Abstract
All metal oxide nanoparticles influence the growth and development of plants. They generally enhance or reduce seed germination, shoot/root growth, biomass production and physiological and biochemical activities. Some plant species have not shown any physiological change, although significant variations in antioxidant enzyme activity and upregulation of heat shock protein have been observed. Plants have evolved antioxidant defence mechanism which involves enzymatic as well as non-enzymatic components to prevent oxidative damage and enhance plant resistance to metal oxide toxicity. The exact mechanism of plant defence against the toxicity of nanomaterials has not been fully explored. The absorption and translocation of metal oxide nanoparticles in different parts of the plant depend on their bioavailability, concentration, solubility and exposure time. Further, these nanoparticles may reach other organisms, animals and humans through food chain which may alter the entire biodiversity. This review attempts to summarize the plant response to a number of metal oxide nanoparticles and their translocation/distribution in root/shoot. The toxicity of metal oxide nanoparticles has also been considered to see if they affect the production of seeds, fruits and the plant biomass as a whole.
Keywords: Metal oxide nanoparticles, Growth response, Antioxidant enzymes, ROS, Phytotoxicity
Review
Introduction
Because of huge production and inadvertent use of nanomaterials, the whole environment is affected. Although, many of them are useful, some are toxic to plants, algae and microorganisms. They may, therefore, pose potential risk to the environment. Nanomaterials are frequently used in plant growth, cosmetics, drug delivery, photonic crystals, analysis, food, coatings, paints, bioremediation, catalysis and material science [1–12]. Keller and Lazareva [13] have reported that about 3000 tons of titanium dioxide (TiO2) nanoparticles are produced every year and more than 50% of which is used in personal care products [14]. Copper oxide (CuO) nanoparticles cause membrane damage to Escherichia coli as demonstrated by K+ leakage [15]. A study on Zebrafish (Danio rerio) has shown that as exposure days of TiO2 nanoparticles were increased, the number of viable embryos was decreased [16]. Some nanomaterials are toxic to flora and fauna as they are used to inhibit their growth to prevent further multiplication [17, 18]. Biouptake and accumulation of nanomaterials in plants may increase shoot length and decrease root length and their proliferation [19, 21]. The toxicity response depends on the concentration, particle size and shape of the nanomaterials [22]. Some studies have demonstrated that nanoparticle exposure improves free-radical scavenging potential and antioxidant enzymatic activities and alters microRNAs expression that regulates different morphological, physiological and metabolic processes in plants [22]. The toxicity of the free metal ions has been shown to be greater than that of the nanoparticles. For instance, silver nanoparticles are less toxic to plants than the silver ions [23] mainly due to greater solubility of AgNO3 and greater mobility of Ag+ ions in aqueous medium. Lipid peroxidation is an important parameter which indicates the cell membrane integrity [24–26]. Reactive oxygen species (ROS) generation is known to damage cell membrane through lipid peroxidation leading to ion leakage and disruption of the cellular metabolism leading to cell death. ROS also cause oxidative damage to photosynthetic apparatuses and biomolecules [27, 28]. Thus, plants protect cellular and sub-cellular system from the cytotoxic effects of active oxygen radicals with antioxidative enzymes (superoxide dismutase, SOD; catalase, CAT; peroxidase, POD and ascorbate peroxidase; etc.) and low molecular weight antioxidants (ascorbate, glutathione, proline, carotenoids, a-tocopherols and phenolics, etc.) and non-enzymatic components (carotenoids, ascorbate and tocopherol, etc.) [26, 29–31]. These components minimize the oxidative damage during exposure to metal oxide nanoparticles [18, 32, 33]. Zea mays exposed to CeO2 nanoparticles [34] did not show lipid peroxidation and any physiological changes, although activity of catalase, ascorbate and upregulation of heat shock proteins was observed. However, no elevation of lipid peroxidation in rice treated with CeO2 nanoparticles (0–500 mg/L) was recorded but ion leakage was observed at higher doses [35].
In recent years, the efficiency of photosystem II (PSII), considered as chlorophyll fluorescence (maximum quantum yield Fv/Fm), has been used as a diagnostic tool in various studies to check the impact of abiotic stress as well as metal or metal oxide nanoparticle toxicity in various plant species [26, 36–40]. Thus, potential variations in Fv/Fm act as an indicator of seedling-stock quality/physiological status of plants, in vivo [30, 41–44]. Shaw et al. [40] have shown that CuO nanoparticles reduced shoot and root growth of Hordeum vulgare seedlings with passage of time in a dose-dependent manner. However, exposure of CuO nanoparticles in barley seedlings exhibited insignificant alteration in the Fv/Fm ratio. They have also reported that the CuO nanoparticles induced the release of ROS, membrane damage and overall enzymatic activity not enough to cope with stress at 20-day exposure.
Superparamagnetic iron oxides, Fe3O4 nanoparticles (SPION) owing to their magnetic properties, are widely used in instruments, medical devices, as drug carrier, and the treatment of many diseases [9]. Like other metal oxide nanoparticles, SPION are cytotoxic to many aquatic organisms and terrestrial plants because they also generate ROS [32]. Bioaccumulation of SPION and other toxic nanoparticles may reach animals through feed and may alter biodiversity. Mushtaq [45] has reported that Fe3O4 nanoparticles inhibited the seed germination and root elongation of cucumber over a wide range of concentration (500, 2500, 5000 μg/mL). In cucurbits, aggregation of Fe3O4 nanoparticles occurred followed by their translocation in stem and roots [46].
Replacing biofertilizers by bionanomaterials may sometime be beneficial if they increase the fruit count, seed and biomass without producing toxic effects. Hydroponically grown soybean plants bioaccumulate metal ions, metal nanoparticle such as Zn/ZnO and CeO2 [47], which influence soil and microbes associated with plants and biomass [48]. The nitrogen-fixing bacteria are most affected because certain nanoparticles (CeO2) eliminate the nitrogen fixation potential and plant growth in soybean. Thus, the soil contaminated with huge quantity of waste material containing a variety of metal/metal oxide nanoparticles may impact both microbes and plants.
Biotransformation of nanomaterials may either enhance toxicity or detoxify the living system [49]. Such transformations are related to redox reaction, sulfidation, phosphorylation and molecular modification [50]. The sulfidation of silver nanoparticles decreased toxicity of E. coli owing to lower solubility of Ag2S. Similarly, the formation of AgCl from AgNO3 in presence of chloride ions also has the same effect.
The plants grown in presence of nanoparticles may absorb and translocate them in different tissues. It has been shown that CuO nanoparticles were reduced to Cu2O and Cu2S in maize plants [51]. Similar transformation and phytotoxicity of La2O3 and Yb2O3 in cucumber have been reported by Ma et al. [52] and Zhang et al. [53]. They were converted to their phosphates in the cucumber roots. The solubility of La2O3 and Yb2O3 was enhanced by the organic acids secreted by the cucumber roots. If there are phosphate salts, the biotransformation of oxides to phosphates is enhanced. From a 3-week study of corn plant grown in presence of CeO2 nanoparticles, Zhao et al. [34] showed that H2O2 was accumulated in phloem, xylem and epidermal cells of shoots. Catalase and ascorbate peroxidase activities were also enhanced in the shoot. Since the plants treated with 400 and 800 mg CeO2/kg triggered the upregulation of heat shock protein 70 (HSP70) in roots, it is believed that it was due to systemic stress response. The increased activities of enzymes and that of HSP70 are due to the induced reaction against CeO2 nanoparticle. CeO2 nanoparticle-plant-root interaction and translocation in hydroponically grown wheat and pumpkin plant for 8 days (17–100 nm) at 100 mg/L in the absence and presence of fulvic acid and gum arabic have been reported by Schwabe et al. [54]. The above plants did not exhibit any reduction in root growth. However, the CeO2 nanoparticles were translocated in pumpkin shoot but not in wheat plants. SEM and TEM images showed the deposition of nanoparticles on the root surfaces of both the plants which suggested that fulvic acid or gum arabic does not interfere with translocation of CeO2 nanoparticles but helps in sticking them to the roots. It has been ascribed to specific alterations in root structure and its interaction with nanoparticles in presence of root exudates [55, 56].
Recently, Rico et al. [57] have shown that CeO2 nanoparticles promoted plant development (H. vulgare) to the extent of 331% increase in shoot biomass without showing any toxic effect; nevertheless, at higher concentration (500 mg/kg), the plant did not produce grain which is a big loss. Effect of a variety of metal nanoparticles (Ag, Co, Ni) and metal oxide nanoparticles (CeO2, Fe3O4, SnO2, TiO2) on the translocation of nutrients in tomato plant has been investigated. Higher concentration of metal nanoparticles was found to be accumulated in roots as well as shoots. However, Fe3O4 nanoparticles promoted root growth and SnO2 reduced it [58]. Plant response against some metal oxide nanoparticles is summarized in Table 1.
Table 1.
Plant response to some metal oxide nanoparticles
| Nanoparticle | Size (nm) | Plant | Concentration | Plant response | Key references |
|---|---|---|---|---|---|
| CeO2 | 7 | Soybean | 0, 500, 1000, 2000, 4000 mg/L | Genotoxicity recoded at 2000 and 4000 mg/L concentration; a new band in the roots’ RAPD profile was observed | [47] |
| 7 | Alfalfa, corn, cucumber, tomato | 0, 500, 1000, 2000, 4000 mg/L | In corn, tomato and cucumber seed germination was reduced at 2000 mg/L; promoted root elongation for corn and cucumber; reduced root growth of alfalfa and tomato | [60] | |
| 8.0 ± 1.0 | Coriander | 125 mg/kg | Increased shoot, root length and biomass; increased ascorbate peroxidase activity in roots and catalase activity in shoots | [175] | |
| <8.0 ± 1.0 | Rice | 0, 62.50, 125, 250, 500 mg/L | Reduced H2O2 generation in shoots and roots; increased electrolyte leakage and lipid peroxidation in shoots | [35] | |
| 8 ± 1 | Corn | 0, 400, 800 mg/kg | No impact on chlorophyll contents and gas exchange | [176] | |
| 8 ± 1 | Barley | 0, 125, 250, 500 mg/kg | Increased the plant height, chlorophyll contents, biomass, reduced spike production; increased Ca, K, Zn, Mg, Cu, Al, Fe, P and S in grains | [57] | |
| 8 ± 1 | Wheat | 0, 100, 400 mg/kg | Changes in microstructure of leaf cells, swollen chloroplasts, squeezed nuclei, bent and loosely arranged thylakoids; decreased chlorophyll contents and exhibits variation in protein content | [177] | |
| 10 ± 3.2 | Bacillus thuringiensis transgenic cotton | 0, 100, 500 mg/L | Swollen and destructed chloroplasts, reduced Zn, Mg, Fe and P levels in xylem sap of cotton | [178] | |
| 50–105 | Tomato | 20 mg/kg | Increased Ca, K, Mg, P in roots; Ca, Mg in stems; decreased Na contents stems; K, Na, P and S in leaves | [58] | |
| 8 ± 1 | Wheat | 0, 125, 250, 500 mg/L | Changes the amounts S and Mn in grains, amino acid composition and linolenic acid contents | [179] | |
| ZnO | 8 | Soybean | 0, 500, 1000, 2000, 4000 mg/L | No change in germination; genotoxicity recoded at 4000 mg/L concentration; a new band in the roots’ RAPD profile was observed | [47] |
| 10 | Soybean | 0–500 mg/kg | Reduced Fe at all treatments; Mg and K were decreased at 500 mg Zn/kg treatment | [180] | |
| <50 | Soybean | 500 mg/kg | Reduced roots and shoots; had smaller surface area and volume; no seed formation | [181] | |
| 20 | Radish, rape, ryegrass, lettuce, corn, cucumber | 2000 mg/L | Reduced root growth and elongation | [19] | |
| <10 | Zucchini | 1000 mg/L | Reduced biomass (78–90%) | [182] | |
| 10 | Cucumber | 400–800 mg/kg | No impact on growth, gas exchange or chlorophyll contents | [183] | |
| 90 | Corn | 800 mg/kg | Reduced growth and inhibition of arbuscular mycorrhizal fungi | [184] | |
| 10 | Alfalfa | 250, 500, 750 mg/kg | Reduced root biomass (80%) | [185] | |
| 44.4 | Arabidopsis | 400, 2000, 4000 mg/L | Reduced seed germination, root elongation and number of leaves | [93] | |
| <100 | Arabidopsis | 100 mg/L | Reduced biomass (81.4%), seed germination, 660 up-regulated genes and 826 down-regulated genes | [92] | |
| <50 | Garden pea | 100–1000 mg/L | No impact on germination; root length, stem length, leaf surface area, transpiration and root nodulation was affected | [186] | |
| 1.2–6.8 | Clusterbean | 10 mg/L | Increased biomass (27.1%), shoot length, root length, root area, chlorophyll content and total soluble leaf protein | [98] | |
| 25 | Tomato | 0–1000 mg/L | Plant height was increased (24%) at 250 mg ZnO/Kg; increased root length in foliar sprayed plants with 250 mg ZnO/L; concentrations above 250 mg ZnO/kg affected root length in both methods of application | [99] | |
| <100 | Wheat | 50 mg/kg | Reduced biomass | [103] | |
| <100 | Wheat | 500 mg/kg | Reduced root growth, increased reactive oxygen species production | [187] | |
| CuO | <50 | Arabidopsis | 0, 0.5, 1, 2, 5, 10, 20, 50, 100 mg/L | Reduced biomass, root growth retardation, increased reactive oxygen species production | [116] |
| <50 | Indian mustard | 0, 20, 50, 100, 200, 400, 500 mg/L | Reduced shoot and root growth | [128] | |
| 10–50 | Mung bean | 0, 20, 50, 100, 200, 500 mg/L | Reduced biomass and root length at all concentrations; reduced chlorophyll content above 100 mg/L; no changes in carotenoid content; increased H2O2 and lipid peroxidation; increased reactive oxygen species production with increase in concentration; modulations in gene expression | [127] | |
| <50 | Wheat | 500 mg/kg | Inhibition in root and shoot growth; produced oxidative stress possibly due to Cu released from nanoparticles, Cu bioaccumulates | [187] | |
| <50 | Squash | 0, 100, 500 mg/L | Reduced growth and transpiration (60–70%) | [188] | |
| <100 | Radish, grasses | 10, 100, 500, 1000 mg/L | Growth inhibition; DNA damage | [21] | |
| TiO2/inorganic bentonite clay | 30/1–60 | Maize | 300, 1000 mg/L | Inhibited hydraulic conductivity, leaf growth and transpiration | [65] |
| Activated carbon-based TiO2 | 30–50 | Tomato | 0–500 mg/L | Improved germination, reduced germination time | [189] |
| 30–50 | Mung bean | 0–500 mg/L | Improved germination, reduced germination time | [189] | |
| TiO2 | – | Soybean | 0, 0.01, 0.03, 0.05% | Increased height (0.05%) and dry weight | [190] |
| <100 | Wheat | ~91 mg/kg | Reduced biomass, nanoparticles found mostly stick on surface of roots | [103] | |
| <25 | Tobacco | 0, 0.1, 1, 2.5, 5% | Reduced biomass, inhibited germination and root length; upregulation of alcohol dehydrogenase and ascorbate peroxidase | [191] | |
| 4–6 | Spinach | 0.25% | Improved growth; increased glutamate dehydrogenase, glutamine synthetase and glutamic piruvic transaminase activity | [146] | |
| 7–40 | Chickpea | 2–10 mg/kg | Reduction in electrolyte leakage and malondialdehyde content at 5 mg/kg treatment | [192] | |
| 6.22 | Ulmus elongata | 0.1–0.4% | Increased Cu accumulation in leaves; reduced net photosynthetic rate; increased carbohydrates and lipids | [193] | |
| 27 ± 4 | Cucumber | 0, 250, 500, 750 mg/kg | Enhanced catalase; activity in leaves; enhanced P and K availability in fruit | [194] | |
| Fe3O4 | 20 | Pumpkin | 500 mg/L | No toxic effect; nanoparticles are translocated throughout the plant tissues, detected in stem and leaves, accumulated on the surface of root | [46] |
| 7 | Cucumber, lettuce | 62, 100, 116 mg/L | Low to zero toxicity on germination | [195] | |
| 6 | Lettuce, radish, cucumber, spinach, tomato, leek, peppers | 0.67 mg/mL | Reduced germination | [196] | |
| 25 | Ryegrass, pumpkin | 30, 100 and 500 mg/L | Increased root elongation; no uptake; block of aquaporins; oxidative stress | [32] | |
| Fe2O3 | 20–100 | Sunflower | 50, 100 mg/L | No uptake and translocation; reduced root hydraulic conductivity | [158] |
| 22–67 | Arabidopsis | 4 mg/kg | Reduced biomass and chlorophyll contents | [197] | |
| – | Soybean | 0, 0.25, 0.5, 0.75, 1.0 g/L | Increased leaf and pod dry weight; increased grain yield (48%) | [167] | |
| 246 | Lettuce, radish, cucumber | 1000 mg/L | Found to be adsorb on the surface of seed | [198] | |
| Al2O3 | 13 | Maize, cucumber, carrots, cabbage | 2000 mg/L | Reduced root growth | [168] |
| – | Corn | 2000 mg/L | Reduced root length | [19] | |
| – | Tobacco | 0, 0.1, 0.5, 1% | Increased root length, biomass; decreased leaf count; the seedlings significantly decreased; 1% Al2O3 exposure has shown extreme increase in microRNA expression | [171] |
The main aim of this review article is to present the impact of a number of metal oxide nanoparticles on plants and their distribution in root/shoot. Their toxic effect has also been considered to see if they produced oxidative stress and inhibited the growth of plant, seed or fruit.
Cerium Oxide Nanoparticles
The CeO2 metal oxide nanoparticles are present in the soil by default due to biosolids disposal from wastewater treatment plant, which are released from the exhaust pipe of automobiles. Priester et al. [59] have studied the impact of nano-CeO2 and ZnO nanoparticles on the growth and yield of soybean which is a major crop containing protein. Hydroponically grown soybean plants accumulate metal and metal oxide nanomaterials. It has been found that TiO2 and ZnO nanomaterials influence the useful microbes and biomass of the plant especially the nitrifying symbiotic bacteria in the root nodules of soybean and many other plants of fabaceae family. Substantial amount of bioaccumulated ZnO nanoparticles was translocated into leaves and beans while CeO2 accumulated into root nodules of soybean plant reduced nitrogen fixation potential and growth. The soil fertility and plant growth are equally affected, and therefore, the controlled amount of metal and metal oxide nanoparticles must only be released in the environment [55]. ZnO and CeO2 nanoparticles affect hydroponic plants [18, 47, 60] and microorganisms [7, 48, 61], but their affect on plant growth and crop yield has not been fully explored [62]. There was an increase in the number of pods of soybean treated with cerium oxide (0.1–1.0 g/kg of soil) and nano-ZnO (0.05–0.5 g/kg of soil). It has been noted that CeO2 generally reduced the pod and biomass while ZnO nanoparticles increased the pod count and had stimulatory effect on soybean.
Zhao et al. [34] have studied the stress response and tolerance of Zea mays to CeO2 nanoparticles. It has been reported that CeO2 nanoparticles are toxic to bacteria, green algae, fish and soybean plants [47, 63, 64]. The plants and fishes are, therefore, equally affected by the toxicity of these nanoparticles due to contamination of water or soil by CeO2. Since the pores of corn primary roots have an average diameter of 6.6 nm, the CeO2 nanoparticles with a diameter [65] smaller than these may penetrate root and could be transferred from root to corn shoots [33]. The nanoparticles attached to the roots of corn plant inhibit the water transpiration to the leaves [65]. The above ground parts of plants showed low toxicity. The maize plants were grown for 20 days in soil with CeO2 at 400 and 800 mg/kg level, and stress-related responses such as H2O2, antioxidant enzyme, heat shock protein 70 (HSP70), lipid peroxidation and cell death were recorded every 5 days. About ten times over production and accumulation of H2O2 in shoots was recorded which indicated a concentration-dependent oxidative stress. In the later stages (after 20 days), the H2O2 production was reduced, perhaps, the plant growth and adaptation prevented over expression of H2O2. The enzyme catalase and ascorbate peroxidase detoxify the plant by converting H2O2 to water and oxygen as shown below:
The catalase activity in shoots of plants at lower CeO2 concentration (400 mg/kg) was 39 times higher but at higher concentration (800 mg/kg), it was reduced to only 30 times. It seems that the enzyme is activated by CeO2 but at higher concentration, the activity gradually decreased. Similarly, the ascorbate peroxidase activity also declined with 800 mg/kg dose with a concomitant decrease in H2O2 level. However, both the enzymes eliminate the excess of H2O2 to prevent the lipid damage by CeO2 nanoparticles.
Biotransformation of CeO2 in cucumber has been thoroughly investigated by Zhang and co-workers [66]. During this process of biotransformation, either the toxicity of the nanoparticles is enhanced or it is detoxified [49]. The environmental and the biological systems together alter the toxicity of nanoparticles to organism [67]. Several steps, such as redox reaction sulfidation, phosphorylation and molecular modification, are involved in biotransformation [50]. Biotransformation of Ni(OH)2 to Ni2+ in plant shoots and leaves was observed by Parsons et al. [68], but no transformation occurred in roots. Some metal nanoparticles or metal oxide nanoparticles are oxidized or reduced depending on the chemical compounds present in certain parts of the plant. For instance, silver nanoparticles were oxidized to Ag(1) by Lolium multiforum [69] while CuO nanoparticles were reduced to Cu2O and Cu2S in maize plants [51]. The toxicity is therefore dependent on the form of element if they are in reduced or oxidized form. Root elongation of cucumber seedlings were inhibited by La2O3 and Yb2O3 nanoparticles as a consequence of their biotransformation to rare earth phosphates in the cucumber roots [52, 53] due to the phosphorous compounds exuded by the root of plant.
The CeO2 nanoparticles are not toxic to cucumber plants up to 2000 mg/L; however, the major part is accumulated in the root, and about 35% is translocated to leaf and stem, perhaps, because of size constraints. The CeO2 nanoparticles mixed with needle-like clusters were found on the outer epidermis of the root. The image of the cluster showed the presence of CePO4. It has been reported that the absorbed Ce3+ ions on the cell wall of the Saccharomyces cerevisiae can react with phosphate released from inside the yeast cells and form Ce(III) phosphate nanocrystallites [70]. Since CePO4 is insoluble, it remains on the surface of the root which has been confirmed from TEM/EDS results. The nanocrystals were found to be deposited on the root epidermis and in the intercellular spaces of the cucumber plant. During the biotransformation of CeO2 to CePO4 in biological system, CeO2 undergoes a valence change from Ce4+ to Ce3+ as a consequence of one electron reduction and formation of CePO4.
Both positive and negative results of the CeO2 nanoparticles on plant system have been reported [71]. Lopez-Moreno et al. [60] have shown that CeO2 nanoparticles of 7 nm were taken up into seedlings of cucumber, alfalfa, tomato and corn at concentrations of up to 4000 mg/L. Particles of 7 to 25 nm were found to be translocated to the shoots of cucumber. It is perhaps the variation in the interaction of nanoparticles with macromolecules present in the root which causes aggregation around the root tips. Besides, uptake and translocation of nanoparticles also depend on the shape, solubility, agglomeration and surface chemistry [5, 22].
Schwabe et al. [54] exposed wheat and pumpkin (hydroponic plant culture) to uncoated CeO2 nanoparticle (of 7–100 nm of 100 mg/L) suspension in the presence of fulvic acid and gum arabic. Although the suspension alone was stable, changes in pH, particle agglomeration rate and hydrodynamic diameter in nanoparticles occurred in presence of wheat and pumpkin plants. CeO2 nanoparticles were found to be translocated into pumpkin shoots but not in wheat plants. However, no toxic effect was observed in both plants. In a recent experiment, Anderson et al. [72] have chosen ten plant species and found that CeO2 and TiO2 nanoparticles do not cause widespread acute toxicity during germination and early growth stage.
Fulvic acid and gum arabic stabilize the CeO2 nanoparticles on one hand and reduce their adsorption by root on the other. However, CeO2 nanoparticles of 17 to >1 μm are partially available for uptake by pumpkin similar to those found by Zhu et al. [46] and Zhang et al. [73]. Translocation of Ce into shoot of wheat was not detected as the monocots are less likely to take up nanoparticles because water uptake by them (wheat) is only 25% to that of pumpkin.
A recent study on the uptake and accumulation of CeO2 nanoparticles in different parts of barley (Hordeum vulgare L.) has been made by Rico et al. [57]. The effect of CeO2 nanoparticles on the vegetative growth and production of barley grown in soil treated with different quantities of CeO2 nanoparticle has been reported (Table 1). It is important to note that at higher dose (500 mg/kg), there occurred rapid shoot development resulting in 331% enhancement of biomass. It is more surprising that at this concentration (500 mg/kg), the barley did not produce grain. It is, however, encouraging that at lower concentration of CeO2-amended soil (125 and 250 mg/kg) and also in control-produced grains with large quantity of Ce accumulated in leaves and grains (Table 2) along with P, K, Ca, Mg, S, Fe, Zn, Cu and Al.
Table 2.
Cerium concentrations (μg/kg dry wt) in different organs of Hordeum vulgare cultivated to grain production in cerium oxide nanoparticles-amended soil
| Soil treatments (mg/kg) | Leaves | Grains |
|---|---|---|
| 0-control | 571 ± 40 | 200 ± 5 c |
| 125-nCeO2-L | 595 ± 140 | 449 ± 51 b |
| 250-nCeO2-M | 524 ± 73 | 787 ± 58 a |
| 500-nCeO2-H | 701 ± 92 | – |
Values are means ± SE (n = 3). Same letters mean no statistical difference between treatments at Tukey’s test (p ≤ 0.05) [57]
Barley treated with CeO2 nanoparticles (250 mg/kg) enhanced methionine, aspartic acid, threonine, arginine and linolenic acid contents in the grain. It is clear that the moderate concentration (125–250 mg/kg) of CeO2 nanoparticles is highly beneficial while the higher doses (500 mg/kg) are toxic to barley. The accumulation of other metal ions in barley leaves and grains (e.g. P, K, Ca, Mg, S, Fe, Zn) is catalyzed by CeO2 nanoparticles. It is perhaps due to the biomolecules coordinated with these metals to make them available as trace elements which also act as nutrient for the plant. CeO2 nanoparticles promoted plant growth in soybean and tomato [59, 74] but did not affect the growth of cucumber.
Cerium uptake in root of rice (Oryza sativa), wheat (Triticum aestivum) and barley (H. vulgare) at different doses has been determined. Although CeO2 are not translocated in shoots of wheat, they are taken up by rice and barley [35] in fairly large quantity. Recent study [57] suggests that rice, wheat and barley could accumulate CeO2 in their root tissues without influencing the rate of germination and root elongation of the seedlings. CeO2 nanoparticles may induce modification in plants at molecular levels [35, 75, 76]. Oxidative stress and membrane damage of rice roots have been observed. It has been found from FTIR spectra of the rice, wheat and barley germinated in cerium oxide suspension that changes in amide I and amide II bands (1700–1600 cm−1 and 1600–1500 cm−1) are significant due to the presence of phenols and proteins [76, 77]. These results suggest that the cerium oxide nanoparticles produce modification in the root xylem of the cereal crops.
The impact of CeO2, Fe3O4, SnO2, TiO2 and metallic Ag-, Co-, Ni-engineered nanoparticle uptake and translocation in tomato plant has thoroughly been investigated in the recent years [58]. The plant exposed to different quantities of nanoparticles showed different vegetative growth (Table 3). CeO2-treated plants showed a very slight increase in stem growth. Fe3O4 nanoparticles significantly promoted the growth and elongation of tomato plant but reduced its green biomass. SnO2 remarkably decreased root growth and the dry stem and leaf weight. Though Fe3O4 was not significantly translocated into stem and leaf, fairly large amount of iron was found to be deposited in fruit and root of tomato plants. However, titanium, tin and cerium were not translocated even under control or treated plants. It is important to note that in all cases of nanoparticles treated tomato plants, the Ca contents in stem and root increased from 25.6 to 69.8% with respect to control. The bioavailability of nanoparticles depends on the coating, original matter in the soil or even clay because they can alter their behaviour leading to aggregation. Thus, the toxicity of nanoparticles is reduced due to their slow release. It is, therefore, concluded that soil polluted by metals can produce adverse effects if they are present above permissible/tolerance limits.
Table 3.
Effect of metal and metal oxide nanoparticles on dry matter of roots, stems and leaves of Lycopersicon esculentum plants grown in pots
| Treatment | Root | Stem | Stem | Root elongation | Plant height | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| g | SD | g | SD | g | SD | cm | SD | cm | SD | |
| Control | 1.9 b | 0.1 | 20.5 b | 0.9 | 25.2 a | 1.1 | 22 ab | 1.3 | 98 a | 3.8 |
| Ag-NPs | 1.6 b | 3.3 | 26.2 a | 1.2 | 24.2 a | 0.9 | 19 b | 1.5 | 82 b | 5.3 |
| Co-NPs | 1.5 b | 0.3 | 10.3 d | 1.1 | 18.3 b | 1.5 | 15 b | 2.8 | 84 b | 4.2 |
| Ni-NPs | 1.0 bc | 0.3 | 26.1 a | 1.2 | 12.1 d | 0.9 | 15 b | 3.2 | 93 ab | 5.1 |
| CeO2-NPs | 2.2 ab | 0.2 | 13.1 cd | 1.4 | 15.7 c | 0.7 | 23 ab | 2.1 | 109 a | 3.1 |
| Fe3O4-NPs | 4.8 a | 0.2 | 18.1 c | 0.8 | 18.9 b | 1.3 | 25 a | 2.3 | 106 a | 3.5 |
| SnO2-NPs | 0.7 c | 0.2 | 5.4 e | 0.7 | 16.8 c | 1.5 | 11 b | 3.7 | 104 a | 3.4 |
| TiO2-NPs | 1.4 b | 0.1 | 19.2 b | 1.1 | 18.8 b | 0.8 | 17 b | 2.1 | 110 a | 4.1 |
Means followed by a different letter within a row are significantly different at p < 0.05 according to Duncan’s multiple range test [58]
Priester et al. [59] have already reported that soybean plants grown in organic farm soil containing ZnO or CeO2 nanoparticles absorb and translocate Zn and Ce in all parts of the plant. The results indicated that the low amount of Ce is translocated in soybean pods [78]. The Ce in pods and nodules exists mainly as Ce4+ and some amount as Ce3+, which suggests that nearly 20% CeO2 is reduced to Ce3+ [34]. Zhao et al. [34] have shown that Ce was coordinated as CeO2 nanoparticles inside the roots of corn plants grown in organic soil amended with alginic acid coated with CeO2 nanoparticles. They also showed, from confocal microscopy images, the presence of CeO2 nanoparticles in the cell wall of the corn root cortex. They termed it passive uptake of the CeO2 nanoparticles. It must be made clear at this juncture that the CeO2 nanoparticles are neutral species which cannot coordinate with any ligand carrying lone pair of electrons or negative charges because these electrons are to be partially donated to an electron pair acceptor. However, it is quite likely that the CeO2 nanoparticles are translocated to the root cortex with support of the alginic acid coating.
Zinc Oxide Nanoparticles
Riesen and Feller [79] have shown the accumulation of zinc in the phloem of wheat plant and also in the soybean grain, but not zinc oxide (ZnO) nanoparticles. Zinc is supposed to be bonded to the oxygen of the carboxyl acid as ZnO [80, 81]. Thangavel et al. [82], from a study of red spruce cell culture, suggested that in living cells, it is more likely that Zn ions bind to the sulphur of phytochelatins rather than the oxygen of organic acids. However, Zn(II) in soybean plants has been found to be associated with oxygen of acids which has also been confirmed on the basis of model compounds [78].
Kopittke and co-workers [83] analyzed cowpea exposed to Zn and found that nearly 65–85% of the zinc was coordinated as a zinc-phytate complex. Phytic acid is present in all beans and is a source of phosphorous storage [84]. It is known that the Zn(II) ions activate the enzyme phytase but it may be bonded to phytic acid to give zinc phytate. Phytic acid is highly unstable and therefore it is stabilized in the form of a metal salt. The free zinc ions are therefore bonded to phytic acid through oxygen giving zinc-phytate complex.
Hu and co-workers [85] have recently investigated the adverse effects of ZnO nanoparticles of 25 nm diameter on the aquatic plant, Salvinia natans (L.). During 7-day exposure of plant to different concentrations of ZnO nanoparticles, no significant difference was observed in growth. However, the ZnSO4-treated plants showed marked decrease in growth. Generally, 50 mg/L ZnO nanoparticles were found to produce oxidative stress and depressed the photosynthetic pigments. SOD and CAT activities were increased but chlorophyll pigments decreased in the leaves of S. natans. It has been already reported that the antioxidant enzymes SOD, CAT and POD can protect plant cells against adverse effects of ROS [26, 28, 29, 32]. While POD acts as scavenger of ROS, SOD and CAT jointly convert O2 − and H2O2 to H2O and O2 and also reduce overall free ·OH radical. Zinc has been reported [86, 87] to increase the biosynthesis of antioxidant enzymes in the duckweed, Spirodela polyrhiza. The POD activity was remarkably inhibited by large quantity of ZnO nanoparticles (50 mg/L). In a recent study Zafar et al. [88] have reported that ZnO nanoparticles (500 to 1500 mg/L) negatively affects the Brassica nigra seed germination and seedling growth; and also increased antioxidative activities and non-enzymatic antioxidants contents. The toxicity of ZnO nanoparticles depends on the quantum of dissolved zinc in the solution [89, 90]. It is true that only a fraction of dissolved zinc is bioavailable which can be absorbed and translocated in different parts of the plants; nevertheless, the solubility of zinc oxide is pH dependent because being amphoteric in nature, it dissolves in both the acidic and alkaline media as shown below:
The ZnO nanoparticles diffuse in the plant cells if size is relatively smaller than the pores in the plant cells. Phytotoxicity of ZnO nanoparticles has been investigated by Watson et al. [91] under both acidic and alkaline soils. In acid soil, inhibition of elongation of roots of wheat (T. aestivum) was observed whereas phytotoxicity was mitigated in the alkaline soil, although absorption of ZnO nanoparticle was doubled even when Zn concentration in soil was low. Soluble zinc in the acid soil was 200-fold higher and shoot levels were tenfold higher than those in the alkaline soil. Phytotoxicity was observed in soil spiked with humic acid but it did not influence the plant responses. The ZnO nanoparticle aggregation with humic acid provides bioavailable zinc. But these nanoparticles may be distributed to the plant only if they are taken up through diffusion. The plant roots are stunted in the acid soil as the quantity of soluble zinc was 100 times higher in acid soil than the alkaline soil. However, higher dose of Zn (500 mg/L) causes phytotoxicity to the plants.
ZnO nanoparticles (100 mg/L) treated Arabidopsis seedlings showed reduced biomass to 81.4 ± 11.5% after 2 weeks [92]. Lee et al. [93] have also reported that ZnO nanoparticle at 400 mg/L inhibited the germination, root growth and leaf development in Arabidopsis similar to other plants [20]. ZnO nanoparticles also caused remarkable transcriptomic changes in terms of number of genes and their expression. It has been suggested that ZnO nanoparticles release Zn2+ ions and damage root tissues. Under such stress conditions, the plant initiates new root growth as an alternative to the damage by ZnO nanoparticles/Zn2+. ZnO nanoparticles promote ROS production in exposed roots [94, 95]. The presence of Zn2+ ions is either due to the presence of zinc salt in the ZnO nanoparticle or due to the conversion of ZnO nanoparticles to Zn2+ ions. It is, therefore, proposed that stress and defence responses of plants are due to a combined effect of ZnO nanoparticles and Zn2+ ions.
Higher toxicity of ZnO nanoparticles with respect to the Zn2+ ion in hydroponic solution using Allium cepa has been attributed to higher release of ROS [96]. Further, a study with Vigna unguiculata in soil amended with either ZnO nanoparticles or Zn2+ showed no difference in plant growth, accumulation or speciation between the zinc ion and ZnO nanoparticle treatment [97]. However, foliar exposure of ZnO nanoparticles to Cyamopsis tetragonoloba and Solanum lycopersicum has revealed a positive response in terms of biomass production, chlorophyll and total soluble leaf protein contents [98, 99].
Effect of citrate-coated Ag and ZnO nanoparticles and uncoated AgNO3 and ZnSO4 on Zea mays L. and Brassica oleracea var. capitata L. has been explored in vitro. The Ag nanoparticles have been shown to be more toxic to plants than free AgNO3. Considerable changes in metaxylem count of maize were observed with Ag nanoparticle, AgNO3 and ZnSO4 treatments. However, ZnO nanoparticles did not show any significant change in maize. In case of cabbage and maize, the germination and root elongation measurements revealed that nanoparticles were more toxic to plants than the free metal ions [100]. ZnO nanoparticles reduce seed germination [19] and damage tissues [20] in hydroponically grown plants. Kim and co-workers [101] have reported that ZnO nanoparticles at 2000 mg/kg did not affect the root length and biomass production of Cucumis sativus grown in a loamy sand soil at pH 5.5. Manzo et al. [102] have reported that ZnO nanoparticles at 286 mg/kg affected the root elongation in Lepidium sativum sown in an artificial standard soil. However, Du et al. [103] reported that at only 45.45 mg/kg (5 g/110 kg soil), these nanoparticles reduced the biomass production of wheat (T. aestivum) cultivated in loamy clay soil at pH 7.36. X-ray absorption spectroscopic studies have shown absence of ZnO nanoparticles in roots [47, 81]. However, confocal microscopic study showed the presence of FITC-stained ZnO nanoparticles in the stele of corn roots, although these particles were not found in the shoots [33]. It indicated that the ZnO nanoparticles were absorbed and incorporated into the plant transport system.
Copper Oxide Nanoparticles
The phytotoxicity of CuO nanoparticles in a 1:1 mixture of CuO and ZnO nanoparticles in plants colonized by Pseudomonas chlororaphis in a sand matrix has been investigated by Dimkpa et al. [104]. Bean root growth was inhibited and shoot was elongated by CuO nanoparticles [105, 106]. The CuO nanoparticles were found to release copper to the shoot where maximum copper loading was noted with the minimum dose of 100 mg/kg CuO nanoparticles and lower level of copper with higher doses of 250 and 500 mg/kg. The accumulation of Cu was 10–20-fold higher than the normal level (10 mg/kg). At higher CuO nanoparticle concentration, the major part was accumulated in the root which inhibited its growth. The other essential metal ions in presence of CuO or Cu2+ ions are either unavailable to the plant or their absorption was reduced. For instance, accumulation of Fe and Ca in the shoot tissue decreased as a consequence of antagonistic effect of Cu on Fe and Ca [107]. In fact, copper increases the absorption of Fe in animals if iron is available in ferrous form. The reduction of ferric to ferrous occurs by the enzyme ferric reductase but if Cu is in excess, it may be bonded to the enzymes making it unavailable for the reduction of Fe3+ to Fe2+. As a result, the iron and calcium accumulation in shoot of plant declines [108, 109]. Dimkpa et al. [104] have suggested that Cu(II) is partially reduced to Cu(I) by citrate present in roots of beans and cucumber but chemically Cu(I) is highly susceptible to oxidation in presence of water and air which cannot be avoided in plant system. The redox process is very rapid and hence, the presence of Cu(I) is extremely difficult. CuO/ZnO nanoparticle exposure of bean plants affected both root and shoot. Improved plant growth has been attributed to lower solubility of CuO nanoparticles. It has been suggested that due to alkaline soil, the other metals (Cu and Fe) may be precipitated as their hydroxides and may not be available for absorption by plants. The reduction and accumulation of iron may be due to its hydroxide formation. The exposure of CuO nanoparticles to bean plants reduced Mn, Zn and Ca concentration and increased Na levels in the shoot tissues without disturbing Mn and K levels. In the bacterial culture medium, CuO nanoparticle treatment showed root growth perhaps due to bacteria which formed a protective layer around the root which does not allow copper to be absorbed. However, the plant cells act against the toxic effects of copper nanoparticles and in doing so, certain metals are absorbed and certain others are precipitated. Phytotoxicity of commercial CuO (<50 nm) and Zn nanoparticles (<100 nm) against sand-grown wheat (T. aestivum) has been investigated. Since these nanoparticles contained some metallic and non-metallic substances, they may also influence the growth rate of the plant. Changes in shape of ZnO nanoparticles were noted when mixed with sand in aqueous medium.
The sand amended with CuO and ZnO (500 mg/kg) significantly reduced root growth. Dissolved Cu from CuO nanoparticles showed toxic behaviour towards wheat plant but zinc did not influence the shoot growth. CuO and Cu(I)-sulphur complexes were found to be accumulated in the shoot while zinc was detected as Zn phosphate. Oxidative stress in the nanoparticle-treated plants was reflected by an increase in lipid peroxidation and oxidized glutathione and higher peroxidase and catalase activities in roots. The solubility of nanoparticles decreased with increasing aggregation causing morphological changes in ZnO nanoparticles [110]. It has been shown that the amount of Cu and Zn ions released from CuO and ZnO are almost negligible to cause phytotoxicity to plants. Plants grown with nanoparticles showed increased accumulation of Cu and Zn (20 fold Cu and 24 fold Zn) which altered root metabolism in wheat plants. Both CuO and ZnO nanoparticles have been detected in shoot of the plants. However, the quantitative difference between the two metals is mainly due to their solubility/diffusion. The zinc as zinc-phytate accumulates in the plants.
CuO nanoparticles have been shown to induce DNA damage in plants [21]. Growth inhibition in radish (Raphanus sativus), perennial ryegrass (Lolium perenne) and annual ryegrass (Lolium rigidum) under laboratory conditions has been reported. Germination of radish seeds in presence of CuO nanoparticles induces substantial accumulation of mutagenic DNA lesions. Radish and similar other plants produce oxygen-derived species (O2 −, H2O2,·OH) during germination [111]. H2O2 enhances seed germination but in presence of peroxidase or transition metal ions such as iron or copper produce an excess of OH via the Fenton reaction [112]. It is therefore suggested that copper ions produced from CuO nanoparticles may catalyze the formation of OH. CuO nanoparticles inhibited the radish root growth to the extent of 79% which is relatively much larger than that observed for Cu2+ ions alone. The stunted growth has been observed mainly in the root/shoot [113].
CuO nanoparticles have been shown to be cytotoxic and genotoxic [114, 115] to mammalian cells. It is thought to be due to nanoparticles which produce oxidative stress within living cells and cause DNA damage in plants or animals. Nair and Chung [116] have studied the impact of CuO nanoparticles on the growth of Arabidopsis thaliana and changes at molecular level. The seedlings were exposed to different concentrations of CuO nanoparticles (0.5 to 100 mg/L) for 3 weeks under laboratory conditions. Total chlorophyll contents were significantly reduced at all concentrations starting from 2 to 100 mg/L. Root growth was reduced even with 0.5 mg/L CuO nanoparticles. Superoxide and hydrogen peroxide increased in roots and leaves with increasing concentration of nanoparticles in plant. Oxidative stress, sulphur assimilation of glutathione and proline biosynthesis were also influenced by CuO nanoparticle exposure. In another study, A. thaliana plants exposed to cerium oxide and indium oxide showed reduction in plant biomass and total chlorophyll contents [117]. The increase in anthocyanin (the flavonoid) concentration in A. thaliana plant exposed to CuO nanoparticles may be due to oxidative stress. Anthocyanin acts as antioxidant to protect the plant cells against ROS-induced oxidative stress [29]. It is obvious that when a foreign matter is absorbed by the plant, it acts against this material through defensive mechanism for protection. Thus, it produces antioxidants which act as scavenger of ROS. As a result of stress by CuO nanoparticles, lignin was also deposited in A. thaliana.
It has been proposed that CuO nanoparticles would have been translocated via the vascular tissues and subsequently dissolved to produce Cu ions which resulted in deposition of lignin. Translocation of CuO nanoparticles is apparent but the production of Cu ions by dissolution is impossible because generation of Cu ions from copper nanoparticles is a redox process which requires a reducing agent such as hydrogen, phenol, protein or an acid. However, CuO being weakly basic dissolves in HCl to give ions as follows:
A comprehensive study of uptake and toxic effects of CuO nanoparticles, Cu2+ ions and also in combination with UV radiation has been done on the aquatic macrophyte, Elodea nuttallii [118]. Growth of the plants was inhibited when treated with CuSO4 or CuO nanoparticles. However, the amount of copper accumulated in E. nuttallii was lower in CuSO4-treated plants than those treated with CuO nanoparticles (Fig. 1). The difference has been attributed to the solubility of Cu2+ in CuO nanoparticle medium. Surprisingly, the relation between accumulated Cu and dissolved Cu2+ was higher in plants exposed to 256 μg/L Cu2+ than those exposed to 10 mg/L CuO nanoparticles containing nearly 2.0 mg/L dissolved Cu (Fig. 1). The accumulated Cu is directly proportional to the amount of Cu dissolved in the medium but it is not related to higher concentration of Cu in the solution [119]. Perhaps at higher concentration, agglomeration occurs which is prevented from absorption. When the dissolved copper is in small quantity, it has greater degree of freedom for movement and can be accumulated in different parts of the plant. However, enhanced Cu uptake in plants exposed to CuO nanoparticles with dissolved Cu concentration has also been reported which is contradictory to above results [120]. It has been suggested that plants exude some acid to dissolve additional Cu from CuO nanoparticles which is transported and accumulated in different parts of the plant [121]. Formation of large agglomerates prevents the dissolution of Cu. It is interesting that Cu accumulation is enhanced, under UV radiation, in shoots after 4 h but there is no direct evidence of enhanced solubility of Cu2+ in CuO nanoparticle suspension. Accumulation of Cd under UV radiation has been ascribed to membrane damage of the plant [122]. Rai et al. [123] suggested altered membrane permeability due to lipid peroxidation in cell membranes of UV-exposed cells in cynobacteria. In plants, under UV radiation, photosynthetic capacity is strongly reduced. When higher quantity of Cu is accumulated in plants, the response of the oxidative stress-related enzymes peroxidase and superoxide dismutase is also high.
Fig. 1.
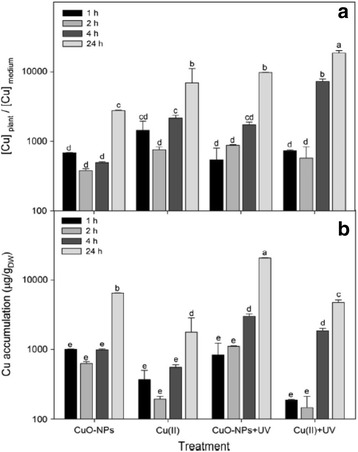
a Accumulation ratios ([Cu]plant/[Cu]medium) and b accumulation of Cu in shoots of Elodea nuttallii exposed to 256 μg/L Cu(II) or 10 mg/L CuO nanoparticles for up to 24 h. UV was applied additionally to test for effects on Cu accumulation. UV exposure lasted maximal 8 h: in the 24-h treatment, a 16-h period without UV followed the 8-h UV treatment before sampling. Different letters indicate statistically significant differences between the values as obtained by ANOVA and Tukey’s post hoc test (p < 0.05), where the letter a is assigned to the groups with the highest mean values [118]
Duckweed exposed to CuO nanoparticles showed inhibition of photosynthetic activity due to the Cu2+ ions released from it [120]. Andreotti et al. [124] have found that Phragmites australis can relocate Cu from roots to shoots. CuO nanoparticle exposure to cotton and Bt cotton has shown significant alterations of the concentrations of indole-3-acetic acid and abscisic acid [125]. Cu-based nanoparticles have been found to increase P and S in alfalfa shoots but decreased Fe and P in lettuce shoots [126]. Carotenoids remained unchanged and chlorophyll reduction began at 100 mg/L CuO nanoparticles in mung beans [127]. Carotenoid contents diminished at 400 mg/L CuO nanoparticles in soybean plants, but chlorophyll started decreasing at 400 mg/L [116]. In another study, CuO nanoparticles reduced carotenoids and chlorophylls in mustard [128] while Cu-based nanoparticles did not affect chlorophyll production in cilantro [129].
Titanium Oxide Nanoparticles
Although TiO2 is used in many consumable materials like cosmetics, sunscreen and colouring matter in medicine, paints, surface coating and water contamination process, it causes pulmonary inflammation in man if inhaled [130–132]. The quantity and exposure time of TiO2 significantly increase toxicity. Clément and co-workers [133] have reported that TiO2 nanoparticles (25 nm) with anatase crystal structure are more toxic to cladocerans, algae, rotifers and plants. At high concentration, they promoted growth of roots in plants. Since the rutile crystalline structures of TiO2 nanoparticles form aggregates in aqueous medium and are less available for absorption, they are less toxic than the anatase TiO2 (1 μm). When the exposure time is increased from 24 to 72 h, toxic effect is enhanced. However, at higher concentration (100 mg/L) of anatase TiO2 nanoparticles, the germination of seeds and root growth of flax seeds was enhanced. The positive effect has been suggested to be due to antimicrobial properties of anatase TiO2 which increases plant resistance to stress. Similar results have also been obtained by Zheng et al. [134].
Larue et al. [135] have studied the effect of anatase and rutile TiO2 on the development and germination of wheat (Triticum aestivum) over a period of 7 days. It has been observed that anatase TiO2 nanoparticle of diameter lower than 140 nm is accumulated in wheat roots. They are translocated to leaves if their diameter is smaller than 36 nm even if it is below the detection limit. TiO2 nanoparticles neither dissolve nor their crystal phase undergoes any change during translocation. The exposure of wheat plant to 14 and 22 nm TiO2 nanoparticles causes enhancement in root elongation without influencing seed germination, vegetative growth, photosynthesis or redox reaction. These are short-term effects of TiO2 nanoparticles but during the whole cycle of the plant, it may have some adverse effects.
Accumulation and translocation of TiO2 nanoparticles in plants suggest that it is not biodegradable. Long-term deposition may interfere with biological function of plants. Servin and co-workers [136] have studied the effect of TiO2 nanoparticles in cucumber plants (Cucumis sativus) over a large accumulation range (0–4000 mg/L). They found that root was significantly increased up to a concentration of 500 mg/L but above this concentration, it ceases to grow further. Nitrogen in the root was converted to organic nitrogen showing an increase of about 51.1%, compared to control. It is thought that TiO2 nanoparticles promote plant root growth by stimulating nitrogen accumulation.
Nanomaterials of the same metal with different structural motifs have different effects on plants, although there is no distinction in their chemical behaviour. Of the three crystalline structures of TiO2 nanoparticles (anatase, rutile and brookite), anatase exhibits the highest catalytic activity [137] and can inhibit the growth of many microbes such as algae, fungi and bacteria. It promotes carotene and chlorophyll synthesis in cucumber. TiO2 nanoparticles increase the Hill reaction and chloroplast activity by enhancing light absorption in chlorophyll a, electron transfer and oxygen evolution rate in spinach leaves [138–142]. Ba [143] showed that nano-TiO2 solution can inhibit the germination and growth of cucumber seedlings due to accumulation of nanoparticles. However, rutile TiO2 nanoparticles can protect chloroplast membrane against reactive oxygen and free radicals and enhance the protective activities of antioxidant enzymes such as SOD, CAT and POD [144, 145].
Nano-anatase TiO2 promotes the activity of spinach nitrate reductase and accelerates the conversion of nitrogen as nitrates or ammonium salts to organic nitrogen (protein, etc.) [146] but the uptake of other essential metals like Mg and Mn is not affected. TiO2 nanoparticles, however, stimulated the synthesis of carbohydrates and lipid as a consequence of stress caused by nanomaterials. Tomato seeds treated with TiO2 nanoparticles and Ag nanoparticles did not influence the germination, perhaps, the thick seed coat did not allow the absorption of nanomaterials. The Ag nanoparticles, at higher concentration (500 to 5000 mg/kg), are toxic to tomato plant during germination and the plant could not grow to full length. Silver nanoparticles in the mature tomato plants showed lower chlorophyll contents, higher SOD activity and less fruit productivity, while nanoTiO2 exhibited higher SOD activity at the highest concentration (5000 mg/kg). Both nano-Ag and nano-TiO2 were also taken up into plant stem, leaves and fruits [147]. The soil fortified with TiO2 nanoparticles enhances the chlorophyll content, POD, CAT and nitrate reductase of many plant species [148, 149].
Iron Oxide Nanoparticles
Hazeem et al. [150] have studied the effect of Fe3O4 nanoparticles on the growth of Picochlorum sp. in aqueous medium. The small (20 and 40 nm) and large (>100 nm) particles at 200 mg/L were used to examine the growth and chlorophyll content at different stages of growth of algae. The nanoparticles of 20 nm with different concentrations promoted the algal growth besides their aggregation and sedimentation. It prompted the authors to believe that this phenomenon can be used in bioremediation of environment from nanoparticles.
The metal oxide nanoparticles are more toxic to microorganisms than the bulk material of the same metal [151], despite the fact that bulk Fe3O4 is used as an algal fertilizer and also a source of iron as nutrient [152]. The smaller nanoparticles penetrate into plant cells or microbial cells but larger ones adhere to cell wall causing agglomeration.
The toxicity of SPION against an aquatic plant Lemna gibba has been investigated [153]. It was found that chlorophyll contents decreased, photosynthetic activity reduced and growth was hampered. The toxicity of nanoparticles is mainly dependent on their size and solubility in aqueous medium. Inhibitory effects of Fe3O4 nanoparticles after 6 days on cucumber (Cucumis sativus) seed germination and root elongation have also been reported [45]. Germination index of seeds was decreased by exposure of Fe3O4 nanoparticles at 500, 2500 and 5000 μg/mL. The effect of manufactured iron oxide nanoparticles on uptake and accumulation in pumpkin (Cucurbita maxima) plants grown hydroponically was investigated by Zhu and co-workers [46]. They showed that different amounts of Fe3O4 nanoparticles were taken up and translocated throughout the root, stem and leaves and indicated the nanoparticle transport pathways and bioaccumulation into the plant system. Chen et al. [154] have shown that Fe3O4 nanoparticles caused a decrease in net photosynthetic rate and chlorophyll a content when alga Chlorella vulgaris was exposed for 72 to 100 h and 200 × 103 μg/L of Fe3O4 nanoparticles. Iron oxide nanoparticles at 3.2 mg/kg showed reduced mycorrhizal clover biomass by 34% by reducing the glomalin content and root nutrient acquisition of Arbuscular mycorrhizal fungi [155]. Wang et al. [32] reported that Fe3O4 nanoparticles induced oxidative stress as compared to Fe3O4 bulk particles in the ryegrass and pumpkin roots and shoots as indicated by increased SOD and CAT activities and lipid peroxidation. Authors have shown that the tested Fe3O4 nanoparticles were unable to translocate in the ryegrass and pumpkin plants. The clogging effects of iron oxide nanoparticles reduce the root hydraulic conductivity by inhibiting the adequate water uptake [117, 156, 157]. Iron oxide nanoparticles have been found to reduce macronutrients such as Ca, K, Mg and S in sunflower’s shoots [158]. It has been suggested that it was due to the water blocking effects of nanoparticles, which altered the dissolved nutrients in water. Wang et al. [32, 159] have reported an increase in lipid peroxidation and attributed to Fe3O4 blockage of the aquaporins and disturbance of the respiration rate in the root. Many other studies on the use of iron oxide nanoparticles have shown reduced accumulation of chlorophylls in the leaves. However, this response is also associated with the reduction of the root hydraulic conductivity and the transport of dissolved nutrients, particularly of Mg since this nutrient is an essential constituent of the chlorophyll pigment [158, 117, 156, 160].
In a recent study, Shankramma et al. [161] have shown enhanced growth parameters of S. lycopersicum exposed to Fe2O3 nanoparticles. They found nanoparticles deposited preferentially in root hairs, root tips followed by nodal and middle zone of plant. They have suggested biomineralization of nanoparticles due to rich phytochemicals in plants. However, in another study, Nhan et al. [162] showed that 1000 mg/L Fe2O3 exposure to Bt transgenic and non-transgenic cotton exhibited presence of dark dots (particles) primarily localized in the endodermis and vascular cylinder (Fig. 2). Absorption of Fe2O3 nanoparticles and their aggregation in the roots were apparent. Iron contents in the shoots and roots increased with increasing concentration of Fe2O3 nanoparticles. It has been suggested that the bioaccumulation of Fe2O3 nanoparticles in Bt transgenic and non-transgenic cotton may cause potential risk for environment and human health. Affect of citrate-coated Fe3O4 nanoparticles on hydroponically grown wheat plant (T. aestivum) has recently been studied by Iannone et al. [163]. TEM images of root section showed the deposition of Fe3O4 in epidermal cell wall via apoplastic route. Fairly large quantities of magnetite (2.01–8.07 mg/g) iron were found in wheat roots. Since no paramagnetic signal was detected in stem and leaves, it suggested that nanoparticles were not translocated through vascular tissues. However, Fe3O4 nanoparticles affect the germination, chlorophyll content and plant growth and also did not produce lipid peroxidation or H2O2 accumulation. Antioxidant enzyme activity of plant reasonably increased in root and shoot which indicates that the Fe3O4 nanoparticles are not toxic to wheat plant under the given experimental conditions. The efficacy of Fe2O3 nanoparticles as iron fertilizer for peanut (Arachis hypogaea) has been studied to check if it can replace the conventional iron fertilizer [164]. The Fe2O3 nanoparticles and Fe2O3-EDTA were found to increase root length, height and biomass of the plant by regulating phytohormones and antioxidant enzyme activity. Fe2O3 nanoparticles are adsorbed onto the soil increasing easy availability of iron to peanut plant. The adsorption of nanoparticles in presence of organic matter is enhanced. It has been demonstrated from hydroponically grown spinach in presence of Fe2O3 nanoparticle and Fe(NO3)3·9H2O salt that the growth rate of plant is dose and time dependent [165]. The lengths of spinach stem under different concentrations of Fe2O3 (100, 150, 200 mg) were 1.45, 1.91 and 2.27-fold greater than those of the control after 45 days. There was, however, no significant change in the plant growth treated with Fe(NO3)3. The vegetative growth in the non-fruit-producing plants/vegetables such as cabbage, radish and beat root is highly useful because they increase the rate of photosynthesis. Hu et al. [166] found uptake of Fe2O3 nanoparticles in plant roots but no translocation from roots to shoots was observed. In the case of soybean, an increase in leaf and pod production due to uptake of Fe2O3 has also been shown in previous studies [167]. The mechanism of uptake of Fe2O3 nanoparticles has been explained in terms of reduction of Fe2O3 to Fe2+. Since Fe3+ is insoluble in aqueous medium, it is converted to Fe2+ in the soil when it became slightly acidic and absorbed. In hydroponic condition, the acidity is produced due to the addition of the nutrient, NH4H2PO4. The presence of iron phosphate has been evidenced from IR spectrum of the nutrient (containing NH4H2PO4 and Fe2O3) which is absorbed and translocated to different parts of the plant.
Fig. 2.
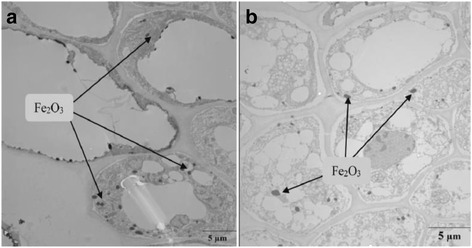
Transmission electron microscopy images of root sections of non-transgenic cotton (a) and Bt transgenic cotton (b) plants after 10 days of treatment with Fe2O3 nanoparticles [162]
Aluminium Oxide Nanoparticles
In the beginning, Yang and Watts [168] reported inhibition of root elongation in maize, cucumber, soybean, cabbage and carrot exposed to 13 nm Al2O3 nanoparticles. Thereafter, Lin and Xing [19] observed no phytotoxicity after 60-nm-sized Al2O3 nanoparticle application in radish, rape, ryegrass, lettuce and cucumber, although root elongation was reduced by 35% in maize. In contrast, studies with Phaseolus vulgaris and Lolium perenne have shown that 100-nm-sized Al2O3 nanoparticles had no adverse effect on plant growth [169]. Phytotoxicity of 150-nm-sized Al2O3 nanoparticles in A. thaliana has been investigated by Lee et al. [93] and no toxic effect was observed. Sadiq et al. [170] have reported the negative effect of below 50-nm-sized Al2O3 nanoparticles on the development of microalgae (Scenedesmus sp. and Chlorella sp.). Effect of Al2O3 on the growth and development of Nicotiana tabacum and role of microRNA has been investigated [171]. It has been observed that as the concentration of Al2O3 nanoparticles increases from 0.1 to 1.0%, the root length, average biomass and the leaf count of each tobacco seedlings decrease. The seedlings form multiple roots with increasing concentration of Al2O3, perhaps, as defensive mechanism to avoid contact with excess nanoparticles. They proposed that the microRNA genes were upregulated and played a key role in plants’ ability to withstand under Al2O3 stress. Al2O3 nanoparticles (40 nm) had no effect on root elongation of Triticum aestivum [172]. However, in a recent study, Yanık and Vardar [173] have reported that Al2O3 nanoparticles inhibit root elongation, callose formation, lignin deposition, cellular deformation, enhancement of peroxidase activity, decrease in total protein content and DNA fragmentation in T. aestivum. It has been suggested that the negative effects of Al2O3 nanoparticles were time and dose dependent. Impact of Al2O3 nanoparticles of 30–60 nm on soybean plant under flooding condition has been investigated by Mustafa and Komatsu [174]. The root length was found to increase while proteins related to glycolysis were suppressed. Al2O3 nanoparticles mediated the scavenging activity of cells by regulating the ascorbate/glutathione pathway. The results suggested that Al2O3 of varying size and shape affects mitochondrial proteins. Since it is a very short-term experiment on soybean seedlings, the plants may recover and tolerate the adverse effects of Al2O3 when they are fully grown.
Conclusions
The inadvertent use and release of nanomaterials into the environment affect plant growth and developmental process from seed germination to crop/fruit production. A variety of metal oxide nanoparticles (CeO2, ZnO, CuO, TiO2, Fe3O4 and Al2O3, etc.) has been examined against seed germination, growth of shoot/root, biomass production and physiological and biochemical activities. They have shown beneficial as well as adverse effects on the plant system and production. Plants absorb them on the surface and subsequently translocated and stored in different tissues. Quite often, the innocuous types of nanoparticles at low concentration have not exhibited any significant adverse effect and seem to be beneficial. However, at higher concentration, it produces stress/toxicity and enhances the generation of reactive oxygen species which results in the disruption of the cellular metabolism. In those conditions, plants protect cellular and sub-cellular system from the cytotoxic effects of active oxygen radicals with antioxidative enzymes and non-enzymatic components. Most of the studies have been carried out on the early developmental stages of the plant which requires full-term study to reach final results. It is hoped that some of the nanomaterials will minimize the use of toxic chemicals and fertilizers in near future. Additionally, detailed research investigations are required to examine the impact of these nanoparticles on the environment and human health.
Acknowledgments
Authors are thankful to the publishers/authors for the permission to adopt the figures in this review.
Authors’ Contributions
AH gathered the research data. AH and KSS analyzed these data findings and wrote this review paper. Both authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Contributor Information
Khwaja Salahuddin Siddiqi, Email: ks_siddiqi@yahoo.co.in.
Azamal Husen, Email: adroot92@yahoo.co.in.
References
- 1.Falcaro P, Ricco R, Yazdi A, Imaz I, Furukawa S, Maspoch D, Ameloot R, Evans JD, Doonan CJ. Application of metal and metal oxide nanoparticles@MOFs. Coord Chem Rev. 2016;307:237–254. doi: 10.1016/j.ccr.2015.08.002. [DOI] [Google Scholar]
- 2.Zuverza-Mena N, Martínez-Fernández D, Du W, Hernandez-Viezcas JA, Bonilla-Bird N, López-Moreno ML, Komárek M, Peralta-Videa JR, Gardea-Torresdey JL. Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses—a review. Plant Physiol Biochem. 2016;110:236–264. doi: 10.1016/j.plaphy.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 3.Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, van Duyne RP. Biosensing with plasmonic nanosensors. Nat Mater. 2008;7:442–453. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 4.Husen A, Siddiqi KS. Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol. 2014;12:16. doi: 10.1186/1477-3155-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husen A, Siddiqi KS. Phytosynthesis of nanoparticles: concept, controversy and application. Nano Res Lett. 2014;9:229. doi: 10.1186/1556-276X-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husen A, Siddiqi KS. Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol. 2014;12:28. doi: 10.1186/s12951-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqi KS, Husen A. Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nano Res Lett. 2016;11:98. doi: 10.1186/s11671-016-1311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi KS, Husen A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nano Res Lett. 2016;11:482. doi: 10.1186/s11671-016-1695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi KS, Rahman A, Tajuddin HA. Biogenic fabrication of iron/iron oxide nanoparticles and their application. Nano Res Lett. 2016;11:498. doi: 10.1186/s11671-016-1714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi KS, Husen A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J Trace Elements Med Biol. 2017;40:10–23. doi: 10.1016/j.jtemb.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Du W, Tan W, Peralta-Videa JR, Gardea-Torresdey JL, Ji R, Yin Y, Guo H. Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol Biochem. 2016;110:210–225. doi: 10.1016/j.plaphy.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Baruah S, Dutta J. Nanotechnology applications in pollution sensing and degradation in agriculture: a review. Environ Chem Lett. 2009;7:191–204. doi: 10.1007/s10311-009-0228-8. [DOI] [Google Scholar]
- 13.Keller AA, Lazareva A. Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett. 2013;1:65–70. doi: 10.1021/ez400106t. [DOI] [Google Scholar]
- 14.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Wang Z, Dai Y, Xing B. Mitigation of CuO nanoparticle-induced bacterial membrane damage by dissolved organic matter. Water Res. 2013;47:4169–4178. doi: 10.1016/j.watres.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 16.Ramsden C, Henry T, Handy R. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat Toxicol. 2013;126:404–413. doi: 10.1016/j.aquatox.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqi KS, Husen A. Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nano Res Lett. 2016;11:363. doi: 10.1186/s11671-016-1580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Lin DH, Xing BS. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol. 2008;42:5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- 21.Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol. 2012;46:1819–1827. doi: 10.1021/es202660k. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqi KS, Husen A. Engineered gold nanoparticles and plant adaptation potential. Nano Res Lett. 2016;11:400. doi: 10.1186/s11671-016-1607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubbins EJ, Batty LC, Lead JR. Phytotoxicity of silver nanoparticles to Lemna minor L. Environ Pollut. 2011;159:1551–1559. doi: 10.1016/j.envpol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 25.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trend Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Husen A. Growth characteristics, physiological and metabolic responses of teak (Tectona grandis Linn.f.) clones differencing in rejuvenation capacity subjected to drought stress. Silvae Gene. 2010;59:124–136. [Google Scholar]
- 27.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 28.Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 29.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Getnet Z, Husen A, Fetene M, Yemata G. Growth, water status, physiological, biochemical and yield response of stay green sorghum (Sorghum bicolor (L.) Moench) varieties—a field trial under drought-prone area in Amhara Regional State, Ethiopia. J Agron. 2015;14:188–202. doi: 10.3923/ja.2015.188.202. [DOI] [Google Scholar]
- 31.Ozyigit II, Filiz E, Vatansever R, Kurtoglu KY, Koc I, Öztürk MX, Anjum NA. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front Plant Sci. 2016;7:301. doi: 10.3389/fpls.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Kou X, Pei Z, Xiao JQ, Shan X, Xing B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology. 2011;5:30–42. doi: 10.3109/17435390.2010.489206. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Peralta-Videa JR, Ren M, Varela-Ramirez A, Li C, Hernandez-Viezcas JA, Aguilera RJ, Gardea-Torresdey JL. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: electron microprobe and confocal microscopy studies. Chem Eng J. 2012;184:1–8. doi: 10.1016/j.cej.2012.01.041. [DOI] [Google Scholar]
- 34.Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR, Tang X, Niu G, Jin L, Varela-Ramirez A, Zhang JY, Gardea-Torresdey JL. Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012;6:9615–9622. doi: 10.1021/nn302975u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang JY, Peralta-Videa JR, Gardea-Torresdey JL. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol. 2013;47:5635–5642. doi: 10.1021/es401032m. [DOI] [PubMed] [Google Scholar]
- 36.Husen A. Growth, chlorophyll fluorescence and biochemical markers in clonal ramets of shisham (Dalbergia sissoo Roxb.) at nursery stage. New For. 2009;38:117–129. doi: 10.1007/s11056-009-9141-z. [DOI] [Google Scholar]
- 37.Jiang HS, Li M, Chang FY, Li W, Yin LY. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem. 2012;31:1880–1886. doi: 10.1002/etc.1899. [DOI] [PubMed] [Google Scholar]
- 38.Husen A, Iqbal M, Aref IM. Growth, water status and leaf characteristics of Brassica carinata under drought and rehydration conditions. Braz J Bot. 2014;37:217–227. doi: 10.1007/s40415-014-0066-1. [DOI] [Google Scholar]
- 39.Husen A, Iqbal M, Aref IM. IAA-induced alteration in growth and photosynthesis of pea (Pisum sativum L.) plants grown under salt stress. J Environ Biol. 2016;37:421–429. [Google Scholar]
- 40.Shaw AK, Ghosh S, Kalaji HM, Bosa K, Brestic M, Zivcak M, Hossain Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.) Environ Exp Bot. 2014;102:37–47. doi: 10.1016/j.envexpbot.2014.02.016. [DOI] [Google Scholar]
- 41.Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Ann Rev Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 42.Husen A. Growth characteristics, biomass and chlorophyll fluorescence variation of Garhwal Himalaya’s fodder and fuel wood tree species at the nursery stage. Open J For. 2013;3:12–16. [Google Scholar]
- 43.Husen A, Khali R, Nautiyal S. Altitudinal variation in chlorophyll fluorescence/photosynthetic efficiency in seedlings of some indigenous fodder species. Ind For. 2004;130:89–94. [Google Scholar]
- 44.Embiale A, Hussein M, Husen A, Sahile S, Mohammed K. Differential sensitivity of Pisum sativum L. cultivars to water-deficit stress: changes in growth, water status, chlorophyll fluorescence and gas exchange attributes. J Agron. 2016;15:45–57. doi: 10.3923/ja.2016.45.57. [DOI] [Google Scholar]
- 45.Mushtaq YK. Effect of nanoscale Fe3O4, TiO2 and carbon particles on cucumber seed germination. J Environ Sci Health. 2011;46:1732–1735. doi: 10.1080/10934529.2011.633403. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H, Han J, Xiao JQ, Jin Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit. 2008;10:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- 47.López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol. 2010;44:7315–7320. doi: 10.1021/es903891g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge Y, Schimel JP, Holden PA. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol. 2011;45:1659–1664. doi: 10.1021/es103040t. [DOI] [PubMed] [Google Scholar]
- 49.Abramowicz DA. Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol. 1990;10:241–251. doi: 10.3109/07388559009038210. [DOI] [Google Scholar]
- 50.Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. Environ Sci Technol. 2012;46:6893–6899. doi: 10.1021/es300839e. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B. Xylem- and Phloem-based transport of CuO nanoparticles in maize (Zea mays L.) Environ Sci Technol. 2012;46:4434–4441. doi: 10.1021/es204212z. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y, He X, Zhang P, Zhang Z, Guo Z, Tai R, Xu Z, Zhang L, Ding Y, Zhao Y. Phytotoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumis sativus) Nanotoxicology. 2011;5:743–753. doi: 10.3109/17435390.2010.545487. [DOI] [PubMed] [Google Scholar]
- 53.Zhang P, Ma Y, Zhang Z, He X, Guo Z, Tai R, Ding Y, Zhao Y, Chai Z. Comparative toxicity of nanoparticulate/bulk Yb2O3 and YbCl3 to cucumber (Cucumis sativus) Environ Sci Technol. 2012;46:1834–1841. doi: 10.1021/es2027295. [DOI] [PubMed] [Google Scholar]
- 54.Schwabe F, Schulin R, Limbach LK, Stark W, Bürge D, Nowack B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere. 2013;91:512–520. doi: 10.1016/j.chemosphere.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 55.Rico CM, Majumdar S, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem. 2011;59:3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Judy JD, Unrine JM, Rao W, Wirick S, Bertsch PM. Bioavailability of gold nanomaterials to plants: importance of particle size and surface coating. Environ Sci Technol. 2012;46:8467–8474. doi: 10.1021/es3019397. [DOI] [PubMed] [Google Scholar]
- 57.Rico CM, Barrios AC, Tan W, Rubenecia R, Lee SC, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL. Physiological and biochemical response of soil-grown barley (Hordeum vulgare L.) to cerium oxide nanoparticles. Environ Sci Pollut Res. 2015;22:10551–10558. doi: 10.1007/s11356-015-4243-y. [DOI] [PubMed] [Google Scholar]
- 58.Vittori Antisari L, Carbone S, Gatti A, Vianello G, Nannipieri P. Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ Sci Pollut Res Int. 2015;22:1841–1853. doi: 10.1007/s11356-014-3509-0. [DOI] [PubMed] [Google Scholar]
- 59.Priester JH, Gea Y, Mielkea RE, Horsta AM, Moritzb SC, Espinosae K, Gelbf J, Walkerg SL, Nisbetb RM, Ani YJ, Schimelb JP, Palmere RG, Hernandez-Viezcasc JA, Zhaoc L, Gardea-Torresdeyc JL, Holdena PA. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci U S A. 2012;109:14734–14735. doi: 10.1073/pnas.1205431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Moreno ML, De La Rosa G, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem. 2010;58:3689–3693. doi: 10.1021/jf904472e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B, Mortensen NP, Allison DP, Joy DC, Allison MR, Brown SD, Phelps TJ, Doktycz MJ. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol. 2010;76:7981–7989. doi: 10.1128/AEM.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gutierrez-Boem FH, Thomas GW. Leaf area development in soybean as affected by phosphorus nutrition and water deficit. J Plant Nutr. 2001;24:1711–1729. doi: 10.1081/PLN-100107308. [DOI] [Google Scholar]
- 63.Kosynkin VD, Arzgatkina AA, Ivanov EN, Chtoutsa MG, Grabko AI, Kardapolov AV, Sysina NA. The study of process production of polishing powder based on cerium dioxide. J Alloys Compd. 2000;303–304:421–425. doi: 10.1016/S0925-8388(00)00651-4. [DOI] [Google Scholar]
- 64.Johnston BD, Scown TM, Moger J, Cumberland SA, Baalousha M, Linge K, van Aerle R, Jarvis K, Lead JR, Tyler CR. Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish. Sci Total Environ. 2010;44:1144–1151. doi: 10.1021/es901971a. [DOI] [PubMed] [Google Scholar]
- 65.Asli S, Neumann M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009;32:577–584. doi: 10.1111/j.1365-3040.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang P, Ma Y, Zhang Z, He X, Zhang J, Guo Z, Tai R, Zhao Y, Chai Z. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano. 2012;6:9943–9950. doi: 10.1021/nn303543n. [DOI] [PubMed] [Google Scholar]
- 67.Van Hoecke K, De Schamphelaere KAC, Van der Meeren P, Smagghe G, Janssen CR. Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, organic matter concentration and ionic strength. Environ Pollut. 2011;159:970–976. doi: 10.1016/j.envpol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Parsons JG, Lopez ML, Gonzalez CM, Peralta-Videa JR, Gardea-Torresdey JL. Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ Toxicol Chem. 2010;29:1146–1154. doi: 10.1002/etc.146. [DOI] [PubMed] [Google Scholar]
- 69.Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES. More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol. 2011;45:2360–2367. doi: 10.1021/es103995x. [DOI] [PubMed] [Google Scholar]
- 70.Jiang M, Ohnuki T, Kozai N, Tanaka K, Suzuki Y, Sakamoto F, Kamiishi E, Utsunomiya S. Biological nano-mineralization of Ce phosphate by Saccharomyces cerevisiae. Chem Geol. 2010;277:61–69. doi: 10.1016/j.chemgeo.2010.07.010. [DOI] [Google Scholar]
- 71.Birbaum K, Brogioli R, Schellenberg M, Martinoia E, Stark WJ, Gunther D, Limbach LK. No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ Sci Technol. 2010;44:8718–8723. doi: 10.1021/es101685f. [DOI] [PubMed] [Google Scholar]
- 72.Andersen CP, King G, Plocher M, Storm M, Pokhrel LR, Johnson MG, Rygiewicz PT (2016) Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ Toxico Chem 35:222–2229 [DOI] [PubMed]
- 73.Zhang ZY, He X, Zhang HF, Ma YH, Zhang P, Ding YY, Zhao YL. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics. 2011;3:816–822. doi: 10.1039/c1mt00049g. [DOI] [PubMed] [Google Scholar]
- 74.Wang Q, Ma XM, Zhang W, Pei HC, Chen YS. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics. 2012;4:1105–1112. doi: 10.1039/c2mt20149f. [DOI] [PubMed] [Google Scholar]
- 75.Rico CM, Morales MI, McCreary R, Castillo-Michel H, Barrios AC, Hong J, Tafoya A, Lee WY, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL. Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ Sci Technol. 2013;47:14110–14118. doi: 10.1021/es4033887. [DOI] [PubMed] [Google Scholar]
- 76.Backmann J, Schultz C, Fabian H, Hahn U, Saenger W, Naumann D. Thermally induced hydrogen exchange processes in small proteins as seen by FTIR spectroscopy. Proteins. 1996;24:379–387. doi: 10.1002/(SICI)1097-0134(199603)24:3<379::AID-PROT11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 77.Fischer G, Braun S, Thissen R, Dott W. FT-IR Spectroscopy as a tool for rapid identification and intra-species characterization of airborne filamentous fungi. J Microbiol Meth. 2006;64:63–77. doi: 10.1016/j.mimet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL. In Situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max) ACS Nano. 2013;7:1415–1423. doi: 10.1021/nn305196q. [DOI] [PubMed] [Google Scholar]
- 79.Riesen O, Feller U. Redistribution of nickel, cobalt, manganese, zinc, and cadmium via the phloem in young and maturing wheat. J Plant Nutr. 2005;28:421–430. doi: 10.1081/PLN-200049153. [DOI] [Google Scholar]
- 80.Serret G, Williams G, Isaure MP, Marcus MA, Fakra SC, Frerot H, Pairis S, Geoffroy N, Manceau A, Saumitou-Laprade P. Zinc distribution and speciation in Arabidopsis halleri x Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol. 2009;184:581–595. doi: 10.1111/j.1469-8137.2009.02996.x. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez-Viezcas JA, Castillo-Michel H, Servin AD, Peralta-Videa JR, Gardea-Torresdey JL. Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora-velutina (velvet mesquite) grown with ZnO nanoparticles. Chem Eng J. 2011;170:346–352. doi: 10.1016/j.cej.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thangavel P, Long S, Minocha R. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell Tissue Organ Cult. 2007;88:201–216. doi: 10.1007/s11240-006-9192-1. [DOI] [Google Scholar]
- 83.Kopittke PM, Menzies NW, de Jonge MD, McKenna BA, Donner E, Webb RI, Paterson DJ, Howard DL, Ryan CG, Glover CJ, Scheckel KG, Lombi E. In situ distribution and speciation of toxic copper, nickel and zinc in hydrated roots of cowpea. J Plant Physiol. 2011;156:663–673. doi: 10.1104/pp.111.173716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr. 1992;56:573–578. doi: 10.1093/ajcn/56.3.573. [DOI] [PubMed] [Google Scholar]
- 85.Hu C, Liu X, Li X, Zhao Y. Evaluation of growth and biochemical indicators of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ Sci Pollut Res. 2014;21:732–739. doi: 10.1007/s11356-013-1970-9. [DOI] [PubMed] [Google Scholar]
- 86.Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 87.Upadhyay R, Panda SK. Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L. J Hazard Mater. 2010;175:1081–1084. doi: 10.1016/j.jhazmat.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 88.Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535 [DOI] [PMC free article] [PubMed]
- 89.Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol. 2007;41:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 91.Watson JL, Fang T, Dimkpa CO, Britt DW, McLean JE, Jacobson A, Anderson AJ. The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals. 2015;28:101–112. doi: 10.1007/s10534-014-9806-8. [DOI] [PubMed] [Google Scholar]
- 92.Landa P, Vankova R, Andrlova J, Hodek J, Marsik P, Storchova H, White JC, Vanek T. Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J Hazard Mater. 2012;241:55–62. doi: 10.1016/j.jhazmat.2012.08.059. [DOI] [PubMed] [Google Scholar]
- 93.Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJ. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem. 2010;29:669–675. doi: 10.1002/etc.58. [DOI] [PubMed] [Google Scholar]
- 94.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 95.Cuypers A, Vangronsveld J, Clijsters H. The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol Biochem. 2001;39:657–664. doi: 10.1016/S0981-9428(01)01276-1. [DOI] [Google Scholar]
- 96.Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater. 2011;190:613–621. doi: 10.1016/j.jhazmat.2011.03.095. [DOI] [PubMed] [Google Scholar]
- 97.Wang P, Menzies NW, Lombi E, McKenna BA, Johannessen B, Glover CJ, Kappen P, Kopittke PM. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata) Environ Sci Technol. 2013;47:13822–13830. doi: 10.1021/es403466p. [DOI] [PubMed] [Google Scholar]
- 98.Raliya R, Tarafdar JC. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.) Agric Res. 2013;2:48–57. doi: 10.1007/s40003-012-0049-z. [DOI] [Google Scholar]
- 99.Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;7:1584–1594. doi: 10.1039/C5MT00168D. [DOI] [PubMed] [Google Scholar]
- 100.Pokhrel LR, Dubey B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ. 2013;452–453:321–332. doi: 10.1016/j.scitotenv.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 101.Kim S, Lee S, Lee I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut. 2012;223:2799–2806. doi: 10.1007/s11270-011-1067-3. [DOI] [Google Scholar]
- 102.Manzo S, Rocco A, Carotenuto R, Picione Fde L, Miglietta ML, Rametta G, Di Francia G. Investigation of ZnO nanoparticles ecotoxicological effects towards different soil organisms. Environ Sci Pollut Res Int. 2011;18:756–763. doi: 10.1007/s11356-010-0421-0. [DOI] [PubMed] [Google Scholar]
- 103.Du WC, Sun YY, Ji R, Zhu JG, Wu JC, Guo HY. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit. 2011;13:822–828. doi: 10.1039/c0em00611d. [DOI] [PubMed] [Google Scholar]
- 104.Dimkpa CO, McLean JE, Britt DW, Anderson AJ. Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology. 2015;24:119–129. doi: 10.1007/s10646-014-1364-x. [DOI] [PubMed] [Google Scholar]
- 105.Adhikari T, Kundu S, Biswas AK, Tarafdar JC, Rao AS. Effect of copper oxide nano particle on seed germination of selected crops. J Agric Sci Technol. 2012;A2:815–823. [Google Scholar]
- 106.Lei X, CeHui M, XiHong L, XiaoLian W, YanWen L, XianPei H, XiangLong Q, Yan H. Toxicity of copper oxide nanoparticles to the seed germination of Chinese cabbage. J Agro Environ Sci. 2011;30:1830–1835. [Google Scholar]
- 107.Yruela I. Copper in plants, acquisition, transport and interactions. Funct Plant Biol. 2009;36:409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]
- 108.Barton LL, Johnson GV, O’nan AG, Wagener BM. Inhibition of ferric chelate reductase in alfalfa roots by cobalt, nickel, chromium and copper. J Plant Nutr. 2000;23:1833–1845. doi: 10.1080/01904160009382146. [DOI] [Google Scholar]
- 109.Welch RM, Norvell WA, Schaefer SC, Shaff JE, Kochian LV. Induction of iron(III) and copper(II) reduction in pea (Pisum sativum L) roots by Fe and Cu status—does the root-cell plasmalemma Fe(III)-chelate reductase perform a general role in regulating cation uptake? Planta. 1993;190:555–561. doi: 10.1007/BF00224795. [DOI] [Google Scholar]
- 110.Voegelin A, Pfister S, Scheinost AC, Marcus MA, Kretzschmar R. Changes in zinc speciation in field soil after contamination with zinc oxide. Environ Sci Technol. 2005;39:6616–6623. doi: 10.1021/es047962g. [DOI] [PubMed] [Google Scholar]
- 111.Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–1602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 113.Fernandes JC, Henriques FS. Biochemical, physiological and structural effects of excess copper in plants. Bot Rev. 1991;57:246–273. doi: 10.1007/BF02858564. [DOI] [Google Scholar]
- 114.Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 115.Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol In Vitro. 2009;23:1365–1371. doi: 10.1016/j.tiv.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nair PMG, Chung IM. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification, and molecular level changes. Environ Sci Pollut Res. 2014;21:12709–12722. doi: 10.1007/s11356-014-3210-3. [DOI] [PubMed] [Google Scholar]
- 117.Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sus Chem Eng. 2013;1:768–778. doi: 10.1021/sc400098h. [DOI] [Google Scholar]
- 118.Regier N, Cosio C, von Moos N, Slaveykova VI. Effects of copper-oxide nanoparticles, dissolved copper and ultraviolet radiation on copper bioaccumulation, photosynthesis and oxidative stress in the aquatic macrophyte Elodea nuttallii. Chemosphere. 2015;128:56–61. doi: 10.1016/j.chemosphere.2014.12.078. [DOI] [PubMed] [Google Scholar]
- 119.Ivask A, Juganson K, Bondarenko O, Mortimer M, Aruoja V, Kasemets K, Blinova I, Heinlaan M, Slaveykova V, Kahru A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology. 2014;8:57–71. doi: 10.3109/17435390.2013.855831. [DOI] [PubMed] [Google Scholar]
- 120.Perreault F, Samadani M, Dewez D. Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology. 2014;8:374–382. doi: 10.3109/17435390.2013.789936. [DOI] [PubMed] [Google Scholar]
- 121.Shi JY, Abid AD, Kennedy IM, Hristova KR, Silk WK. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut. 2011;159:1277–1282. doi: 10.1016/j.envpol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agrawal SB, Mishra S. Effects of supplemental ultraviolet-B and cadmium on growth, antioxidants and yield of Pisum sativum L. Ecotoxicol Environ Saf. 2009;72:610–618. doi: 10.1016/j.ecoenv.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Rai LC, Tyagi B, Mallick N, Rai PK. Interactive effects of UV-B and copper on photosynthetic activity of the cyanobacterium Anabaena doliolum. Environ Exp Bot. 1995;35:177–185. doi: 10.1016/0098-8472(94)00046-8. [DOI] [Google Scholar]
- 124.Andreotti F, Mucha AP, Caetano C, Rodrigues P, Gomes CR, Almeida CMR. Interactions between salt marsh plants and Cu nanoparticles effects on metal uptake and phytoremediation processes. Ecotoxicol Environ Saf. 2015;120:303–309. doi: 10.1016/j.ecoenv.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 125.Le Van N, Ma C, Shang J, Rui Y, Liu S, Xing B. Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere. 2016;144:661–670. doi: 10.1016/j.chemosphere.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 126.Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL. Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa) Environ Sci Process Impact. 2016;17:177–185. doi: 10.1039/C4EM00551A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nair PMG, Kim SH, Chung IM. Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant. 2014;36:2947–2958. doi: 10.1007/s11738-014-1667-9. [DOI] [Google Scholar]
- 128.Nair PMG, Chung IM. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.) Ecotoxicol Environ Saf. 2015;113:302–313. doi: 10.1016/j.ecoenv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 129.Zuverza-Mena N, Medina-Velo IA, Barrios AC, Tan W, Peralta-Videa JR, Gardea-Torresdey JL. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum) Environ Sci Process Impact. 2015;17:1783–1793. doi: 10.1039/C5EM00329F. [DOI] [PubMed] [Google Scholar]
- 130.Warheit DB, Brock WJ, Lee WJ, Webb KP, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci. 2005;88:514–524. doi: 10.1093/toxsci/kfi331. [DOI] [PubMed] [Google Scholar]
- 131.Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006;91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- 132.Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine—TiO2 particles: differential responses related to surface properties. Toxicology. 2007;230:90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Clément L, Hurel C, Marmier N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—effects of size and crystalline structure. Chemosphere. 2013;90:1083–1090. doi: 10.1016/j.chemosphere.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 134.Zheng L, Hong F, Lu S, Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res. 2005;104:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- 135.Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank AM, Brisset F, Carriere M. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ. 2012;431:197–208. doi: 10.1016/j.scitotenv.2012.04.073. [DOI] [PubMed] [Google Scholar]
- 136.Servin AD, Castillo-Michel H, Hernandez-Viezcas JA, Diaz BC, Peralta-Videa JR, Gardea-Torresdey JL. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ Sci Technol. 2012;46:7637–7643. doi: 10.1021/es300955b. [DOI] [PubMed] [Google Scholar]
- 137.Yin HC, Liu QJ, Lin Q, Chen SN, Zhou LJ. Inhibitory effects of nano-TiO2 loaded Pd on cyanobacteria growth. Acta Bot Boreali-Occidentalia Sin. 2005;25:1884–1887. [Google Scholar]
- 138.Hong FS, Yang P, Gao FQ, Liu C, Zheng L, Zhou J. Effect of nano-anatase TiO2 on spectral characterization of photosystem II particles from spinach. Chem Res Chin Univ. 2005;21:196–200. [Google Scholar]
- 139.Su MY, Hong FS, Liu C, Wu X, Liu XQ, Chen L, Gao FQ, Yang F, Li Z. Effects of nano-anatase TiO2 on absorption, distribution of light, and photoreduction activities of chloroplast membrane of spinach. Biol Trace Elem Res. 2007;131:101. doi: 10.1007/s12011-009-8430-x. [DOI] [PubMed] [Google Scholar]
- 140.Zheng L, Su MY, Chao L, Liang C, Hao H, Xiao W, Liu XQ, Yang F, Gao FQ, Hong FS. Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol Trace Elem Res. 2007;119:68–76. doi: 10.1007/s12011-007-0047-3. [DOI] [PubMed] [Google Scholar]
- 141.Zheng L, Su MY, Xiao W, Chao L, Qu CX, Chen L, Hao H, Liu XQ, Hong FS. Effects of nano-anatase on spectral characteristics and distribution of LCHII on the thylakoid membranes of spinach. Biol Trace Elem Res. 2007;120:273–283. doi: 10.1007/s12011-007-8025-3. [DOI] [PubMed] [Google Scholar]
- 142.Wang XM, Gao FQ, Ma LL, Liu J, Yin ST, Yang P, Hong FS. Effects of nano-anatase on ribulose-1, 5-bisphosphate carboxylase/oxygenase mRNA expression in spinach. Biol Trace Elem Res. 2008;126:280–289. doi: 10.1007/s12011-008-8203-y. [DOI] [PubMed] [Google Scholar]
- 143.Ba CL (2010) Study on the uptake, transport, and accumulation of nano-TiO2 in plants and its protein mechanism. Master dissertation, Hebei University
- 144.Hong F, Yang F, Liu C, Gao Q, Wan Z, Gu F, Wu C, Ma Z, Zhou J, Yang P. Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol Trace Elem Res. 2005;104:249–260. doi: 10.1385/BTER:104:3:249. [DOI] [PubMed] [Google Scholar]
- 145.Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 146.Yang F, Hong FS, You WJ, Liu C, Gao FQ, Wu C, Yang P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res. 2006;110:179–190. doi: 10.1385/BTER:110:2:179. [DOI] [PubMed] [Google Scholar]
- 147.Song U, Jun H, Waldman B, Roh J, Kim Y, Yi J, Lee EJ. Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum) Ecotoxicol Environ Saf. 2013;93:60–67. doi: 10.1016/j.ecoenv.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 148.Hruby M, Cigler P, Kuzel S. Contribution to understanding the mechanism of titanium action in plant. J Plant Nutr. 2002;25:577–598. doi: 10.1081/PLN-120003383. [DOI] [Google Scholar]
- 149.Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol Trace Elem Res. 2012;146:101–106. doi: 10.1007/s12011-011-9222-7. [DOI] [PubMed] [Google Scholar]
- 150.Hazeem LJ, Waheed FA, Rashdan S, Bououdina M, Brunet L, Slomianny C, Boukherroub R, Elmeselmani WA. Effect of magnetic iron oxide (Fe3O4) nanoparticles on the growth and photosynthetic pigment content of Picochlorum sp. Environ Sci Pollut Res. 2015;22:11728–11739. doi: 10.1007/s11356-015-4370-5. [DOI] [PubMed] [Google Scholar]
- 151.Manzo S, Miglietta M, Rametta G, Buono S, Francia G. Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tortiolecta. Sci Total Environ. 2013;445–446:371–376. doi: 10.1016/j.scitotenv.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 152.WHOI (Woods Hole Oceanographic Institution) (2007) Iron fertilization in southern ocean increased growth of algae that absorb greenhouse gases, and could cool climate. Available at: http://www.whoi.edu/main/news-releases/1995-2004?tid=3622&cid=971
- 153.Barhoumi L, Oukarroum A, Taher LB, Smiri LS, Abdelmelek H, Dewez D. Effects of superparamagnetic iron oxide nanoparticles on photosynthesis and growth of the aquatic plant Lemna gibba. Arch Environ Contam Toxicol. 2015;68:510–520. doi: 10.1007/s00244-014-0092-9. [DOI] [PubMed] [Google Scholar]
- 154.Chen X, Zhu X, Li R, Yao H, Lu Z, Yang X. Photosynthetic toxicity and oxidative damage induced by nano-Fe3O4 on Chlorella vulgaris in aquatic environment. Op J Ecol. 2012;2:21–28. doi: 10.4236/oje.2012.21003. [DOI] [Google Scholar]
- 155.Feng Y, Cui X, He S, Dong G, Chen M, Wang J, Lin X. The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ Sci Technol. 2013;47:9496–9504. doi: 10.1021/es402109n. [DOI] [PubMed] [Google Scholar]
- 156.Ma X, Gurung A, Deng Y. Phytotoxicity and uptake of nanoscale zerovalent iron (nZVI) by two plant species. Sci Total Environ. 2013;443:844–849. doi: 10.1016/j.scitotenv.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 157.Martínez-Fernández D, Vítková M, Bernal MP, Komárek M. Effects of nano-maghemite on trace element accumulation and drought response of Helianthus annuus L. in a contaminated mine soil. Water Air Soil Pollut. 2015;226:1–4. doi: 10.1007/s11270-015-2365-y. [DOI] [Google Scholar]
- 158.Martínez-Fernández D, Barroso D, Komárek M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ Sci Pollut Res. 2016;23:1732–1741. doi: 10.1007/s11356-015-5423-5. [DOI] [PubMed] [Google Scholar]
- 159.Wang Z, Li J, Zhao J, Xing B. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ Sci Technol. 2011;45:6032–6040. doi: 10.1021/es2010573. [DOI] [PubMed] [Google Scholar]
- 160.Trujillo-Reyes J, Majumdar S, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL. Exposure studies of core—shell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard? J Hazard Mater. 2014;267:255–263. doi: 10.1016/j.jhazmat.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 161.Shankramma K, Yallappa S, Shivanna MB, Manjanna J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl Nanosci. 2016;6:983–990. doi: 10.1007/s13204-015-0510-y. [DOI] [Google Scholar]
- 162.Nhan LV, Ma C, Rui Y, Cao W, Deng Y, Liu L, Xing B. The effects of Fe2O3 nanoparticles on physiology and insecticide activity in non-transgenic and Bt-transgenic cotton. Front Plant Sci. 2016;6:1263. doi: 10.3389/fpls.2015.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iannone MF, Groppa MD, de Sousa ML, Fernández van Raap MB, Benavides MP. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: evaluation of oxidative damage. Environ Exp Bot. 2016;131:77–88. doi: 10.1016/j.envexpbot.2016.07.004. [DOI] [Google Scholar]
- 164.Rui M, Ma C, Hao Y, Guo J, Rui Y, Tang X, Zhao Q, Fan X, Zhang Z, Hou T, Zhu S. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea) Fron Plant Sci. 2016;7:815. doi: 10.3389/fpls.2016.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jeyasubramanian K, Thoppey UUG, Hikku GS, Selvakumar N, Subramania A, Krishnamoorthy K. Enhancement in growth rate and productivity of spinach grown in hydroponics with iron oxide nanoparticles. RSC Adv. 2016;6:15451–15459. doi: 10.1039/C5RA23425E. [DOI] [Google Scholar]
- 166.Hu J, Guo H, Li J, Gan Q, Wang Y, Xing B (2017) Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ Pollut 221:199–208 [DOI] [PubMed]
- 167.Sheykhbaglou R, Sedghi M, Tajbakhsh Shishevan M, Seyed Sharifi R. Effects of nano-iron oxide particles on agronomic traits of soybean. Not Sci Biol. 2010;2:112–113. [Google Scholar]
- 168.Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxico Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 169.Doshi R, Braida W, Christodoulatos C, Wazne M, O’Connor G. Nano-aluminum: transport through sand columns and environmental effects on plant and soil communication. Environ Res. 2008;106:296–303. doi: 10.1016/j.envres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 170.Sadiq IM, Pakrashi S, Chandrasekaran N, Mukherjee A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J Nanopar Res. 2011;13:3287–3299. doi: 10.1007/s11051-011-0243-0. [DOI] [Google Scholar]
- 171.Burklew CE, Ashlock J, Winfrey WB, Zhang B. Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum) PLoS One. 2012;7:e34783. doi: 10.1371/journal.pone.0034783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Riahi-Madvar A, Rezaee F, Jalali V. Effects of alumina nanoparticles on morphological properties and antioxidant system of Triticum aestivum. Iran J of Plant Physiol. 2012;3:595–603. [Google Scholar]
- 173.Yanık F, Vardar F. Toxic effects of aluminum oxide (Al2O3) nanoparticles on root growth and development in Triticum aestivum. Water Air Soil Pollut. 2015;226:296. doi: 10.1007/s11270-015-2566-4. [DOI] [Google Scholar]
- 174.Mustafa G, Komatsu S. Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J Proteome Res. 2016;15:4464–4475. doi: 10.1021/acs.jproteome.6b00572. [DOI] [PubMed] [Google Scholar]
- 175.Morales MI, Rico CM, Hernandez-Viezcas JA, Nunez JE, Barrios AC, Tafoya A, Flores-Marges JP, Peralta-Videa JR, Gardea-Torresdey JL. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J Agric Food Chem. 2013;61:6224–6230. doi: 10.1021/jf401628v. [DOI] [PubMed] [Google Scholar]
- 176.Zhao LJ, Sun Y, Hernandez-Viezcas JA, Hong J, Majumdar S, Niu GH, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL. Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environ Sci Technol. 2015;49:2921–2928. doi: 10.1021/es5060226. [DOI] [PubMed] [Google Scholar]
- 177.Du WC, Gardea-Torresdey JL, Ji R, Yin Y, Zhu JG, Peralta-Videa JR, Guo HY. Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: a life cycle field study. Environ Sci Technol. 2015;49:11884–11893. doi: 10.1021/acs.est.5b03055. [DOI] [PubMed] [Google Scholar]
- 178.Nhan LV, Ma CX, Rui YK, Liu ST, Li XG, Xing BS, Liu LM. Phytotoxic mechanism of nanoparticles: destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci Rep. 2015;5:11618. doi: 10.1038/srep11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rico CM, Lee SC, Rubenecia R, Mukherjee A, Hong J, Peralta-Videa JR, Gardea-Torresdey JL. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.) J Agric Food Chem. 2014;62:9669–9675. doi: 10.1021/jf503526r. [DOI] [PubMed] [Google Scholar]
- 180.Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L, Diaz BC, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem. 2014;80:128–135. doi: 10.1016/j.plaphy.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 181.Yoon SJ, Kwak JI, Lee WM, Holden PA, An YJ. Zinc oxide nanoparticles delay soybean development: a standard soil microcosm study. Ecotoxicol Environ Saf. 2014;100:131–137. doi: 10.1016/j.ecoenv.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 182.Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;43:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- 183.Zhao L, Sun Y, Hernandez-Viezcas JA, Servin AD, Hong J, Niu G, Peralta-Videa JR, Duarte-Gardea M, Gardea-Torresdey JL. Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J Agric Food Chem. 2013;61:11945–11951. doi: 10.1021/jf404328e. [DOI] [PubMed] [Google Scholar]
- 184.Wang F, Liu X, Shi Z, Tong R, Adams CA, Shi X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—a soil microcosm experiment. Chemosphere. 2016;147:88–97. doi: 10.1016/j.chemosphere.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 185.Bandyopadhyay S, Plascencia-Villa G, Mukherjee A, Rico CM, José-Yacamán M, Peralta-Videa JR, Gardea-Torresdey JL. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci Total Environ. 2015;515:60–69. doi: 10.1016/j.scitotenv.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 186.Huang YC, Fan R, Grusak MA, Sherrier JD, Huang CP. Effects of nano-ZnO on the agronomically relevant Rhizobiumelegume symbiosis. Sci Total Environ. 2014;497:78–90. doi: 10.1016/j.scitotenv.2014.07.100. [DOI] [PubMed] [Google Scholar]
- 187.Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res. 2012;14:1–15. doi: 10.1007/s11051-012-1125-9. [DOI] [Google Scholar]
- 188.Musante C, White JC. Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles. Environ Toxicol. 2012;27:510–517. doi: 10.1002/tox.20667. [DOI] [PubMed] [Google Scholar]
- 189.Singh P, Singh R, Borthakur A, Srivastava P, Srivastava N, Tiwary D, Mishra PK. Effect of nanoscale TiO2-activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energ Ecol Environ. 2016;1:131–140. doi: 10.1007/s40974-016-0009-8. [DOI] [Google Scholar]
- 190.Rezaei F, Moaveni P, Mozafari H. Effect of different concentrations and time of nano TiO2 spraying on quantitative and qualitative yield of soybean (Glycine max L.) at Shahr-e-Qods, Iran. Biol Forum. 2015;7:957–964. [Google Scholar]
- 191.Frazier TP, Burklew CE, Zhang B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum) Funct Integr Genomics. 2014;14:75–83. doi: 10.1007/s10142-013-0341-4. [DOI] [PubMed] [Google Scholar]
- 192.Mohammadi R, Maali-Amiri R, Abbas A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol Trace Elem Res. 2013;152:403–410. doi: 10.1007/s12011-013-9631-x. [DOI] [PubMed] [Google Scholar]
- 193.Gao J, Xu G, Qian H, Liu P, Zhao P, Hu Y. Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ Pollut. 2013;176:63–70. doi: 10.1016/j.envpol.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 194.Servin AD, Morales MI, Castillo-Michel H, Hernandez-Viezcas JA, Munoz B, Zhao LJ, Nunez JE, Peralta-Videa JR, Gardea-Torresdey JL. Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ Sci Technol. 2013;47:11592–11598. doi: 10.1021/es403368j. [DOI] [PubMed] [Google Scholar]
- 195.Barrena R, Casals E, Colon J, Font X, Sanchez A, Puntes V. Evaluation of the ecotoxicity of model nanoparticles. Chemo. 2009;75:850–857. doi: 10.1016/j.chemosphere.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 196.García A, Espinosa R, Delgado L, Casals E, González E, Puntes V, Carlos B, Font X, Sánchez A. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination. 2011;269:136–141. doi: 10.1016/j.desal.2010.10.052. [DOI] [Google Scholar]
- 197.Marusenko Y, Shipp J, Hamilton GA, Morgan JLL, Keebaugh M, Hill H, Dutta A, Zhuo X, Upadhyay N, Hutchings J, Herckes P, Anbar AD, Shock E, Hartnett HE. Bioavailability of nanoparticulate hematite to Arabidopsis thaliana. Environ Pollut. 2013;174:150–156. doi: 10.1016/j.envpol.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 198.Wu SG, Huang L, Head J, Chen DR, Kong IC, Tang YJ. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J Pet Environ Biotechnol. 2012;3:1–6. [Google Scholar]


