Highlights
-
•
We presented two esophageal cancer patients performed thoracoscopic esophagectomy.
-
•
These two cases have lymph node metastasis of dorsal area of thoracic aorta (DTA).
-
•
We performed successfully underwent the dissection of lymph node of DTA.
-
•
The bilateral thoracoscopic approach performedsafely in the prone position.
-
•
The long-term outcome of lymphadenectomy in the DTA among esophageal cancer patients remain controversial.
Abbreviations: DTA, dorsal area of the thoracic aorta; CT, computed tomography; PET, 18F 2-fluoro-2-deoxy-d-glucose positron emission tomography; SUVmax, maximum standardised uptake value
Keywords: Esophagus, Lymph node metastasis, Left thoracic cavity, Thoracoscopy, Thoracoscopic esophagectomy
Abstract
Introduction
The incidence of lymph node metastasis in the dorsal area of the thoracic aorta (DTA) is relatively low in patients with esophageal cancer. It is difficult to approach the DTA using surgical procedures, such as an open thoracotomy and thoracoscopy in the left decubitus position.
Case presentation
Case 1: A 70-year-old man with esophageal cancer underwent thoracoscopic esophagectomy with mediastinal lymph node dissection via a right thoracoscopic approach, followed by lymphadenectomy in the DTA via left thoracoscopy in the prone position. Microscopic findings revealed two metastatic lymph nodes in the DTA. The definitive diagnosis was squamous cell carcinoma of the esophagus, and the pathological stage was T2N3M0 (Union for International Cancer Control [UICC], 7th edition). The patient showed lung metastasis 8 months after the surgery. Case 2: A 72-year-old man with esophageal cancer underwent esophagectomy via a bilateral approach in the prone position, using a similar procedure as in case 1. The definitive diagnosis was squamous cell carcinoma of the esophagus, and the pathological stage was T3N2M0. The patient showed a metastatic mediastinal lymph node 4 months after the surgery.
Conclusion
Bilateral thoracoscopic esophagectomy in the prone position can be safely performed, and it might be an alternative curative surgery for esophageal cancer. However, both our cases showed metastasis in the early postoperative period. The long-term outcome and significance of dissection of lymph nodes in the DTA in patients with esophageal cancer remains controversial. Further studies are required to establish the indications and efficacy of this therapeutic approach.
1. Introduction
Lymph node metastasis in the dorsal area of the thoracic aorta (DTA) in patients with esophageal cancer is relatively rare [1], and thoracoscopic esophagectomy with lymph node dissection is difficult via a standard right thoracic approach. Thoracoscopic surgery for esophageal cancer via the left thoracic cavity has been reported in patients with situs inversus totalis [2], [3], [4]. However, there have been only few reports on thoracic surgery via a left thoracic approach for dissection of lymph nodes in the DTA among esophageal cancer patients [5]. Here, we present two cases of esophageal cancer with lymph node metastasis in the DTA that successfully underwent thoracoscopic esophagectomy via a bilateral thoracoscopic approach performed in the prone position. Furthermore, we have discussed and reviewed relevant literature regarding the long-term outcome and significance of dissection of lymph nodes in the DTA among esophageal cancer patients.
2. Case presentation
2.1. Case 1
A 70-year-old man visited a physician with a complaint of difficulty in swallowing and was referred to our hospital. His medical history included hypertension, hyperlipidemia, and a vocal cord polyp. Upper gastrointestinal tract endoscopy showed a type 3 tumor in the upper thoracic esophagus. Endoscopic biopsy samples were obtained from the tumor, and analysis revealed squamous cell carcinoma of the esophagus. Computed tomography (CT) showed a thick wall in the upper esophagus and lymph node swelling around the bilateral recurrent laryngeal nerves and DTA (Fig. 1a, b). Positron emission tomography (PET) was performed, and the maximum standardized uptake value of the lymph nodes in the DTA was 3.1. The clinical TNM staging (TNM 7th edition) was T3N3M0 stage IIIB.
Fig. 1.
Computed tomography images.
Computed tomography images show a thick wall in the upper thoracic esophagus (a) and lymph node swelling in the dorsal area of the thoracic aorta (b).
Two cycles of 5-fluorouracil (800 mg/m2 from days 1–5) and cisplatin (80 mg/m2 on day 1) were administered intravenously as neo-adjuvant chemotherapy [6], without any adverse events. We evaluated the effect of neo-adjuvant chemotherapy according to the tumor response to chemotherapy based on the response evaluation criteria in solid tumors (RECIST) [7], and noted stable disease (SD) for the primary tumor and the lymph nodes in the DTA. On PET, F-fluoro-2-deoxy-d-glucose accumulation in the lymph nodes in the DTA was not noted. CT showed significant shrinking one of three lymph nodes and no change in the size of the other two lymph nodes in the DTA. Thoracoscopic esophagectomy with lymph node dissection was planned. General anesthesia was administered with single-lumen endotracheal intubation for bilateral lung ventilation. Thoracoscopic esophagectomy with lymph node dissection via a bilateral thoracic approach was performed in the prone position under 8 mmHg of artificial pneumothorax. Thoracic esophagectomy and mediastinal lymph node dissection were performed using five ports via the right thoracic cavity. The thoracic duct was preserved. The operating field was changed from the right to the left thoracic cavity, and the left arm was raised, while the right arm was pulled down. The procedure via the left side approach was performed using four ports (Fig. 2) to dissect around the DTA according to the preoperative navigation CT findings (Fig. 3a). The thoracoscopic operational view is shown in Figs. 3b and c. Lymph nodes around the DTA were close to two left posterior intercostal arteries; therefore, these arteries were divided. Hand-assisted gastric tube mobilization was performed in the supine position. The gastric tube was inserted through the post mediastinal route, and hand-sewn anastomosis was performed between the gastric tube and the esophagus in the neck. Chest tubes in both thoracic cavities were removed 2 days post-surgery. The definitive diagnosis was pathological T2N3M0 (G2, stage IIIC) squamous cell carcinoma of the esophagus. Barium studies revealed no leakage in the anastomosis; however, resumption of diet was delayed due to the endoscopic finding of delayed wound healing in the anastomotic area. The patient was discharged to home 26 days after the surgery (Table 1). CT and PET performed 8 months after the surgery revealed lung metastasis in the left lung, and the patient is being administered chemotherapy intravenously.
Fig. 2.
Placement of the ports.
①: A 5-mm port is placed in the 5th intercostal space along the middle axillary line (grasping forceps). ②: A 5-mm port is placed in the 7th intercostal space along the middle axillary line (operator’s use). ③: A 12-mm port is placed in the 8th intercostal space along the posterior axillary line (assistant’s use). ④: A 12-mm port is placed in the 9th intercostal space along the posterior axillary line (camera port).
Fig. 3.
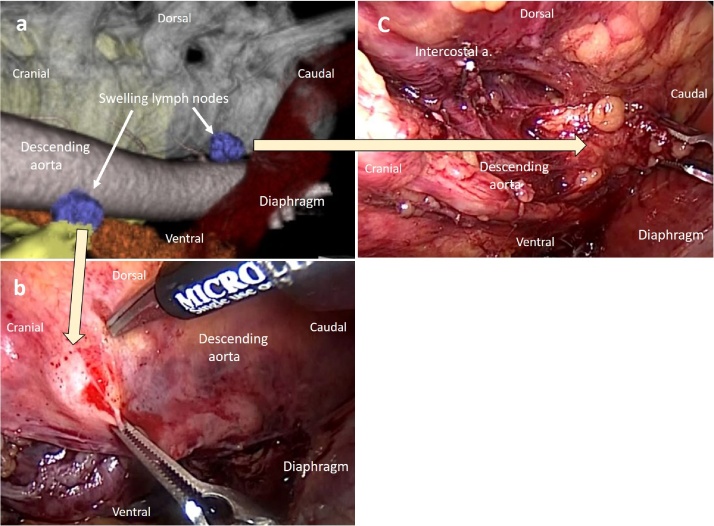
Images of case 1.
a: A preoperative three-dimensional computed tomography image shows the relationship between lymph node swelling in the dorsal area of the thoracic aorta and the neighboring structures.
b: A lymph node swelling in the cranial side of the dorsal area of the thoracic aorta (DTA) is removed using a left thoracoscopic procedure.
c: A lymph node swelling in the caudal side of the DTA is removed using a left thoracoscopic procedure.
Table 1.
Perioperative outcomes.
| Case | Duration of the right thoracic procedure (min) | Duration of the left thoracic procedure (min) | Surgical duration of the thoracic procedure (min) | Blood loss during the thoracic procedure (g) | Hospital stay after the operation (days) |
|---|---|---|---|---|---|
| 1 | 249 | 59 | 308 | 36 | 26 |
| 2 | 519 | 29 | 548 | 140 | 27 |
| Shimada et.al. [5] | 220 | 40 | 260 | <50 | 23 |
2.2. Case 2
A 72-year-old man with complaints of dysphagia and vomiting visited our hospital for the treatment of esophageal cancer. He had undergone appendectomy at 30 years of age.
Upper gastrointestinal tract endoscopy revealed esophageal stenosis due to a tumor present 36 cm from his incisor. Endoscopic biopsy indicated squamous cell carcinoma of the esophagus. CT showed a thick wall in the lower esophagus and lymph node swelling in the DTA (Fig. 4a). The clinical TNM staging (TNM 7th edition) was T3N2M0 stage IIIB. Neoadjuvant chemotherapy was performed according to the same protocol as in case 1, and no adverse events were noted. We evaluated the tumor response to chemotherapy based on RECIST [7], and noted SD for both the primary tumor and the lymph nodes in the DTA. Subtotal esophagectomy with lymph node dissection was planned. General anesthesia was administered as in case 1. Subtotal esophagectomy and lymph node dissection were performed via the right thoracic cavity, followed by dissection of the lymph nodes around the DTA. The procedure via the right thoracic approach was longer in case 2 than in case 1 owing to the hard adhesion of the right lung and pleura. The lymph nodes in the DTA were dissected via the left thoracic cavity, and the intercostal arteries were identified using preoperative three-dimensional CT for reference (Figs. 4b, c). The definitive diagnosis was pathological T3N2M0 (G2, stage IIIB) squamous cell carcinoma of the esophagus. The perioperative outcomes are shown in Table 1. The patient had melena on day 19 due to the sigmoid diverticulitis, which was subsequently treated conservatively. The patient was discharged 27 days after the surgery. CT and PET performed 4 months after the surgery revealed lymph node metastasis around the lower mediastinum and the DTA. The patient is currently being administered chemotherapy intravenously.
Fig. 4.
Images of case 2.
a: A computed tomography image shows a thick wall in the esophagus and metastatic lymph node swelling in the dorsal area of the thoracic aorta (DTA).
b: A three-dimensional computed tomography image shows lymph node swelling in the DTA (arrows) and the course of left intercostal artery (arrow-head).
c: Left thoracoscopic intraoperative view. A metastatic lymph node in the DTA is dissected via the left thoracic cavity.
3. Discussion
The incidence of lymph node metastasis in the DTA in esophageal cancer patients is relatively low. Hatooka et al. reported that this condition was found in 3 of 1029 esophageal cancer patients [1].
Thoracoscopic esophagectomy in the prone position was first reported in 1994 by Cuschieri [8], and it was thought to be superior to thoracoscopic surgery in the lateral decubitus position or open thoracotomy in terms of lower blood loss and fewer pulmonary complications [9], [10]. Shimada et al. reported a bilateral thoracic approach for a patient with lymph node metastasis in the DTA, and the authors mentioned that minimal conversion of the surgical position was the advantage of surgery in the prone position [5]. During the left thoracoscopic procedure, the course of the descending aorta could be recognized and a sufficient surgical field could be obtained in the left thoracic cavity. Both hospitalization and surgical duration of the cases discussed here tended to be longer compared to those in the case reported by Shimada et al. [5] (Table 1). However, this approach is expected to be longer in patients with pleural adhesions, and blood loss is also expected to be more than usual. The procedure is thought to be a safe alternative for patients with metastasis in the DTA; however, only a few cases have been studied in the recent past.
The transesophageal hiatal approach was reported as another approach for the dissection of posterior mediastinal lymph nodes. Shiozaki et al. reported that the posterior mediastinal lymph nodes, including those around the anterior area of thoracic aorta, could be dissected using the pneumomediastinum method [11]. Ninomiya et al. reported on dissection of lymph nodes in the DTA performed using transhiatal mediastinoscopy [12]. During the pneumomediastinal procedure, the operating field is deep and narrow, and the mediastinoscopic view would be more limited with this procedure than with the thoracoscopic procedure. The left thoracic approach appears to be superior to their method, as it provides a wider surgical view. However, the benefit of the mediastinoscopic procedure is that it can be performed for lymphadenectomy in the DTA, followed by the laparoscopic procedure sequentially without any change in the surgical position.
Thoracic esophagectomy is usually performed via the right thoracic approach, and the left thoracic approach is rare in esophageal surgery. We performed three-dimensional CT imaging in order to simulate and understand the relation between the swollen lymph nodes and the surrounding tissues. It is thought that recognition of the anatomy for preoperative navigation is useful to perform the procedure safely [13], [14], [15].
The long-term prognosis of patients who have undergone dissection of metastatic lymph nodes in the DTA is unclear. Shimada et al. reported a case that did not show recurrence 7 months after the operation [5]. However, Nakagawa et al. reported a case that underwent DTA dissection and died 10 months after the surgery owing to recurrence around the trachea [16]. Kaisaki et al. reported two patients with dissection of lymph nodes in the DTA, who showed prolonged survival of 60 months and 12 months [17]. Hatooka et al. reported two esophageal cancer patients with metastasis in the DTA, who died 19 months and 23 months after chemoradiotherapy, and another patient treated with neoadjuvant chemotherapy, followed by esophagectomy and dissection of lymph nodes in the DTA, who survived for 46 months after the operation without any recurrence [1]. Further studies are required to reach consensus on guidelines that may inform clinical management in proceeding with DTA lymphadenectomy after stable disease post neoadjuvant chemotherapy, or neoadjuvant chemoradiotheapy. Some reports have presented patients with long-term survival after dissection of lymph nodes in the DTA, while other reports have presented patients with recurrence in the early period after surgery. Case 1 of our report developed lung metastasis 8 months after the operation, and case 2 showed recurrence of lymph node metastasis around the reconstructed gastric tube and DTA 4 months after the surgery. The long-term outcome and significance of dissection of lymph nodes in the DTA among esophageal cancer patients remain controversial. Therefore, the surgical indication should be decided carefully. Thoracoscopy could be considered, which would be followed by esophagectomy; however, further studies are required to establish the indications and efficacy of our therapeutic approach. This work has been reported in line with the SCARE criteria [18].
4. Conclusion
We reported two cases of esophageal cancer with lymph node metastasis in the DTA that safely underwent thoracoscopic esophagectomy with lymphadenectomy via a bilateral approach in the prone position. The procedure could be performed without a complicated change in the surgical position. Although bilateral thoracoscopic esophagectomy in the prone position for dissection of lymph nodes in the DTA might be an alternative curative surgery for esophageal cancer, more studies are needed in the future to elucidate the safety and efficacy thereof.
Conflicts of interest
No.
Funding
No.
Ethical approval
This work does not require ethical approval.
Consent for publication
Written informed consents were obtained from the patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Authors’ contributions
Onodera Y is the first author of this article; Nakano T, Onodera Y and Heishi T drafted the manuscript; Nakano T, Kamei T, Sakurai T, Taniyama Y, Sato C, and Ohuchi N were attending doctors and performed clinical treatment including surgical operation; Onodera Y constructed imaging and prepared figures; all authors have read and approved the final manuscript.
Guarantor
Toru Nakano accept full responsibility for this work.
References
- 1.Hatooka S., Abe T., Saito T., Mitsudomi T., Shinoda M. Lymph node metastasis of an esophageal cancer behind the thoracic descending aorta. Esophagus. 2010;7:111–114. [Google Scholar]
- 2.Peel J., Darling G. Left video-assisted thoracoscopic surgery esophagectomy in a patient with situs inversus totalis and Kartagener syndrome. Ann. Thorac. Surg. 2014;98:706–708. doi: 10.1016/j.athoracsur.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 3.Singh G., Costa J., Bessler M., Sonett J. Minimally invasive Ivor Lewis oesophagogastrectomy in a patient with situs inversus totalis. Interact. Cardiovasc. Thorac. Surg. 2016;22:235–237. doi: 10.1093/icvts/ivv299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ujiie N., Nakano T., Kamei T., Ichikawa H., Miyata G., Onodera K. Thoracoscopic esophagectomy for esophageal cancer with situs inversus totalis: a case report and literature review. Gen. Thorac. Cardiovasc. Surg. 2016;64:359–362. doi: 10.1007/s11748-016-0639-y. [DOI] [PubMed] [Google Scholar]
- 5.Shimada Y., Kawabe A., Nakajima S., Hata K., Takahashi Y., Kume M. A bilateral thoracic approach for esophageal cancer in the prone position. Surg. Today. 2015;45:91–95. doi: 10.1007/s00595-013-0738-7. [DOI] [PubMed] [Google Scholar]
- 6.Ando N., Kato H., Igaki H., Shinoda M., Ozawa S., Shimizu H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann. Surg. Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri A. Thoracoscopic subtotal oesophagectomy. Endosc. Surg. Allied Technol. 1994;2:21–25. [PubMed] [Google Scholar]
- 9.Biere S.S., van Berge Henegouwen M.I., Maas K.W., Bonavina L., Rosman C., Garcia J.R. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 10.Teshima J., Miyata G., Kamei T., Nakano T., Abe S., Katsura K. Comparison of short-term outcomes between prone and lateral decubitus positions for thoracoscopic esophagectomy. Surg. Endosc. 2015;29:2756–2762. doi: 10.1007/s00464-014-4003-y. [DOI] [PubMed] [Google Scholar]
- 11.Shiozaki A., Fujiwara H., Murayama Y., Komatsu S., Kuriu Y., Ikoma H. Posterior mediastinal lymph node dissection using the pneumomediastinum method for esophageal cancer. Esophagus. 2012;9:58–64. [Google Scholar]
- 12.Ninomiya I., Okamoto K., Tsukada T., Saito H., Fushida S., Ikeda H. Thoracoscopic radical esophagectomy and laparoscopic transhiatal lymph node dissection for superficial esophageal cancer associated with lymph node metastases in the dorsal area of the thoracic aorta. Surg. Case Rep. 2015;1:25. doi: 10.1186/s40792-015-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita Y., Takase K., Yamada T., Sato A., Higano S., Takahashi S. Virtual CT thoracoscopy: preoperative simulation for thoracoscopic surgery of esophageal cancer. Abdom. Imaging. 2007;32:679–687. doi: 10.1007/s00261-006-9165-1. [DOI] [PubMed] [Google Scholar]
- 14.Morita Y., Takase K., Ichikawa H., Yamada T., Sato A., Higano S. Bronchial artery anatomy: preoperative 3D simulation with multidetector CT. Radiology. 2010;255:934–943. doi: 10.1148/radiol.10081220. [DOI] [PubMed] [Google Scholar]
- 15.Nakano T., Sakurai T., Maruyama S., Ozawa Y., Kamei T., Miyata G. Indocyanine green fluorescence and three-dimensional imaging of right gastroepiploic artery in gastric tube cancer. World J. Gastroenterol. 2015;21:369–372. doi: 10.3748/wjg.v21.i1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa S., Nishimaki T., Suzuki T., Kanda T., Kuwabara S., Hatakeyama K. Tumor angiogenesis as an independent prognostic factor after extended radical esophagectomy for invasive squamous cell carcinoma of the esophagus. Surgery. 2001;129:302–308. doi: 10.1067/msy.2001.111122. [DOI] [PubMed] [Google Scholar]
- 17.Kaisaki S., Kitayama J., Ishigami H., Nagawa H. Solitary nodal recurrence in the dorsal area of the thoracic aorta after a curative resection of esophageal cancer: report of two cases. Surg. Today. 2007;37:243–247. doi: 10.1007/s00595-006-3349-8. [DOI] [PubMed] [Google Scholar]
- 18.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 doi: 10.1016/j.ijsu.2016.08.014. (article in press) [DOI] [PubMed] [Google Scholar]