Abstract
A variety of non-covalent interactions (including hydrogen bonding, ionic interactions, metal coordination and desolvation/solvation) have been utilized to organize oligomers into well-defined structures. Herein is described a survey of aromatic foldamers that capitalize on electrostatic complementarity of substituted aromatic units to drive folding and assembly in aqueous environments. A brief description of recent advances in the understanding of aromatic interactions is provided, followed by examples of foldamers that exploit interactions between aromatic units to drive their assembly in predictable fashion. The history of our aromatic foldamers is traced from the first structure designed to fold into a pleated structure in an aqueous environment to a heteroduplex system more related to nucleic acids. Taken together, the results demonstrate that electrostatic complementarity of aromatic units provides a versatile framework for driving predictable folding and assembly in aqueous environments.
INTRODUCTION
A well-established principle in biological chemistry is that the structure of large, complex biomolecules is crucial for their function. The intricate array of competing and interacting non-covalent interactions (hydrogen bonding, ionic interactions, Van der Waals, and especially desolvation in water, to name a few) constitutes the driving force for the folding and assembly of biochemically useful structures. These non-covalent inter- and intramolecular interactions introduced by different combinations of monomeric units along an oligomer or polymer chain are able to precisely form complex higher order molecular architectures such as the DNA double-helix or protein secondary, tertiary and quaternary structures. How these non-covalent interactions work together to form such complex and highly-ordered architectures is of particular interest for organic chemists and biochemists who desire to improve, mimic, or manipulate natural biological systems.
Through the investigation of these higher order architectures driven by non-covalent interactions in solution, the field of “foldamer”1 chemistry emerged. Through the exploration of oligomeric β-peptides, Gellman et al.2-4 and Seebach et al.5-8 were able to construct and characterize helical and sheet architectures. From there, several other groups have explored other well-defined secondary structures generated by various non-covalent interactions. For example, Moore and co-workers have developed stable helical structures from the solvophobically driven folding of meta-phenylene ethynylene oligomers.9 Additionally, Lehn et al. has established several helical folded conformations of polyheterocyclic oligomers.10-12 Recently, Huc et al. synthesized and characterized aromatic oligoamides that fold into multi-stranded artificial β-sheet architectures utilizing a 4,6-dinitro-1,3-phenylenediamine rigid turn unit.13
Our lab has undertaken the study of the specific non-covalent interactions between aromatic units, particularly in the study of 1,5-dialkoxynaphthalene (DAN) and 1,4,5,8-naphthalenetetracarboxylic acid diimide (NDI) units in the construction of foldamers and other assemblies in aqueous environments. Based upon early work demonstrating that the relatively electron-rich DAN and relatively electron-deficient NDI associate in solution, the first aromatic foldamer operating in water (called an “aedamer” at the time) was designed and synthesized by linking DAN and NDI units in an alternating fashion with flexible peptide linkers.14 This became the foundation of several other foldamer designs as well as efforts to use aromatic interactions to drive aromatic supramolecular assembly. Since then, the understanding of aromatic interactions has been further refined through computational as well as experimental work, which has enabled more intentional designs of aromatic foldamers and associations. Herein is described some of the work our lab has contributed to the field of aromatic foldamers. First, however, the current models of aromatic interactions will be presented and then the significance of solvation/desolvation for aromatic molecules in water.
“POLAR/PI” MODEL
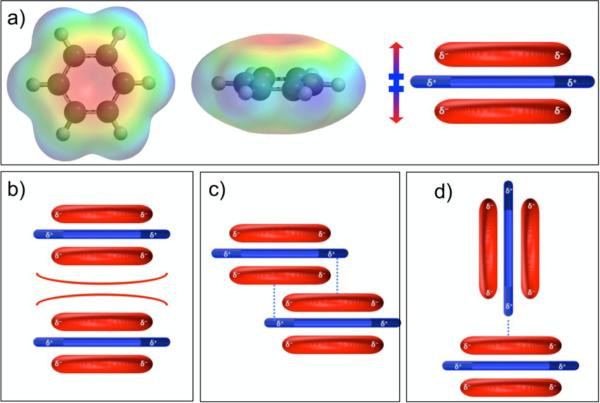
A model to predict aromatic stacking geometry was proposed by Hunter and Sanders in the 1990s, focused on the overall polarization of the aromatic π-electron cloud.15 By treating the electron-rich π-cloud of aromatic molecules as separate from the σ-framework, they proposed a model in which the partially positively-charged σ-framework around the periphery of the molecule is “sandwiched” between two partially negatively-charged π-electron clouds (Figure 1a), revealing a quadrupole moment when visualized from a side-on perspective. The geometry observed for interacting aromatic molecules is therefore predicted by favorable or unfavorable interactions of the “aromatic core” (the π-electron clouds) and the periphery of the aromatic molecules.
Figure 1.
a) Electrostatic potential map of benzene with cartoon depicting quadrupole moment described in the polar/pi model. b) Repulsion of face-centered stacking of benzene. c) Possible off-centered stacking of benzene. d) Preferred T-shaped, or “herringbone” interaction of benzene.
The terminology of the “polar/pi” model was set forth by Cozzi and Siegel.16-20 Through a series of experiments utilizing a 1,8-diarylnaphthalene system, they concluded that the interaction of aromatic molecules can be predicted in terms of the polarization of π-electron density. Both this and the Hunter-Sanders approach offer an explanation for the general tendency of neutral, non-substituted aromatic molecules, such as benzene or naphthalene, to interact with each other in a T-shaped, or “herringbone” geometry (Figure 1d) or possibly an off-set stacked geometry (Figure 1c) rather than a face-centered stacked geometry (Figure 1b). The partially negatively-charged π-electron clouds are predicted to repel one another, disrupting a face-centered interaction for most aromatic units.21
Importantly, the polar/pi model also predicts that when strongly electron-withdrawing functional groups are placed on the periphery of an aromatic molecule, the π-electron cloud can be polarized such that the quadrupole moment is essentially reversed, creating a generally electron-deficient aromatic core and a partially negatively-charged periphery. A reversed polarization of the aromatic core is used to explain why aromatics with strongly electron-withdrawing substituents prefer to stack in an alternating face-centered geometry when associating with relatively electron-rich aromatics, for example in the solid-state complex of benzene with hexafluorobenzene.22, 23
“LOCAL, DIRECT INTERACTION” MODEL
An alternative view of the interaction between substituted aromatic molecules was suggested through the early experimental work of Rashkin and Waters.24 They noted that the orientation of stacked substituted aromatic molecules plays a role in the magnitude of observed interactions between aromatic units. It was suggested that direct, through-space electrostatic interactions of polarized substituents around the periphery of the aromatic molecules provide a stabilizing effect.
Using calculated interaction energies between substituted benzene dimers, Wheeler and Houk investigated the idea that direct complementary interactions between the substituents of aromatic units provide a driving force for stacking interactions.25 For example, the computed interaction energies between a mono-substituted benzene (C6H5-X) and unsubstituted benzene (C6H6) were compared to the energies calculated for the corresponding H-X analogue with benzene. The resulting trends in interaction energies indicated that no significant additional stabilizing energy was afforded by the aromatic core of the substituted benzene relative to the H-X analogue, even when the substituents (X) demonstrated similar trends in interaction energies. This led to the conclusion that “substituent effects in the sandwich configuration of the benzene dimer do not involve the π-system of the substituted benzene”.25 This observation was further investigated with experimental work by Houk et al.26 and computational work by Wheeler27, 28 to develop a refined model to predict the interaction of substituted aromatic units. This model, deemed the “local, direct interaction” model,27 proposes that the local, direct through-space electrostatic attraction/repulsion between highly polarized substituents around the periphery of aromatic units is the dominant factor determining stacking geometry. Furthermore, the work of Sherill et al.29-31, Snyder et al.32, Lee et al.33, and Grimme34 have also indicated the importance of the interactions of the substituents around the periphery of aromatic molecules.
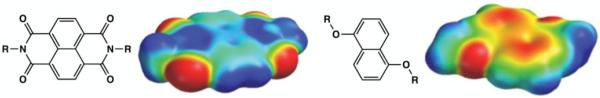
Our work in the field of aromatic interactions is based on the use of substituted naphthyl aromatic units (DAN and NDI). The electrostatic potential surface maps of these two molecules reveal the polarization of the aromatic core and substituents derived from electron-donating alkoxy groups and electron-withdrawing imide carbonyl groups, respectively (Figure 2). Therefore, an alternating, face-centered geometry is predicted for these two complementary aromatic molecules in the solid state as well as in solution, and is indeed observed (Figure 3a).35 Note that face-centered stacking is predicted by either the “polar/pi” or “local, direct interaction” models. Additionally, it has been observed that the π-orbital mixing introduced by this face-centered geometry results in a charge-transfer absorbance and thus a characteristic red-purple color once the colorless solutions of DAN and NDI are mixed. For situations in which complexes demonstrate charge-transfer absorbance, an alternative aromatic donor-acceptor terminology has been historically used. The electron-rich aromatics such as DAN are referred to as a “donor” while electron-deficient aromatics such as NDI are referred to as an “acceptor” because the charge-transfer absorbance occurs when an electron from the HOMO of the donor is excited to the LUMO of the adjacent acceptor.
Figure 2.
Molecular structures and electrostatic potential surfaces calculated for (left) NDI and (right) DAN using DFT (B3LYP/6-31G*) as implemented in Spartan (Wavefunction, Inc.)
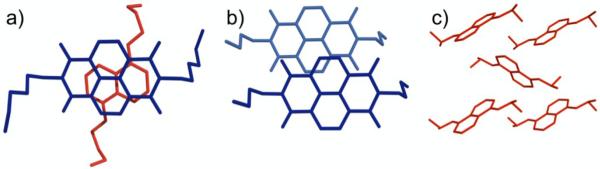
Figure 3.
X-ray crystal structures of (a) DAN-NDI face-centered stacked monomers, (b) NDI-NDI offset stacked monomers, and (c) DAN herringbone geometry in the solid state. Figure reprinted from reference 73.
A sample of pure NDI crystallizes in an off-set stacking mode (Figure 3b).36 Closer examination of NDI-NDI self-stacked crystal structure reveals that the carbonyl oxygen atom of one NDI is in close proximity to the carbonyl carbon of the adjacent NDI unit, suggesting a qualitative explanation for the solid-state geometry that is best predicted by the “local, direct interaction” model. Note that a sample of pure DAN crystallizes in a herringbone arrangement in the solid state (Figure 3c).36
You will notice that “pi-stacking” and/or “pi-pi interactions” were not mentioned despite the fact that stacked aromatics are being discussed. As described in the recent article by Martinez and Iverson37, these terms do not have meaning when discussing energetically important interactions despite their common use (or misuse in our opinion) in the literature. When it comes to aromatic units, stacking is a geometry, not a particular interaction.
While dispersion does contribute to the stability of stacked aromatic molecules (and is even the dominant attractive contribution to the interaction energy for the benzene dimer in certain configurations as calculated by energy decomposition analysis)38, it has also been calculated that the favorable dispersion forces seen in the stacked benzene dimer are also equally important in the stacked cyclohexane dimer, further suggesting that aromaticity in monomeric units is not a requirement for the stacked geometry. The association of substituted aromatic units of the size involved with most biological and supramolecular systems (one to four fused aromatic rings) is driven primarily by electrostatic attraction combined with desolvation (see below), not any special interactions such as dispersion associated with the presence of aromatic π-electrons on the face of aromatic units.
AROMATIC INTERACTIONS IN STRONGLY INTERACTING SOLVENTS
Thus far, aromatic interactions have been discussed without considering solvent. However, solvation/desolvation effects have considerable impact on the association of aromatic molecules, especially in aqueous environments. In general, the electrostatic driving force described above is thought to be important and in fact dominant in low polarity, weakly interacting solvents. However, in strongly interacting polar solvents, solvophobic effects dominate. In strongly interacting polar solvents, especially water, aromatic molecules will tend to prefer geometries of maximal surface overlap thereby reducing the amount of surface area in contact with solvent. The important implication is that desolvation considerations alone favor face-centered stacking of aromatic units. Balanced against this preference for a face-centered stacking can be electrostatic interactions favoring other geometries such as off-set stacking or even a herringbone type of geometry. The result is an interesting interplay of forces in which the electrostatic interactions between associated aromatic molecules influence the solvophobic driving force.
In order to probe the influence of solvophobic interactions on aromatic association, monomeric DAN-DAN, NDI-NDI and DAN-NDI interactions were analyzed in solvents of different polarities.39 The association constants for the three different combinations of aromatic units were calculated from 1H NMR chemical shift data (Table 1). The results demonstrated a significant solvent dependence, with associations being by far the strongest in water. Importantly, the DAN-NDI association constant was the strongest by an order of magnitude. The second strongest interaction was seen with the NDI-NDI interactions, and the DAN-DAN complex showed the weakest interaction in every solvent. Taken together, it is clear that in water, the driving force for association must derive from solvophobic interactions of the relatively non-polar aromatic units in a strongly interacting polar solvent, i.e. the hydrophobic effect. However, this is not the entire story.
Table 1.
Association constants (M−1) of monomeric DAN and NDI in their self-association or complementary association in various solvents.
| Solvent | DAN:NDI | NDI:NDI | DAN:DAN |
|---|---|---|---|
| D2O | 2045 | 245 | 20 |
| CD3OD | 30 | 8 | 1 |
| CD3CN | 11 | 3 | 1 |
| DMSO-d6 | 3 | 2 | 1 |
| Acetone-d6 | 8 | 1 | 1 |
| CDCl3 | 2 | negligible | negligible |
Shaded data provides evidence for desolvation as a driving force for association. Boxed data provides evidence for an electrostatic driving force. Adapted with permission from J. Am. Chem. Soc., 2001, 123, 7560-7563. Copyright 2001 American Chemical Society.
Solid-state structural preferences, derived largely from interactions of highly polarized substituents on the periphery of the aromatic rings (local, direct interaction model), are predictive of overall association constants in water when the amount of solvent-exposed surface area is considered. The DAN-NDI interaction, known to prefer alternating face-centered stacking in the solid state, shows the highest association constant, presumably because maximum hydrophobic surface area is buried due to the face-centered complex geometry. The NDI-NDI complex, known to prefer an off-set stacking geometry in the solid state that would lead to an intermediate level of hydrophobic surface area exposed, exhibits an intermediate association. The DAN-DAN complex, known to prefer a herringbone arrangement in the solid state that would lead to the most hydrophobic surface area left exposed to solvent, displayed the lowest association constants. The bottom line here is that both experimental and theoretical studies of interacting aromatic molecules have revealed a range of behaviours,21 leading to an increasing number of rationally designed foldamers and higher order architectures.
SURVEY OF IVERSON LAB AROMATIC FOLDAMERS
AROMATIC FOLDAMER AND ASSEMBLY
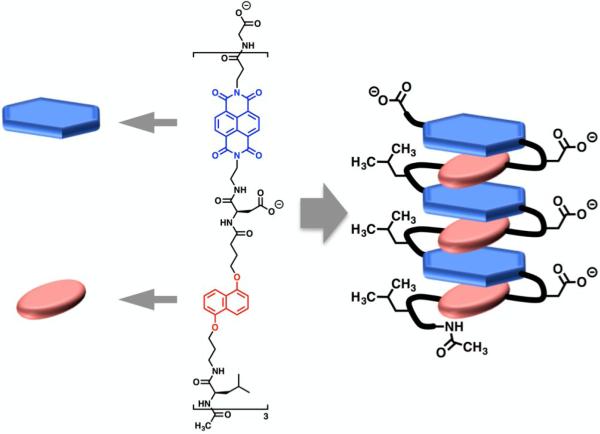
In 1995, Lokey and Iverson described the first aromatic foldamer in an aqueous environment (Figure 4).14 In an alternating fashion, DAN and NDI units were strung together with flexible peptide linkers. Glutamic acid residues were chosen to link the aromatic units together in order to impart water solubility and also to prevent intermolecular aggregation. The molecule was designed to adopt a pleated secondary structure in aqueous solution driven by the face-centered stacking of the alternating DAN and NDI units.
Figure 4.
Molecular structure and cartoon diagram of folded conformation of Lokey's first aromatic foldamer.
Comparison of 1H NMR chemical shifts and especially hypochromism in the UV-Vis absorbance spectra (which is characteristic of face-centered aromatic stacking) revealed that the DAN-NDI foldamer was indeed folded into the predicted pleated structure, placing the aromatic moieties in a face-centered stacked conformation. The aromatic stacked geometry was further affirmed by the resulting plum color characteristic of the formation of charge-transfer (CT) absorption bands when DAN and NDI are stacked in a face-centered fashion.
Being peptide-based, this new class of foldamers could be easily synthesized using standard solid phase peptide synthesis, which was a favorable characteristic for exploring the effects of the nature of the linkers on structure and foldamer properties. Additionally, with the use of several different spectroscopic handles (UV-Vis spectroscopy, 1H NMR chemical shifts, and 2-dimensional COSY data) the folding conformation of these aromatic foldamers was easily confirmed.
The successful formation of the organized architecture gave credence to the pleated secondary structure design generated by aromatic units within an aqueous environment. This first-generation aromatic foldamer has resemblance to the DNA double helix, but is so entirely distinct that it is reasonable to claim an entirely new type of higher order architecture for these folded systems, the first such synthetic system operating in water.
AMPHIPHILIC AROMATIC FOLDAMER
The predictable higher-order architecture and facile synthetic route of our first aromatic foldamer opened the door for deeper investigations of aromatic foldamers, their properties, and the factors that contributed to their ordered structure. Because the linkers that strung together the DAN and NDI units were peptides, the character of the aromatic foldamer could be changed depending on which amino acid residues were chosen. The length of the linker and order of aromatic units could also be changed, leading to aromatic foldamers that were not based on alternating DAN-NDI sequences, yet nevertheless folded in unique topologies that assumed an alternating DAN-NDI stacking arrangement.40
A series of amphiphilic DAN-NDI foldamers were designed and synthesized. When folded, hydrophilic aspartic acid residues were predicted to be on one side of the structure, while hydrophobic leucine residues were located on the other side (Figure 5).41 The structure was designed to be conceptually similar to the leucine zipper motif found in proteins.42 Spectroscopic analysis of this new amphiphilic aromatic foldamer proved to have similar hypochromism and charge-transfer absorbance in aqueous buffer. However, the unresolved 1H NMR spectra of the molecule in D2O gave evidence for extensive aggregation, which was further confirmed with dynamic light scattering experiments.
Figure 5.
Molecular structure and cartoon depicting the folded state of the amphiphilic aromatic foldamer.
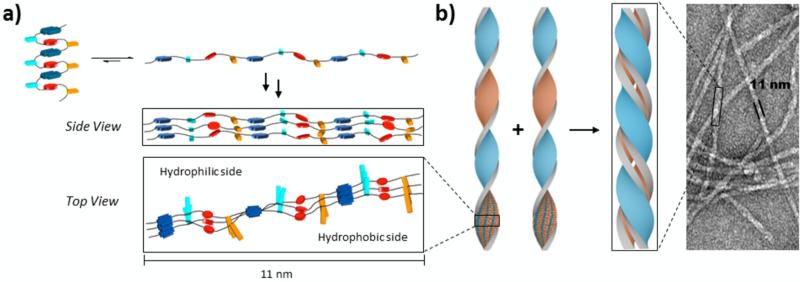
Surprisingly, when the aqueous solution of this amphiphilic foldamer was heated above 80°C, the dark red-purple color changed to a pale-yellow and the viscosity of the solution notably increased. Further spectroscopic investigation of the resulting hydrogel demonstrated the loss of the charge-transfer band, an increased UV-Vis baseline and higher degree of aggregation. The hydrogel persisted for extended periods, not returning back to the original state after even months at room temperature, demonstrating that an irreversible conformational change had occurred upon heating. Additionally, CD spectroscopy determined that the aggregated state of the hydrogel was actually a highly-ordered structure. This intriguing behavior not only demonstrated that changing the linkers connecting these aromatic units can have a profound effect on its physical properties, but it also bore striking similarities to the process and properties of natural amyloid formation.43
A detailed model was proposed for the hydrogel formation of the amphiphilic aromatic foldamer.44 The work of Parquette et al.45-47, Matile et al.48, 49, Govindaraju et al.50, 51, and Ghosh et al.52 involving NDI-driven supramolecular assemblies, have provided additional insight in to the formation of these hydrogel aggregates. Characteristic UV and CD spectroscopic traces indicative of NDI off-set, twisted self-assembly was identified in the aggregated sample. Further, TEM and AFM images revealed fibrils of uniform width and regular helicities (Figure 6b). Therefore, it was proposed that the formation of this newly aggregated structure was due to a conformational switching of the DAN-NDI intramolecular stacked foldamer to an NDI-NDI intermolecular off-set stacked fibril aggregate (Figure 6a).44 The NDI-NDI interactions form the basis for an amphiphilic tape-like structure, which then pairs together to occlude the hydrophobic faces of each. The result is a bilayer tape-like assembly, with only hydrophilic surfaces exposed on either side. The amount of hydrophobic surface area buried by this bilayer assembly is extensive, explaining the irreversible nature of its formation. Being the first synthetic analog with behavior analogous to the natural amyloid fibrils observed in many diseases including Alzheimer's disease, the amphiphilic aromatic foldamer has shed considerable light on the energetics and even mechanism of amyloid fibril assembly.
Figure 6.
Proposed model depicting the conformational shift to form one-dimensional fibrils. Adapted with permission from Chemistry, 2013, 19, 11598-11602. Copyright 2013 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
The amphiphilic foldamer studies, along with additional support from other NDI driven supramolecular structures, have confirmed that the NDI-NDI off-set stacking interaction should also be considered as a significant interaction, capable of forming the basis of higher-order architectures in aqueous solution.
HETERODUPLEX ASSEMBLY
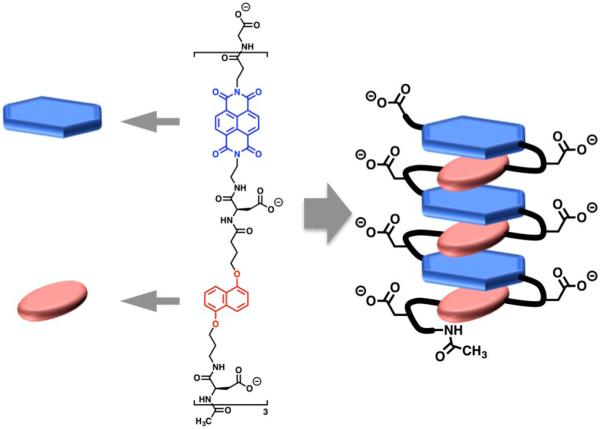
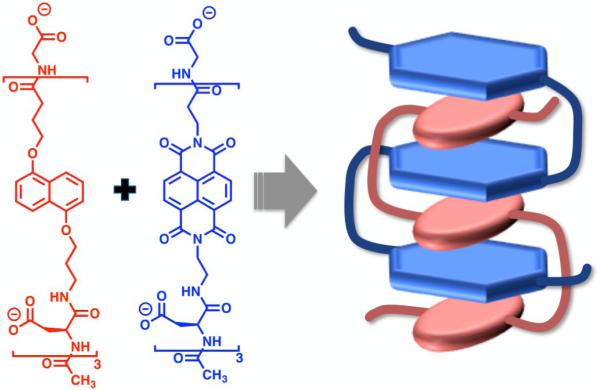
The aromatic interactions that drove the formation of pleated architectures in our early foldamer designs were then exploited to drive two oligomers (oligo-NDI and oligo-DAN) into an intertwined heteroduplex in aqueous solution (Figure 7).53 These aromatic units were linked together using aspartic acid residues, imparting water solubility. These acidic amino acids also reduced the aggregation of like-charged chains, enhancing the specificity of the complementary DAN-NDI association driving force. Similar interactions have also been demonstrated to drive heteroduplex formation in organic media by Li and co-workers in both zipper54 and intertwined55 modes. Heteroduplexes have also been constructed utilizing various other non-covalent interactions to fold in a variety of ways. Hydrogen bond-driven heteroduplex formation has been demonstrated by Gong and co-workers in the formation of linear oligoamide duplexes56 and also Krische and co-workers in the construction of duplex forming oligoaminotriazines57 for example. Combinations of both aromatic interactions and hydrogen bonding were exploited in the formation of heteroduplexes formed by Bisson and co-workers in a zipper-like fashion58 and also by Lehn and co-workers in a double-helical conformation.59, 60 Additionally, Lehn and co-workers have developed metal-coordinating foldamer heteroduplexes of a helical conformation.61, 62
Figure 7.
Molecular structure and cartoon depicting the formation of the intertwined heteroduplex.
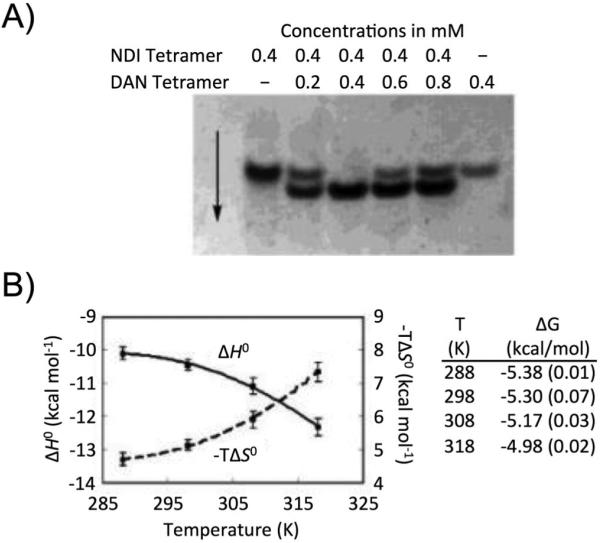
Heteroduplex formation was supported by a series of NMR, polyacrylamide gel electrophoresis (PAGE) and ITC experiments (Figure 8). The expected face-centered NDI-DAN interaction was confirmed through 1H NMR peak shift experiments by comparison with the association of the monomeric DAN and NDI units. A 1:1 duplex formation was confirmed by the PAGE gel experiments in analogy to DNA double helix formation, as a fast-moving oligo-DAN-oligo-NDI duplex was observed as the only band when a 1:1 mixture of the oligo-DAN and oligo-NDI units were mixed. Additionally, a surprising temperature independence in the association constant for the NDI-dimer with its complementary DAN-dimer was observed, indicating an enthalpy-entropy compensation effect was operating.
Figure 8.
(A) Gel-shift assay to demonstrate duplex formation. (B) Thermodynamic data of DAN-dimer and NDI-dimer association. Adapted with permission from J. Am. Chem. Soc., 2002, 124, 15174-15175. Copyright 2002 American Chemical Society.
NDI AND DAN DNA
The intertwined assembly of two negatively-charged chains driven by desolvation and interactions of aromatic units naturally turned attention to the biologically relevant context of DNA. Within the realm of “nucleotidomimetic” foldamers, two general approaches have been reported; modifications to the aromatic units or modifications to the backbone. From this research, a number of modified backbones have emerged that form well-defined secondary structures: peptide nucleic acids (PNA), threofuranosyl nucleic acids (TNA), and glycol nucleic acids (GNA) to name a few. Additionally, modified aromatic units have been also explored that could alter DNA stability and structure63-65, possibly expand the genetic code66-70, or even be assembled by the highly predictable double-helical structure of DNA71, 72.
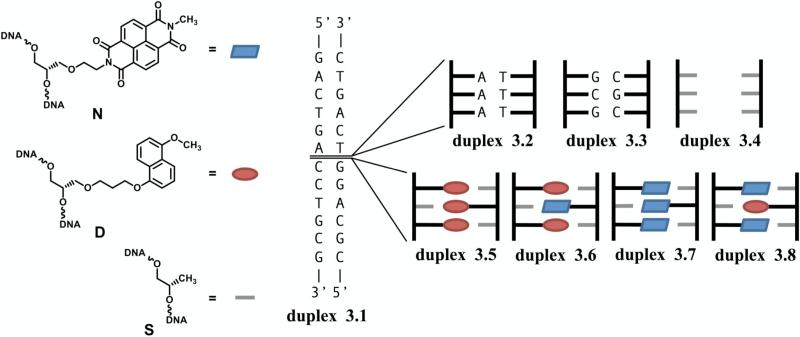
Our particular interest is in the stabilizing effect of complementary stacked aromatic units within the context of the DNA duplex. Initial investigations of this concept involved inserting DAN and NDI aromatic units into a three-base-pair region within the interior of a 12-mer DNA duplex to allow the natural DNA base flanking sequences to direct the DAN-NDI interactions (Figure 9).73 The design of this system was such that the DAN and NDI units should be pre-organized into their preferred stacked orientation, directed by the flanking natural DNA sequences (Figure 10). Phosphoramidite monomers of DAN and NDI were synthesized and incorporated into oligomer strands of DNA. The well-defined double helical structure of DNA with predictable properties such as melting temperature based on the sequence of nucleotide base pairs provided a standard reference for the stabilizing effect of the DAN and NDI units.
Figure 9.
Modified DNA base surrogates and a cartoon representing the control DNA duplex 1 as well as the insertions of natural DNA bases (duplex 2 and duplex 3), spacer units (duplex 4) and the four NDI and DAN modified units (duplexes 5-8). Figure reprinted from reference 73.
Figure 10.
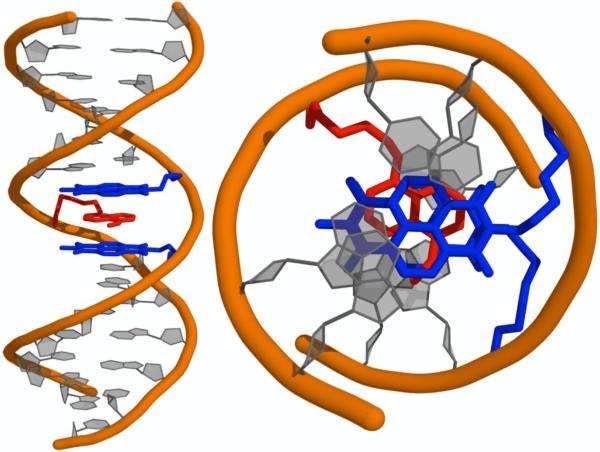
Model of the predicted NDI-DAN interaction flanked by natural DNA bases (left) side view; (right) top-down view. NDI and DAN are shown in blue and red respectively. Figure reprinted with permission from reference 73.
After annealing these complementary modified oligonucleotides, CD spectroscopy revealed that the overall B-form double-helical structure was relatively unaffected by the insertion of the NDI and DAN aromatic units.
Through UV melting temperature experiments, it was found that the NDI-DAN-NDI interaction served to stabilize the duplex melting temperature comparable to three A-T base pairs inserted into the same sequence. Additionally, the trends in varying the combinations of DAN and NDI affirmed the prediction that the strongest association of substituted aromatic molecules would be those in which electrostatic complementarity of the aromatic substituents favor face-centered stacking geometries, presumably allowing for the greatest desolvation driving force. One can imagine how NDI and DAN might be utilized to stabilize a poly-phosphodiester heteroduplex on its own, without the aid of the pre-organization provided by flanking DNA base pairs.
OUTLOOK
The electrostatically-complementary aromatic DAN and NDI units have proven to be of unprecedented versatility for the assembly of various aromatic foldamers and other assemblies in aqueous environments. Folding and assembly based on the DAN-NDI interaction has ranged from a pleated secondary structure to higher order architectures of one-dimensional fibrils, and ultimately the assembly of heteroduplexes. Not mentioned here is our extensive work with DNA-binding oligo-NDI molecules.74-76 Considering the complex nature of aromatic interactions, the predictable stacking geometry of DAN and NDI in water provides a well-defined recognition motif to achieve higher order architectures. Because the stacking of electrostatically complementary aromatic units is driven in part by polar solvents, the construction of aromatic foldamers and more complex aromatic assemblies in water can be more rationally designed. Therefore, one can predict a very bright future for the further development of new generations of aromatic-based foldamers and more complex assemblies in water.
Supplementary Material
Acknowledgements
This work was supported by the Robert A. Welch Foundation (F1188) and the National Institutes of Health (GM-069647).
References
- 1.Gellman SH. Acc. Chem. Res. 1998;31:173–180. [Google Scholar]
- 2.Appella DH, Christianson LA, Klein DA, Powell DR, Huang XL, Barchi JJ, Gellman SH. Nature. 1997;387:381–384. doi: 10.1038/387381a0. [DOI] [PubMed] [Google Scholar]
- 3.Dado GP, Gellman SH. J. Am. Chem. Soc. 1994;116:1054–1062. [Google Scholar]
- 4.Krauthauser S, Christianson LA, Powell DR, Gellman SH. J. Am. Chem. Soc. 1997;119:11719–11720. [Google Scholar]
- 5.Hintermann T, Seebach D. Synlett. 1997:437–438. [Google Scholar]
- 6.Seebach D, Gademann K, Schreiber JV, Matthews JL, Hintermann T, Jaun B, Oberer L, Hommel U, Widmer H. Helv. Chim. Acta. 1997;80:2033–2038. [Google Scholar]
- 7.Seebach D, Matthews JL. Chem. Commun. 1997:2015–2022. [Google Scholar]
- 8.Seebach D, Overhand M, Kuhnle FNM, Martinoni B, Oberer L, Hommel U, Widmer H. Helv. Chim. Acta. 1996;79:913–941. [Google Scholar]
- 9.Stone MT, Moore JS. Org. Lett. 2004;6:469–472. doi: 10.1021/ol036238k. [DOI] [PubMed] [Google Scholar]
- 10.Bassani DM, Lehn JM, Baum G, Fenske D. Angewandte Chemie-International Edition in English. 1997;36:1845–1847. [Google Scholar]
- 11.Gardinier KM, Khoury RG, Lehn JM. Chemistry-a European Journal. 2000;6:4124–4131. doi: 10.1002/1521-3765(20001117)6:22<4124::aid-chem4124>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Ohkita M, Lehn JM, Baum G, Fenske D. Chemistry-a European Journal. 1999;5:3471–3481. [Google Scholar]
- 13.Sebaoun L, Maurizot V, Granier T, Kauffmann B, Huc I. J. Am. Chem. Soc. 2014;136:2168–2174. doi: 10.1021/ja412729s. [DOI] [PubMed] [Google Scholar]
- 14.Scott Lokey R, Iverson BL. Nature. 1995;375:303–305. [Google Scholar]
- 15.Hunter CA, Sanders JK. J. Am. Chem. Soc. 1990;112:5525–5534. [Google Scholar]
- 16.Cozzi F, Annunziata R, Benaglia M, Cinquini M, Raimondi L, Baldridge KK, Siegel JS. Org Biomol Chem. 2003;1:157–162. doi: 10.1039/b208871a. [DOI] [PubMed] [Google Scholar]
- 17.Cozzi F, Cinquini M, Annunziata R, Dwyer T, Siegel JS. J. Am. Chem. Soc. 1992;114:5729–5733. [Google Scholar]
- 18.Cozzi F, Cinquini M, Annuziata R, Siegel JS. J. Am. Chem. Soc. 1993;115:5330–5331. [Google Scholar]
- 19.Cozzi F, Ponzini F, Annunziata R, Cinquini M, Siegel JS. Angewandte Chemie International Edition in English. 1995;34:1019–1020. [Google Scholar]
- 20.Cozzi F, Siegel JS. Pure Appl. Chem. 1995;67:683–689. [Google Scholar]
- 21.Hunter CA, Lawson KR, Perkins J, Urch CJ. Journal of the Chemical Society-Perkin Transactions 2. 2001:651–669. DOI: 10.1039/b008495f. [Google Scholar]
- 22.Williams JH, Cockcroft JK, Fitch AN. Angewandte Chemie-International Edition in English. 1992;31:1655–1657. [Google Scholar]
- 23.Patrick CR, Prosser GS. Nature. 1960;187:1021–1021. [Google Scholar]
- 24.Rashkin MJ, Waters ML. J. Am. Chem. Soc. 2002;124:1860–1861. doi: 10.1021/ja016508z. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler SE, Houk KN. J. Am. Chem. Soc. 2008;130:10854–10855. doi: 10.1021/ja802849j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler SE, McNeil AJ, Muller P, Swager TM, Houk KN. J. Am. Chem. Soc. 2010;132:3304–3311. doi: 10.1021/ja903653j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler SE. J. Am. Chem. Soc. 2011;133:10262–10274. doi: 10.1021/ja202932e. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler SE, Bloom JW. J. Phys. Chem. A. 2014;118:6133–6147. doi: 10.1021/jp504415p. [DOI] [PubMed] [Google Scholar]
- 29.Ringer AL, Sinnokrot MO, Lively RP, Sherrill CD. Chemistry – A European Journal. 2006;12:3821–3828. doi: 10.1002/chem.200501316. [DOI] [PubMed] [Google Scholar]
- 30.Sinnokrot MO, Sherrill CD. The Journal of Physical Chemistry A. 2003;107:8377–8379. [Google Scholar]
- 31.Arnstein SA, Sherrill CD. PCCP. 2008;10:2646–2655. doi: 10.1039/b718742d. [DOI] [PubMed] [Google Scholar]
- 32.Snyder SE, Huang B-S, Chu YW, Lin H-S, Carey JR. Chemistry – A European Journal. 2012;18:12663–12671. doi: 10.1002/chem.201202253. [DOI] [PubMed] [Google Scholar]
- 33.Lee EC, Kim D, Jurečka P, Tarakeshwar P, Hobza P, Kim KS. The Journal of Physical Chemistry A. 2007;111:3446–3457. doi: 10.1021/jp068635t. [DOI] [PubMed] [Google Scholar]
- 34.Grimme S. Angew. Chem. Int. Ed. 2008;47:3430–3434. doi: 10.1002/anie.200705157. [DOI] [PubMed] [Google Scholar]
- 35.Reczek JJ, Villazor KR, Lynch V, Swager TM, Iverson BL. J. Am. Chem. Soc. 2006;128:7995–8002. doi: 10.1021/ja061649s. [DOI] [PubMed] [Google Scholar]
- 36.Alvey PM, Reczek JJ, Lynch V, Iverson BL. J. Org. Chem. 2010;75:7682–7690. doi: 10.1021/jo101498b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez CR, Iverson BL. Chemical Science. 2012;3:2191–2201. [Google Scholar]
- 38.Alonso M, Woller T, Martin-Martinez FJ, Contreras-Garcia J, Geerlings P, De Proft F. Chemistry. 2014;20:4931–4941. doi: 10.1002/chem.201400107. [DOI] [PubMed] [Google Scholar]
- 39.Cubberley MS, Iverson BL. J. Am. Chem. Soc. 2001;123:7560–7563. doi: 10.1021/ja015817m. [DOI] [PubMed] [Google Scholar]
- 40.Gabriel GJ, Sorey S, Iverson BL. J. Am. Chem. Soc. 2005;127:2637–2640. doi: 10.1021/ja046722y. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen JQ, Iverson BL. J. Am. Chem. Soc. 1999;121:2639–2640. [Google Scholar]
- 42.O'Shea EK, Klemm JD, Kim PS, Alber T. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 43.Bradford VJ, Iverson BL. J. Am. Chem. Soc. 2008;130:1517–1524. doi: 10.1021/ja0780840. [DOI] [PubMed] [Google Scholar]
- 44.Peebles C, Piland R, Iverson BL. Chemistry. 2013;19:11598–11602. doi: 10.1002/chem.201302009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao H, Gao M, Kim SH, Jaroniec CP, Parquette JR. Chemistry – A European Journal. 2011;17:12882–12885. doi: 10.1002/chem.201102616. [DOI] [PubMed] [Google Scholar]
- 46.Shao H, Nguyen T, Romano NC, Modarelli DA, Parquette JR. J. Am. Chem. Soc. 2009;131:16374–16376. doi: 10.1021/ja906377q. [DOI] [PubMed] [Google Scholar]
- 47.Shao H, Parquette JR. Chem. Commun. 2010;46:4285–4287. doi: 10.1039/c0cc00701c. [DOI] [PubMed] [Google Scholar]
- 48.Talukdar P, Bollot G, Mareda J, Sakai N, Matile S. Chemistry – A European Journal. 2005;11:6525–6532. doi: 10.1002/chem.200500516. [DOI] [PubMed] [Google Scholar]
- 49.Talukdar P, Bollot G, Mareda J, Sakai N, Matile S. J. Am. Chem. Soc. 2005;127:6528–6529. doi: 10.1021/ja051260p. [DOI] [PubMed] [Google Scholar]
- 50.Avinash M, Govindaraju T. Nanoscale. 2011;3:2536–2543. doi: 10.1039/c0nr00766h. [DOI] [PubMed] [Google Scholar]
- 51.Pandeeswar M, Avinash MB, Govindaraju T. Chemistry – A European Journal. 2012;18:4818–4822. doi: 10.1002/chem.201200197. [DOI] [PubMed] [Google Scholar]
- 52.Kar H, Molla MR, Ghosh S. Chem. Commun. 2013;49:4220–4222. doi: 10.1039/c2cc36536g. [DOI] [PubMed] [Google Scholar]
- 53.Gabriel GJ, Iverson BL. J. Am. Chem. Soc. 2002;124:15174–15175. doi: 10.1021/ja0275358. [DOI] [PubMed] [Google Scholar]
- 54.Zhou QZ, Jiang XK, Shao XB, Chen GJ, Jia MX, Li ZT. Org. Lett. 2003;5:1955–1958. doi: 10.1021/ol034549p. [DOI] [PubMed] [Google Scholar]
- 55.Zhou QZ, Jia MX, Shao XB, Wu LZ, Jiang XK, Li ZT, Chen GJ. Tetrahedron. 2005;61:7117–7124. [Google Scholar]
- 56.Zeng H, Ickes H, Flowers RA, 2nd, Gong B. J. Org. Chem. 2001;66:3574–3583. doi: 10.1021/jo010250d. [DOI] [PubMed] [Google Scholar]
- 57.Gong H, Krische MJ. J. Am. Chem. Soc. 2005;127:1719–1725. doi: 10.1021/ja044566p. [DOI] [PubMed] [Google Scholar]
- 58.Bisson AP, Carver FJ, Hunter CA, Waltho JP. J. Am. Chem. Soc. 1994;116:10292–10293. [Google Scholar]
- 59.Berl V, Huc I, Khoury RG, Krische MJ, Lehn JM. Nature. 2000;407:720–723. doi: 10.1038/35037545. [DOI] [PubMed] [Google Scholar]
- 60.Berl V, Huc I, Khoury RG, Lehn JM. Chemistry-a European Journal. 2001;7:2810–2820. doi: 10.1002/1521-3765(20010702)7:13<2810::aid-chem2810>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 61.Smith VCM, Lehn JM. Chem. Commun. 1996:2733–2734. DOI: DOI 10.1039/cc9960002733. [Google Scholar]
- 62.Stiller R, Lehn JM. Eur. J. Inorg. Chem. 1998:977–982. [Google Scholar]
- 63.Wang W, Wan W, Zhou H-H, Niu S, Li ADQ. J. Am. Chem. Soc. 2003;125:5248–5249. doi: 10.1021/ja0341900. [DOI] [PubMed] [Google Scholar]
- 64.Zheng Y, Long H, Schatz GC, Lewis FD. Chem. Commun. (Cambridge, U. K.) 2005:4795–4797. doi: 10.1039/b509754a. DOI: 10.1039/b509754a. [DOI] [PubMed] [Google Scholar]
- 65.Brotschi C, Mathis G, Leumann CJ. Chem. - Eur. J. 2005;11:1911–1923. doi: 10.1002/chem.200400858. [DOI] [PubMed] [Google Scholar]
- 66.Schweitzer BA, Kool ET. J. Am. Chem. Soc. 1995;117:1863–1872. doi: 10.1021/ja00112a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wojciechowski F, Leumann CJ. Chem. Soc. Rev. 2011;40:5669–5679. doi: 10.1039/c1cs15027h. [DOI] [PubMed] [Google Scholar]
- 68.Leconte AM, Hwang GT, Matsuda S, Capek P, Hari Y, Romesberg FE. J. Am. Chem. Soc. 2008;130:2336–2343. doi: 10.1021/ja078223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamashige R, Kimoto M, Takezawa Y, Sato A, Mitsui T, Yokoyama S, Hirao I. Nucleic Acids Res. 2012;40:2793–2806. doi: 10.1093/nar/gkr1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piccirilli JA, Krauch T, Moroney SE, Benner SA. Nature. 1990;343:33–37. doi: 10.1038/343033a0. [DOI] [PubMed] [Google Scholar]
- 71.Wagenknecht H-A. Angew. Chem., Int. Ed. 2009;48:2838–2841. doi: 10.1002/anie.200900327. [DOI] [PubMed] [Google Scholar]
- 72.Endo M, Fujitsuka M, Majima T. J. Org. Chem. 2008;73:1106–1112. doi: 10.1021/jo7025004. [DOI] [PubMed] [Google Scholar]
- 73.Ikkanda BA, Samuel SA, Iverson BL. J. Org. Chem. 2014;79:2029–2037. doi: 10.1021/jo402704z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rhoden Smith A, Iverson BL. J. Am. Chem. Soc. 2013;135:12783–12789. doi: 10.1021/ja4057344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith AR, Ikkanda BA, Holman GG, Iverson BL. Biochemistry. 2012;51:4445–4452. doi: 10.1021/bi300317n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holman GG, Zewail-Foote M, Smith AR, Johnson KA, Iverson BL. Nature Chemistry. 2011;3:875–881. doi: 10.1038/nchem.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.