Abstract
The paper deals with application of three nanomaterial systems: undoped TiO2, chromium-doped TiO2:Cr and TiO2-SnO2 synthesized by flame spray synthesis (FSS) technique for hydrogen sensing. The emphasis is put on the role of anatase and rutile polymorphic forms of TiO2 in enhancing sensitivity towards reducing gases. Anatase-to-rutile transformation is achieved by annealing of undoped TiO2 in air at 700 °C, specific Cr doping and modification with SnO2. Undoped TiO2 and TiO2-SnO2 exhibit n-type behaviour and while TiO2: 5 at.% Cr is a p-type semiconductor. X-ray diffraction (XRD) has been applied to determine anatase-to-rutile weight ratio as well as anatase and rutile crystal size. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) have been used to characterize the structure and morphological parameters. Optical reflectometry enabled to find and compare the band gaps E g of anatase and rutile predominated compositions. Electrical properties, i.e. the electrical conductivity and values of constant phase element (CPE), have been established on the basis of impedance spectroscopy. Dynamic responses of the electrical resistance as a function of hydrogen concentration revealed that predominance of rutile in anatase/rutile mixture is beneficial for gas sensing. Partial transformation to rutile in all three material systems under study resulted in an increased sensitivity towards hydrogen. It is proposed that this effect can be explained in a similar way as in photocatalysis, i.e. by specific band alignment and electron transfer from rutile to anatase to facilitate oxygen preadsorption on the surface of anatase grains.
Keywords: Nanopowders, Anatase, Rutile, TiO2, TiO2-SnO2, Gas sensing
Background
Anatase and rutile are the most frequently encountered polymorphic forms of titanium dioxide and usually co-exist for samples prepared in typical technologies such as sputtering [1–4], flame spray synthesis [4–7] and sol-gel [8]. It has been demonstrated that for the purposes of photocatalysis, a certain ratio of anatase to rutile, corresponding to anatase content extending from 40 to 80%, is desired as it causes a synergetic effect [9]. Some authors [10, 11] attribute this effect to the specific parameters of electronic structure such as electron affinity, work function and, in a consequence, a flat band potential (see Table 1) [12–14].
Table 1.
| Property | Anatase | Rutile |
|---|---|---|
| Density (g/cm3) | 3.894 | 4.250 |
| Space group | I41/amd | P42/mnm |
| Symmetry | tetragonal | tetragonal |
| Lattice parameters (nm) | a = 0.3784 c = 0.9515 | a = 0.4594 c = 0.2959 |
| Molecules/cell | 4 | 2 |
| Volume/molecule (·10−3nm3) | 34.061 | 31.2160 |
| Average static dielectric constant | 48 | 110–117 |
| Band gap energy [eV] | 3.2–3.26 | 3.02–3.05 |
| Work function [eV] | 5.1 | 4.9 |
| Transformation temperature | 700–900 °C | |
In our opinion, it is highly probable that the phenomena observed in catalysts and gas sensors composed of anatase-rutile mixtures may have the same physical basis. Therefore, they may be interpreted in terms of complementary behaviour of both constituents towards oxygen adsorption and electron transfer to the surface due to differences in band alignment [10, 11, 15].
The advantages of using anatase or rutile in gas sensing have been discussed since decades. Rutile as the most stable form of TiO2 has been proved to be useful for hydrogen [16] and high temperature oxygen detection [17]. Applications of anatase in gas sensors [18–20] are inherently related to the advent of nanotechnology that, due to a substantial increase in surface-to-volume ratio, enables low temperature operation. Not only nanotubes [21] but other different and sometimes even exotic nanoforms have been tested as gas sensors [20].
In the past years, quite extensive research has been devoted to the mechanism and basic factors affecting the irreversible transformation from anatase to rutile [22–26]. In the case of bulk polycrystalline materials, temperature at which transformation takes place is of about 600 °C but its range can be much wider especially for nanomaterials. Transition from anatase to rutile is not instantaneous [25] because it involves a substantial structural reconstruction. The fundamental factors affecting the rate and temperature of transition are initial grain size, chemical surroundings and impurities. It has been demonstrated that this transformation can be achieved not only by annealing [25] or athermal illumination [22] but also by incorporation of aliovalent (Cr) dopant [5] as well as isovalent (Sn) additives [27]. From the thermodynamic point of view [24], rutile is the most stable of all polymorphic, macrocrystalline forms of TiO2 but the stability of anatase is particle-size dependent in the case of nanomaterials. It has been demonstrated in [23] that at particle diameters below ca. 14 nm, anatase is more stable than rutile.
Under experimental conditions discussed here, both anatase and rutile grains are present which allows to study their influence on gas-sensing properties of TiO2-based nanomaterials. The aim of this work is to show beneficial effect of contribution of both forms on the sensitivity to hydrogen at relatively low temperature (below 400 °C). Interpretation of this effect that takes into account electron transfer from rutile to anatase grains is proposed.
Methods
For the purposes of this work, the following nanomaterial systems prepared by flame spray synthesis (FSS) were studied:
undoped TiO2
chromium-doped TiO2:Cr
TiO2-SnO2 nanopowders
Titanium diisopropoxide bis (TDIP) and/or titanium isopropoxide (TTIP), chromium acetylacetonate and tetramethyltin were used as Ti, Cr and Sn precursors, respectively. The method and preparation conditions have been previously described in detail [6, 7, 28].
Selected samples were exposed to heat treatment at 700 °C in air.
X-ray diffraction (XRD) studies were carried out with X’Pert MPD Philips diffractometer in the Bragg–Brentano geometry. Weight percentage of rutile f R and crystallite diameters of anatase d A and rutile d R were determined in a standard way.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were employed to get an insight into morphology of sample. NOVA NANO SEM 200 (FEI Europe Company) and HR-TEM FEI TECNAI TF 20 X-TWIN microscopes were used, respectively.
Impedance spectroscopy was performed with the Solartron system (1260 + 1294 dielectric interface). The experimental parameters and data acquisition were controlled by means of the FRA software. A frequency range of 1–106 Hz was covered, with 10 mV amplitude. The impedance spectra were analyzed using the ZView software. An equivalent circuit comprising one or two parallel resistors and a constant phase element (CPE) was used in the fitting procedure.
Optical spectra of diffuse reflectance R Diff(λ) were acquired using a double beam Lambda 19 Perkin Elmer spectrophotometer equipped with an integrating sphere and operating within a wavelength range of 250–2000 nm. Calibration of reflectance spectra was performed using a SRS-99-010 Spectralon standard.
Gas-sensing measurements were performed for tablets prepared from nanopowders compressed at 25 MPa and then annealed at 400 °C and covered with planar silver electrodes. Dynamic changes in the electrical resistance response, or , depending on the type of conductivity, were detected over low-to-medium concentrations of 50–3000 ppm H2 at a constant temperature chosen within the range of 250–400 °C. R 0 denotes electrical resistance in air as a reference gas, and R is its value upon interaction with hydrogen. Homemade set-up described in [29] was used for gas-sensing measurements.
Results
Figure 1 illustrates the most important experimental results concerning the first nanomaterial system—undoped n-type TiO2. XRD (Fig. 1a) for as-prepared TiO2 nanopowder indicates the predominance of anatase polymorphic form. The content of rutile is low as determined from the analysis of this XRD pattern and amounts to f R = 0.07. As expected, annealing of this powder at 700 °C created conditions favourable for the transformation of anatase to rutile as seen in Fig. 1a. However, as one can note, this transformation is incomplete. The sample is composed of rutile/anatase mixture with predominating rutile contribution (f R = 0.75).
Fig. 1.
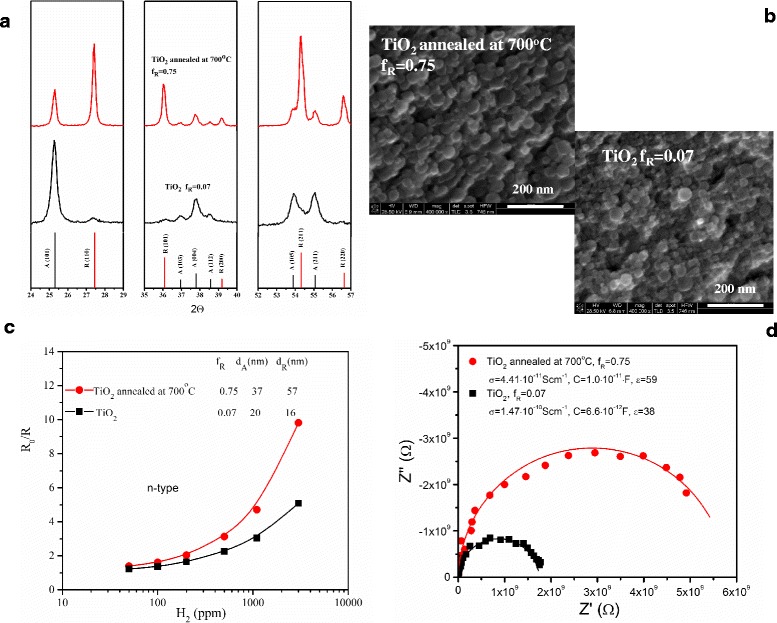
Comparison between anatase- and rutile-predominated undoped n-type TiO2 nanopowders obtained by flame spray synthesis (FSS): a X-ray diffraction patterns, b SEM images, c resistive-type response R 0/R to hydrogen at 300 °C, d Nyquist plots recorded at 400 °C illustrating imaginary part of impedance Z″ as a function of its real part Z′: f R, rutile content; d A, diameter of anatase crystallite; d R, diameter of rutile crystallite; R 0, electrical resistivity in the reference atmosphere (air); R, electrical resistivity under exposure to detected gas; σ, electrical conductivity; C, capacitance and ε, dielectric constant
Annealing is accompanied by crystallite growth as determined from XRD pattern analysis and clearly seen in SEM micrographs presented in Fig. 1b. As found from XRD, both anatase and rutile crystallites increase their respective diameters from d A = 20 nm, d R = 16 nm (before annealing) to d A = 37 nm, d R = 57 nm (after annealing). However, spherical shape of nanograins is preserved with larger agglomeration due to annealing as concluded on the basis of SEM images.
This process has significant consequences as far as gas sensing (Fig. 1c) and electrical properties (Fig. 1d) are concerned. Figure 1c demonstrates the resistive-type response to hydrogen at 300 °C expressed in terms of the relative change of R 0/R. This is suitable for n-type semiconductors as their electric resistance R decreases when reducing gas (hydrogen) is introduced. TiO2 annealed at 700 °C has better response than as-prepared sample.
On the other hand, a careful look into the electrical properties before exposure to the detected gas (Fig. 1d) indicates that the impedance increases considerably due to annealing. Parameters derived from the analysis of the impedance spectra: electrical conductivity σ, capacitance C and dielectric constant ε remain in accordance with the observed transformation from anatase to rutile.
Figure 2 summarizes the experimental results pertaining to the second system under study—Cr-doped p-type TiO2. Our intention was to contrast two samples with different anatase to rutile ratio with otherwise similar aspect, i.e. the same doping level and very close crystallite size. In order to obtain such different anatase to rutile ratio, slightly different technological procedures were employed [5, 6, 30]. In the case of anatase predominating sample, the total precursor mole number per minute was lower than that for rutile predominating sample which resulted in higher specific surface area SSA and smaller grain size as seen in Table 2. As mentioned in “Background” section, it is well known that anatase is stabilized when the crystallite diameter decreases. XRD patterns of TiO2: 5 at.% Cr for f R = 0.16 and f R = 0.77 clearly illustrate the predominance of either one (anatase) or another (rutile) polymorphic form as shown in Fig. 2a. Both anatase and rutile crystallites have comparable diameter for anatase dominated sample (d A = 9 nm, d R = 8 nm for f R = 0.16). The same applies for rutile dominated one (d A = 12 nm, d R = 11 nm when f R = 0.77). Morphology of both samples is quite similar as evidenced by SEM/TEM images (Fig. 2b).
Fig. 2.
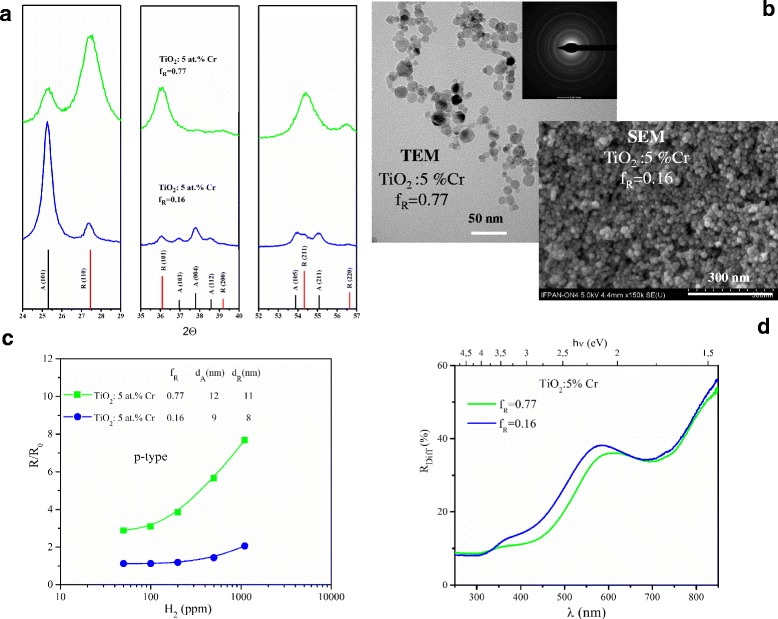
Comparison between anatase- and rutile-predominated p-type chromium Cr-doped TiO2 nanopowders obtained by flame spray synthesis (FSS): a X-ray diffraction patterns, b TEM/SEM images, c resistive-type response R/R 0 to hydrogen at 300 °C, d diffused reflectance R Diff as a function of wavelength λ; f R, rutile content; d A, diameter of anatase crystallite; d R, diameter of rutile crystallite; R 0, electrical resistivity in the reference atmosphere (air); R, electrical resistivity under exposure to detected gas and hν, photon energy
Table 2.
Summary of the experimental results of three different systems studied in this work
| System | Sample | SSA (m2/g) | f R | d A (nm) | d R (nm) | R 0/R at 300 °C, 3000 ppm H2 | Figure |
|---|---|---|---|---|---|---|---|
| Undoped TiO2 | As-obtained | 67.7 | 0.07 | 20 | 16 | 4 | 1 |
| Annealed at 700 °C | – | 0.75 | 37 | 57 | 10 | ||
| TiO2:5 at.% Cr | Mostly anatase | 126.6 | 0.16 | 9 | 8 | 3 | 2 |
| Mostly rutile | 102.9 | 0.77 | 12 | 11 | 12 | ||
| TiO2-SnO2 | TiO2 | 57 | 0.05 | 25 | 9 | 2 | 3 |
| 10% SnO2 | 60 | 0.73 | 28 | 14 | 8.5 |
As chromium doping transfers conductivity of TiO2 to p-type, the definition of response has to be inverted as compared with n-type semiconductors because electrical resistance R increases upon hydrogen admission. Sample with higher rutile content exhibits visibly higher response in terms of R/R 0 (Fig. 2c).
Different polymorphic phase composition finds its confirmation in optical properties of TiO2: 5 at.% Cr as well (Fig. 2d). As the band gap of rutile is by 0.2 eV smaller than that of anatase (see Table 1), its fundamental absorption edge in the spectral dependence of diffused reflectance shifts to longer wavelength. Additional absorption feature attributed to Cr band formation within the fundamental band gap of TiO2 is also displaced towards visible.
Figure 3 resumes the most significant data for TiO2-SnO2 system under investigation. As seen in Fig. 3a, even a relatively small amount of SnO2 additive to TiO2 results in a dramatic reconstruction of crystallographic structure. Rutile contribution increases from f R = 0.05 for pure TiO2 to f R = 0.73 for TiO2: 10% SnO2. The crystallite size of anatase remains almost without changes (d A = 25 nm for TiO2 and d A = 28 nm for TiO2:10% SnO2). In contrast, diameter of rutile crystallites increases from d R = 9 nm for TiO2 to d R = 14 nm for TiO2:10% SnO2. No evidence of precipitation of SnO2 phase and detailed analysis of XRD patterns [28] indicates substitutional doping of Sn into TiO2 lattice.
Fig. 3.
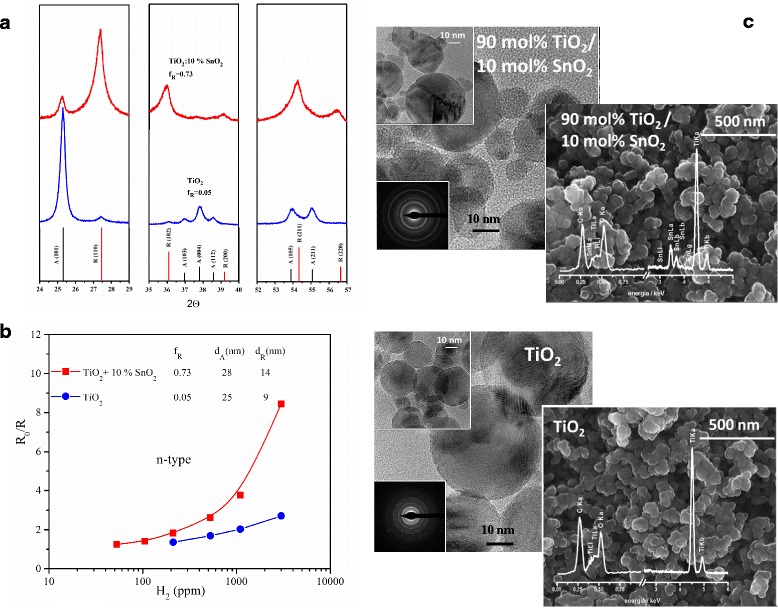
Comparison between anatase- and rutile-predominated n-type TiO2-SnO2 nanopowders obtained by flame spray synthesis (FSS): a X-ray diffraction patterns, b resistive-type response R 0/R to hydrogen at 300 °C, c TEM and SEM images: f R, rutile content; d A, diameter of anatase crystallite; d R, diameter of rutile crystallite; R 0, electrical resistivity in the reference atmosphere (air) and R, electrical resistivity under exposure to detected gas
As n-type conductivity is preserved upon incorporation of aliovalent additives, the sensor response is defined as R 0/R in Fig. 3b. Much higher sensitivity is obtained in the case of TiO2:10% SnO2.
TEM and SEM images are given in Fig. 3c, for both TiO2 and TiO2:10% SnO2. Spherically shaped nanograins of TiO2 probably composed of smaller crystallites are clearly seen in TEM. SEM images reveal agglomeration process taking place.
Discussion
Recapitulation of the sensing performance of all material systems studied in this work is given in Fig. 4 for a fixed hydrogen concentration (1000 ppm H2) at a constant temperature of 300 °C. Two material systems, namely, undoped TiO2 and TiO2-SnO2 exhibit n-type response towards reducing gases, i.e. their electrical resistance decreases upon interaction with hydrogen. Only TiO2:5 at.% Cr behaves as a p-type semiconductor with its electrical resistance increase when reducing gas is introduced.
Fig. 4.
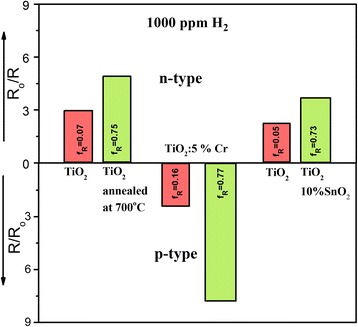
Comparison of resistive type responses towards 1000 ppm H2 at 300 °C for all TiO2 - based nanomaterial systems (n- and p-type) studied in this work; f R rutile content
The reasons for such behaviour are very well known. Gas-solid interactions leading to the physical adsorption and chemisorption modify the electron/hole density in a relatively shallow region near the surface [31]. In the case of chemisorption of the reducing gas on the surface of an n-type semiconductor, a two-step interaction mechanism has been proposed [32]. The first step is considered to be an oxygen adsorption at the surface of the n-type semiconductor already exposed to the oxidizing atmosphere. In the second step, the surface reduction by the detected gas, e.g. hydrogen, methane and carbon oxide, takes place.
The chemisorption of oxygen may be described by the following reaction:
| 1 |
This reaction results in a decrease in the surface conductivity. Upon exposure to a reducing gas such as H2, the following counter process takes place:
| 2 |
and electrons are reintroduced into the conduction band so that the surface conductivity is increased. As the reaction described by Eq. (2) is reversible, reducing gases in air can be detected by monitoring the change in the surface conductivity of metal oxides.
Eq. (2) is, in fact, an oversimplified description of the surface reaction, since it may also proceed via O2 − or O−2. The doubly charged oxygen ion is in general excluded from the considerations [33], because such a high charge on the ion may lead to instability, unless the adsorption site has a high Madelung potential. Higher reactivity of O− as compared with O2 − makes the former one more probable [32, 33].
However, quite surprising conclusion can be drawn suggesting that rutile-dominated TiO2 nanomaterials exhibit higher sensitivity towards hydrogen than those with the prevailing anatase.
We propose to account for that in a similar way as it was done in the case of photocatalytic properties of TiO2. In fact, the fundamental phenomena such as surface oxidation in the first step of gas sensing have the same physical basic as in photocatalysis.
Synergetic effect in photocatalysis, discussed at the beginning of this work, is interpreted by some authors [9] as a result of specific electronic band alignment of anatase and rutile crystals leading to, for instance, successful space separation of photoexcited electron-hole pairs.
Two opposite cases of band alignment presented in Fig. 5 reflect the state of the art of the studies on the electronic structure of anatase and rutile polymorphic forms of TiO2 [10, 11]. Historically, first was the model presented in Fig. 5a that has been derived from the measurements of flat band potential V 0 Fb. Our own results of V 0 Fb determination [3] as a function of anatase content remain in accordance with the literature data [34–36]. It has been shown that V 0 Fb is by 0.2 more negative in anatase than in rutile which makes the conduction band minimum of rutile below that of anatase (Fig. 5a). In such a case, the electrons are injected to rutile. However, recent theoretical calculation by Scanlon et al. [10] and XPS studies [11] revealed that the opposite case is more probable, i.e. the conduction band minimum of rutile above that of anatase (Fig. 5b). Two arguments speak in favour of this picture. The first one is the difference in work function (5.1 eV for anatase and 4.9 for rutile, as shown in Table 1). The second one is related to the stability of rutile to adsorption of oxygen species [19] that makes the surface of anatase grains more active in the first step of gas sensing (Eq. 1).
Fig. 5.
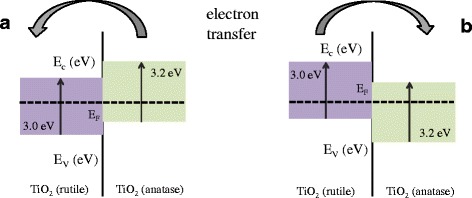
Two proposed valence and conduction band alignment mechanisms for the anatase-rutile mixture. a Electron injection from anatase to rutile. b Electron injection from rutile to anatase. The present study supports model b
In the proposed model of specific alignment of bands in rutile and anatase shown in Fig. 5b, the electrons are injected from rutile to anatase participating in more active oxidation reaction at the anatase grain surface. This phenomenon has been observed previously and used to explain significant improvement in gas sensing of TiO2-SnO2 nanocomposites [29]. Now, it seems that this mechanism can be successfully applied to rutile-anatase mixtures.
Conclusions
The following nanomaterial systems prepared by flame spray synthesis (FSS) were studied:
undoped TiO2
chromium-doped TiO2:Cr
TiO2-SnO2 nanopowders
Sample characterization was performed using standard methods such as XRD, SEM, TEM, impedance spectroscopy and optical reflectometry. In all cases, the crystallite size was below 60 nm which correlated very well with the possibility of gas-sensing measurements at relatively low temperatures of 300 °C. For each of the studied systems, we were able to discuss the cases of low and high rutile content. Moreover, n-type or p-type conductivity was observed. It turned out that rutile-dominated TiO2 nanomaterials exhibited higher sensitivity towards hydrogen than those with the prevailing anatase. This phenomenon could be accounted for in a similar way as in photocatalysis, i.e. by specific band alignment and electron transfer from rutile to anatase to facilitate oxygen preadsorption.
Acknowledgements
KZ acknowledges the financial support of the National Science Centre NCN, Poland, grant decision DEC-2011/03/B/ST7/01840 (project entitled: Nanosensor array as a new tool in studies of gas-solid interactions - selectivity problem). MR thanks the National Science Centre NCN, Poland, grant decision DEC-2012/07/B/ST8/03879 (project entitled: Electronic structure and conductivity mechanism of metal oxide nanocomposites in photoelectrochemistry). We are grateful to Prof. Thomas Graule from EMPA, Switzerland, and Dr. K. Michalow-Mauke for providing us with nanopowders for this research. We also thank Dr. M. Gajewska for the TEM measurements, Dr. M. Ziabka for the SEM images and Dr. B. Lyson-Sypien for the participation in the experimental work and data preparation.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contribution
Both authors contributed equally to the final version of the manuscript. KZ has been responsible for the interpretation and description of gas-sensing measurements while MR for the part concerning photocatalytic properties and synergetic effect. Impedance spectroscopy studies have been performed by MR. The text of the manuscript was discussed and written by both authors working in close collaboration. Both authors read and approved the final manuscript.
Abbreviations
- FSS
Flame spray synthesis
- SEM
Scanning electron microscopy
- TEM
Transmission electron microscopy
- XRD
X-ray diffraction
References
- 1.Wicaksana D, Kobayashi A, Kinbara A. Process effects on structural properties on TiO2 thin films by reactive sputtering. J. Vac. Sci. Technol. A. 1992;10:1479–1482. doi: 10.1116/1.578269. [DOI] [Google Scholar]
- 2.Löbl P, Huppertz M, Mergel D (1994) Nucleation and growth in TiO2 films prepared by sputtering and evaporation. Thin Solid Films 251:72–79
- 3.Brudnik A, Gorzkowska-Sobas A, Pamuła E, Radecka M, Zakrzewska K (2007) Thin film TiO2 photoanodes for water photolysis prepared by dc magnetron sputtering. J Power Sources 173:774–780
- 4.Radecka M, Rekas M, Kusior E, Zakrzewska K, Heel A, Michalow KA, Graule T. TiO2-based nanopowders and thin films for photocatalytical applications. J. Nanosci Nanotechno. 2010;10:1032–1042. doi: 10.1166/jnn.2010.1865. [DOI] [PubMed] [Google Scholar]
- 5.Trenczek-Zajac A, Radecka M, Jasinski M, Michalow KA, Rekas M, Kusior E, Zakrzewska K, Heel A, Graule T, Kowalski K (2009) Influence of Cr on structural and optical properties of TiO2:Cr nanopowders prepared by flame spray synthesis. J Power Sources 194:104–111
- 6.Michalow KA, Otal EH, Burnat D, Fortunato G, Emerich H, Ferri D, Heel A, Graule T. Flame-made visible light active TiO2:Cr photocatalysts: correlation between structural, optical and photocatalytic properties. Catalysis Today. 2013;209:47–53. doi: 10.1016/j.cattod.2012.10.007. [DOI] [Google Scholar]
- 7.Lyson-Sypien B, Radecka M, Rekas M, Swierczek K, Michalow-Mauke K, Graule T, Zakrzewska K. Grain-size-dependent gas-sensing properties of TiO2 nanomaterials. Sens. Actuators B. Chem. 2015;211:67–76. doi: 10.1016/j.snb.2015.01.050. [DOI] [Google Scholar]
- 8.Kusior A, Klich-Kafel J, Trenczek-Zajac A, Swierczek K, Radecka M, Zakrzewska K. TiO2–SnO2 nanomaterials for gas sensing and photocatalysis. J. Eur. Ceram. Soc. 2013;33:2285–2290. doi: 10.1016/j.jeurceramsoc.2013.01.022. [DOI] [Google Scholar]
- 9.Su R, Bechstein R, So L, Vang RT, Sillassen M, Esbjornsson B, Palmqvist A, Besenbacher F (2011) How the anatase-to-rutile ratio influences the photoreactivity of TiO2. J Phys Chem C 115:24287–24292
- 10.Scanlon DO, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlow CR, Powell MJ, Palgrave RG, Parkin IP, Watson GW, Keal TW, Sherwood P, Walsh A, Sokol AA. Band alignment of rutile and anatase TiO2. Nat Mater. 2013;12:798–801. doi: 10.1038/nmat3697. [DOI] [PubMed] [Google Scholar]
- 11.Mi Y, Weng Y (2015) Band alignment and controllable electron migration between rutile and anatase TiO2. Sci Rep 5:11482. doi:10.1038/srep11482 [DOI] [PMC free article] [PubMed]
- 12.Mo SD, Ching WY (1995) Electronic and optical properties of three phases of titanium dioxide: rutile, anatase, and brookite. Phys Rev B 51:13023-13032 [DOI] [PubMed]
- 13. Madelung O (ed) (1983) Landolt - Boernstein Numerical data and functional relationships in science and technology, vol 17. Springer, Berlin, Germany pp 266-277
- 14.Xiong G, Shao R, Droubay TC, Joly AG, Beck KM, Chambers SA, Hess WP. Photoemission electron microscopy of TiO2 anatase films embedded with rutile nanocrystals. Adv Funct Mater. 2007;17:2133–2138. doi: 10.1002/adfm.200700146. [DOI] [Google Scholar]
- 15.Li X, Ramasamy R, Dutta PK. Study of the resistance behavior of anatase and rutile thick films towards carbon monoxide and oxygen at high temperatures and possibilities for sensing applications. Sens. Actuators B. Chem. 2009;143:308–315. doi: 10.1016/j.snb.2009.09.021. [DOI] [Google Scholar]
- 16.Harris LA. A titanium dioxide hydrogen sensor. J. Electroch. Soc. 1980;127:2657–2662. doi: 10.1149/1.2129567. [DOI] [Google Scholar]
- 17.Ramamoorthy R, Dutta PK, Akbar SA. Oxygen sensors: materials, methods, designs and applications. J. Mater. Sci. 2003;38:4271–4282. doi: 10.1023/A:1026370729205. [DOI] [Google Scholar]
- 18.Tang H, Prasad K, Sanjines R, Levy F. TiO2 anatase thin films as gas sensors. Sens. Actuators B. Chem. 1995;26–27:71–75. doi: 10.1016/0925-4005(94)01559-Z. [DOI] [Google Scholar]
- 19.Sclafani A, Herrmann JM (1996) Comparison of photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J Phys Chem 100:13655–13661
- 20.Yang G, Hu P, Cao Y, Yuan F, Xu R. Fabrication of porous TiO2 hollow spheres and their application to gas sensing. Nanoscale Res. Lett. 2010;5:1437–1441. doi: 10.1007/s11671-010-9658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galstyan V, Comini E, Faglia G, Sberveglieri G. TiO2 nanotubes: recent advances in synthesis and gas sensing properties. Sensors. 2013;13:14813–14838. doi: 10.3390/s131114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricci PC, Carbonaro CM, Stagi L, Salis M, Casu A, Enzo S, Delogu F (2013) Anatase-to-rutile phase transformation in TiO2 nanoparticles irradiated by visible light. J Phys Chem C 117:7850–7857
- 23.Zhang H, Banfield JF. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998;8:2073–2076. doi: 10.1039/a802619j. [DOI] [Google Scholar]
- 24.Zhang H, Banfield JF. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. J. Phys. Chem. B. 2000;104:3481–3487. doi: 10.1021/jp000499j. [DOI] [Google Scholar]
- 25.Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile phase transformation. J Mater Sci 46:855–874
- 26.Muscat J, Swamy V, Harrison NM (2002) First-principles calculations of the phase stability of TiO2. Phys Rev B 65:224112–1 224112–15
- 27.Radecka M, Rekas M, Trenczek-Zajac A, Zakrzewska K. Importance of the band energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources. 2008;181:46–55. doi: 10.1016/j.jpowsour.2007.10.082. [DOI] [Google Scholar]
- 28.Lyson Sypien B, Kusior A, Rekas M, Zukrowski J, Gajewska M, Michalow Mauke K, Graule T, Radecka M, Zakrzewska K. Nanocrystalline TiO2/SnO2 heterostructures for gas sensing. Beilstein J. Nanotechnol. 2017;8:108–122. doi: 10.3762/bjnano.8.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusior A, Radecka M, Zych L, Zakrzewska K, Reszka A, Kowalski BJ. Sensitization of TiO2/SnO2 nanocomposites for gas detection. Sens. Actuators B. Chem. 2013;189:251–259. doi: 10.1016/j.snb.2013.07.029. [DOI] [Google Scholar]
- 30.Lyson Sypien B, Czapla A, Lubecka M, Gwizdz P, Schneider K, Zakrzewska K, Michalow K, Graule T, Reszka A, Rekas M, Lacz A, Radecka M (2012) Nanopowders of chromium doped TiO2 gas sensors. Sens Actuators B Chem 175:163–172
- 31.Göpel W, Lampe U. Influence of defects on the electronic structure of zinc oxide surfaces. Phys.Rev. B. 1980;22:6447–-6462. doi: 10.1103/PhysRevB.22.6447. [DOI] [Google Scholar]
- 32.Azad AM, Akbar SA, Mhaisalkar SG, Birkefeld LD, Goto KS. Solid‐state gas sensors: a review. J. Electrochem. Soc. 1992;139:3690–3704. doi: 10.1149/1.2069145. [DOI] [Google Scholar]
- 33.Bielanski A, Haber J. Oxygen in catalysis on transition metal oxides. Catal. Rev. Sci. Eng. 1979;19:1–41. doi: 10.1080/03602457908065099. [DOI] [Google Scholar]
- 34.Kavan L, Gratzel M. Highly efficient semiconducting TiO2 photoelectrodes prepared by aerosol pyrolysis. Electrochim. Acta. 1995;40:643–652. doi: 10.1016/0013-4686(95)90400-W. [DOI] [Google Scholar]
- 35.Watanabe T, Fujishima A, Honda K-I. Photoelectrochemical reaction at SrTiO3 single crystal electrode. Bulletin of the Chemical Society of Japan. 1976;49(2):355–358. doi: 10.1246/bcsj.49.355. [DOI] [Google Scholar]
- 36.Berger T, Lana-Villarreal T, Monllor-Satoca D, Gómez R. The electrochemistry of transparent quantum size rutile nanowire thin films prepared by one-step low temperature chemical bath deposition. Chem. Phys. Lett. 2007;447:91–95. doi: 10.1016/j.cplett.2007.08.087. [DOI] [Google Scholar]


