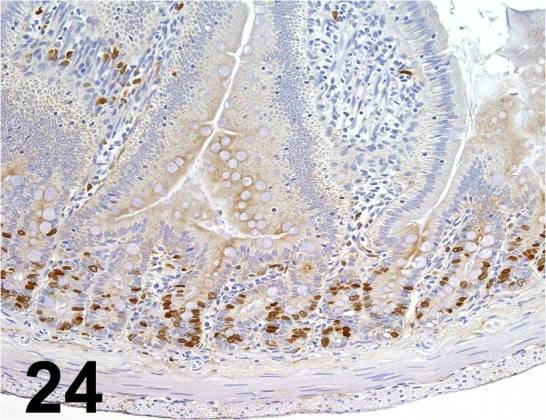
BrdU/ Bu20a/ Dako/ M0744, Duodenum/ Rat.
Fig. 25.
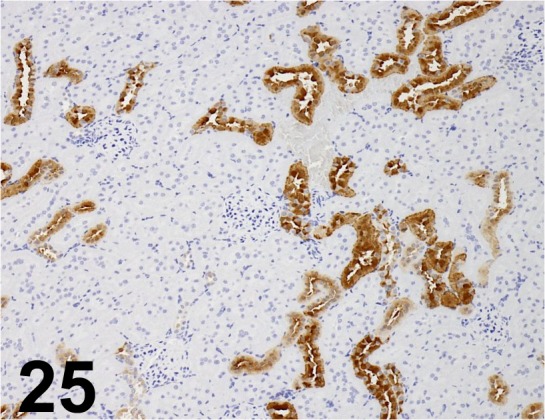
Calbindin D28K/ CB-955/ Abcam/ ab25085, Kidney/ Rat.
Fig. 26.
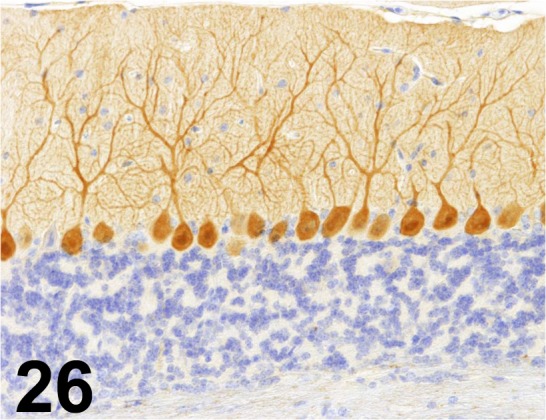
Calbindin D28K/ CB-955/ Sigma-Aldrich/ C9848, Cerebellum/ Mouse.
Fig. 27.
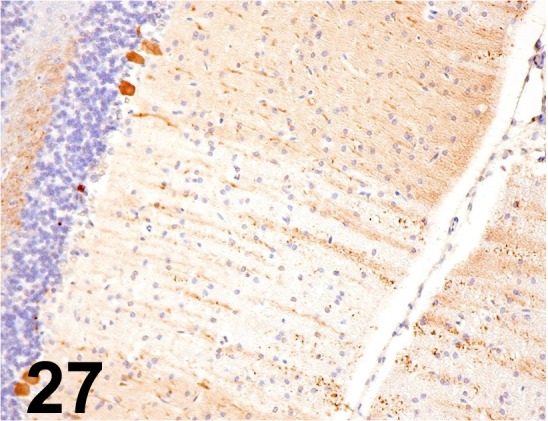
Calbindin D28K/ CB-955/ Sigma-Aldrich/ C9848, Cerebellum/ Rat.
Fig. 28.
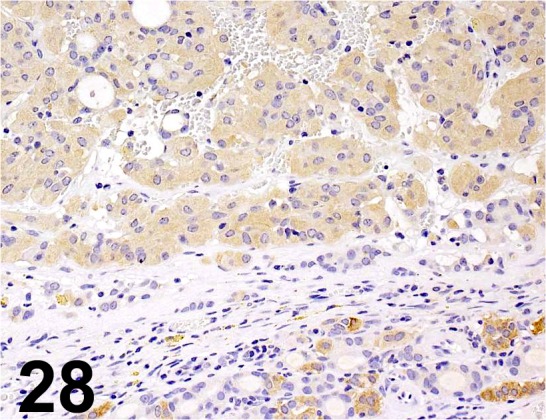
Calcitonin/ - / GeneTex/ GTX28553,Thyroid gland/ Rat.
Fig. 29.
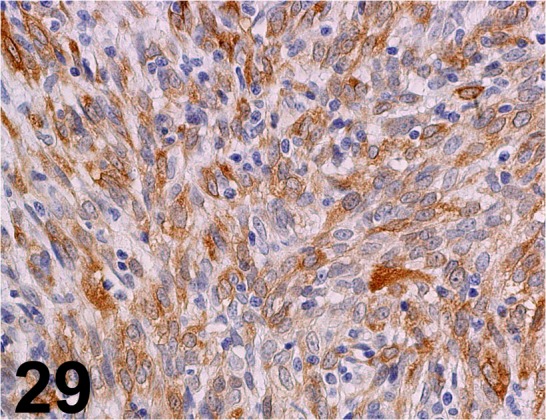
Calponin/ hCP/ Sigma-Aldrich/ C2687, Complex carcinoma/ Dog.
Fig. 30.
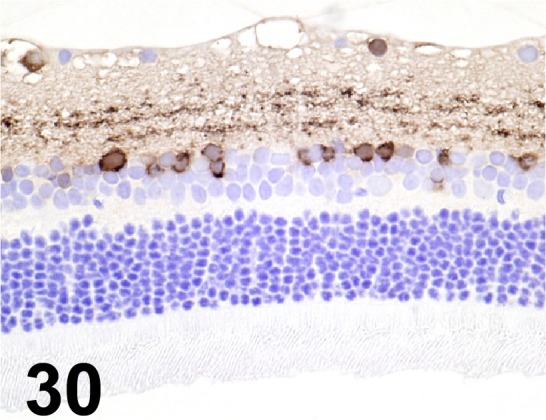
Calretinin/ 6B8.2/ Millipore/ MAB1568, Eye/ Rat.
Fig. 31.
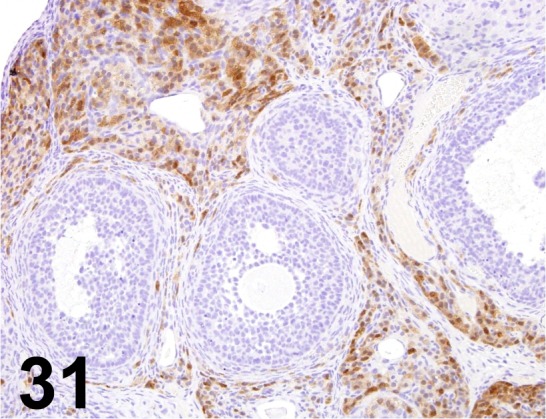
Calretinin/ - / Acris Antibodies GmbH/ DP043-05, Ovary/ Rat.
Fig. 32.
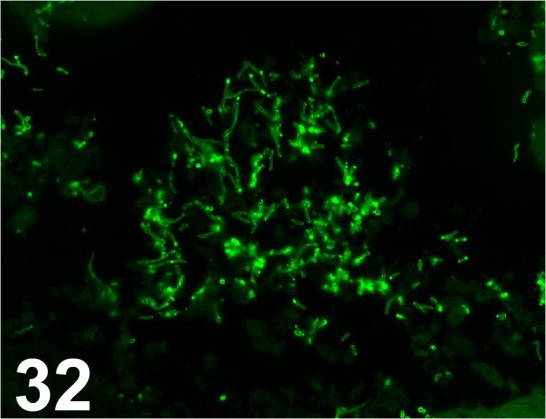
Candida albicans/ - / Abnova/ PAB14205, Liver/ Rabbit.
Fig. 33.
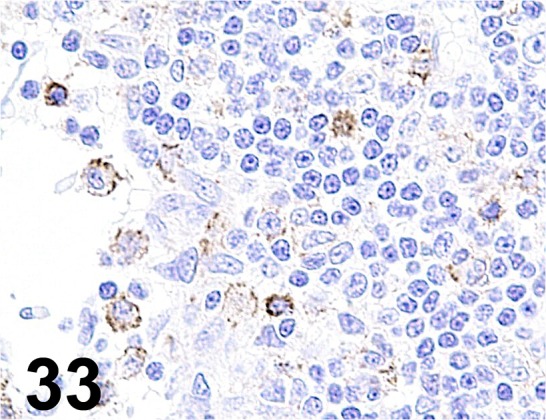
Caspase-3/ - / Promega/ G748, Lymph node/ Rat.
Fig. 34.
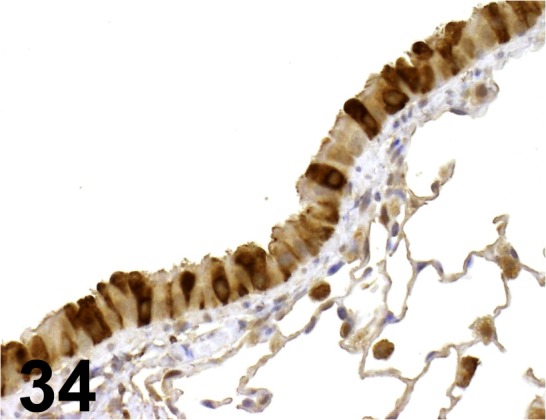
CC10/ - / Santa Cruz/ sc-9772, Lung/ Mouse.
Fig. 35.
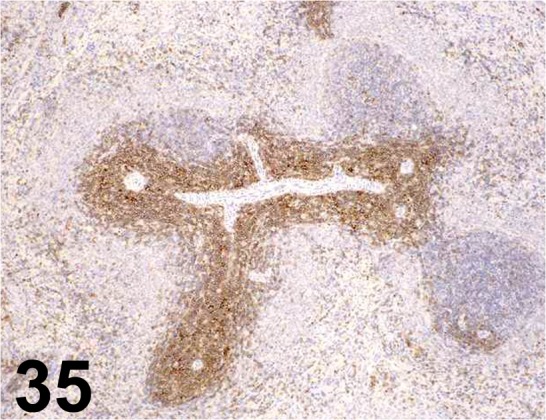
CD3/ 1F4/ AbD serotec/ MCA772, Spleen/ Rat.
Fig. 36.
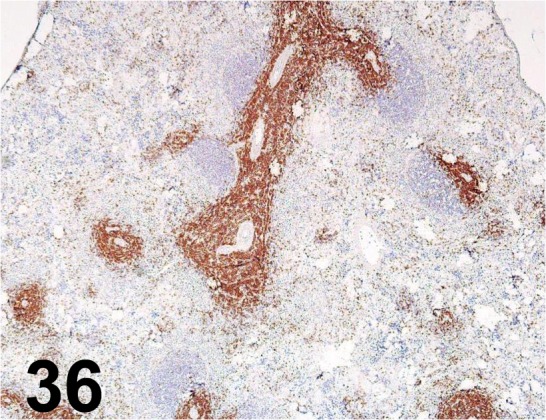
CD3/ G4, 18/ BD Biosciences/ 550295, Spleen/ Rat.
Fig. 37.
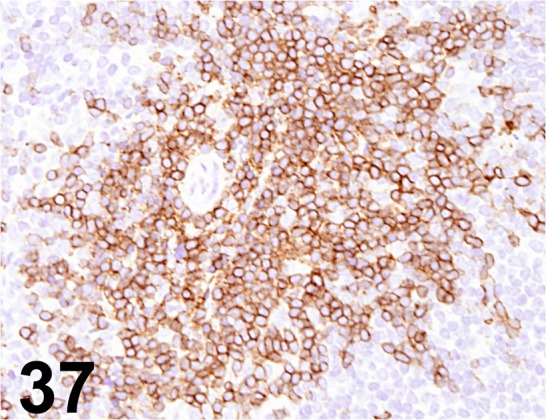
CD3/ SP7/ Abcam/ ab16669, Lymph node/ Mouse.
Fig. 38.
CD3/ - / Abcam/ ab5690, Spleen/ Mouse.
Fig. 39.
CD3/ - / Dako/ IR503, Spleen / Monkey.
Fig. 40.
CD3/ - / Invitrogen/ 18-0102, Spleen/ Monkey.
Fig. 41.
CD3/ - / Santa Cruz/ sc-1127, Spleen/ Rat.
Fig. 42.
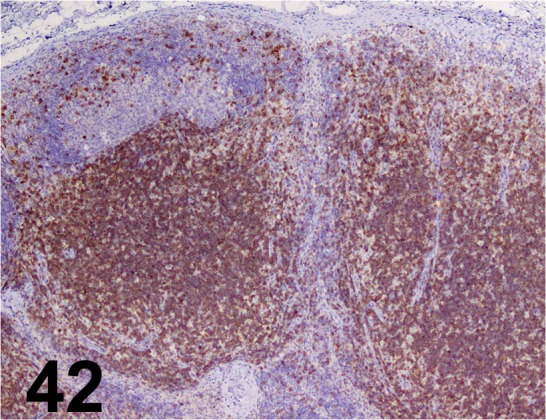
CD3/ - / Sigma-aldrich/ C7930, Lymph node/ Dog.
Fig. 43.
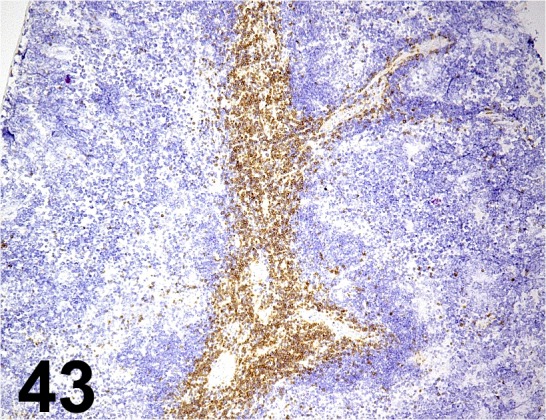
CD3e/ 145-2C11/ BD Biosciences/ 550275, Spleen/ Mouse.
Fig. 44.
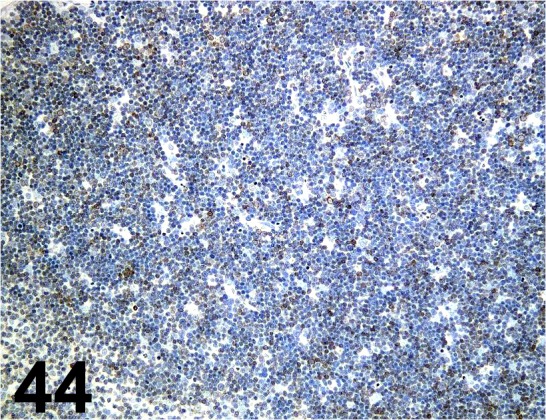
CD3e/ SP7/ Nichirei/ 413591, Thymus/ Mouse.
Fig. 45.
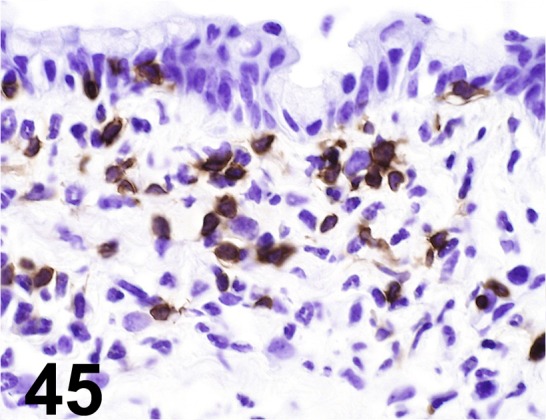
CD4/ GK1.5/ Santa Cruz/ sc-13573, Lymph node/ Mouse.
Fig. 46.
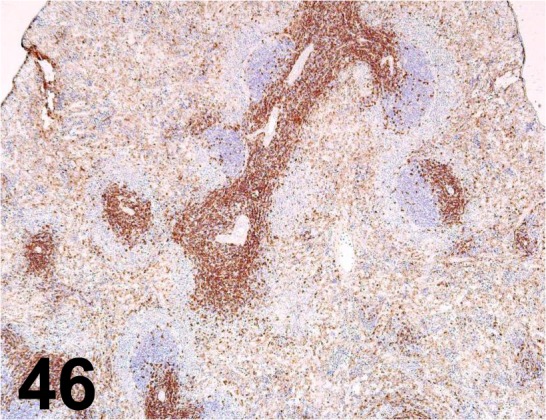
CD4/ OX-38/ BD Biosciences/ 550297, Spleen/ Rat.
Fig. 47.
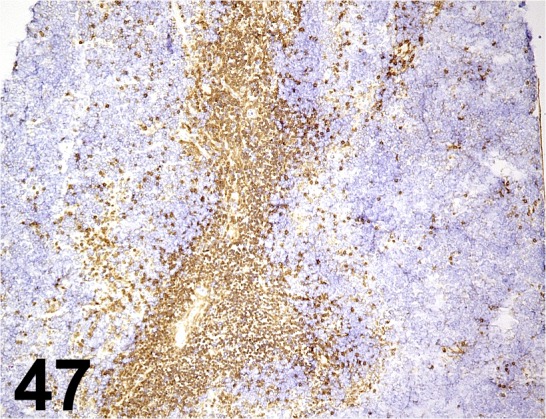
CD4/ RM4-5/ BD Biosciences/ 550280, Spleen/ Mouse.
Fig. 48.
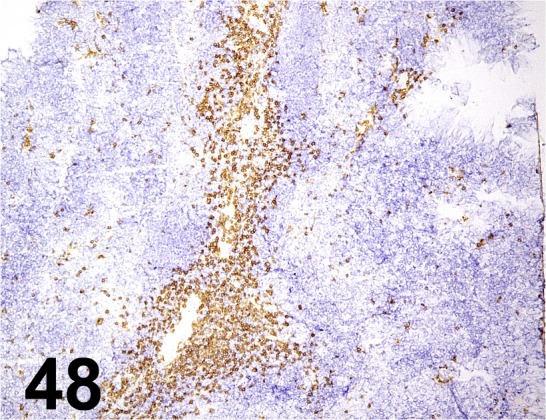
CD8α/ 53-6.7/ BD Biosciences/ 550281, Spleen/ Mouse.
Fig. 49.
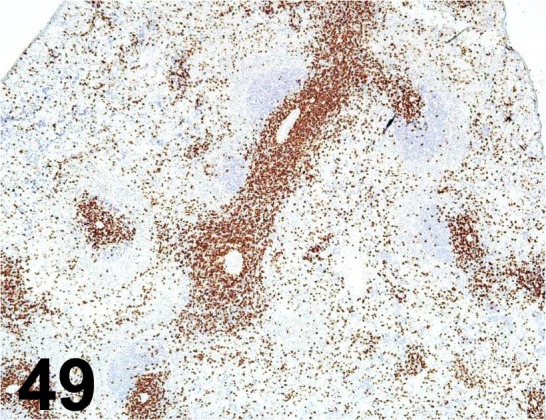
CD8α/ OX-8/ BD Biosciences/ 550298, Spleen/ Rat.
Fig. 50.
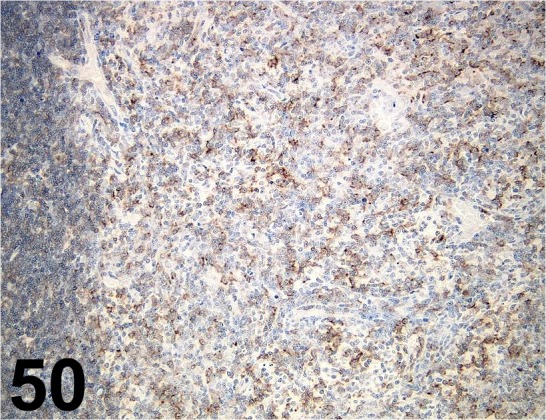
CD8α/ OX-8/ AbD serotec/ MCA48GA, Lymph node/ Rat.
Fig. 51.
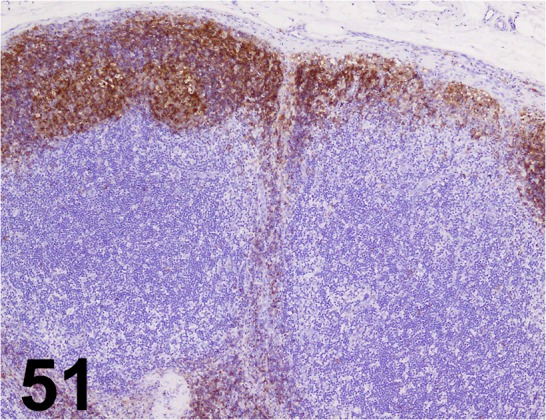
CD20/ E17-P/ Acris Antibodies GmbH/ 24828-1-AP, Lymph node/ Dog.
Fig. 52.
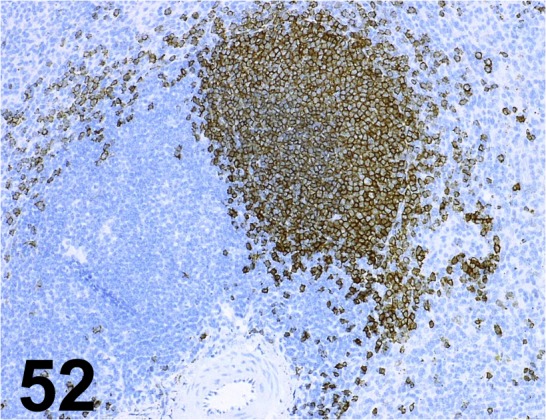
CD20cy/ L26/ Dako/ IR604, Spleen/ Monkey.
Fig. 53.
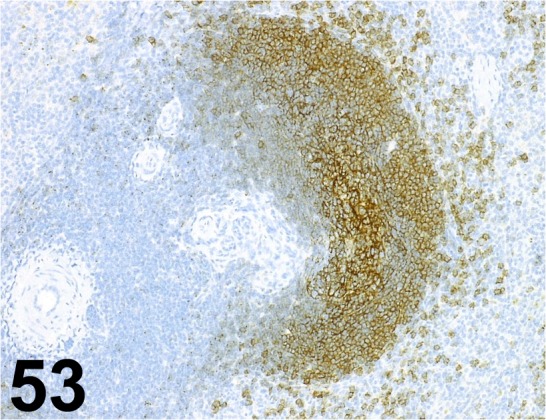
CD21/ EP3093/ Abcam/ ab75985, Spleen/ Monkey.
Fig. 54.
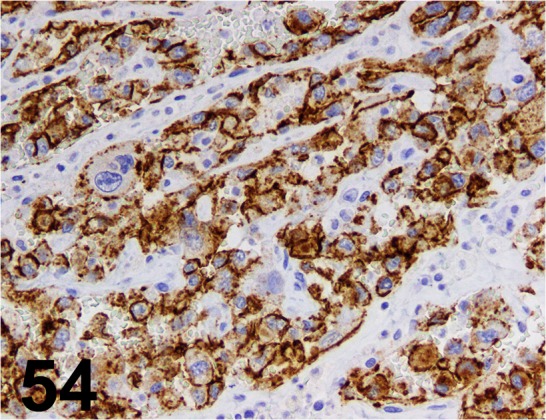
CD31/ JC70A/ Dako/ N1596, Hemangiosarcoma/ Dog.
Fig. 55.
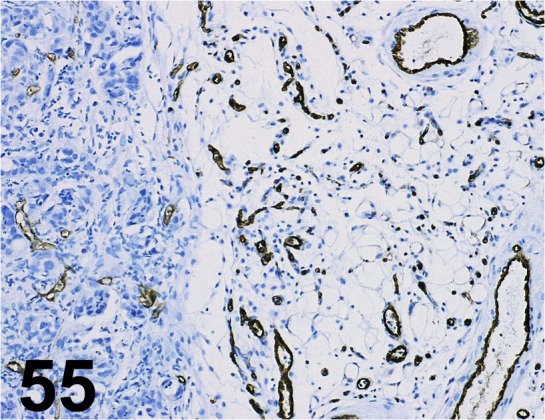
CD31/ SZ31/ dianova GmbH/ DIA 310, Human pancreatic cancer cell xenograft/ -.
Fig. 56.
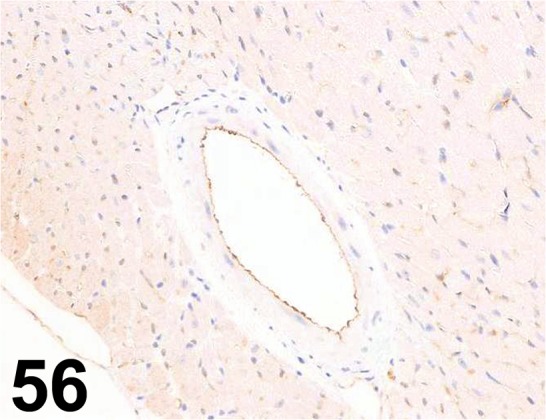
CD31/ - / Abcam/ ab28364, Heart/ Mouse.
Fig. 57.
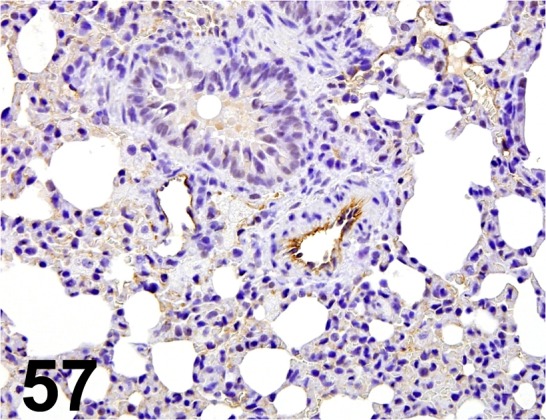
CD31/ - / Santa Cruz/ sc-1506, Lung/ Rat.
Fig. 58.
CD34/ - / R&D Systems/ AF4117, Lung/ Rat.
Fig. 59.
CD45R/ RA3-6B2/ BD Biosciences/ 550286, Spleen/ Mouse.
Fig. 60.
CD45RA/ OX33/ AbD serotec/ MCA340GA, Lymph node/ Rat.
Fig. 61.
CD45RA/ OX33/ BD Biosciences/ 554882, Spleen/ Rat.
Fig. 62.
CD45RA/ OX33/ Bio-Rad Laboratories/ MCA340G, Spleen/ Rat.
Fig. 63.
CD68/ ED1/ AbD serotec/ MCA341, Spleen/ Rat.
Fig. 64.
CD68/ ED1/ Acris Antibodies GmbH/ BM4000, Osteosarcoma (infiltrating macrophages)/ Rat.
Fig. 65.
CD68/ ED1/ BMA Biomedicals/ T-3003, Spleen/ Rat.
Fig. 66.
CD68/ ED1/ Millipore/ MAB1435, Liver/ Rat
Fig. 67.
CD68/ KP1/ Dako/ M0814, Lung/ Monkey.
Fig. 68.
CD77/ 38.13/ Beckman Coulter/ IM0175, Kidney/ Mouse.
Fig. 69.
CD79α/ HM57/ Nichirei/ 413171, Lacrimal gland/ Cat.
Fig. 70.
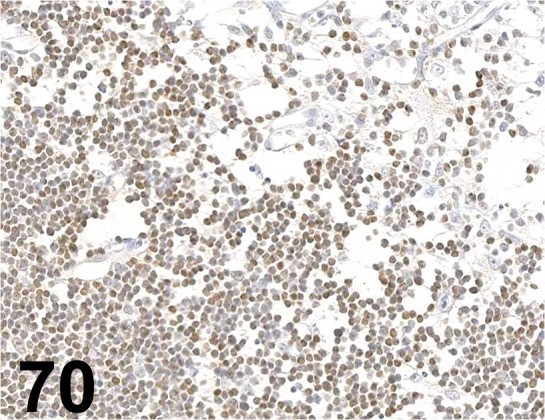
CD90/ Thy 1/ OX-7/ Cedarlane Laboratories/ CL005AP/−2, Thymus/ Rat.
Fig. 71.
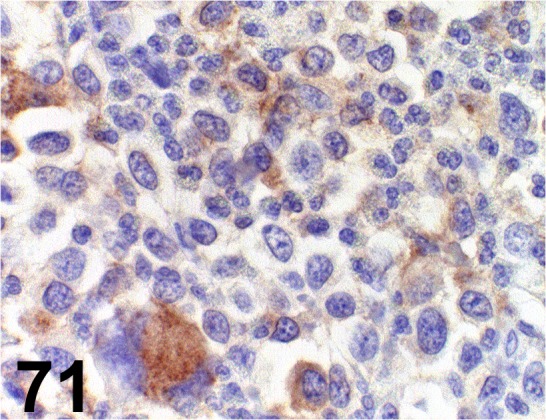
CD117/c-kit/ - / Dako/ A4502, Mastocytoma/ Dog.
Fig. 72.
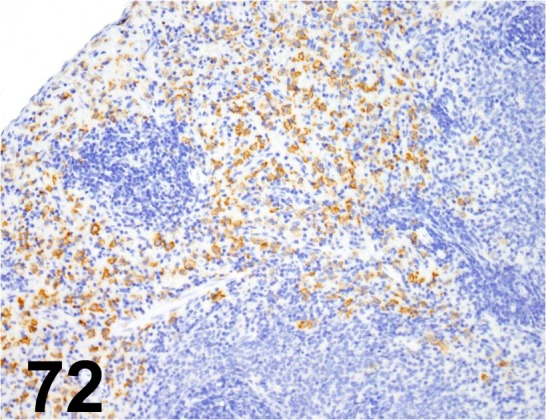
CD163/ ED2/ Acris Antibodies GmbH/ BM4001, Spleen/ Rat.
Fig. 73.
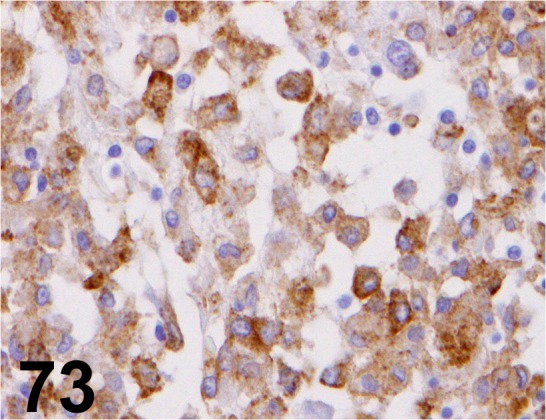
CD204/ SRA-E5/ Trans Genic/ KT022, Histiocytic sarcoma/ Dog.
Fig. 74.
Clusterin/ - / R&D Systems/ AF2937, Kidney/ Rat.
Fig. 75.
Collagen type I/ - / BioLogo/ CO20141, Lung/ Rat.
Fig. 76.
Collagen type II/ II-4C11/ Daiichi Fine Chemical/ F-57, Joint/ Minipig.
Fig. 77.
Collagen type III/ - / BioLogo/ CO20341, Lung/ Rat.
Fig. 78.
Cyclin D1/ SP4/ Nichirei/ 413521, Stomach/ Rat.
Fig. 79.
Cystatin C/ EPR4413/ Abcam/ ab109508, Kidney/ Rat.
Fig. 80.
Cytochrome P450 3A1/ - / Millipore/ AB1253, Liver/ Mouse.
Fig. 81.
Cytokeratin/ AE1/ AE3/ Dako/ IR053, Skin/ Rat.
Fig. 82.
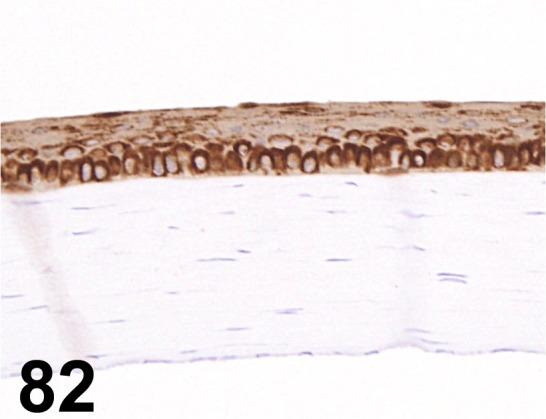
Cytokeratin/ AE1/ AE3/ Dako/ M3515, Eye/ Rat
Fig. 83.
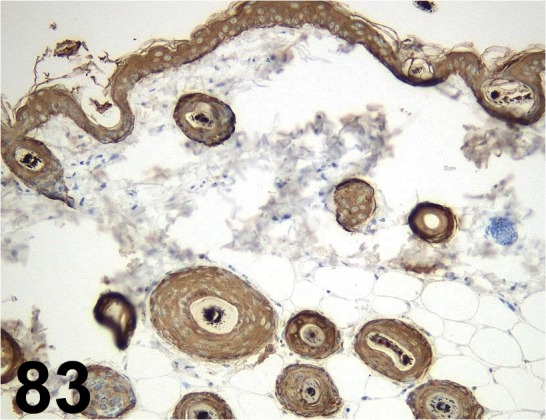
Cytokeratin/ - / Nichirei/ 422061, Skin/ Mouse.
Fig. 84.
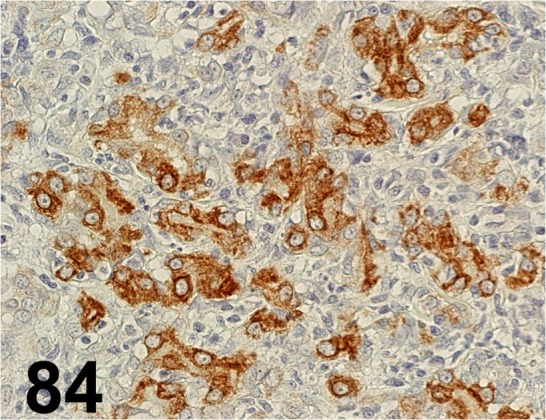
Cytokeratin Low Molecular Weight/ CAM 5.2/ BD Biosciences/ 349205, Complex carcinoma/ Dog.
Fig. 85.
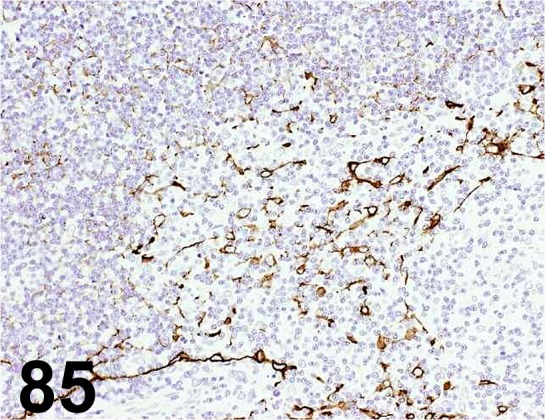
Cytokeratin High Molecular Weight/ 34βE12/ Dako/ M0630, Thymus/ Rat.
Fig. 86.
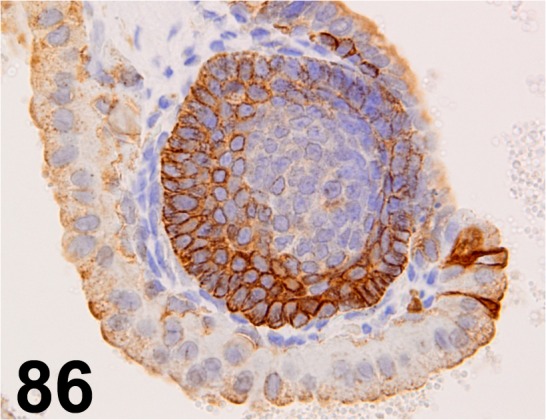
Cytokeratin 5/ - / Abcam/ ab53121, Seminal vesicle/ Rabbit.
Fig. 87.
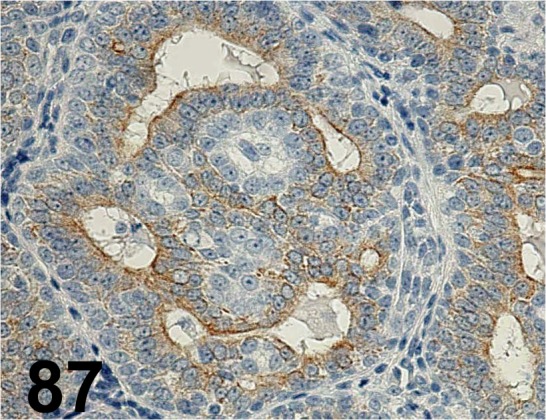
Cytokeratin 8/ Ks8.7/ Progen/ 65138, Mammary gland/ Rat.
Fig. 88.
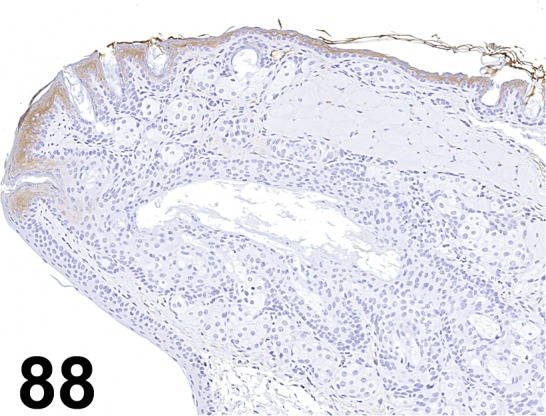
Cytokeratin 10/ DE-K10/ Abcam/ ab9026, Eyelid/ Mouse.
Fig. 89.
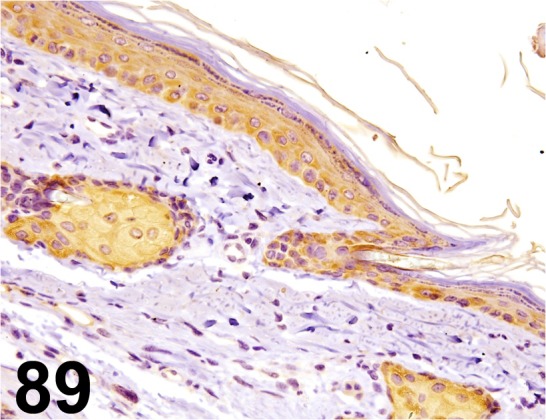
Cytokeratin 14/ LL002/ Acris Antibodies GmbH/ SM1359P, Skin/ Rat.
Fig. 90.
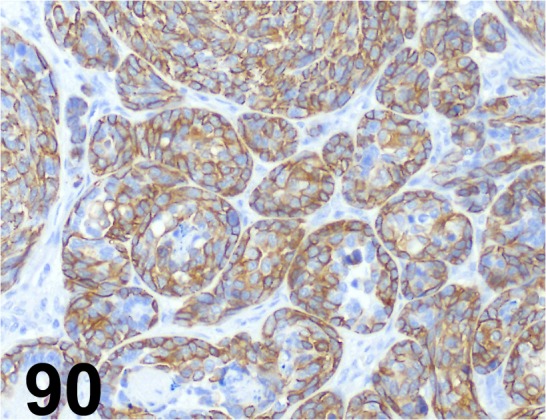
Cytokeratin 14/ LL002/ BioGenex/ AM146-5M, Mammary gland/ Dog.
Fig. 91.
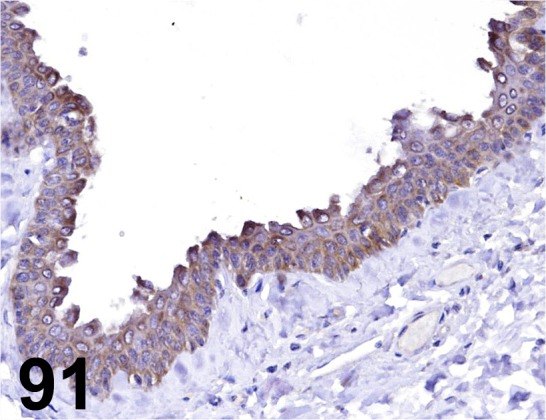
Cytokeratin 14/ RCK107/ Millipore/ MAB3232, Skin/ Cow.
Fig. 92.
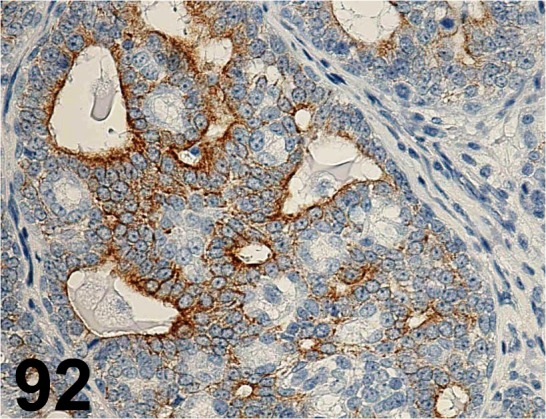
Cytokeratin 18/ C-04/ Santa Cruz/ sc-51582, Mammary gland/ Rat.
Fig. 93.
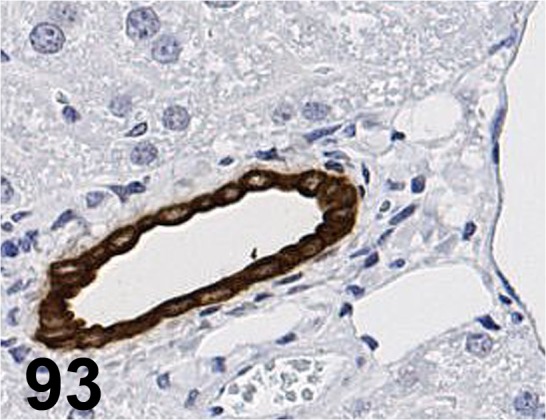
Cytokeratin 19/ EPNCIR127B/ Epitomics/ 3863-1, Liver/ Mouse.
Fig. 94.
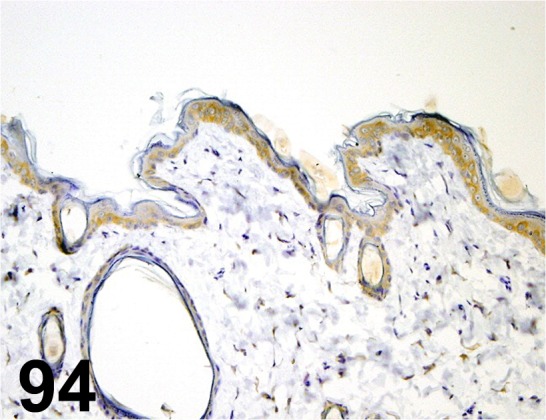
Cytokeratin 19/ - / Abcam/ ab15463, Skin/ Rat.
Fig. 95.
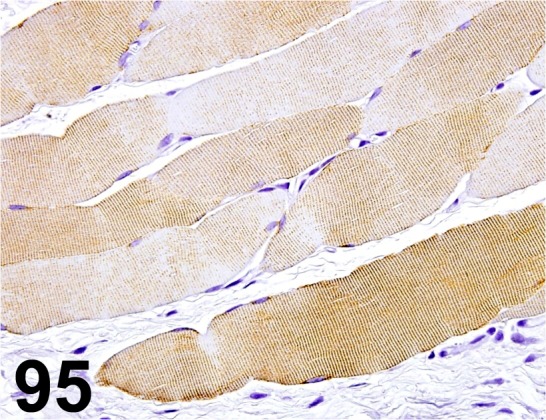
Desmin/ D33/ Dako/ IR606, Muscle/ Rat.
Fig. 96.
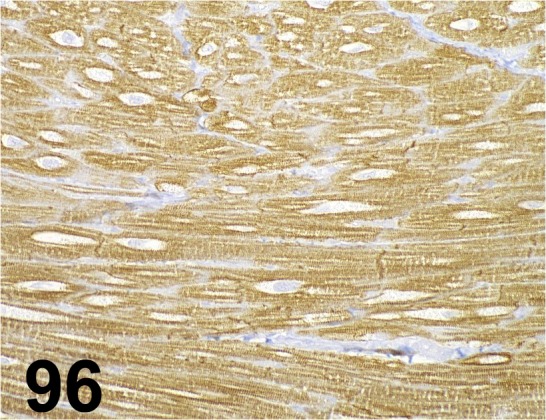
Desmin/ - / Abcam/ ab15200, Heart/ Rat.
Fig. 97.
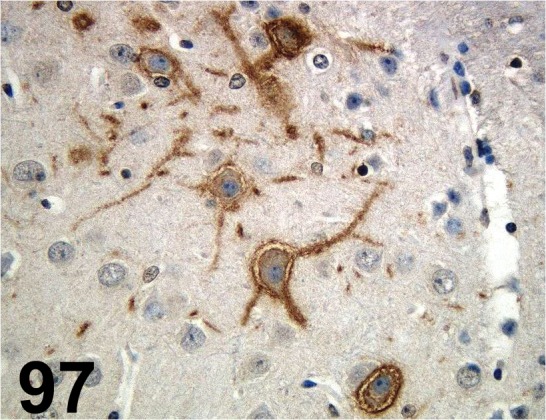
Doublecortin/ - / Santa Cruz/ sc-8066, Cerebrum/ Rat.
Fig. 98.
E-cadherin/ 36/ E-Cadherin/ BD Biosciences/ C20820, Stomach/ Rat.
Fig. 99.
E-cadherin/ NCH-38/ Dako/ M3612, Human gastric cancer cell xenograft/ -.
Fig. 100.
EGF Receptor/ D38B1/ Cell Signaling Technology/ 4267, Human squamous cancer cell xenograft/ -.
Fig. 101.
ERCC/ - / Santa Cruz/ sc-56386, Stomach/ Rat.
Fig. 102.
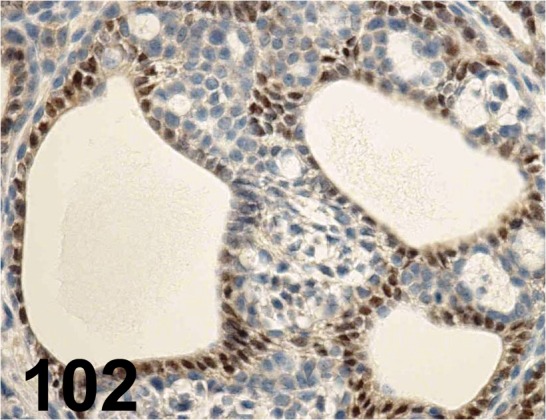
Estrogen Receptor α/ - / Santa Cruz/ sc-543, Mammary gland/ Rat.
Fig. 103.
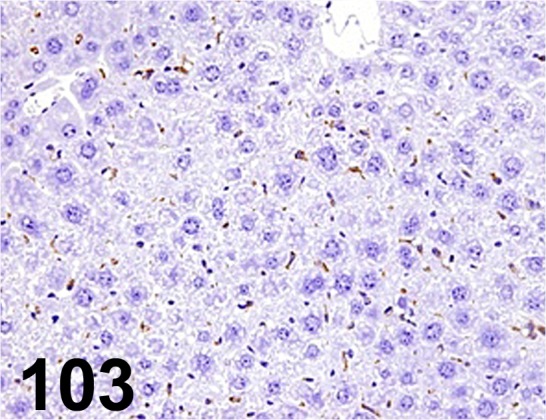
F4/ 80/ Al-3/ Acris Antibodies GmbH/ BM4008, Liver/ Mouse.
Fig. 104.
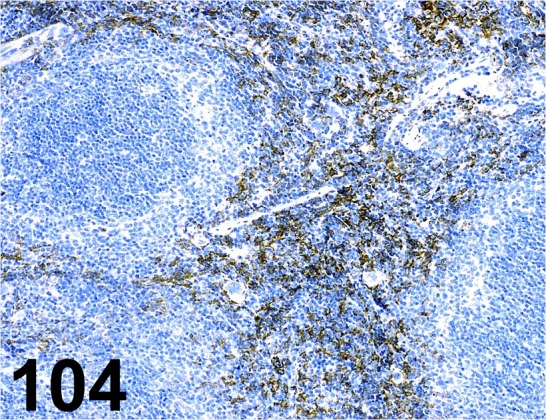
F4/ 80/ BM8/ BMA Biomedicals/ T-2028, Spleen/ Mouse.
Fig. 105.
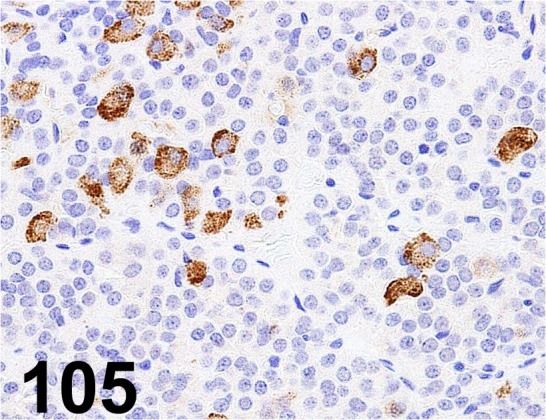
Follicle Stimulating Hormone/ - / AbD serotec/ 4561-6959, Pituitary gland/ Rat.
Fig. 106.
GADD 153/ - / Santa Cruz/ sc-575, Pancreas/ Mouse.
Fig. 107.
Glial Fibrillary Acidic Protein/ 6F2/ Dako/ M0761, Eye/ Rat.
Fig. 108.
Glial Fibrillary Acidic Protein/ - / Dako/ IR524, Cerebrum/ Rat.
Fig. 109.
α2µ-globulin/ 129736/ R&D Systems/ BAM586, Kidney/ Rat.
Fig. 110.
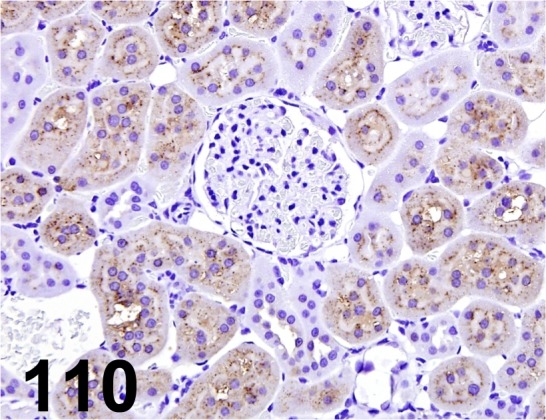
α2µ-globulin/ - / R&D Systems/ AF586, Kidney/ Rat.
Fig. 111.
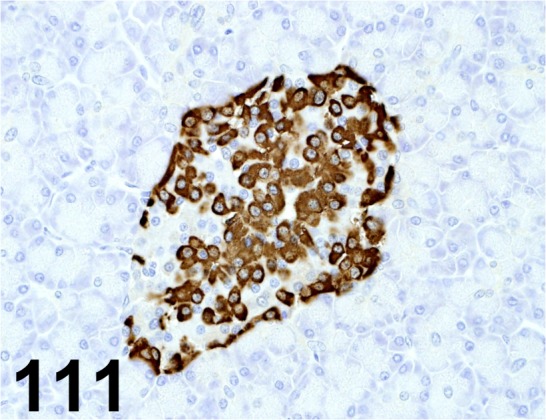
Glucagon/ K79bB10/ Abcam/ ab10988, Pancreas/ Monkey.
Fig. 112.
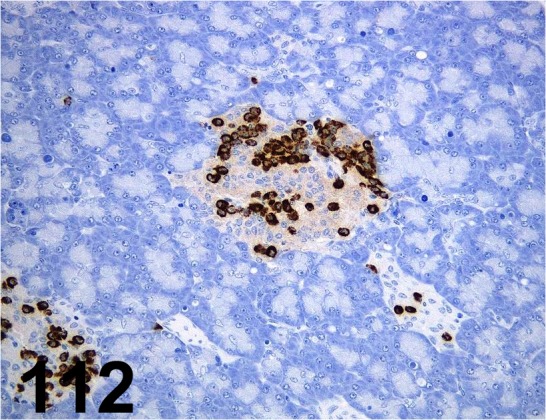
Glucagon/ K79bB10/ Sigma-Aldrich/ G2654, Pancreas/ Dog.
Fig. 113.
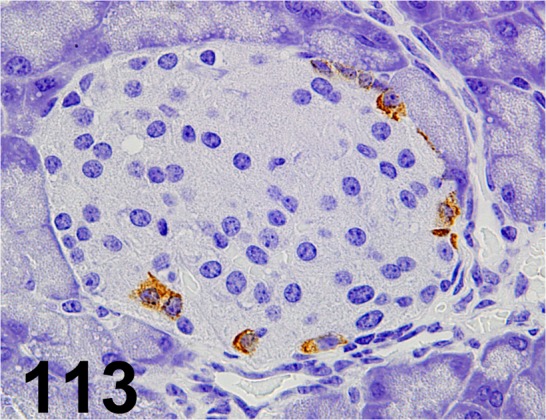
Glucagon/ - / Nichirei/ 422271, Pancreas/ Mouse.
Fig. 114.
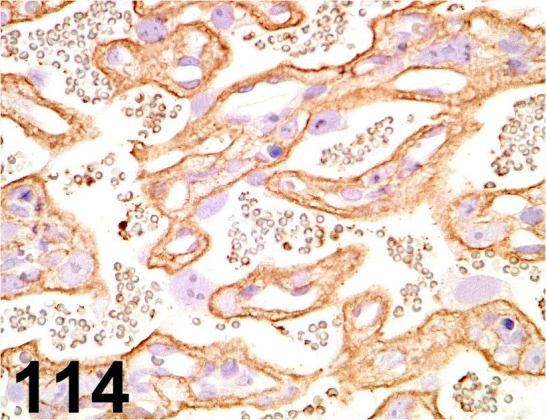
Glucose Transporter type 1/ - / Abcam/ ab14683, Placenta/ Rat.
Fig. 115.
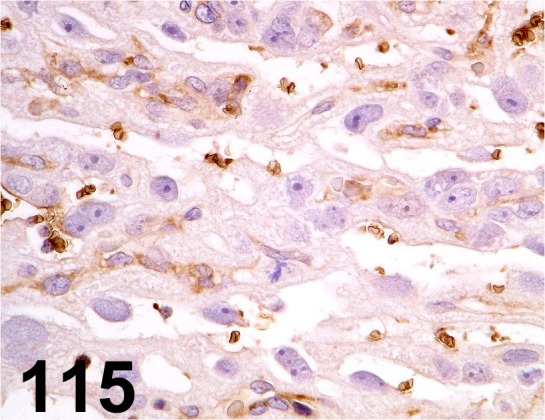
Glucose Transporter type 3/ - / GeneTex/ GTX15311 Placenta/ Rat.
Fig. 116.
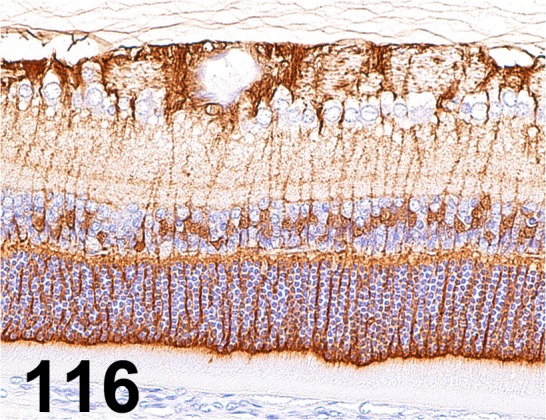
Glutamine Synthetase/ GS-6/ Millipore/ MAB302, Eye/ Rat.
Fig. 117.
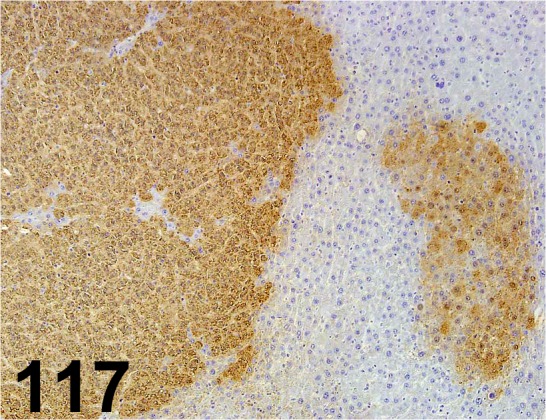
Glutathione S-transferase Placental type/ - / MBL/ 311, Liver/ Rat.
Fig. 118.
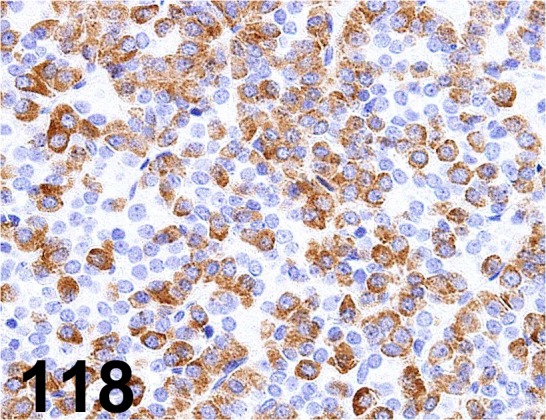
Growth Hormone/ - / R&D Systems/ MAB1566, Pituitary gland/ Rat.
Fig. 119.
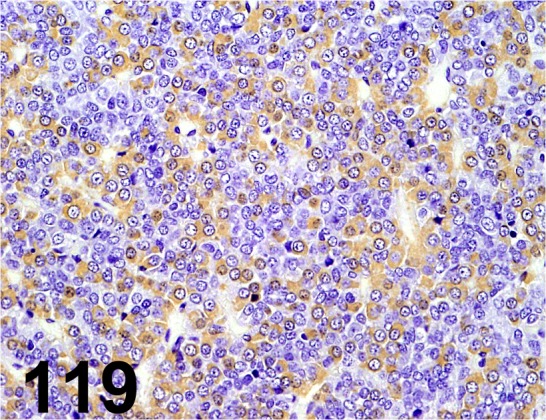
Growth Hormone/ 222540/ Millipore/ AB940, Pituitary gland/ Rat.
Fig. 120.
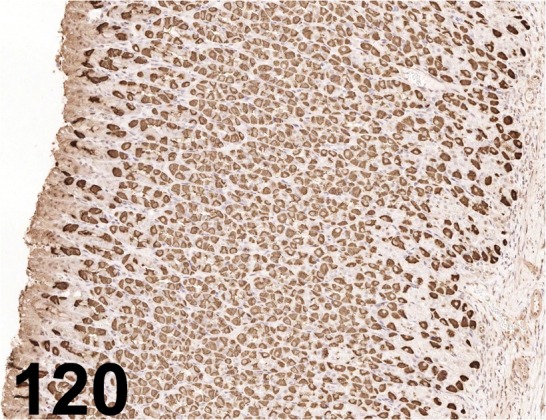
H+-K+-ATPase/ - / Millipore/ 119102, Stomach/ Rat.
Fig. 121.
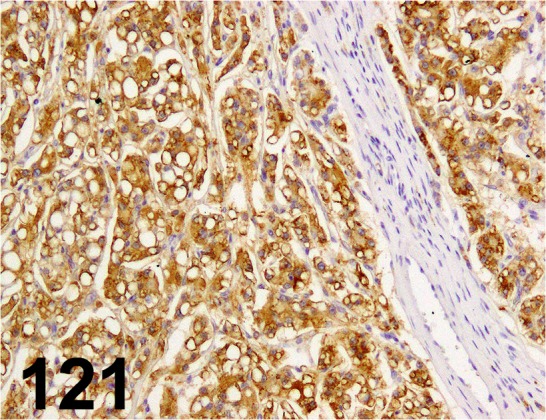
Hepatocyte/ OCH1E5/ Dako/ M7158, Hepatocellular carcinoma/ Dog.
Fig. 122.
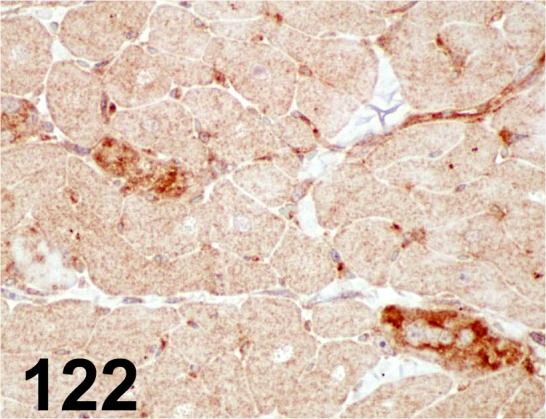
4-Hydroxy-2-nonenal/ HNEJ2/ JalCA/ MHN-020P, Heart/ Dog.
Fig. 123.
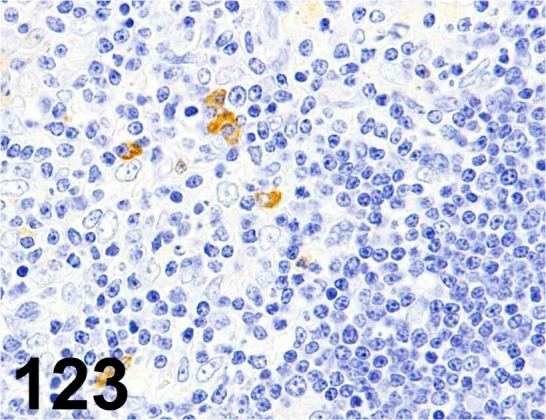
Iba 1/ - / Bioss/ bs-1363R, Thymus/ Rat.
Fig. 124.
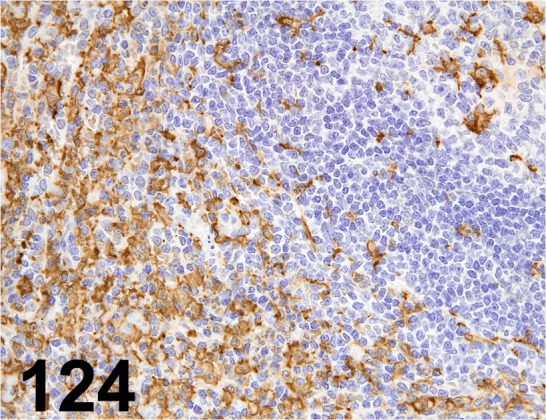
Iba 1/ - / Wako/ 019-19741, Spleen/ Monkey.
Fig. 125.
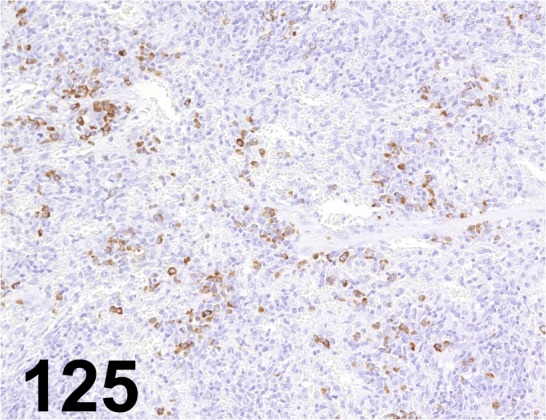
IgG, Fc(γ)/ - / Jackson ImmunoResearch Laboratories/ 112-005-008, Spleen/ Rat.
Fig. 126.
IgM, (µ)/ - / Jackson ImmunoResearch Laboratories/ 112-005-020, Spleen/ Rat.
Fig. 127.
Inhibin α/ R1/ AbD serotec/ MCA951S, Testis/ Dog.
Fig. 128.
Insulin/ K36aC10/ Abcam/ ab6995, Pancreas/ Monkey.
Fig. 129.
Insulin/ K36aC10/ Sigma-Aldrich/ I2018, Pancreas/ Dog.
Fig. 130.
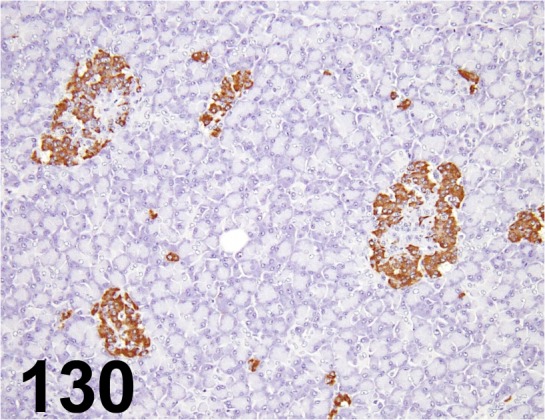
Insulin/ - / Dako/ IR002, Pancreas/ Monkey.
Fig. 131.
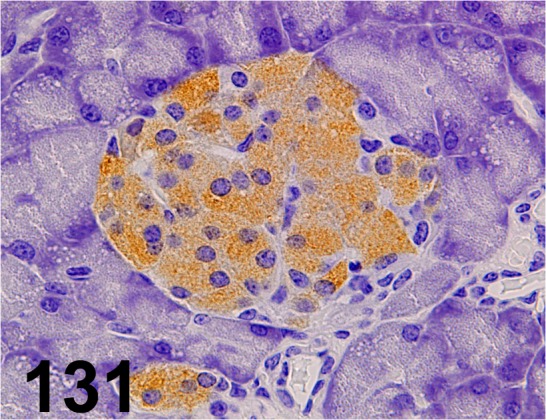
Insulin/ - / Santa Cruz/ sc-9168, Pancreas/ Mouse.
Fig. 132.
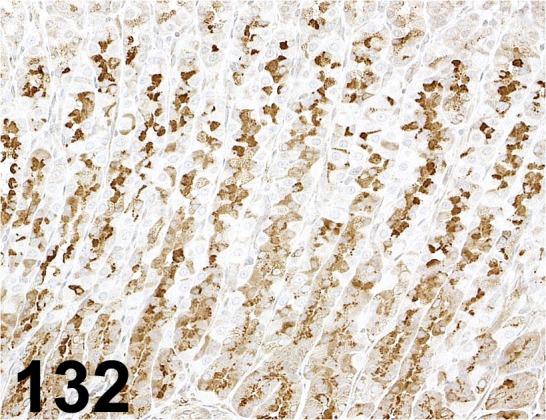
Intrinsic Factor/ - / Fitzgerald/ 20-IR51, Stomach/ Rat.
Fig. 133.
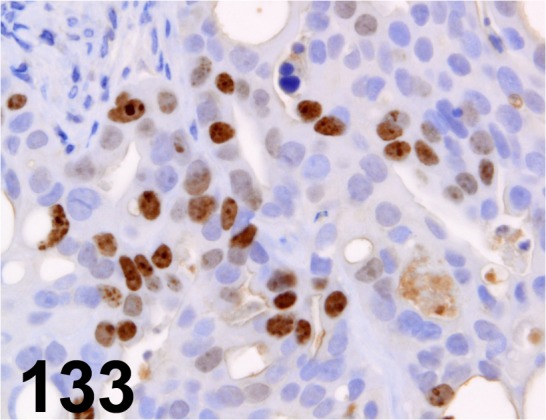
Ki-67/ MIB-1/ Dako/ IR056, Human gastric carcinoma cell line/-.
Fig. 134.
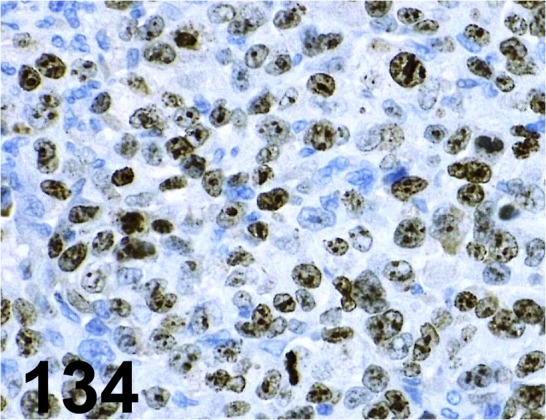
Ki-67/ MIB-1/ Dako/ IR626, Human gastric cancer cell xenograft/-.
Fig. 135.
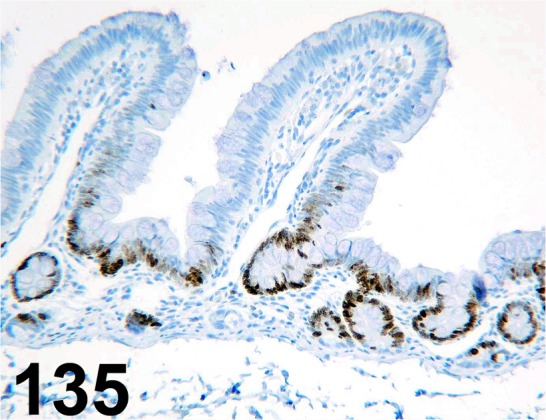
Ki-67/ MIB-1/ Dako/ M7240, Small intestine/ Monkey.
Fig. 136.
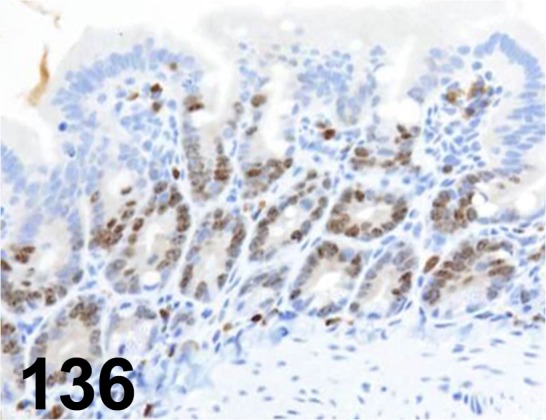
Ki-67/ MIB-5/ Dako/ M7248, Duodenum/ Rat.
Fig. 137.
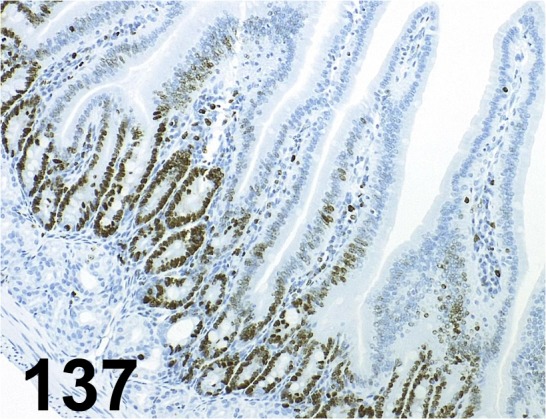
Ki-67/ SP6/ Abcam/ ab16667, Duodenum/ Mouse.
Fig. 138.
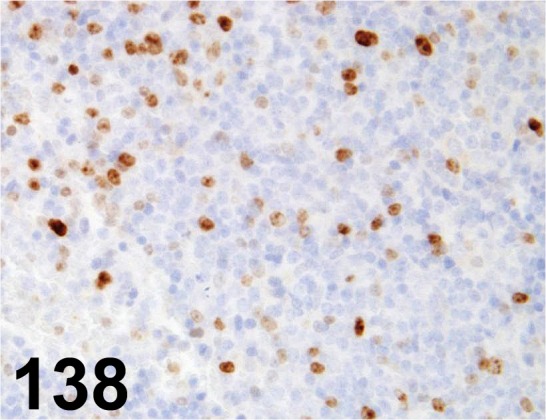
Ki-67/ - / Abcam/ ab15580, Spleen/ Mouse.
Fig. 139.
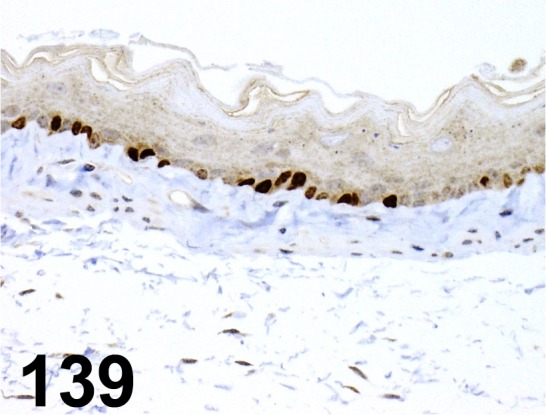
Ki-67/ - / Novus Biologicals/ NB110-89717, Esophagus/ Mouse.
Fig. 140.
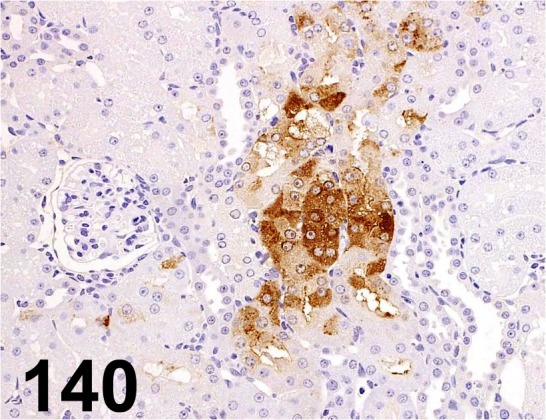
KIM-1/ Tim-1/ - / R&D Systems/ AF3689, Kidney/ Rat.
Fig. 141.
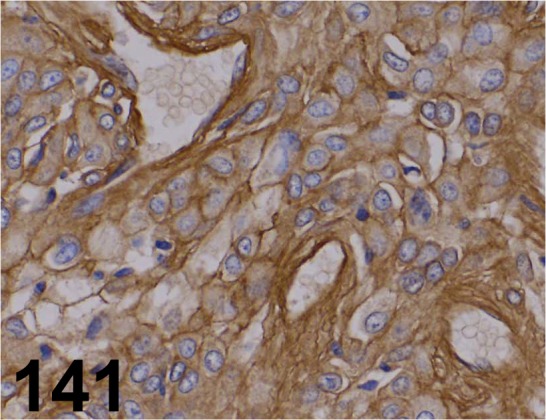
Laminin/ - / LSL/ LB-1013, Stomach/ Rat.
Fig. 142.
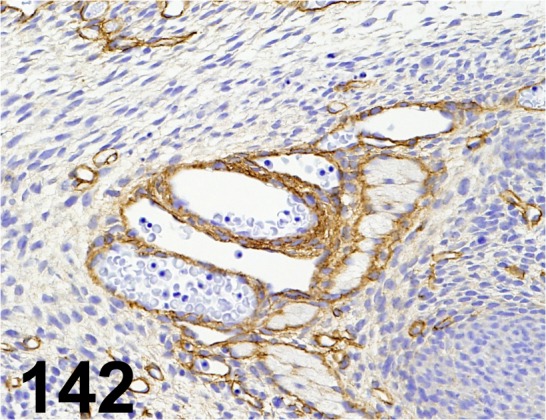
Laminin/ - / Sigma-Aldrich/ L9393, Blood vessel/ Rat (fetus).
Fig. 143.
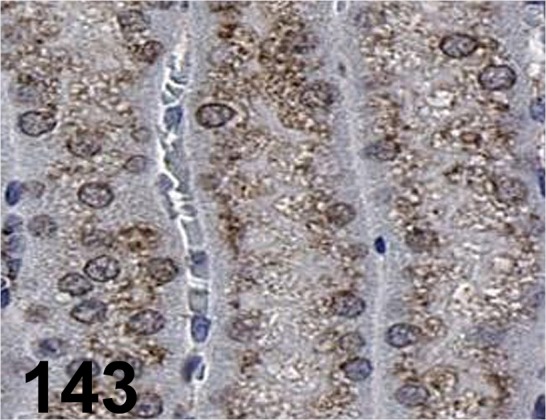
LAMP-2/ M3/ 84/ Santa Cruz/ sc-19991, Kidney/ Mouse.
Fig. 144.
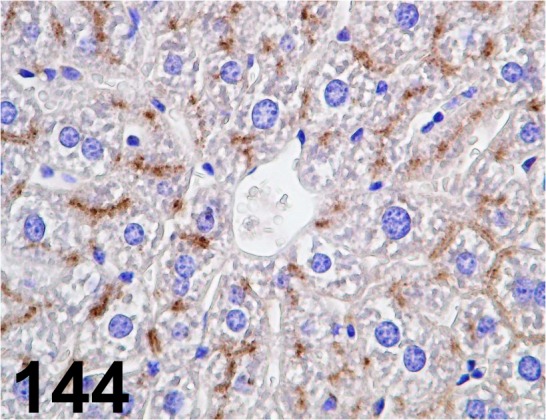
LAMP-2/ - / Invitrogen/ 51-2200, Liver/ Mouse.
Fig. 145.
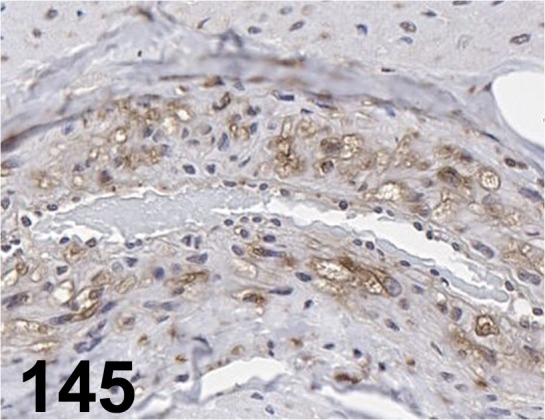
LAMP-2/ - / LifeSpan BioSciences/ LS-B3144, Aorta/ Dog.
Fig. 146.
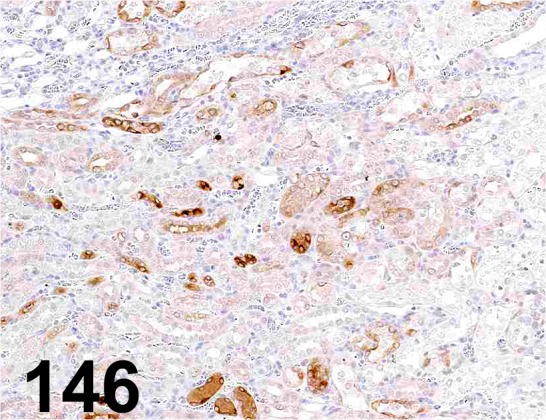
Lipocalin-2/ NGAL/ - / R&D Systems/ AF1757, Kidney/ Rat.
Fig. 147.
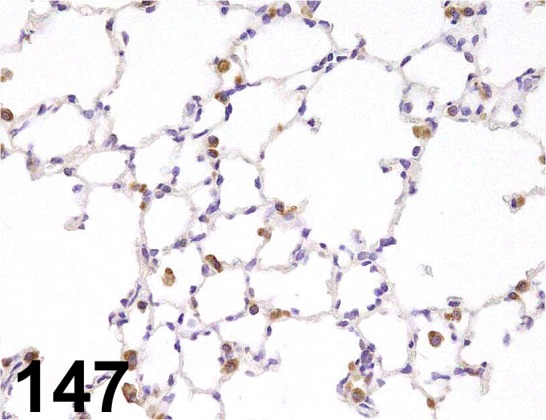
Lysozyme/ - / Dako/ A0099, Lung/ Rat.
Fig. 148.
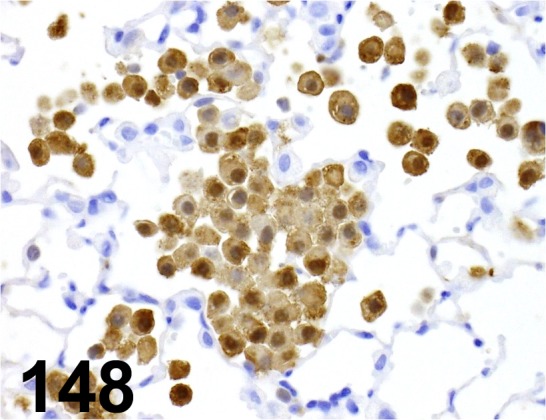
MAC-2/ M3/38/ Cedarlane/ CL8942AP, Macrophage/ Mouse.
Fig. 149.
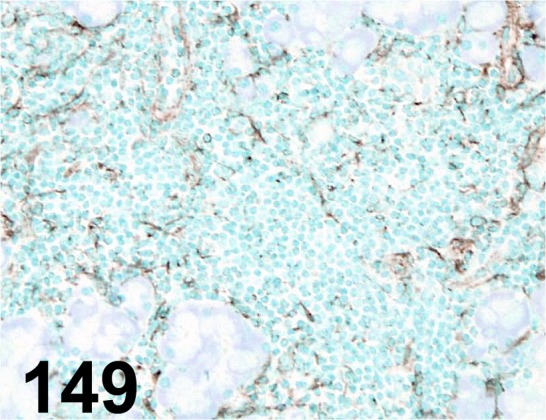
Macrophage/ RM0029-11H3/ Abcam/ ab56297, Lacrimal gland/ Mouse.
Fig. 150.
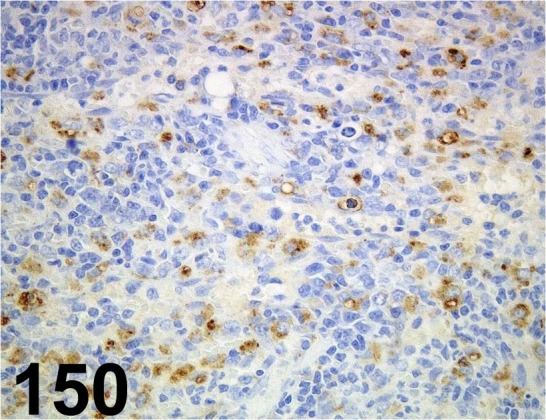
Macrophage/ Dendritic Cells/ RM-4/ Trans Genic/ KT014, Kidney/ Rat.
Fig. 151.
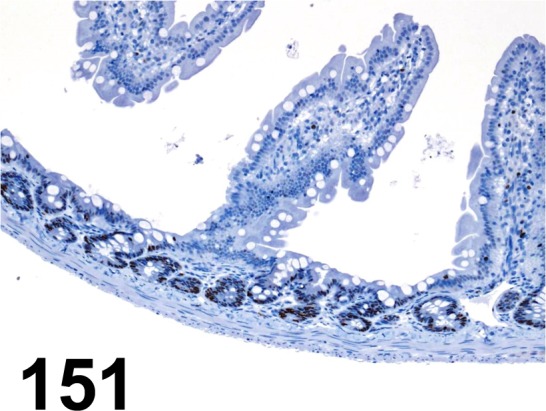
MCM7/ 47DC141/ DCS-141/ Abcam/ ab52489, Small intestine/ Rat.
Fig. 152.
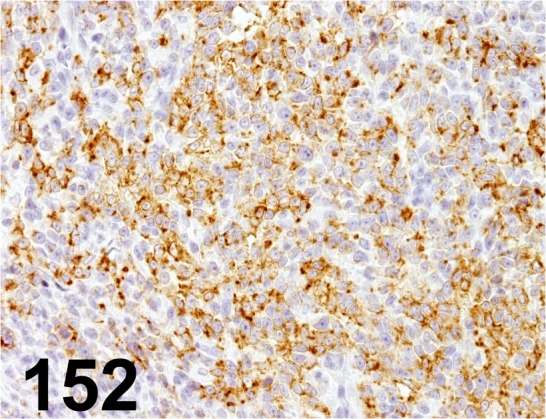
Melan A/ A103/ Dako/ M7196, Melanoma/ Dog.
Fig. 153.
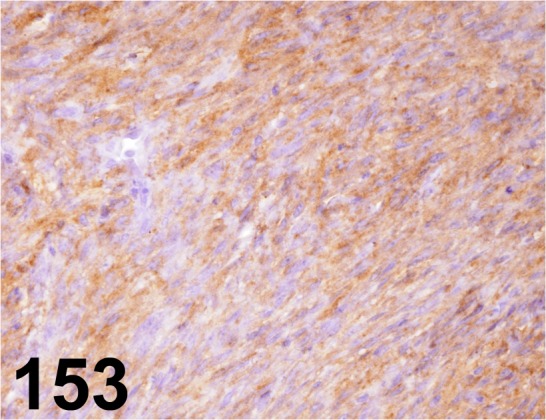
Melanoma/ PNL2/ Abcam/ ab12502, Melanoma/ Rat.
Fig. 154.
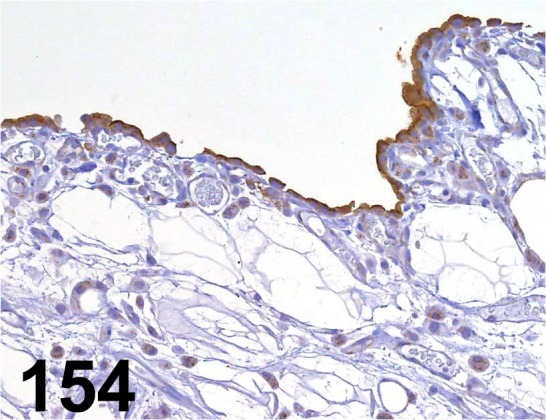
Mesothelin/ - / IBL/ 28001, Adipose tissue/ Rat.
Fig. 155.
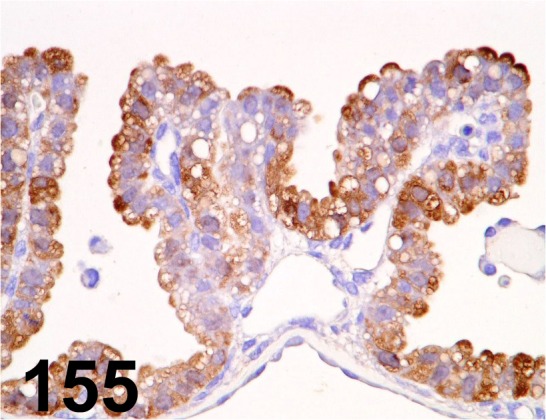
Metallothionein/ E9/ Dako/ M0639, Yolk sac/ Rat.
Fig. 156.
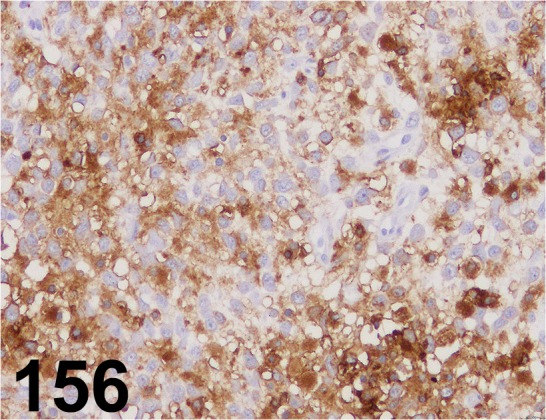
MHC class II (Human HLA-DR)/ TAL.1B5/ Dako/ M0746, Histiocytic sarcoma/ Dog.
Fig. 157.
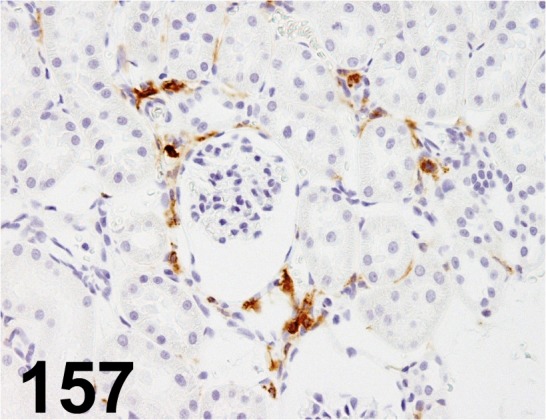
MHC class II (RT1B)/ OX-6/ OX-6/ MCA46GA, Kidney/ Rat.
Fig. 158.
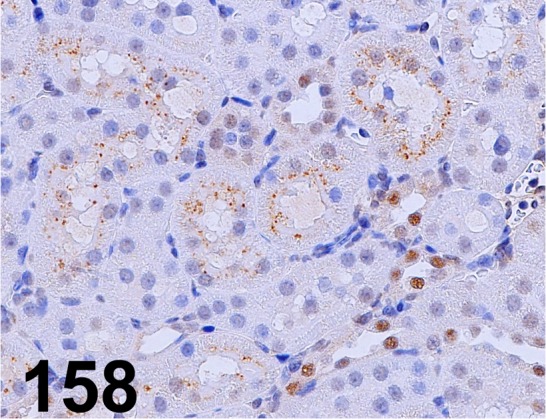
β2-microglobulin/ - / Lifespan Biosciences/ LS-B33, Kidney/ Rat.
Fig. 159.
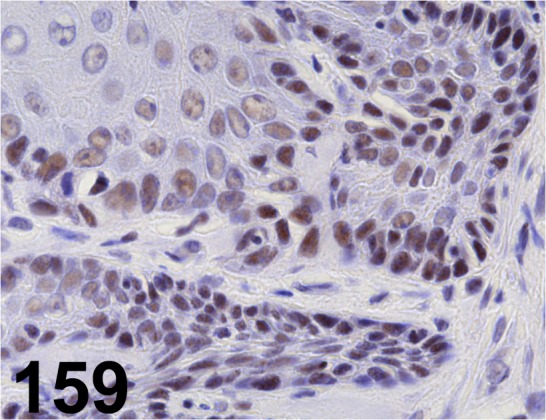
MLH1/ EPR3894/ Abcam/ ab92312, Stomach/ Rat.
Fig. 160.
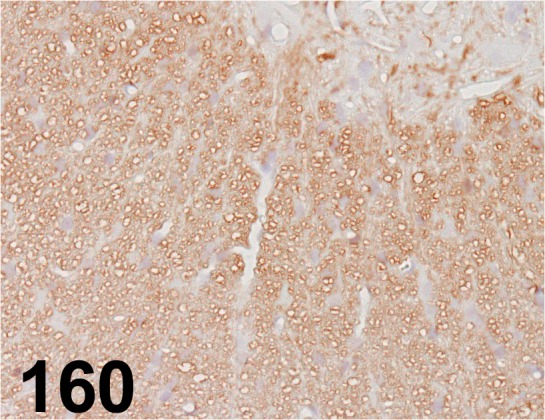
Myelin Basic Protein/ 26/ Millipore/ MAB384, Spinal cord/ Rat.
Fig. 161.
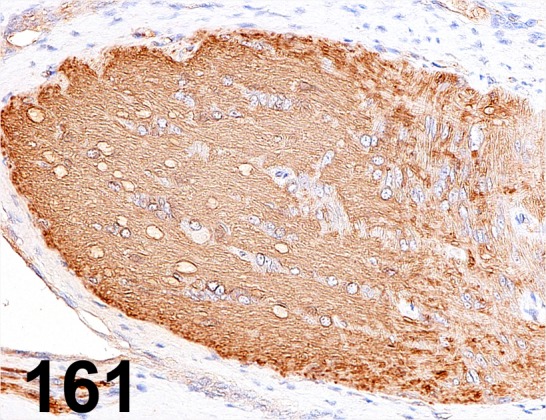
Myelin Basic Protein/ - / Abcam/ ab40390, Optic nerve/ Rat.
Fig. 162.
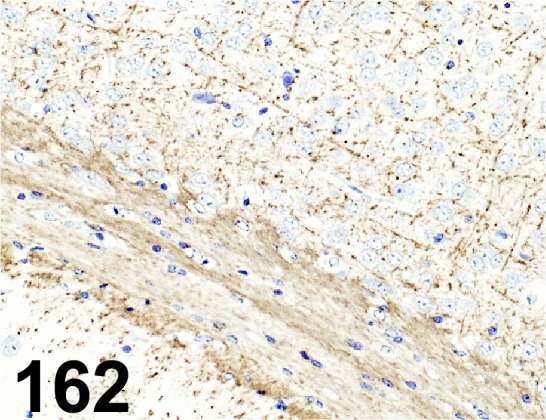
Myelin Basic Protein/ - / AbFrontier/ LF-PA50045, Brain/ Mouse.
Fig. 163.
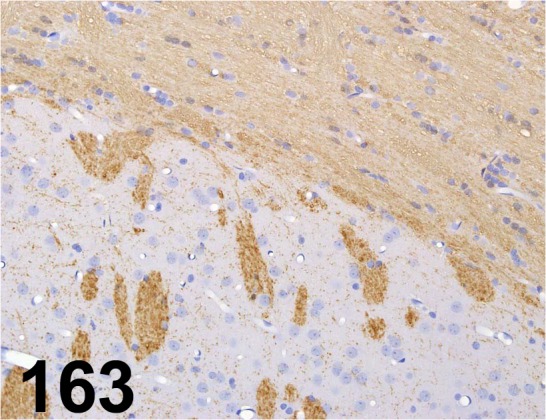
Myelin Basic Protein/ - / Sigma-Aldrich/ M3821, Cerebrum/ Rat.
Fig. 164.
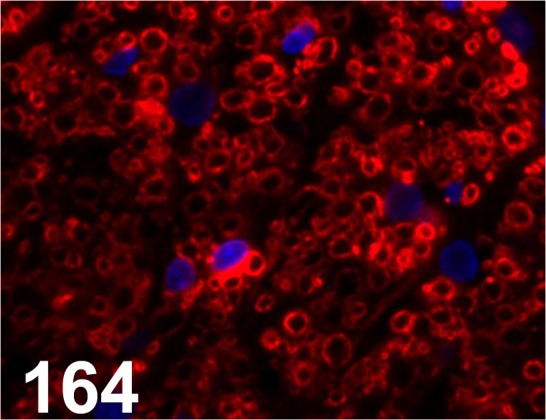
Myelin Proteolipid Protein/ PLPC1/ PLPC1/ Millipore, Spinal cord/ Rat.
Fig. 165.
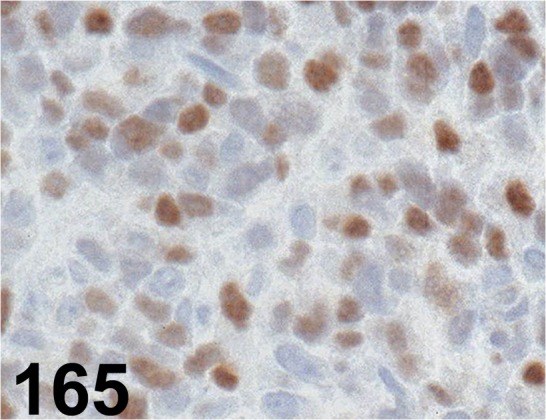
MyoD1/ 5.8A/ Dako/ M3512, Rhabdomyosarcoma/ Dog
Fig. 166.
Myogenin/ F5D/ Abcam/ ab1835, Myoblast/ Rat.
Fig. 167.
Myogenin/ F5D/ Dako/ M3559, Rhabdomyosarcoma/ Cow.
Fig. 168.
Nephrin C/ - / IBL/ 29070, Kidney/ Rat.
Fig. 169.
Nestin/ Rat-401/ Millipore/ MAB353, Skin/ Rat
Fig. 170.
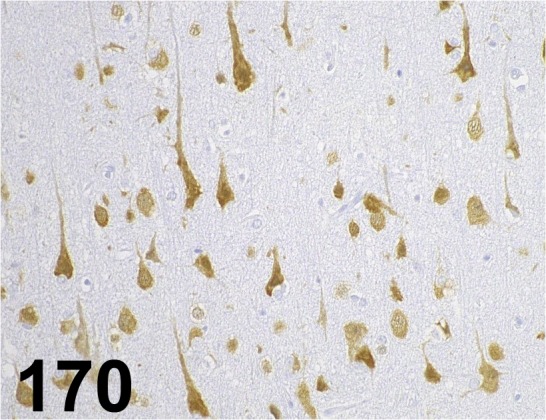
NeuN/ A60/ Millipore/ MAB377, Cerebrum/ Monkey.
Fig. 171.
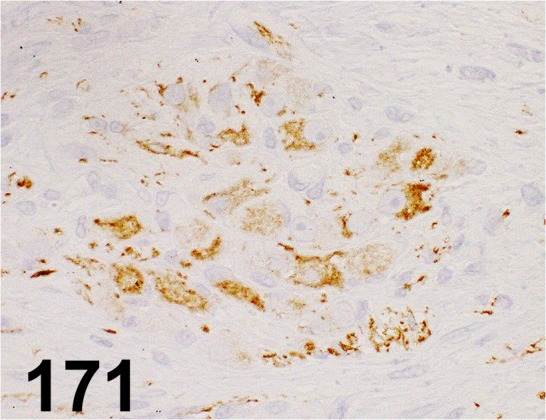
Neurofilament/ 2F11/ Dako/ IR607, Ganglioneuroma/ Dog.
Fig. 172.
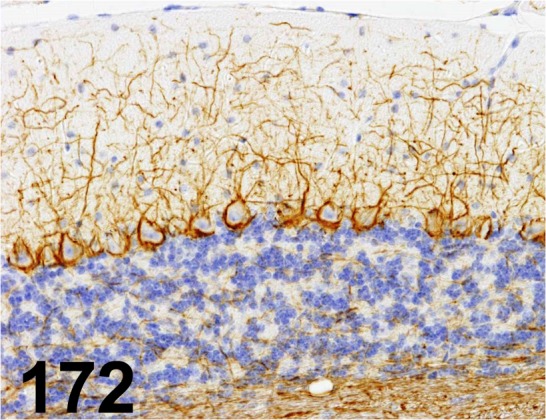
Neurofilament/ 2F11/ Dako/ M0762, Cerebellum/ Mouse.
Fig. 173.
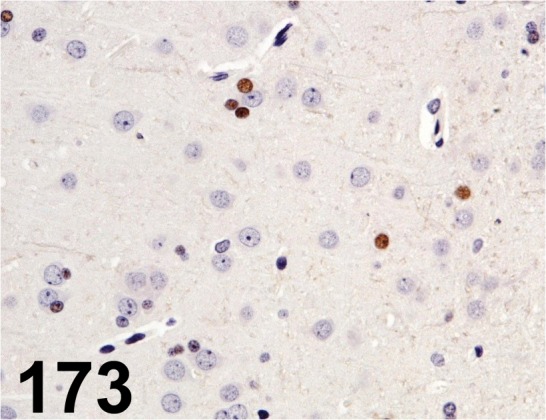
Olig2/ - / IBL/ 18953, Brain/ Rat.
Fig. 174.
Olig2/ - / Novus Biologicals/ NBP1-28667, Cerebrum/ Mouse.
Fig. 175.
Osteocalcin/ OCG3/ Abcam/ ab13420, Sternum/ Rat.
Fig. 176.
Osteocalcin/ OCG3/ Takara Bio/ M043, Parietal bone/ Rat.
Fig. 177.
Osterix/ - / Abcam/ ab22552, Sternum/ Rat.
Fig. 178.
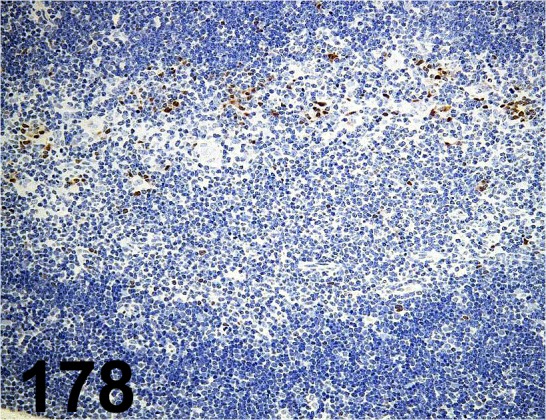
Pax5/ EPR3730(2)/ Abcam/ ab109443, Thymus/ Mouse.
Fig. 179.
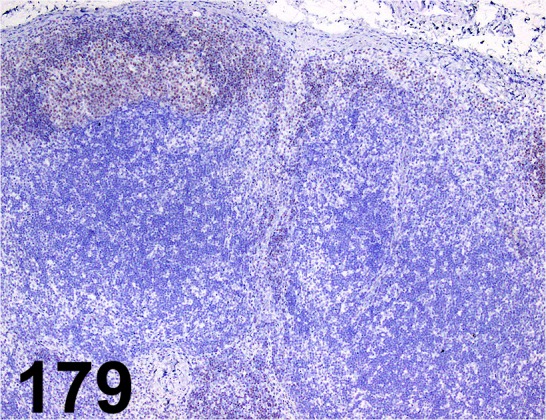
Pax5/ - / Abcam/ ab15164, Lymph node/ Dog.
Fig. 180.
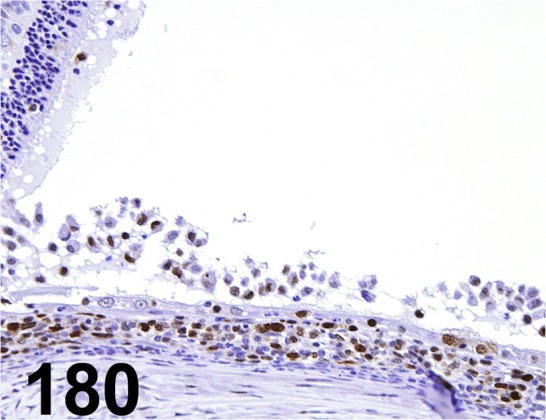
PCNA/ PC10/ Dako/ M0879, Eye/ Rat.
Fig. 181.
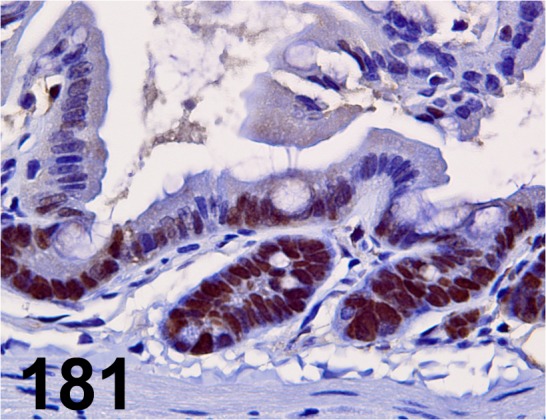
PCNA/ PC10/ Nichirei/ 412801, Ileum/ Mouse.
Fig. 182.
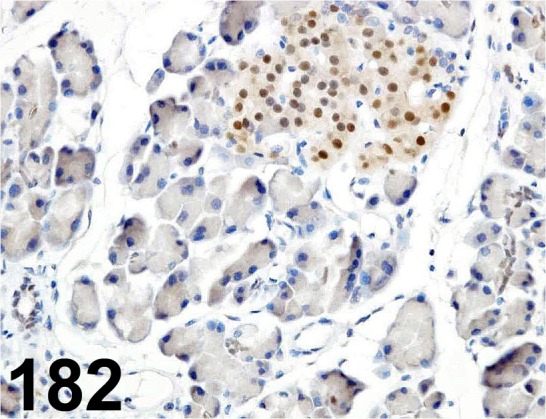
PDX1/ - / Trans Genic/ KR059, Pancreas/ Rat.
Fig. 183.
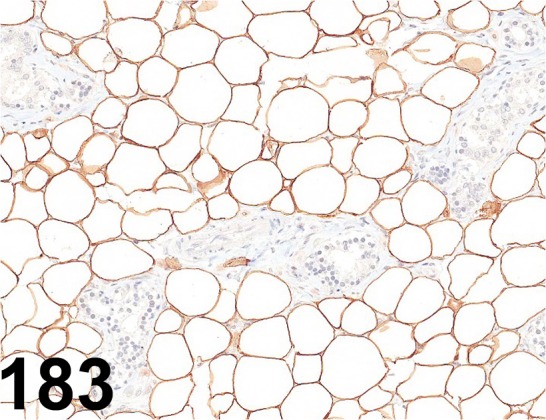
Perilipin 1/ - / Progen/ GP29, Adipose tissue/ Rat.
Fig. 184.
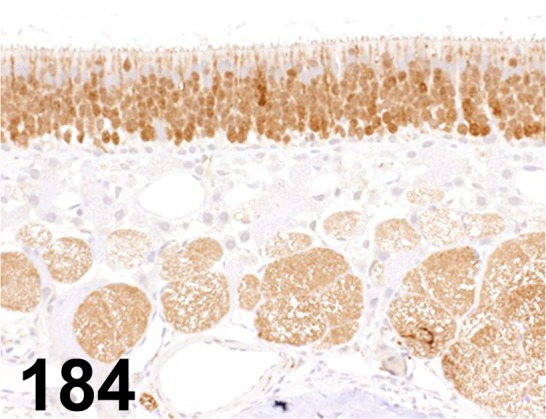
PGP-9.5/ - / Dako/ Z5116, Olfactory/ Rat.
Fig. 185.
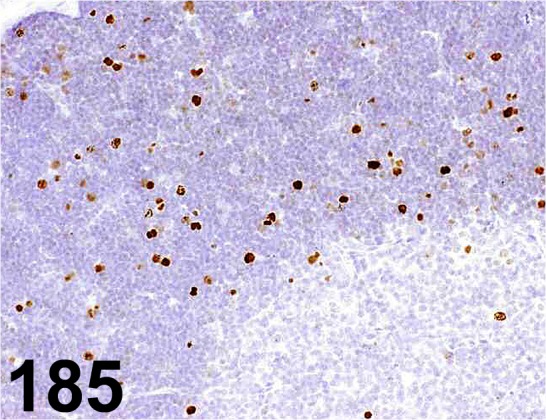
Phospho-histone H3/ - / Cell Signaling Technology/ 9701, Thymus/ Rat.
Fig. 186.
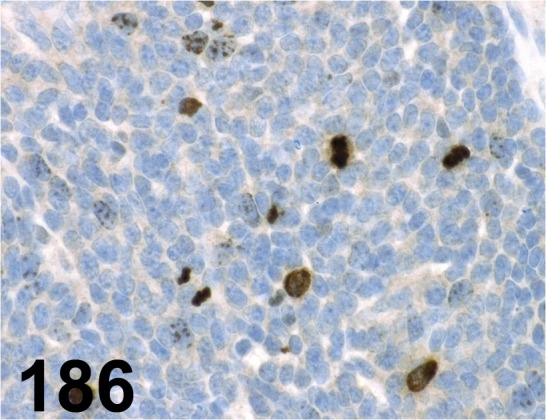
Phospho-histone H3 (Ser10)/ - / Millipore/ 06-570, Carcinoma/ Mouse.
Fig. 187.
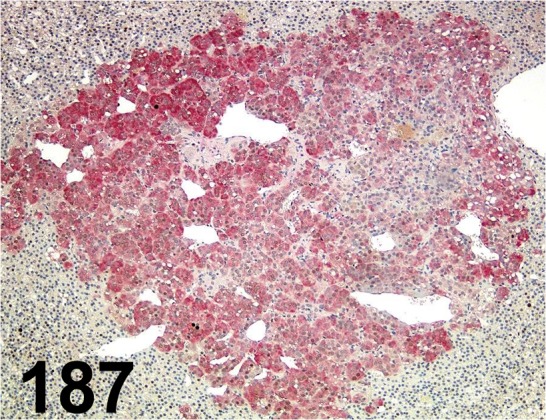
PNMT/ 1D2/ Abcam/ ab119784, Adrenal gland/ Rat.
Fig. 188.
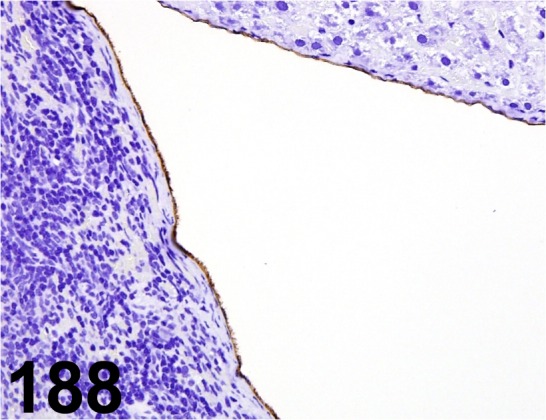
Podoplanin/ - / AngioBio/ 11035, Mesothelium / Rat
Fig. 189.
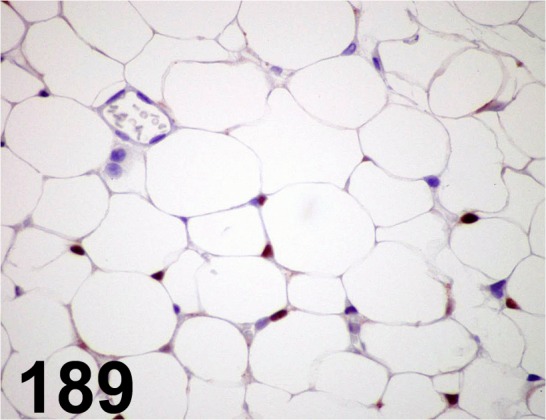
PPARγ/ C26H12/ Cell Signaling Technology/ 2435S, Adipose tissue/ Mouse.
Fig. 190.
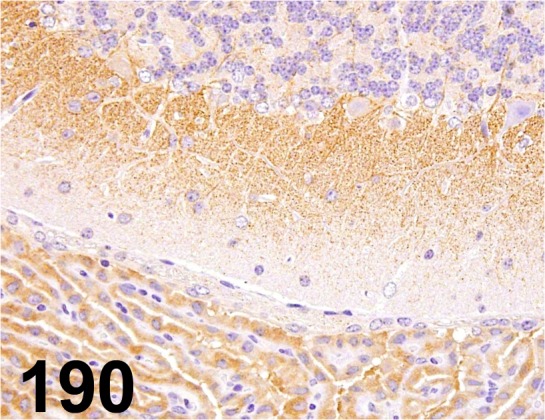
PPARγ2/ - / Thermo Fisher Scientific/ PA1-824, Brain/ Mouse.
Fig. 191.
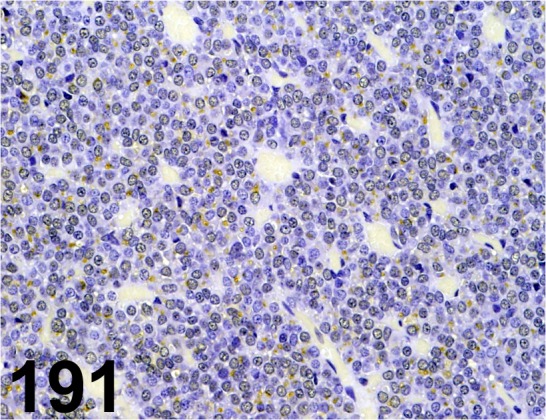
Prolactin/ - / Millipore/ AB960, Pituitary gland/ Rat.
Fig. 192.
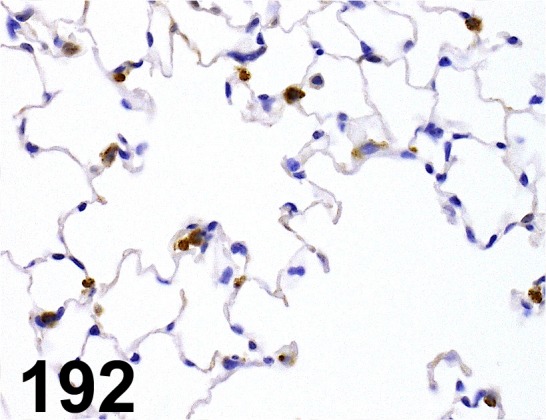
Prosurfactant Protein C/ - / Abcam/ ab90716, Lung/ Mouse.
Fig. 193.
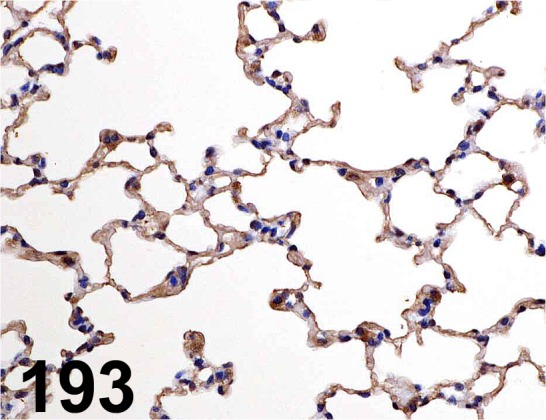
Pulmonary Surfactant-associated Protein D/ - / Bioss/ bs-1583R, Lung/ Mouse.
Fig. 194.
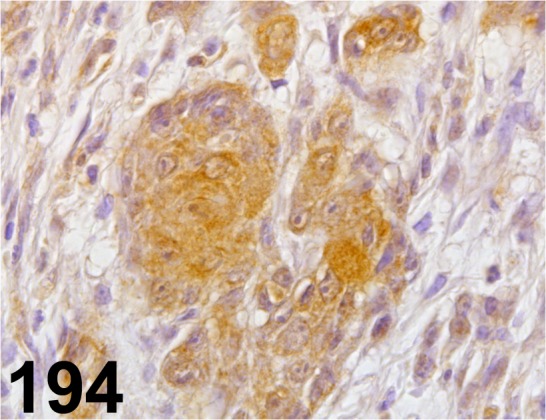
RASSF1/ - / Abcam/ ab110900, Stomach/ Rat.
Fig. 195.
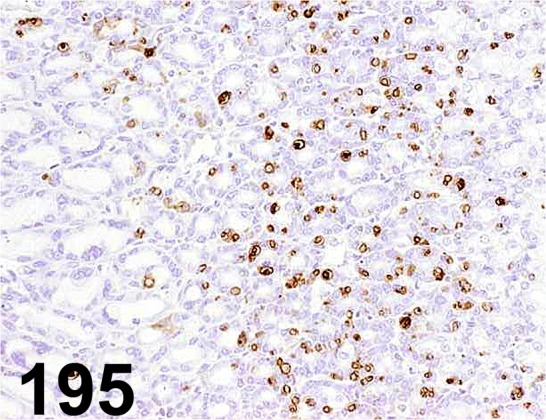
Rat Mast Cell Protease 2/ - / Moredun scientific/ MS-RM4, Glandular stomach/ Rat.
Fig. 196.
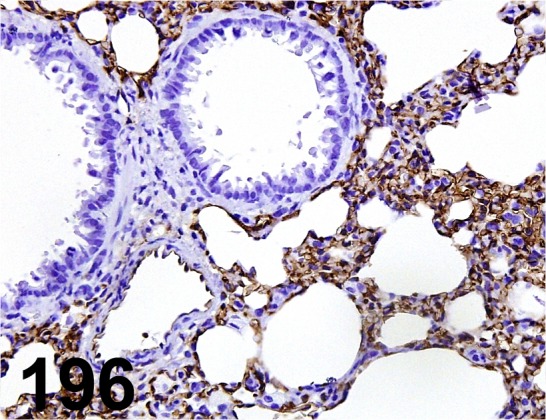
Rat MFH/ A3/ Trans Genic/ KJ091, Lung/ Rat.
Fig. 197.
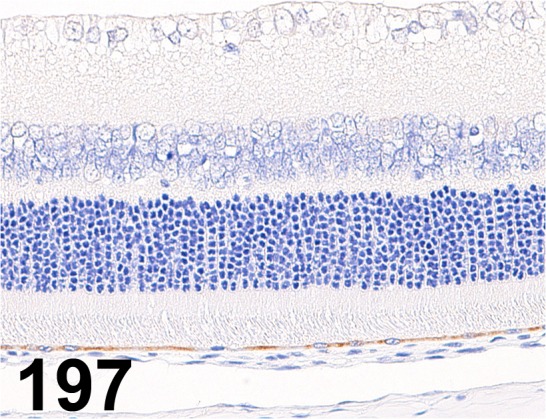
Retinal Pigment Epithelium-specific 65 kDa Protein/ - / Abcam/ ab77381, Eye/ Rat.
Fig. 198.
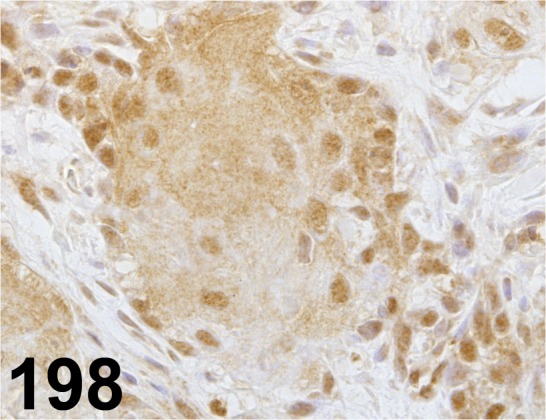
Retinoic Acid Receptor β/ - / Abcam/ ab53161, Stomach/ Rat.
Fig. 199.
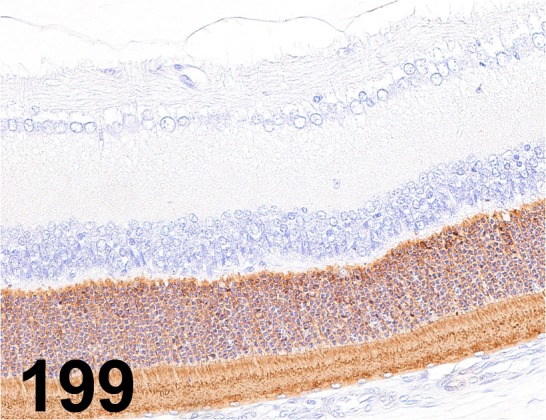
Rhodopsin/ RET-P1/ Millipore/ MAB5316, Eye/ Rat.
Fig. 200.
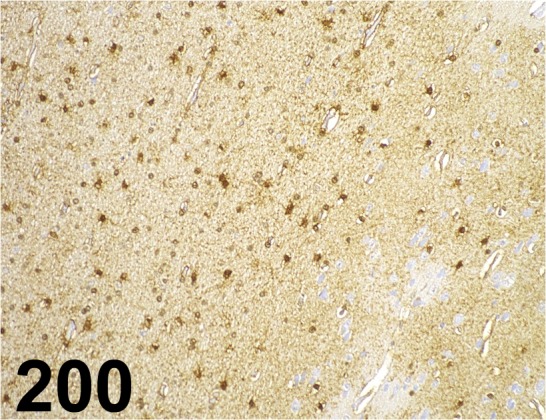
S100/ B32.1/ Abcam/ ab7852, Cerebrum/ Monkey.
Fig. 201.
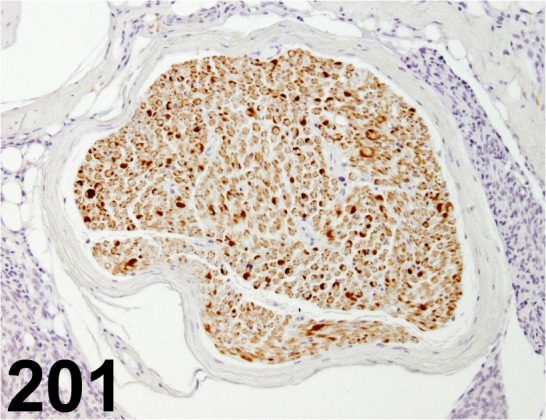
S100/ - / Dako/ IR504, Peripheral nerve/ Rat.
Fig. 202.
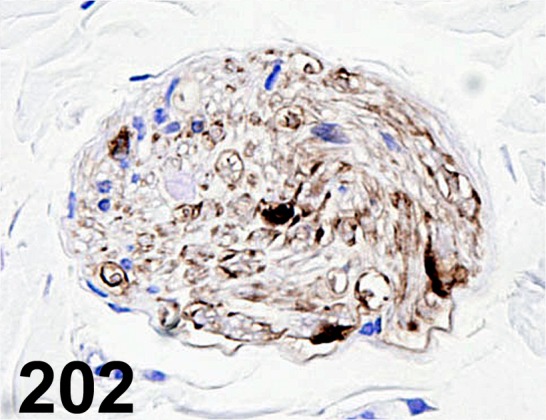
S100/ - / Nichirei/ 422091, Peripheral nerve/ Rat.
Fig. 203.
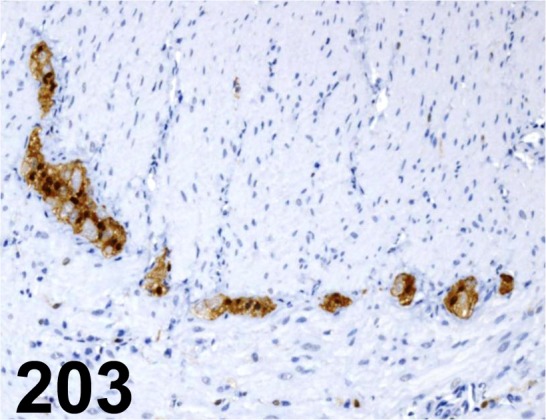
S100a/ - / Dako/ Z0628, Large intestine/ Rat.
Fig. 204.
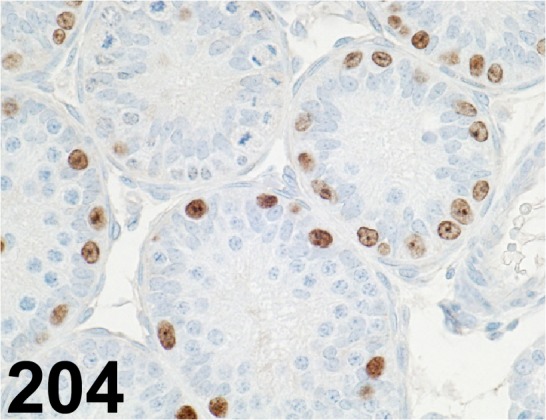
Sall4/ EE-30/ Santa Cruz/ sc-101147, Testis/ Rat.
Fig. 205.
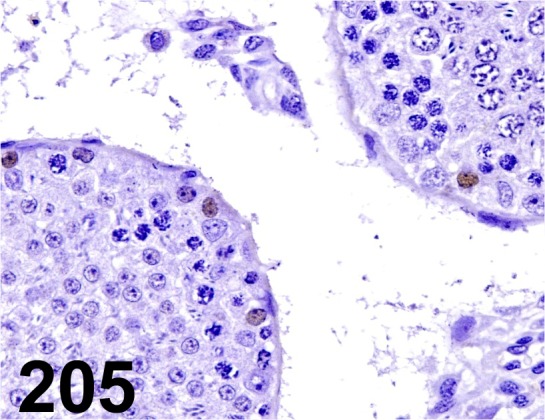
Sall4/ - / Abcam/ ab57577, Testis/ Rat.
Fig. 206.
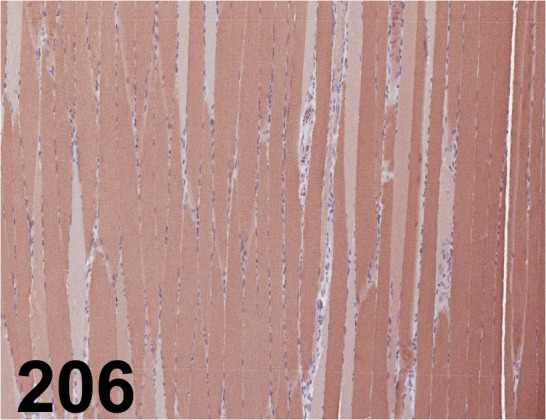
Sarcoplasmic/ Endoplasmic Reticulum Calcium ATPase 2/ - / Abcam/ ab3625, Muscle/ Rat.
Fig. 207.
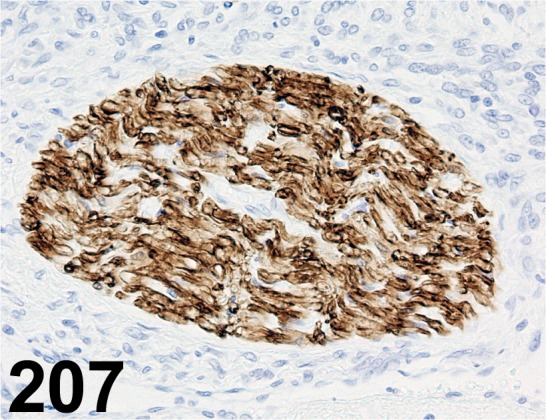
Schwann Cell/ Peripheral Myelin/ Schwann/ 2E/ Cosmo Bio/ GU01-M01AS-A, Peripheral nerve/ Rat.
Fig. 208.
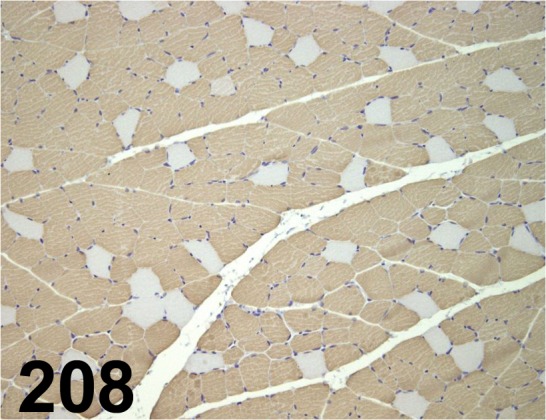
Slow Skeletal Myosin Heavy Chain/ NOQ7.5.4D/ Abcam/ ab11083, Muscle/ Rat
Fig. 209.
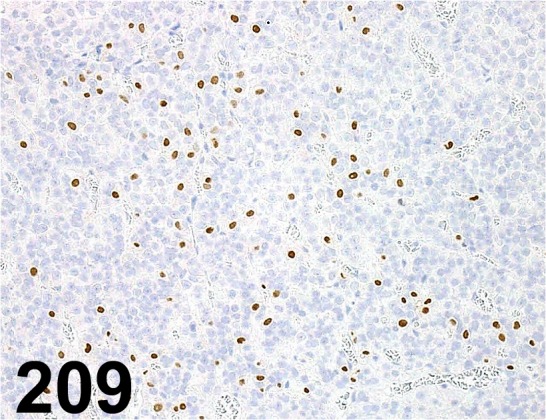
Steroidogenic Factor 1/ N1665/ Perseus Proteomics Inc./ PP-N1665-00, Pituitary gland/ Rat.
Fig. 210.
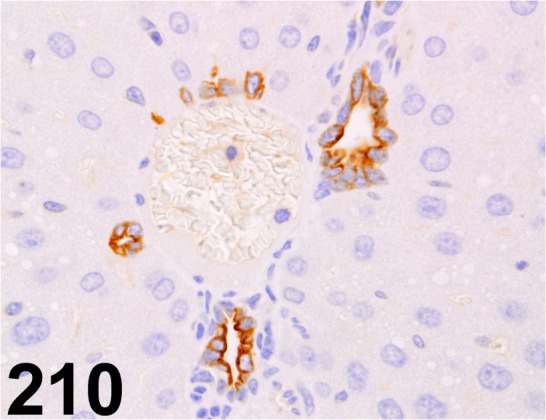
Stromal Cell-derived Factor 1/ CXCL12/ 79018.111/ R&D Systems/ MAB350, Liver/ Mouse.
Fig. 211.
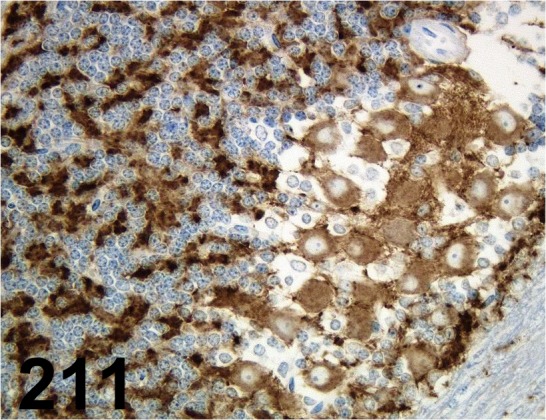
Synaptophysin/ 27G12/ Leica/ NCL-L-SYNAP-299, Cerebellum/ Rat.
Fig. 212.
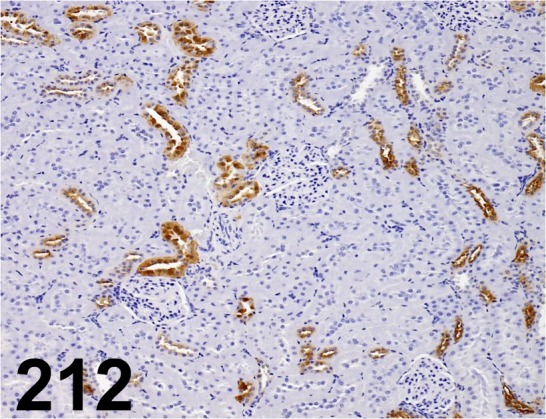
Tamm-Horsfall Urinary Glycoprotein/ - / Santa Cruz/ sc-20631, Kidney/ Rat.
Fig. 213.
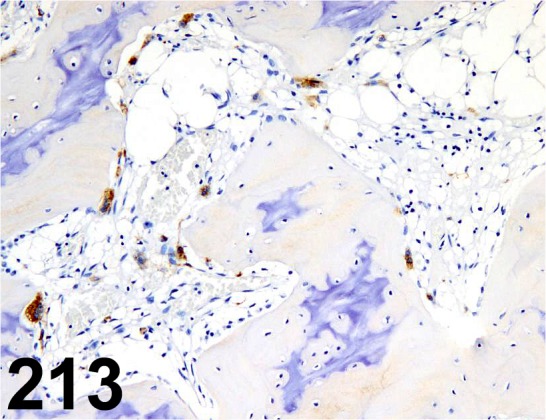
Tartrate-resistant Acid Phosphatase/ - / Takara Bio/ M183, Femur/ Monkey.
Fig. 214.
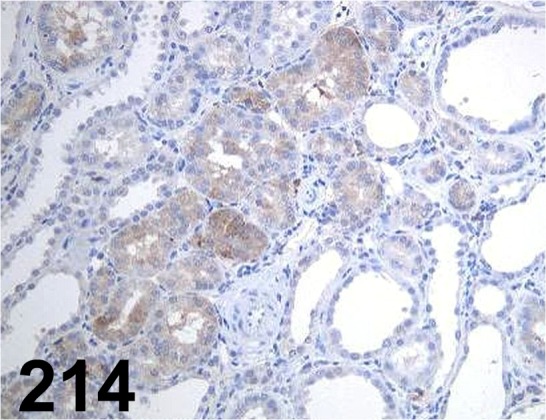
TGFβ1/ - / Santa Cruz/ sc-146, Kidney/ Rat.
Fig. 215.
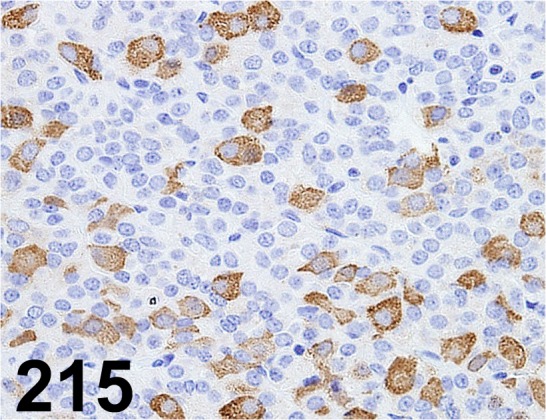
Thyroid Stimulating Hormone/ - / Biogenesis/ 8926-0004, Pituitary gland/ Rat
Fig. 216.
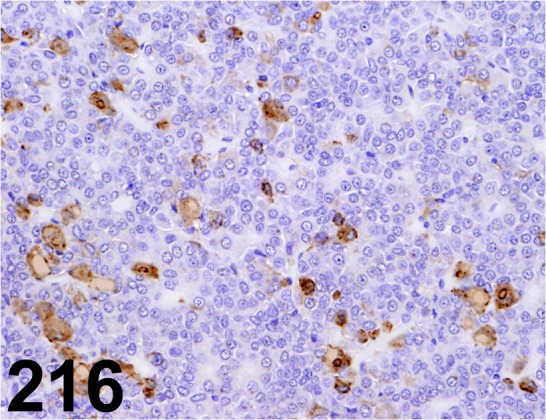
Thyroid Stimulating Hormone/ - / Millipore/ AB976, Pituitary gland/ Rat.
Fig. 217.
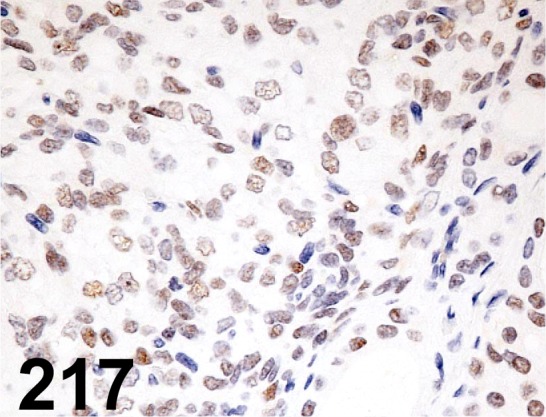
Thyroid Transcription Factor 1/ 8G7G3/1/ Progen/ 16108, Thyroid/ Rat.
Fig. 218.
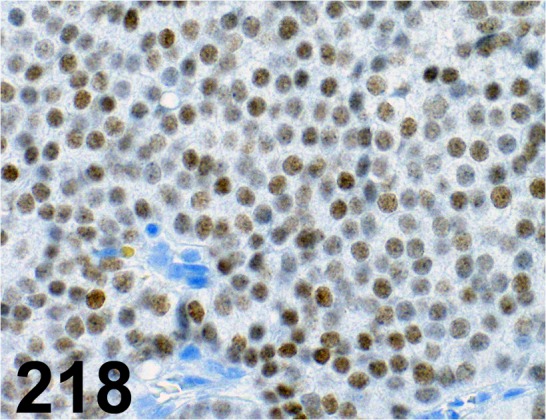
Thyroid Transcription Factor 1/ 8G7G3/1/ Dako/ M3575, Lung/ Dog.
Fig. 219.
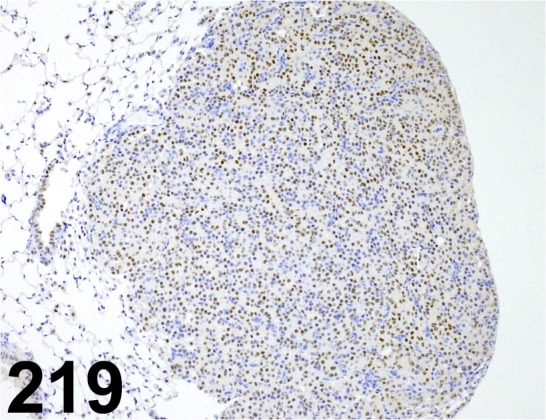
Thyroid Transcription Factor 1/ EP1584Y/ Abcam/ ab76013, Lung/ Mouse.
Fig. 220.
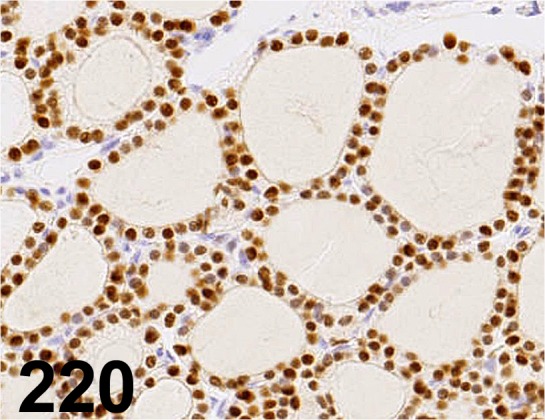
Thyroid Transcription Factor 1/ SPT24/ Leica/ NCL-L-TTF-1, Thyroid gland/ Rat.
Fig. 221.
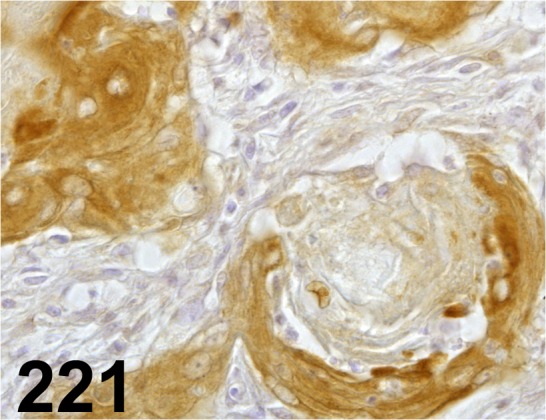
Tissue Inhibitor of Metalloproteinase-3/ - / Proteintech/ 10858-1-AP, Stomach/ Rat.
Fig. 222.
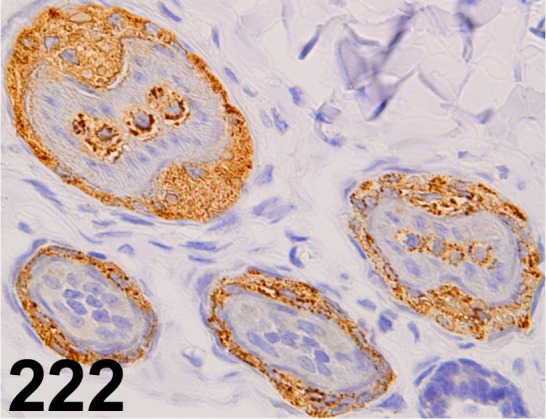
Trichohyalin/ AE15/ Abcam/ ab58755, Skin/ Rabbit.
Fig. 223.
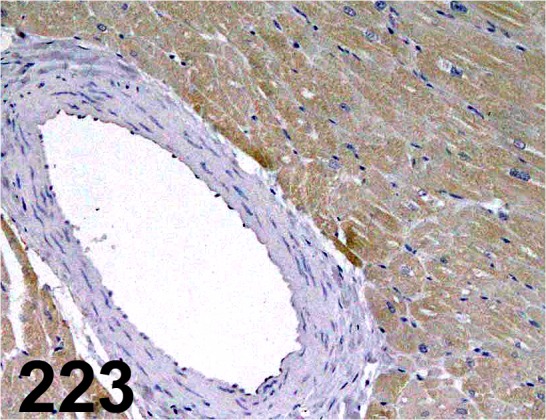
Troponin I, Cardiac/ 2D5/ Affinity Bioreagents/ MA1-34958, Heart/ Monkey.
Fig. 224.
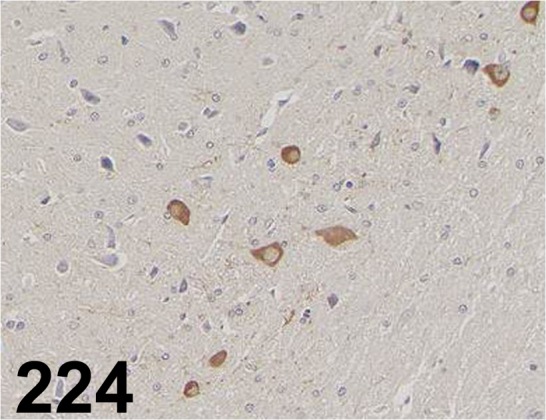
Tyrosine Hydroxylase/ - / Enzo Life Sciences/ BML-TZ1010, Midbrain/ Rat.
Fig. 225.
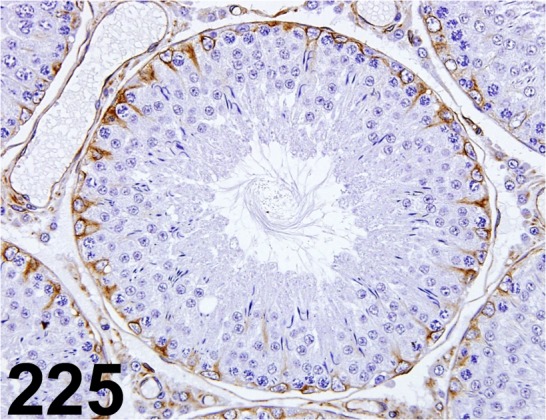
Tyrosine Tubulin/ TUB-1A2/ Sigma-Aldrich/ T9028, Testis/ Rat.
Fig. 226.
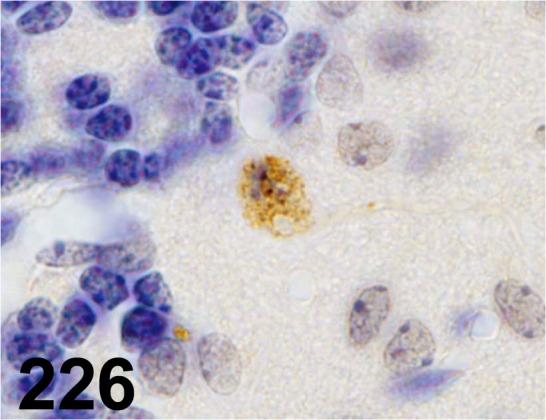
Ubiquitin/ - / Dako/ Z0458, Cerebellum/ Mouse.
Fig. 227.
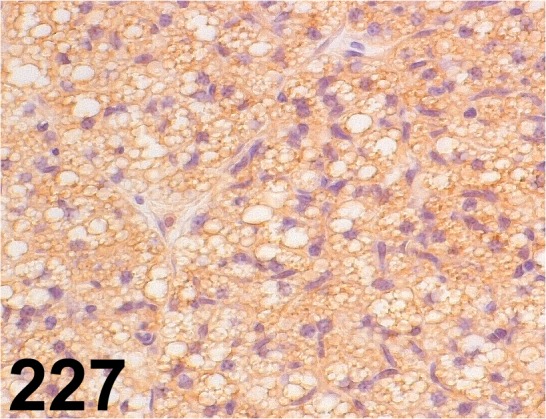
Uncoupling Protein 1/ - / Abcam/ ab10983, Brown adipose tissue/ Mouse.
Fig. 228.
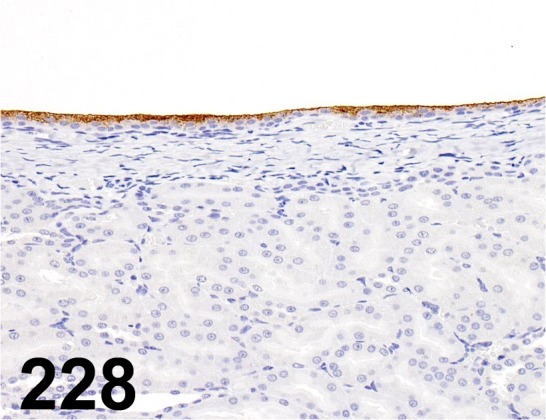
Uroplakin III/ AU1/ Progen/ 651108, Kidney/ Rat.
Fig. 229.
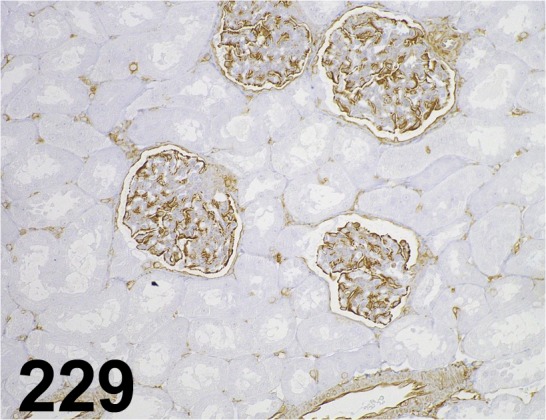
Vimentin/ V9/ Dako/ IR630, Kidney/ Monkey.
Fig. 230.
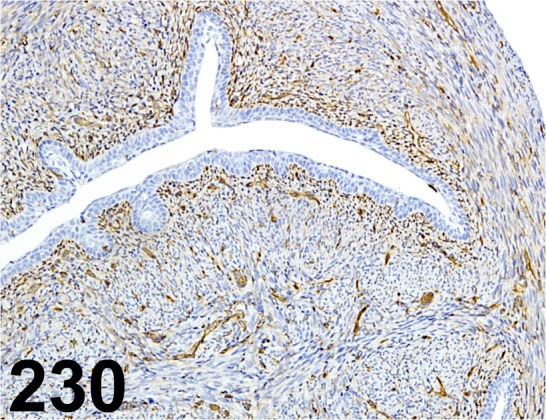
Vimentin/ Vim 3B4/ Dako/ CBL202, Uterus/ Mouse.
Fig. 231.
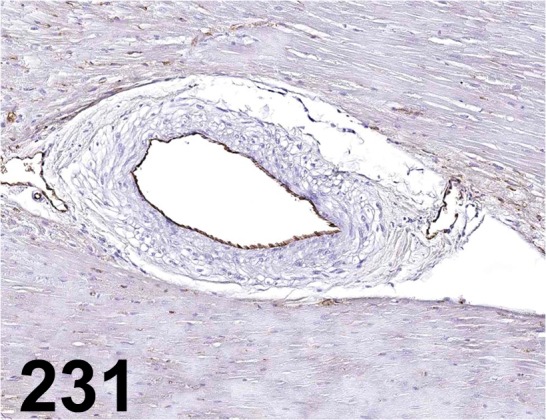
Von Willebrand Factor/ - / Abcam/ ab6994, Heart/ Dog.
Fig. 232.
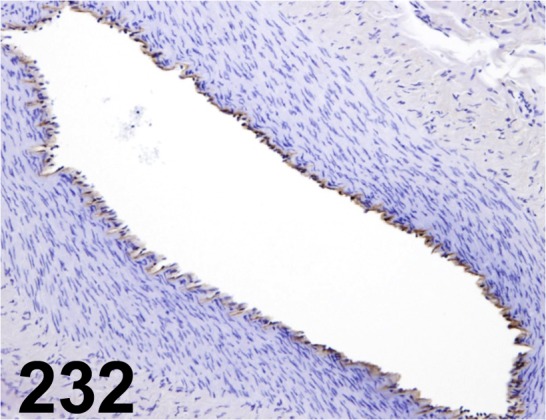
Von Willebrand Factor/ - / Thermo Fisher Scientific/ 18-0018, Uterus/ Dog.
Fig. 233.
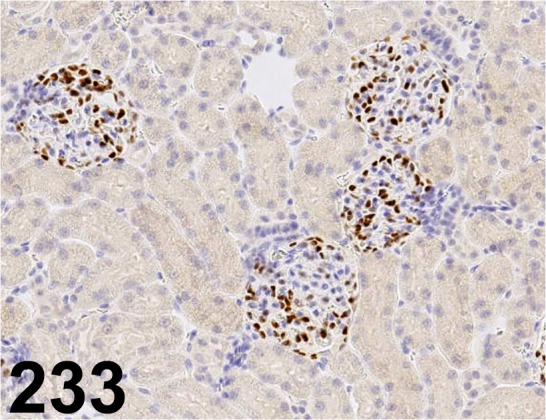
Wilms’ Tumor 1/ 6F-H2/ Dako/ M3561, Kidney/ Rat.
Fig. 234.
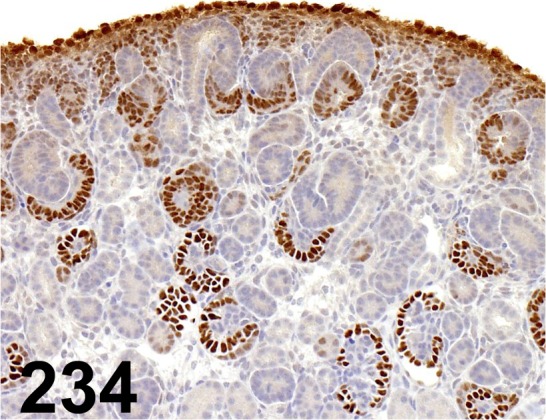
Wilms’ Tumor 1/ - / Santa Cruz/ sc-192, Kidney/ Rat.
Fig. 235.
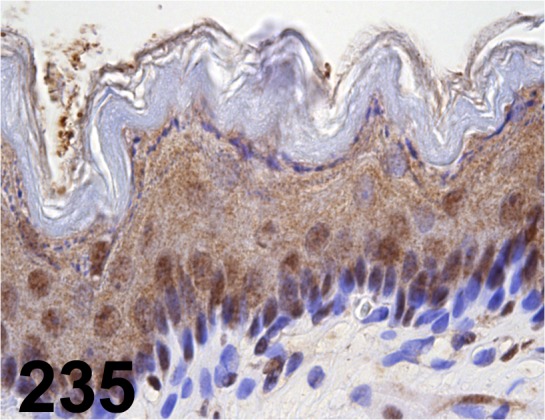
X-ray Repair Cross-complementing 1/ - / Santa Cruz/ sc-11429, Stomach/ Rat.
Supplementary Material
Acknowledgments
The authors would like to thank the cooperating research institutes belonging to the CEAH for generously providing the data and Dr. Naho Tsuji (Nissan Chemical Industries, Ltd.) for the technical support.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that there is no conflict of interest.
Reference
- 1.Ramos-Vara JA, and Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol. 51: 42–87. 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


