Abstract
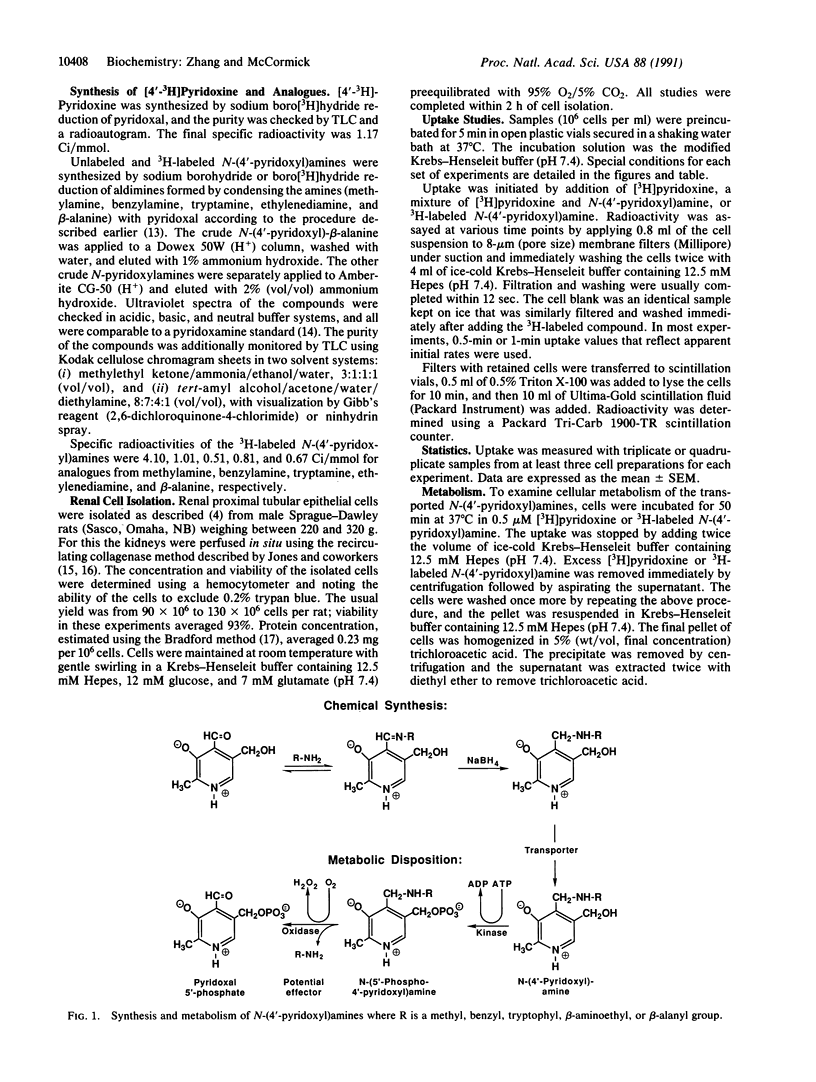
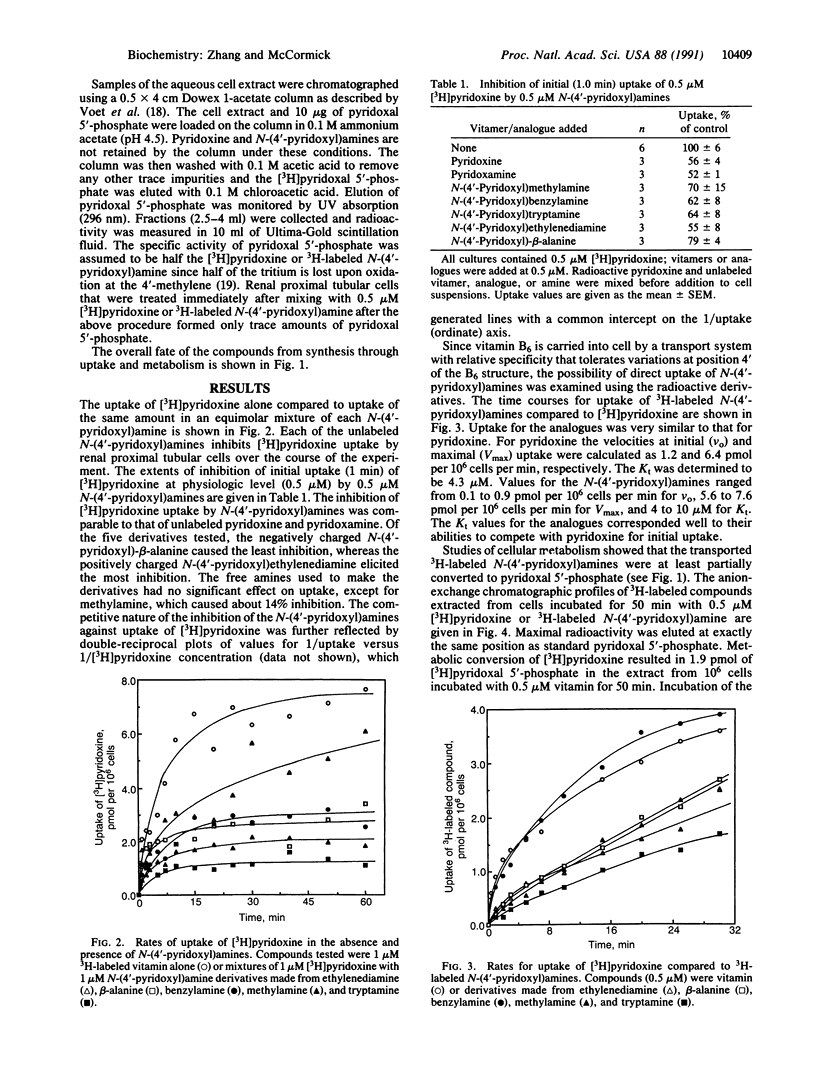
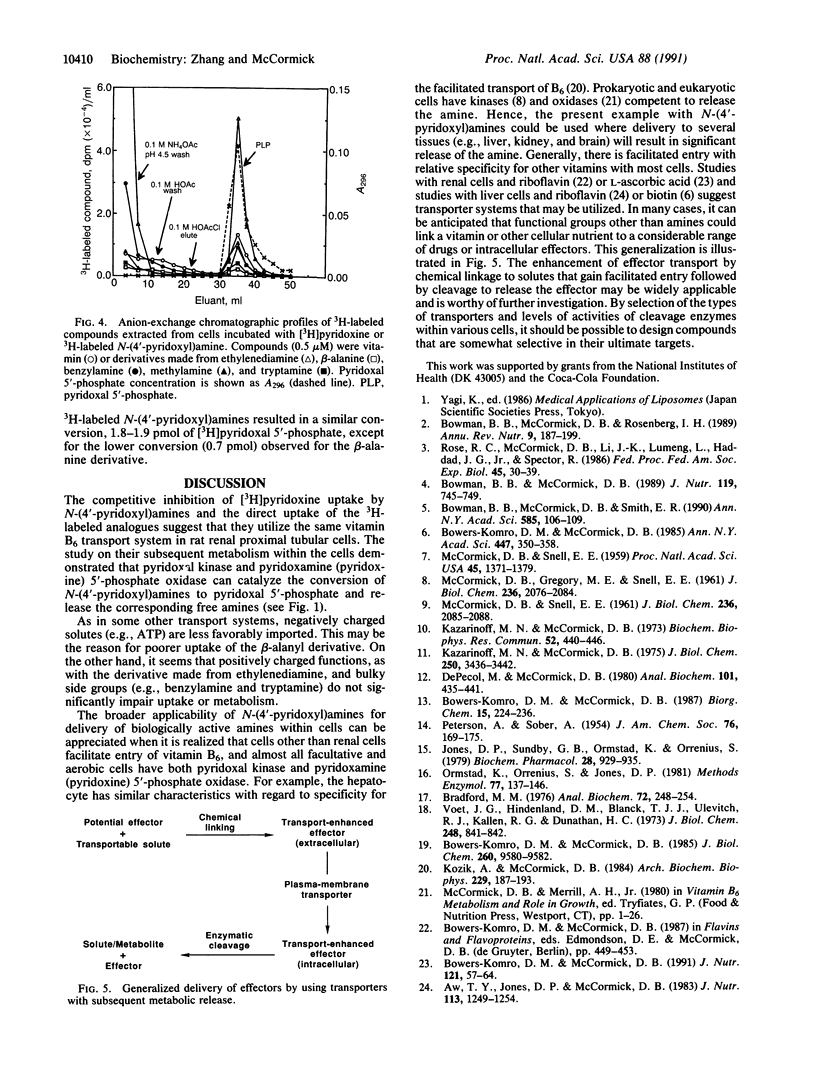
The importing of vitamin B6 by renal proximal tubular cells from the rat is facilitated and Na(+)-dependent and reflects specificity for the meta-phenolate pyridinium structure with a 5-hydroxymethyl function. This transporter can, however, accept competitively each of the natural nonphosphorylated vitamers (pyridoxine, pyridoxamine, and pyridoxal) and other B6 analogues differing only in the groups at position 4. A series of N-(4'-pyridoxyl)amines was synthesized by sodium borohydride or boro[3H]hydride reduction of aldimines formed by condensing the amines with pyridoxal. The unlabeled B6-secondary amine compounds were found to competitively inhibit the uptake of [4'-3H]pyridoxine by the renal cells. Moreover, the 3H-labeled N-(4'-pyridoxyl)amines were shown to enter the cells by the process facilitated by the B6 transporter. Upon entry the labeled compounds were converted to N-(5'-phospho-4'-pyridoxyl)amines in a reaction catalyzed by pyridoxal kinase, an enzyme that tolerates considerable functional variation in position 4 of the B6 structure. The 5'-phosphates were subsequently converted within the cell to pyridoxal 5'-phosphate with liberation of the original amine in a reaction catalyzed by pyridoxamine (pyridoxine) 5'-phosphate oxidase, an enzyme with broad specificity for 4'-substituted amines on the 5'-phospho-B6 structure. This system illustrates how knowledge of transporter specificity can permit design of a compound with potential biologic activity. A drug or other intracellular effector may be piggybacked onto a transported solute (e.g., vitamin or other nutrient) that gains facilitated entry to a cell and is, thereafter, metabolized to release the active compound.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aw T. Y., Jones D. P., McCormick D. B. Uptake of riboflavin by isolated rat liver cells. J Nutr. 1983 Jun;113(6):1249–1254. doi: 10.1093/jn/113.6.1249. [DOI] [PubMed] [Google Scholar]
- Bowers-Komro D. M., McCormick D. B. Biotin uptake by isolated rat liver hepatocytes. Ann N Y Acad Sci. 1985;447:350–358. doi: 10.1111/j.1749-6632.1985.tb18450.x. [DOI] [PubMed] [Google Scholar]
- Bowers-Komro D. M., McCormick D. B. Characterization of ascorbic acid uptake by isolated rat kidney cells. J Nutr. 1991 Jan;121(1):57–64. doi: 10.1093/jn/121.1.57. [DOI] [PubMed] [Google Scholar]
- Bowers-Komro D. M., McCormick D. B. Pyridoxamine-5'-phosphate oxidase exhibits no specificity in prochiral hydrogen abstraction from substrate. J Biol Chem. 1985 Aug 15;260(17):9580–9582. [PubMed] [Google Scholar]
- Bowman B. B., McCormick D. B. Pyridoxine uptake by rat renal proximal tubular cells. J Nutr. 1989 May;119(5):745–749. doi: 10.1093/jn/119.5.745. [DOI] [PubMed] [Google Scholar]
- Bowman B. B., McCormick D. B., Rosenberg I. H. Epithelial transport of water-soluble vitamins. Annu Rev Nutr. 1989;9:187–199. doi: 10.1146/annurev.nu.09.070189.001155. [DOI] [PubMed] [Google Scholar]
- Bowman B. B., McCormick D. B., Smith E. R. Vitamin B6 uptake by rat kidney cells and brush-border membrane vesicles. Ann N Y Acad Sci. 1990;585:106–109. doi: 10.1111/j.1749-6632.1990.tb28046.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DePecol M. E., McCormick D. B. Syntheses, properties, and use of fluorescent N-(5'-phospho-4'-pyridoxyl)amines in assay of pyridoxamine (pyridoxine) 5'-phosphate oxidase. Anal Biochem. 1980 Jan 15;101(2):435–441. doi: 10.1016/0003-2697(80)90210-9. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Sundby G. B., Ormstad K., Orrenius S. Use of isolated kidney cells for study of drug metabolism. Biochem Pharmacol. 1979 Mar 15;28(6):929–935. doi: 10.1016/0006-2952(79)90378-2. [DOI] [PubMed] [Google Scholar]
- Kazarinoff M. N., McCormick D. B. N-(5'-phospho-4'-pyridoxyl)amines as substrates for pyridoxine (pyridoxamine) 5'-phosphate oxidase. Biochem Biophys Res Commun. 1973 May 15;52(2):440–446. doi: 10.1016/0006-291x(73)90731-6. [DOI] [PubMed] [Google Scholar]
- Kazarinoff M. N., McCormick D. B. Rabbit liver pyridoxamine (pyridoxine) 5'-phosphate oxidase. Purification and properties. J Biol Chem. 1975 May 10;250(9):3436–3442. [PubMed] [Google Scholar]
- Kozik A., McCormick D. B. Mechanism of pyridoxine uptake by isolated rat liver cells. Arch Biochem Biophys. 1984 Feb 15;229(1):187–193. doi: 10.1016/0003-9861(84)90143-7. [DOI] [PubMed] [Google Scholar]
- MCCORMICK D. B., GREGORY M. E., SNELL E. E. Pyridoxal phosphokinases. I. Assay, distribution, I. Assay, distribution, purification, and properties. J Biol Chem. 1961 Jul;236:2076–2084. [PubMed] [Google Scholar]
- MCCORMICK D. B., SNELL E. E. Pyridoxal phosphokinases. II. Effects of inhibitors. J Biol Chem. 1961 Jul;236:2085–2088. [PubMed] [Google Scholar]
- McCormick D. B., Snell E. E. PYRIDOXAL KINASE OF HUMAN BRAIN AND ITS INHIBITION BY HYDRAZINE DERIVATIVES. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1371–1379. doi: 10.1073/pnas.45.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormstad K., Orrenius S., Jones D. P. Preparation and characteristics of isolated kidney cells. Methods Enzymol. 1981;77:137–146. doi: 10.1016/s0076-6879(81)77018-6. [DOI] [PubMed] [Google Scholar]
- Rose R. C., McCorrmick D. B., Li T. K., Lumeng L., Haddad J. G., Jr, Spector R. Transport and metabolism of vitamins. Fed Proc. 1986 Jan;45(1):30–39. [PubMed] [Google Scholar]
- Voet J. G., Hindenlang D. M., Blanck T. J., Ulevitch R. J., Kallen R. G., Dunathan H. C. The stereochemistry of pyridoxal phosphate enzymes. The absolute stereochemistry of cofactor C' 4 protonation in the transamination of holoserine hydroxymethylase by D-alanine. J Biol Chem. 1973 Feb 10;248(3):841–842. [PubMed] [Google Scholar]



