Abstract
Graft failure due to de-differentiation of the chondrocytes during in vitro culture and after transplantation is a major hurdle in Autologous Chondrocyte Implantation (ACI). We, herein, report the transplantation of autologous chondrocytes ex vivo expanded using a Thermo-reversible Gelation Polymer (TGP) in a rabbit model. A full thickness chondral defect was created in one of the knee joints in each of the six rabbits of the study and autologous chondrocytes in vitro expanded using TGP scaffold were transplanted after 10 weeks. H & E staining of the biopsy after 6 months revealed maintenance of articular cartilage phenotype.
Keywords: Autologous chondrocyte implantation (ACI), Articular cartilage, Thermo-reversible gelation polymer (TGP), De-differentiation, Tissue engineering
Autologous Chondrocyte Implantation (ACI) is an established two-step procedure for treating articular cartilage injuries. One of the major factors contributing to its success is the ability to maintain the chondrocytes in the native articular cartilage state without de-differentiation during the process of ex vivo expansion. To overcome this de-differentiation, different scaffolds have been employed both natural and synthetic for enabling 3D growth of chondrocytes. While natural scaffolds have the risk of biological contamination, many of the synthetic scaffolds do not provide proper cell attachment. The Thermoreversible Gelation Polymer (TGP) (Commercial Name: Mebiol Gel) is a chemically synthesized biocompatible copolymer which supports the in vitro expansion of different kinds of cells, stem cells and cell lines.1 Since TGP is thermo-responsive, the cells can be detached from the culture without the use of enzymes.1 TGP does not alter the gene expression of the cells grown on it.1 Yasuda et al. in 2006 reported that TGP acts as a suitable 3-D hydrogel scaffold for bovine chondrocytes2 and Arumugam et al. have earlier demonstrated the successful use of TGP for the in vitro expansion of human articular chondrocytes for a longer period of 16 weeks with maintenance of the hyaline phenotype.3 In this study, we report the preliminary result of the experimental in vivo transplantation of autologous chondrocytes ex vivo expanded using TGP in a rabbit model of experimental articular cartilage defect.
1. The study
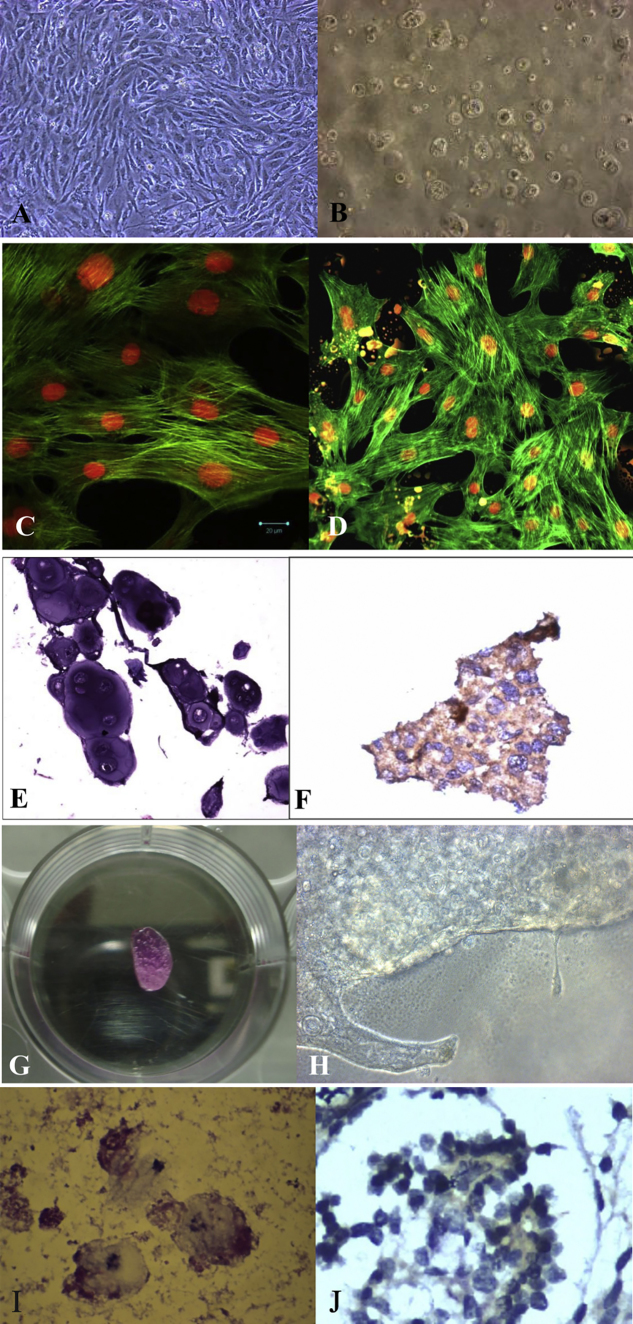
Six “Russian chinchilla” rabbits were included in this study, after approval by the ethics committee of Sri Ramachandra University, Chennai, India. Animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee and following the Indian Council of Medical Research (ICMR) guidelines on the care of experimental animals, as the study was conducted in India. The rabbits were anesthetized and a full thickness chondral defect of 4 mm diameter was created in the center of the trochlear groove in the knee joint. The cartilage sample taken from the knee was sent to the lab for isolation of chondrocytes. After receiving the samples in the laboratory, the samples were washed and then subjected to 0.3% Collagenase Type II overnight at 37° C, 5% CO2 for digestion. After digestion, DMEM containing FBS was added for arresting the enzyme activity. The cell viability was checked by using trypan blue dye exclusion method. An average of 0.3 × 106 cells were obtained in each sample. The cells were seeded in T25 flasks and DMEM/F12 medium with 10% FBS, Pen/step, Gentamicin (50 mg/ml) and l-Ascorbic acid (50 mg/ml) were added. The cultures were incubated at 37 °C with 5% CO2. The non −adherent cells were removed after 48 h incubation. After the first passage, the cells were taken and seeded in 0.2 ml of TGP scaffold for expansion following the methodology of Arumugam et al.3 The culture in TGP was performed for 10 weeks. The cells were subjected to H & E staining followed by characterization for S-100 using Immuno-histochemistry. The cells were also stained with Phalloidin and Propodium Iodide (PI) staining. The defect earlier created in the knee in each rabbit was transplanted with its autologous cartilage tissue engineered from in vitro expanded chondrocytes after 10 weeks of tissue engineering in vitro. At end of 6 months after in vivo transplantation, a biopsy of the cartilage from the transplanted site was taken for all six rabbits and stained with haematoxylin and eosin. Before seeding into TGP, the cells were observed to have spindle-shaped fibroblast like morphology (de-differentiated chondrocytes) (Fig. 1A) which were again converted into round- and oval-shaped chondrocytes which closely resembled native hyaline cartilage phenotype in the TGP scaffold (Fig. 1B). The cytoskeleton network of chondrocytes during the culture was appreciated by using Confocal and Florescent microscopes (Fig. 1C and D). H & E staining and immuno-histochemistry confirmed the morphological appearance of chondrocytes (Fig. 1E and F). A macroscopic structure which resembled cartilage was appreciated in the TGP construct in vitro after 64 days (Fig. 1G and H). The post-implantation biopsy tissues showed good repair and filling of defects in the TGP embedded in vitro expanded chondrocytes' transplanted tissue. The transplanted tissue exhibited round morphology of chondrocytes implicating hyaline cartilage in H & E staining (Fig. 1I) and were positive for S-100 (Fig. 1J).
Fig. 1.
A: In vitro appearance of the chondrocytes during culture: De-differentiation to spindle shaped fibroblast morphology in monolayer culture; B: Re-differentiation to rounded hyaline morphology after seeding in TGP; C: Confocal microscopy, D Florescent microscopy images of the in vitro cultured chondrocytes using Phalloidin and Propodium Iodide (PI) staining; E: H & E staining showing rounded hyaline morphology of cultured chondrocytes; F: Immuno-histochemical staining positive for S-100 marker in the cultured chondrocytes; G, H: Macroscopic structure resembling cartilage appreciated in the TGP construct during in vitro culture; I: H & E staining of the of the tissue biopsy, post-transplantation; J: Immuno-histochemistry using S-100 of the tissue biopsy, post-transplantation showing rounded chondrocytes of hyaline phenotype.
Continuous efforts are being taken to improve the quality of the repaired tissue in ACT. Different types of materials are being employed as during culture and as carriers during transplantation in the form of Matrix-assisted autologous chondrocyte transplantation (MACT) to provide ease of handling for the surgeon and to hold the cells in place during the repair process. Most of the MACT procedures use collagen scaffold. Even approved products such as Japan's JACC® (Japan Tissue Engineering Co., Ltd.) uses atellocollagen from animal sources (J-tec).4 Collagen from animal sources carry the risk of biological contamination. TGP is purely synthetic and hence is of great advantage. Magnussen et al.5 suggested that any difference in the outcome of articular cartilage defect treatment procedure based on hyaline or fibrocartilage may actually reveal themselves only after many years of follow-up. Approved products like JACC4 grow the chondrocytes up to 4–5 weeks only. Yasuda et al.2 and Arumugam et al's3 studies based on which the present study was designed and our present experiment of being able to grow the cartilage in vitro for nearly 10 weeks encourage us to hypothesize that use of TGP can help in maintenance of phenotype for longer period of time. Further, our present study demonstrates the potentials of TGP in maintenance of the quality of chondrocytes before transplantation and the quality of tissue formed after transplantation making it an ideal scaffold for autologous chondrocyte implantation. Similar procedures can be tried in select group of human patients to confirm the replicability and efficacy before starting to implement it as a routine clinical procedure.
Declaration of conflicting interests
Authors Yuichi Mori, Hiroshi Yoshioka and Samuel JK Abraham are applicants to a patent on use of Thermo-reversible Gelation Polymer (TGP) for the in vitro culture-expansion of chondrocytes.
Institute where the study was conducted
Sri Ramachandra Arthroscopy & Sports Sciences Centre (SRASSC), Sri Ramachandra University, Chennai, India
Grants
The study was performed using the funds granted by the Department of Science and Technology (DST), India – Grant no: DST No: SR/SO/AS-0064/2008 & Arthroscopy Study Group Foundation, India.
Acknowledgements
The authors acknowledge
1. M/s Chennai Cell Cluster (CCC) for technical advice.
2. Loyola ICAM College of Engineering Technology (LICET) and Loyola Institute of Frontier Energy (LIFE) for their support to our research work.
3. Hope Foundation, Chennai, India for their support throughout the study.
4. Capt. A. Nagarajan, Chennai, India for his advice to the project.
Contributor Information
Sivaraman Arumugam, Email: drarumugam@srassc.in.
Balasubramanyan Bhupesh Karthik, Email: drbhupeshkarthik@gmail.com.
Rajeswar Chinnuswami, Email: rajeswarc@yahoo.co.in.
Yuichi Mori, Email: mori@mebiol.co.jp.
Hiroshi Yoshioka, Email: yoshioka@mebiol.co.jp.
Rajappa Senthilkumar, Email: rsk@nichimail.jp.
Rajmohan Mathaiyan, Email: mrm@nichimail.jp.
Karthick Ramalingam, Email: mrk@nichimail.jp.
Preethy Senthilkumar, Email: drspp@nichimail.jp.
Samuel J.K. Abraham, Email: drsam@nichimail.jp.
References
- 1.Kataoka K., Huh N. Application of a thermo-reversible gelation polymer, mebiol gel, for stem cell culture and regenerative medicine. J Stem Cells Regen Med. 2010;6:10–14. doi: 10.46582/jsrm.0601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasuda A., Kojima K., Tinsley K.W., Yoshioka H., Mori Y., Vacanti C.A. In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors. Tissue Eng. 2006;12:1237–1245. doi: 10.1089/ten.2006.12.1237. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam S., Manjunath S., Senthilkumar R. In vitro expansion and characterization of human chondrocytes using a novel thermoreversible gelation polymer (TGP) J Orthop. 2011;8:1–10. [Google Scholar]
- 4.Japan Tissue Engineering Co., Ltd. (J-TEC). http://www.jpte.co.jp/english/business/Regenerative/cultured_cartilage.html. (Accessed 9 December 2016).
- 5.Magnussen R.A., Dunn W.R., Carey J.L., Spindler K.P. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466:952–962. doi: 10.1007/s11999-007-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]