Abstract
Today much is known about cytochrome P450 (P450) enzymes and their catalytic specificity, but the range of reactions catalyzed by each still continues to surprise. Historically P450s had been considered to be involved in either the metabolism of xenobiotics or endogenous chemicals, in the former case playing a generally protective role and in the latter case a defined physiological role. However, the line of demarcation is sometimes blurred. It is difficult to be completely specific in drug design, and some P450s involved in the metabolism of steroids and vitamins can be off-targets. In a number of cases, drugs have been developed that act on some of those P450s as primary targets, e.g., steroid aromatase inhibitors. Several of the P450s involved in the metabolism of endogenous substrates are less specific than once thought and oxidize several related structures. Some of the P450s that primarily oxidize endogenous chemicals have been shown to oxidize xenobiotic chemicals, even in a bioactivation mode.
1. INTRODUCTION
Historically the field of cytochrome P450 (P450) research developed from early work on the metabolism of carcinogens,1,2 drugs,3,4 and steroids.5 The biochemical studies were initiated with investigations on the pigmented proteins in rat liver.6,7 Through extensive biochemical studies in the 1960s–1980s, an extensive knowledge base on P450s developed. Biochemical and recombinant DNA studies on the human P450s led to increased understanding of these enzymes and their relationships to their animal orthologues. With the Human Genome Project and knowledge of the signature cysteine sequence of P450s, the number of human P450 genes is set at 57 (Table 1).
Table 1.
Classification of Human P450s Based on Major Substrate Class8
| sterols | xenobiotics | fatty acids | eicosanoids | vitamins | unknown |
|---|---|---|---|---|---|
| 1B1* | 1A1* | 2J2 | 4F2 | 2R1* | 2A7 |
| 7A1* | 1A2* | 2U1 | 4F3 | 24A1** | 2S1 |
| 7B1 | 2A6* | 4A11 | 4F8 | 26A1 | 2W1 |
| 8B1 | 2A13* | 4B1** | 5A1 | 26B1 | 4A22 |
| 11A1* | 2B6* | 4F11 | 8A1* | 26C1 | 4X1 |
| 11B1 | 2C8* | 4F12 | 27B1 | 4Z1 | |
| 11B2* | 2C9* | 4F22 | 27C1 | 20A1 | |
| 17A1* | 2C18 | 4V2 | |||
| 19A1* | 2C19* | ||||
| 21A2* | 2D6* | ||||
| 27A1 | 2E1* | ||||
| 39A1 | 2F1 | ||||
| 46A1* | 3A4* | ||||
| 51A1* | 3A5 | ||||
| 3A7 | |||||
| 3A43 |
X-ray crystal structure(s) reported (for human enzyme).9
Rat or rabbit X-ray crystal structure reported.
As the field developed, thoughts about the functions of P450s developed around two main themes. One was the roles of P450 in the metabolism of endogenous compounds, as exemplified by steroids.10 Indeed, some inborn errors of metabolism could be related to deficiencies in these enzymes.10–12 The other general function of P450s was in the metabolism of xenobiotics, predominantly in detoxication13,14 but sometimes bioactivation.2,15 In general, these two groups of P450s were considered almost unrelated except in their overall structural similarity.
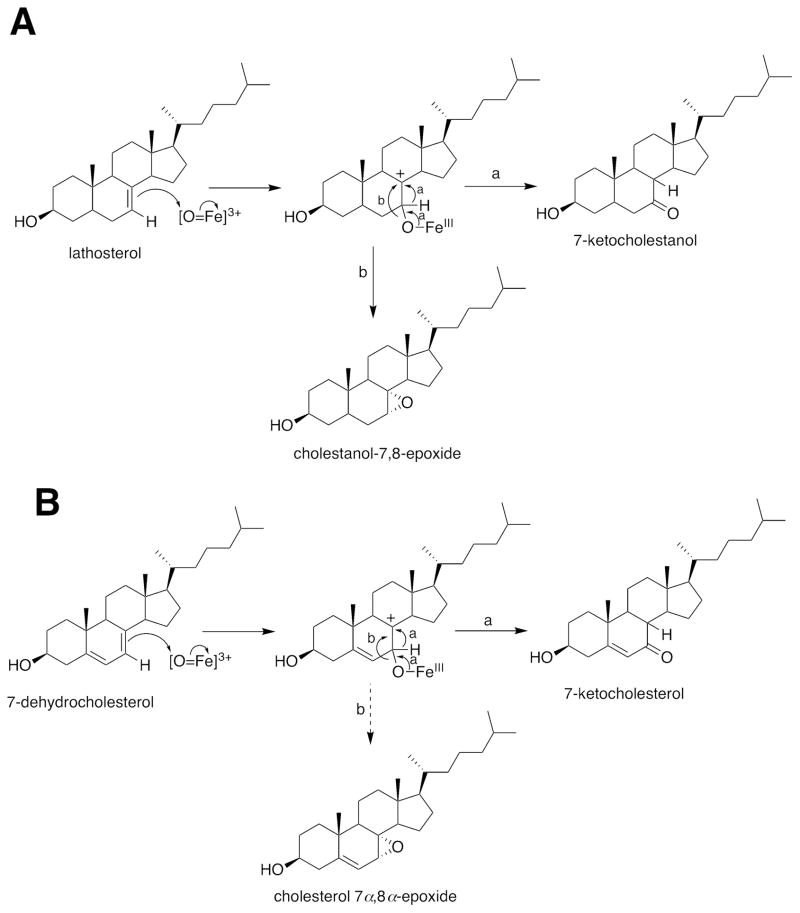
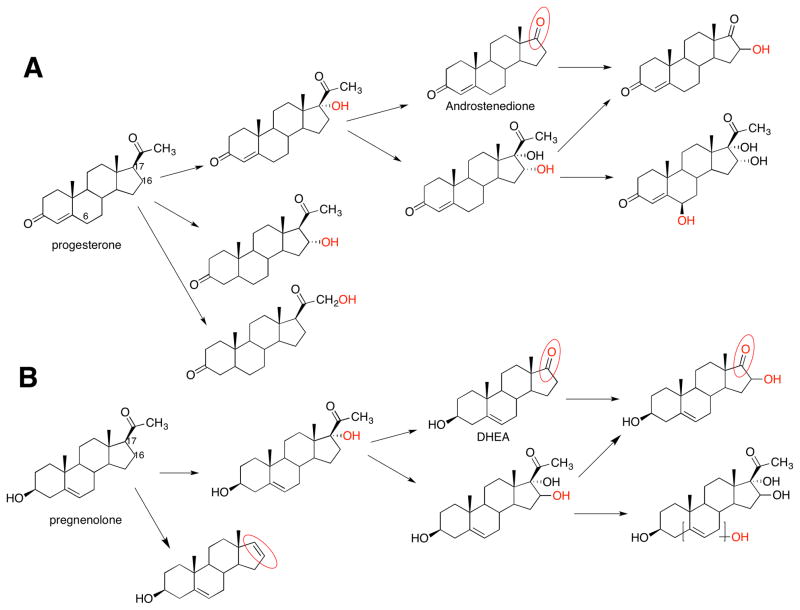
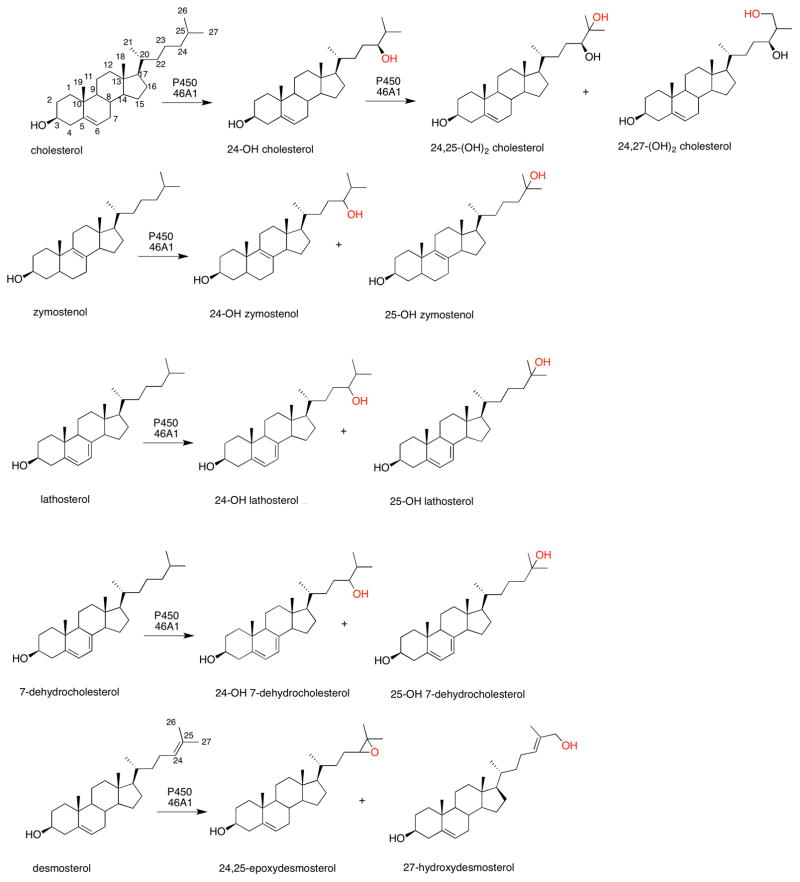
However, more recently two general concepts have changed. One is that many of the P450s involved in the metabolism of endogenous substrates are not as highly specific as once thought. For example, some of the P450s that oxidize cholesterol will also utilize several related sterols (Table 2).16,17 7-Dehydrocholesterol is a substrate for P450 7A1 and is oxidized to 7-ketocholesterol (Figure 1).18 Recently a number of additional oxidation reactions have been attributed to P450 17A1 (Figure 2)19 and P450 46A1 (Figure 3).16,17 The second point is that the line of demarcation between endogenous and xenobiotic substrates is not so sharp as once thought, a topic which will be the subject of the remainder of this Perspective.
Table 2.
| P450 | sterolsa | products |
|---|---|---|
| P450 7A1 | zymostenol | (not determined) |
| lathosterol | 7-ketocholestanol | |
| cholestanol-7α,8α -epoxide | ||
| 7-dehydrocholesterol | 7-ketocholesterol | |
| desmosterol | 7α-hydroxydesmosterol | |
| cholesterol | 7α-hydroxycholesterol | |
| P450 11A1 | zymostenol | (not determined) |
| lathosterol | (not determined) | |
| 7-dehydrocholesterol | 7-dehydropregnenolone | |
| desmosterol | pregnenolone | |
| cholesterol | pregnenolone | |
| P450 27A1 | zymostenol | 25-hydroxyzymostenol |
| 27-hydroxyzymostenol | ||
| lathosterol | 25-hydroxylathosterol | |
| 27-hydroxylathosterol | ||
| 7-dehydrocholesterol | 25-hydroxy-7-dehydrocholesterol | |
| 27-hydroxy-7-dehydrocholesterol | ||
| desmosterol | 27-hydroxydesmosterol | |
| cholesterol | 27-hydroxycholesterol | |
| P450 46A1 | zymostenol | 24-hydroxyzymostenol |
| 25-hydroxyzymostenol | ||
| lathosterol | 24-hydroxylathosterol | |
| 25-hydroxylathosterol | ||
| 7-dehydrocholesterol | 24-hydroxy-7-dehydrocholesterol | |
| 25-hydroxy-7-dehydrocholesterol | ||
| desmosterol | 24S,25-epoxycholesterol | |
| 27-hydroxydesmosterol | ||
| cholesterol | 24S-hydroxycholesterol |
Depending on whether cholesterol synthesis occurs via the Bloch or the Kandutsch-Russell pathway (i.e., whether reduction of the 24,25 double bond is early or late), the pathway from lanosterol will either involve the steps desmosterol—> cholesterol or zymostenol —> lathosterol—> 7-dehydrocholesterol —> cholesterol, in terms of the substrates considered here.17
Figure 1.
Oxidations of lathosterol and 7-dehydrocholesterol by P450 7A1.18 (A) Lathosterol; (B) 7-dehydrocholesterol. The a and b pathways indicate hydride transfer and closure to an epoxide, respectively.
Figure 2.
Multiple oxidations catalyzed by P450 17A1.19 Sites of oxidation are indicted in red. The site of oxidation in the structure in the lower right corner has not been ascertained.
Figure 3.
Multiple oxidations of sterols catalyzed by P450 46A1.16,17 Sites of oxidation are indicted in red. Sterol numbering is shown in the structure of cholesterol (upper left structure). The oxidations with 7-dehydrocholesterol had not been detected in a previous study, presumably due to limited sensitivity.20
2. P450S THAT OXIDIZE BOTH ENDOGENOUS AND XENOBIOTIC SUBSTRATES
In a sense the P450s that oxidize both endogenous and xenobiotic substrates are like centaurs in Greek mythology, who had the upper body of a human fused to the body of a horse. They operated in two worlds at once, although the P450s are not exactly the same in that respect—they are doing the same chemistry in both worlds, and how they act is apparently only determined by the shape (and size) of what they encounter. A list of P450s in this category is presented in Table 3. The point can be made that numerous P450s have been demonstrated to catalyze fatty acid hydroxylation,8 but the physiological relevance of most of these reactions is unknown with the possible exception of the ω-hydroxylation of arachidonate (P450 4A11).23,24
Table 3.
Some Human P450s that Oxidize Both Endogenous and Xenobiotic Substrates
P450 1B1 was first characterized in adrenals and was of interest due to its ability to oxidize polycyclic hydrocarbons.25 Subsequently it was shown to be capable of activating a large variety of procarcinogens,26 to be the major “aryl hydrocarbon hydroxylase” involved in the trimodal induction response in human lymphocytes,27–29 and to be the major estrogen 4-hydroxylase,30 which has implications of its own in chemical carcinogenesis.31 Exactly what the most critical physiological role of P450 1B1 is remains unknown, but genetic deficiency is related to congenital glaucoma.32,33
P450s 1A2 and 3A4 are best known for their abilities to oxidize drugs and carcinogens34,35 but also oxidize estrogens.36,37 P450 3A4 oxidizes Δ4 steroids, including progesterone, testosterone, and androstenedione.38 For instance, P450 3A4 catalyzes 1β-, 2 β-, 6 β-, and 15 β-hydroxylations of testosterone39 and 4β-hydroxylation of cholesterol.40,41 The in vivo significance of these reactions with the steroids is not clear, particularly in light of the inter-individual variation of over an order of magnitude in the levels of these enzymes.42
P450 11A1 has been documented to oxidize a drug candidate, which is discussed later in this article.21
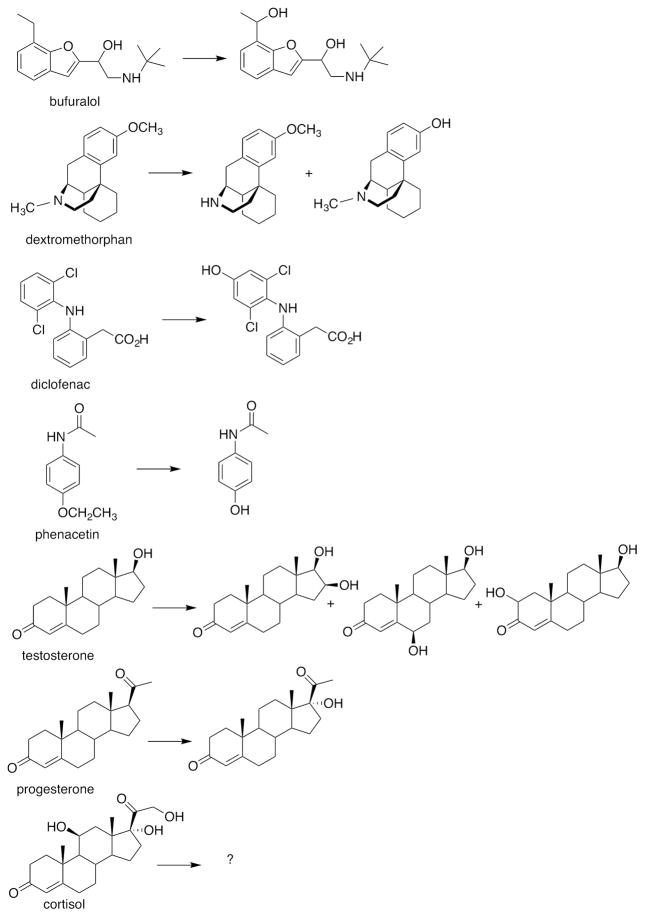
P450 46A1 is a cholesterol 24-hydroxylase localized in brain, and transgenic mice missing the enzyme have learning defects.43 Pikuleva’s group has demonstrated that this enzyme binds a number of drugs tightly and oxidizes them (albeit slowly), including dextromethorphan, diclofenac, and phenacetin, as well as progesterone and testosterone (Figure 4).22 Voriconazole is an effective inhibitor, with a Ki of 11 nM.44 Crystal structures are now available with P450 46A1 bound to the drugs voriconazole, tranylcypromine, thioperamide, and clotrimazole.45 The nitrogen-containing heterocyclic rings of these drugs bond to the heme iron. An unusual mode of drug binding to P450 46A1 was seen in the crystal structures with bicalutamide, in which a water molecule is sandwiched between the heme iron and a nitrile on the drug.46 Interestingly, some drugs were found to be positive effectors, and efavirenz was shown to produce this effect on cholesterol turnover in vivo (in mice).47 A combination of hydrogen-deuterium exchange kinetics and other methods was used to conclude that the effector (efavirenz) site borders that occupied by the redox partner NADPH-P450 reductase.48
Figure 4.
Multiple oxidations of non-sterol compounds catalyzed by P450 46A1.22
3. DRUGS THAT INHIBIT P450S
3.1. Drug-metabolizing P450s
The matter of inhibition of drug metabolizing P450s (Table 1) has received considerable attention for many years and will not be treated in depth here. The major concern is drug-drug interactions (of course, drug-drug interactions can also result from enzyme induction).49 Extensive reviews of the mechanisms and consequences of drug-drug interactions have been published.50–53
3.2. Carcinogen-metabolizing P450s
Shortly after the discovery that P450s were involved in the bioactivation of chemical carcinogens, efforts at chemoprevention were initiated. The concept is to develop drugs as inhibitors or to identify foods that contain such inhibitors. This field of “chemoprevention” is considerable54–56 and, as in the case of drug-drug interactions, it will not be reviewed in detail.
In reviewing Table 1, the P450s that have been of most interest in this field are 1A1, 1A2, 1B1, 2A6, 2A13, 2E1, and 3A4, understandably because they are involved in the bulk of activation reactions with carcinogens.34 Both reversible and irreversible inhibitors have long been considered for some time, particularly with the Family 1 P450s.57,58 Oltipraz is an inhibitor of P450 1A2 and can block aflatoxin metabolism.59,60 Watercress and several vegetables, which contain isothiocyanates, have been considered for inhibition of P450 2E1.61 There has been interest in inhibiting P450s 2A6 and 2A13 because of their relevance in tobacco-specific nitrosamine activation.62–64 Much of the work has been done in vitro, and the relevance of in vivo work with animal models has to be considered carefully. Some of the inhibitors show strong inhibition, e.g., certain stilbenes with P450 1B1.65 An issue of concern is that, depending on the situation, P450s are also prominent in the detoxication of many carcinogens.34 Realistically it will be difficult to have new chemopreventive agents approved for use, and there is merit in finding foods that contain natural inhibitors, e.g., grapefruit with intestinal P450 3A4.66,67
3.3. P450s That Catalyze Important Endogenous Reactions
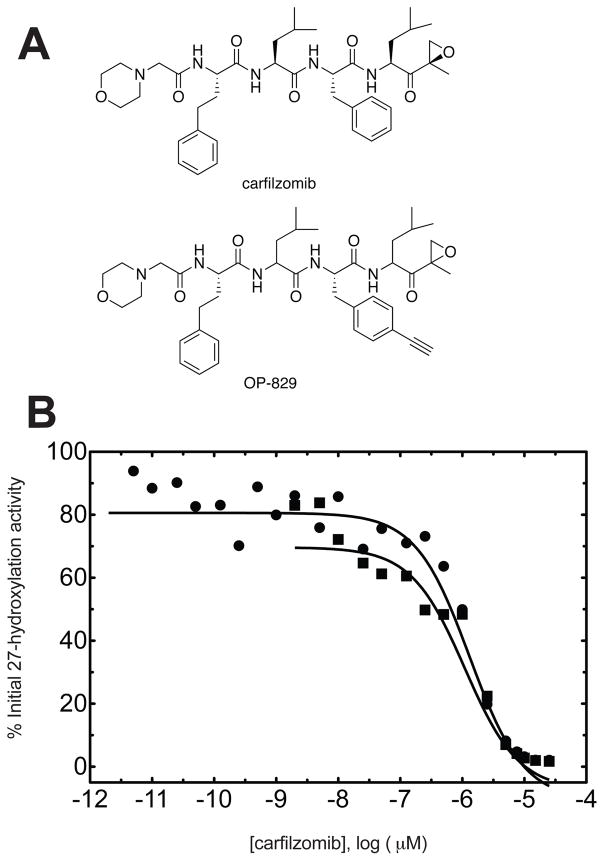
In looking at Table 1, P450s that one would not want a new drug candidate to inhibit include most of those in the columns with the headings of steroids, eicosanoids, and vitamins. There are exceptions relative to certain disease states, but in general these enzymes are involved in physiological processes (Table 4). Some inhibition can be tolerated, however, and as in all toxicology and safety assessment it is the dose that matters. For instance, a proteasome inhibitor in development for cancer (carfilzomib) was found to covalently bind to and inhibit P450 27A1 (Table 5)68 However, the IC50 was ~ 1 μM, which is slightly lower than the initial plasma concentration of this drug following i.v. infusion at the maximum tolerated dose (3 μM), but the t1/2 is ~ 1 h.69,70 Carfilzomib (Figure 5) is an epoxide that also reacts with other targets. To identify targets in cell culture, an analog with an acetylene side chain (OP-829, Figure 5A) was synthesized and “click chemistry” was used to recover the adducted proteins. In this case P450 27A1, a sterol 27-hydroxylase (Table 2), and GSH transferase O1–1 were identified; both purified enzymes were also inhibited. The inhibition of P450 27A1 was not enhanced by pre-incubation of the enzyme with the epoxide for 60 min prior to the assay (Figure 5B). This may mean that the extent of inhibition was maximal during the incubation. The covalent binding did not appear to be specific, in that P450 27A1 residues Cys-127, Cys-426, and Cys-475 were all modified.68
Table 4.
Human P450s That Oxidize Endogenous Substrates and Should Not Be Inhibited Under Normal Physiological Conditions
| P450 | substrate |
|---|---|
| 8A1 | prostagalandin H2 |
| 11A1 | cholesterol |
| 11B1 | 11-deoxycortisol |
| 11B2 | corticosterone |
| 17A1 | pregnenolone, progesterone |
| 19A1 | testosterone, androstenedione |
| 21A2 | 17α-hydroxyprogesterone |
| 24A1 | 25-hydroxyvitamin D3 |
| 26A1 | retinoic acid |
| 26B1 | retinoic acid |
| 26C1 | retinoic acid |
| 27A1 | vitamin D3, cholesterol |
| 27B1 | vitamin D3 |
| 39A1 | 24-hydroxycholesterol |
| 51A1 | lanosterol |
Table 5.
Human P450s Known to be Covalently Modified and Inhibited by Drugs
Figure 5.
Human P450 27A1 and carfilzomib.68 (A) Structure of carfilzomib and acetylenic analog (OP-829). (B) Inhibition of P450 27A1 by carfilzomib with (filled circles) and without (filled squares) preincubation (60 min at 37 °C, no cofactors present). IC50 with pre-incubation: 1.3 ± 0.1 μM; IC50 without pre-incubation: 1.1 ± 0.1 μM.
Several P450s involved in steroid metabolism in the adrenals have been shown to be inhibited by drugs or other xenobiotic chemicals and to cause adrenal toxicity. The concept of drugs causing adrenal toxicity, or at least inhibiting adrenal steroidogenesis, is not new and goes back >50 years.71 A list21 includes aminoglutethimide/P450 11A1,72 metyrapone and etomidate/P450 11B1,73,74 etomidate/P45011B2,74 atrazine and letrozole/P450 19A1,73,75 and ketoconazole/P450s 17A1 and 11B1 (11β-hydroxylase).76 Examination of the structures of these inhibitors shows a distinct lack of similarity to sterols. In some cases the adrenal P450s not only bind xenobiotic molecules but also bioactivate them. P450 11B1 has been reported to activate several compounds (to cause adrenal toxicity), including mitotane,77,78 a methylsulfone derivative of 4,4′-dichlorodiphenyldichloroethylene (DDE),79,80, and 7,12-dimethylbenz[a]anthracene.81,82
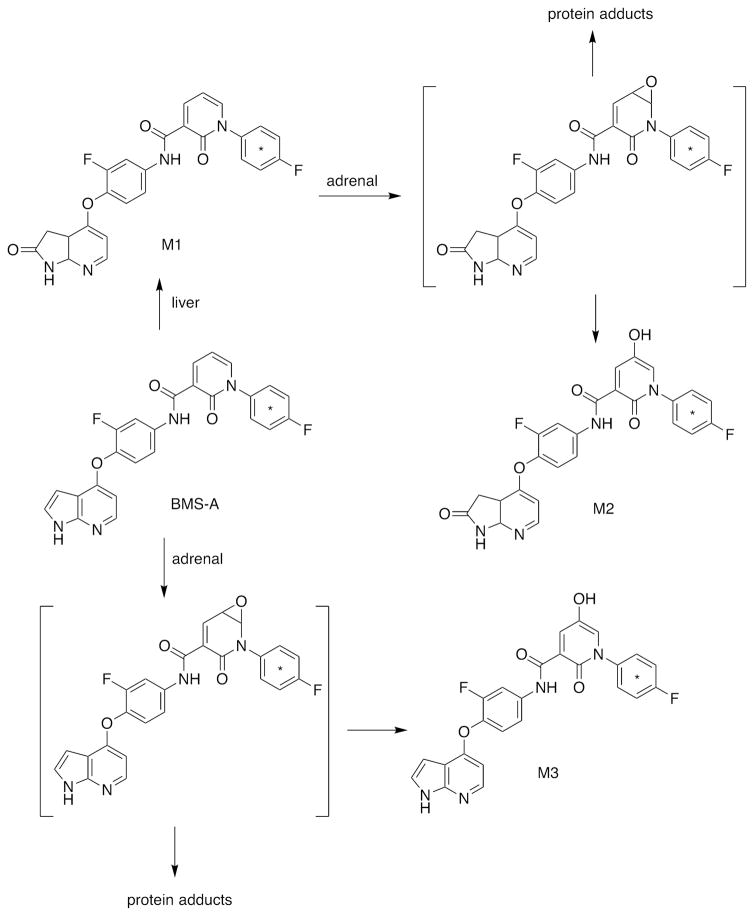
One of the more unusual chemicals bioactivated by both rat and human P450 11A1 is a Bristol-Myers Squibb compound (“BMS-A”) considered for development (Figure 6).21 Covalent binding to the protein was demonstrated, and a proposed pathway involves an epoxide (in a heterocyclic ring) (Figure 6). Adrenal toxicity of the compound was demonstrated in rats. The binding was considerably less in adrenal cells of human origin (H295R) than mouse cells, suggesting a major species difference. The relevance to any possible human adrenal toxicity has not been established.
Figure 6.
Activation of “BMS A” by P450 11A1.21 The site of the 14C label is indicated by an asterisk (*). Proposed reactive products are indicated in brackets.
3.4. P450s as Drug Targets
Several P450s are established drug targets, including P450s 5A1, 17A1, and 19A1 (Table 4). In particular, excellent third-generation inhibitors of P450 19A1 (the steroid aromatase) are widely used in estrogen receptor-positive breast cancer (e.g., letrozole, anastrozole, exemestane).83 Thromboxane levels can be reduced by inhibitors of P450 5A1, often known by its common name of thromboxane synthase. Inhibition of P450 17A1 is a relatively new area, mainly related to prostate cancer, which is often androgen-stimulated. An issue in castration-resistant prostate cancer is the extra-testicular supply of androgens. Inhibition of P450 17A1 can be achieved with drugs (e.g., abiraterone).84,85 This is problematic in that P450 17A1 catalyzes two reactions, the 17α-hydroxylation of pregnenolone and progesterone and the subsequent 17α,20-lyase step that converts these to androgens (Figure 2). 17α-Hydroxy steroids are also utilized for synthesis of glucocorticoids and mineralocorticoids, however, and therefore the use of P450 17A1 inhibitors has side effects. One goal is the selective inhibition of the lyase step of P450 17A1, therefore blocking androgen production but maintaining levels of other steroids. Claims of selective inhibition with the drug candidate orteronel (TAK-700, Takeda) have been published86 but apparently the candidate was dropped from development in Phase II clinical trials. Another candidate is VT-464 (Viamet/Innocrin).87 One issue in the matter is whether the two major reactions of P450 17A1 (17α-hydroxylation and 17,20-lyase action) are processive or distributive (i.e. the question is whether the 17α-hydroxy products leave the enzyme and re-bind).88 Our own results on the topic indicate a rather distributive reaction, for both the fish enzyme89 and human P450 17A1 (Gonzalez, E., and Guengerich, F. P., in preparation) which may suggest that reaction-specific drugs are possible.
P450 3A4 has been proposed as a drug target (Table 6), for different reasons. The enzyme is the major one involved in drug metabolism,35 and the concept is to retard metabolism, particularly of expensive drugs. This practice has already been used for 20 years with the drugs ritonavir and cobicistat, especially with drugs used to treat HIV patients.92 Rational design approaches are underway with ritonavir analogs.93
Table 6.
Some Human P450s That Are Established or Proposed Targets for Drugs8
| established targets
|
|
|---|---|
| P450 | desired effect |
| 5A1 | decreased thromboxane levels (anti-platelet aggregation (thrombosis)) |
| 11B1 | block cortisol production in Cushing’s syndrome |
| 17A1 | decreased androgen levels (prostate cancer) |
| 19A1 | decreased estrogen levels (several hormonal cancers, e.g., breast, ovarian) |
|
| |
| targets in development
|
|
| P450 | intended effect |
| 3A4 | decreased drug metabolism and higher drug levels90 |
|
| |
| proposed targets
|
|
| P450 | intended effect |
| 1A1, 1A2, 1B1 | block carcinogen activation, cancer prevention |
| 2A6, 2A13 | block carcinogen activation, cancer prevention |
| 4A11 | block arachidonic acid ω-hydroxylation, treat hypertension |
| 11A1 | block androgen production in prostate cancer |
| 11B2 | treatment for elevated aldosterone levels |
| 24A1 | raise level of active vitamin D metabolites |
| 26A1 | block degradation of endogenous retinoids |
| 26B1 | block degradation of endogenous retinoids |
| 26C1 | block degradation of endogenous retinoids |
| 51A1 | block cholesterol synthesis (cancer)91 |
In addition to the above P450s, a number of others have been proposed as targets (Table 6). Some of these involve cancer chemoprevention, related to blocking bioactivation of chemical carcinogens, as mentioned earlier. Most of the remainder are involved in the production of steroids or vitamin D products that stimulate tumors.8 Another goal is blocking the metabolism of vitamin A, as an alternative to supplementing with the vitamin (Table 6).
4. SUMMARY
There are a number of implications of the findings presented here. In 1980 Jakoby presented an overview of the enzymes involved in detoxication.94 At that time the general consensus was that some of these enzymes, including P450s, had defined substrates and functions, e.g., metabolism of steroids, eicosanoids, and vitamins (Table 1). The question was what the rest are really for. One school held that these enzymes had “true” physiological substrates, which would ultimately be discovered. The other view, held by Jakoby and to which I have also adhered, is that animals (including humans) have this battery of lower-selectivity enzymes as a general defense mechanism against xenobiotics.94 Our food is not a simple mixture of amino acids, simple carbohydrates, and lipids (actually these would not have much taste). We consume gram amounts daily of a mixture of terpenes, alkaloids, flavones, and other assorted natural products.
Cells have two major lines of defense against these “unnatural” natural compounds, materials that are not expected in the body. The cells can pump them out (transporters)95 or they can break them down (metabolize them using enzymes). In the 1980s, I began to follow the literature on a phenomenon termed multiple drug resistance, which had first been observed in tumors, and I even incorporated some of this material into my medical student lectures. The issue was a very practical one in that tumor cells became resistant to many drugs because of the induction of drug efflux transporters, either due to gene amplification or transcriptional regulation.95 As our own work on P450 3A4 developed, I realized that there was considerable overlap between the substrate repertoires of the newly discovered P450 3A4 and what was then called MDR-1.96,97 The MDR-1 protein turned out not be only associated with tumors but was also a normal plasma membrane constituent in liver, intestine, and brain.98,99 Since then, a number of other efflux transporters have been found to exist and pump many chemicals out of cells.100,101 Like P450s, there are a number of these proteins with overlapping substrate specificity. The substrates, as with the P450s, include both xenobiotic and endogenous substrate. Some of these transporters are regulated by the same compounds and elements, e.g., PXR. With regard to drugs, there is an interesting balance between intestinal transporters and P450s and hepatic transporters and P450s, as discussed elsewhere.102,103 Some of these interactions are probably also relevant to endogenous chemicals.
Thus, the role of the xenobiotic-metabolizing P450s can be seen as an adaptive response.94 This seems to make good sense, but in light of what we know now even that may be too simple a view of the P450 world.
What should be done in pharmaceutical development? Are issues such as those raised here in Tables 4 and 5 serious enough to require extra screens in the discovery/early development phases? The answer, in the author’s opinion, is to be realistic and not add such extra assays immediately in screening. One of the good points of our current P450 knowledge is that a set of only a few P450s dominate the metabolism of xenobiotics, including drugs, and thus are a focal point in screening.35 One has to have priorities. A logical approach is that used at Bristol-Myers Squibb: when adrenal toxicity was seen in an animal model, investigators identified the P450 11A1 role.21 This is an example of a new strategy discussed by Blomme and Will in their review article in a recent Special Issue of Chem. Res. Toxicol.,104 which can be summarized as “testing the right things at the right time.”
In conclusion, we know a considerable amount about P450s, including human P450s, but there continue to be surprises. The P450s that have been characterized for their roles in the metabolism of steroids and fat-soluble vitamins are less specific than originally thought, and many interact with drugs and other xenobiotic chemicals. In the design of drugs, off-target interactions are hard to completely avoid (hitting one target out of > 20,000 is the issue). Thus, it is not surprising that some drugs interact with that set of P450s. Some of the P450s that are normally involved in the metabolism of endogenous compounds have become drug targets, especially for cancers. Surprisingly, some of the “endogenous” substrate P450s even activate drugs.
Acknowledgments
Funding
This work was supported, in whole or in part, by National Institutes of Health grants R01 GM118122 and R01 GM103937 (F.P.G.). The author declares that he has no conflicts of interest with the contents of this article. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
I thank K. Trisler for her help in the preparation of the manuscript. This Perspective is dedicated to Prof. Michael R. Waterman, who was a major force in characterization of the P450s involved in steroid oxidation, Chairman of the Department of Biochemistry at Vanderbilt for 18 years, and a good friend and colleague. He set the stage for many of the P450 studies my laboratory is involved in today, including several mentioned here.
ABBREVIATIONS
- P450
cytochrome P450
- MDR
multiple drug resistance
Footnotes
The author declares no competing financial interests, although he is involved in consulting for several pharmaceutical companies.
References
- 1.Mueller GC, Miller JA. The metabolism of 4-dimethylaminoazobenzene by rat liver homogenates. J Biol Chem. 1948;176:535–544. [PubMed] [Google Scholar]
- 2.Miller EC, Miller JA. Mechanisms of chemical carcinogenesis: nature of proximate carcinogens and interactions with macromolecules. Pharamcol Rev. 1966;18:805–838. [PubMed] [Google Scholar]
- 3.Gillette JR, Brodie BB, La Du BN. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957;119:532–540. [PubMed] [Google Scholar]
- 4.Axelrod J. The enzymatic deamination of amphetamine. J Biol Chem. 1955;214:753–763. [PubMed] [Google Scholar]
- 5.Ryan KJ. Biological aromatization of steroids. J Biol Chem. 1959;234:268–272. [PubMed] [Google Scholar]
- 6.Omura T, Sato R. A new cytochrome in liver microsomes. J Biol Chem. 1962;237:1375–1376. [PubMed] [Google Scholar]
- 7.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 8.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 4. Springer; New York: 2015. pp. 523–785. [Google Scholar]
- 9.Guengerich FP, Waterman MR, Egli M. Structural insights into cytochrome P450 function. Trends Pharmacol Sci. 2016 doi: 10.1016/j.tips.2016.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auchus RJ, Miller WL. P450 enzymes in steroid processing. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 4. Springer; New York: 2015. pp. 851–879. [Google Scholar]
- 11.Miller WL, Morel Y. The molecular genetics of 21-hydroxylase deficiency. Annu Rev Genetics. 1989;23:371–393. doi: 10.1146/annurev.ge.23.120189.002103. [DOI] [PubMed] [Google Scholar]
- 12.New MI. Inborn errors of adrenal steroidogenesis. Mol Cell Endocrinol. 2003;211:75–83. doi: 10.1016/j.mce.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Williams RT. Detoxication Mechanisms. 1. Wiley; New York: 1947. [Google Scholar]
- 14.Williams RT. Detoxication Mechanisms. 2. Wiley; New York: 1959. [Google Scholar]
- 15.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 16.Goyal S, Xiao Y, Porter NA, Xu L, Guengerich FP. Oxidation of 7-dehydrocholesterol and desmosterol by human cytochrome P450 46A1. J Lipid Res. 2014;55:1933–1943. doi: 10.1194/jlr.M051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D. Cytochrome P450 metabolism of the post-lanosterol intermediates expains enigmas of cholesterol synthesis. Sci Reports. 2016;6:28462. doi: 10.1038/srep28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto FK, Gonzalez E, Auchus RJ, Guengerich FP. Mechanism of 17α,20-lyase and new hydroxylation reactions of human cytochrome P450 17A1. 18O-labeling and oxygen surrogate evidence for a role of a perferryl oxygen. J Biol Chem. 2016;291 doi: 10.1074/jbc.A116.732966. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björkhem I, Starck L, Andersson U, Lutjohann D, von Bahr S, Pikuleva I, Babiker A, Diczfalusy U. Oxysterols in the circulation of patients with the Smith-Lemli-Opitz syndrome: Abnormal levels of 24S- and 27-hydroxycholesterol. J Lipid Res. 2001;42:366–371. [PubMed] [Google Scholar]
- 21.Zhang D, Flint O, Wang L, Gupta A, Westhouse RA, Zhao W, Raghavan N, Caceres-Cortes J, Marathe P, Shen G, Zhang Y, Allentoff A, Josephs J, Gan J, Borzilleri R, Humphreys WG. Cytochrome P450 11A1 bioactivation of a kinase inhibitor in rats: Use of radioprofiling, modulation of metabolism, and adrenocortical cell lines to evaluate adrenal toxicity. Chem Res Toxicol. 2012;25:556–571. doi: 10.1021/tx200524d. [DOI] [PubMed] [Google Scholar]
- 22.Mast N, Norcross R, Andersson U, Shou M, Nakayama K, Björkhem I, Pikuleva IA. Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain. Biochemistry. 2003;42:14284–14292. doi: 10.1021/bi035512f. [DOI] [PubMed] [Google Scholar]
- 23.Savas Ü, Wei S, Hsu MH, Falck JR, Guengerich FP, Capdevila JH, Johnson EF. 20-Hydroxyeicosatetrenoic acid (20-HETE) dependent hypertension in human cytochrome P450 (CYP) 4A11 transgenic mice. Normalization of blood pressure by sodium restriction, hydrocholorthiazide, or blockade of the Type 1 angtiotensin receptor. J Biol Chem. 2016;291 doi: 10.1074/jbc.M116.732297. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Wu CC, Garcia V, Dimitrova I, Weidenhammer A, Joseph G, Zhang F, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. 20-HETE induces remodeling of renal resistance arteries independent of blood pressure elevation in hypertension. Am J Physiol Renal Physiol. 2013;305:F753–763. doi: 10.1152/ajprenal.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto S, Marcus C, Pidgeon C, Jefcoate C. A novel adrenocorticotropin-inducible cytochrome P450 from rat adrenal microsomes catalyzes polycyclic aromatic hydrocarbon metabolism. Endocrinology. 1991;129:970–982. doi: 10.1210/endo-129-2-970. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- 27.Kellerman G, Luyten-Kellerman M, Shaw CR. Genetic variation of aryl hydrocarbon hydroxylase in human lymphocytes. Am J Human Genet. 1973;25:327–331. [PMC free article] [PubMed] [Google Scholar]
- 28.Kellerman G, Shaw CR, Luyten-Kellerman M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. New Engl J Med. 1973;298:934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- 29.Toide K, Yamazaki H, Nagashima R, Itoh K, Iwano S, Takahashi Y, Watanabe S, Kamataki T. Aryl hydrocarbon hydroxylase represents CYP1B1, and not CYP1A1, in human freshly isolated white cells: Trimodal distribution of Japanese population according to induction of CYP1B1 mRNA by environmental dioxins. Cancer Epidemiol Biomarkers Prev. 2003;12:219–222. [PubMed] [Google Scholar]
- 30.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17β-Estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liehr JG, Ricci MJ. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proc Natl Acad Sci U S A. 1996;93:3294–3296. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoilov I, Akarsu AN, Alozie I, Child A, Barsoum-Homsy M, Turacli ME, Or M, Lewis RA, Ozdemir N, Brice G, Aktan SG, Chevrette L, Coca-Prados M, Sarfarazi M. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Human Genet. 1998;62:573–584. doi: 10.1086/301764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Human Mol Genet. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 34.Rendic S, Guengerich FP. Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol. 2012;25:1316–1383. doi: 10.1021/tx300132k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rendic S, Guengerich FP. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol. 2015;28:38–42. doi: 10.1021/tx500444e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shou M, Korzekwa KR, Brooks EN, Krausz KW, Gonzalez FJ, Gelboin HV. Role of human hepatic cytochrome P450 1A2 and 3A4 in the metabolic activation of estrone. Carcinogenesis. 1997;18:207–214. doi: 10.1093/carcin/18.1.207. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki H, Shaw PM, Guengerich FP, Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol. 1998;11:659–665. doi: 10.1021/tx970217f. [DOI] [PubMed] [Google Scholar]
- 38.Waxman DJ, Attisano C, Guengerich FP, Lapenson DP. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6β-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988;263:424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- 39.Krauser JA, Voehler M, Tseng LH, Schefer AB, Godejohann M, Guengerich FP. Testosterone 1β-hydroxylation by human cytochrome P450 3A4. Eur J Biochem. 2004;271:3962–3969. doi: 10.1111/j.1432-1033.2004.04339.x. [DOI] [PubMed] [Google Scholar]
- 40.Diczfalusy U, Kanebratt KP, Bredberg E, Andersson TB, Bottiger Y, Bertilsson L. 4β-Hydroxycholesterol as an endogenous marker for CYP3A4/5 activity. Stability and half-life of elimination after induction with rifampicin. Br J Clinical Pharmacol. 2009;67:38–43. doi: 10.1111/j.1365-2125.2008.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinkyo R, Guengerich FP. Inhibition of human cytochrome P450 3A4 by cholesterol. J Biol Chem. 2011;286:18426–18433. doi: 10.1074/jbc.M111.240457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 43.Russell DW, Halford RW, Ramirez DM, Shah R, Kotti T. Cholesterol 24-hydroxylase: An enzyme of cholesterol turnover in the brain. Annu Rev Biochem. 2009;78:1017–1040. doi: 10.1146/annurev.biochem.78.072407.103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafaati M, Mast N, Beck O, Nayef R, Heo GY, Bjorkhem-Bergman L, Lutjohann D, Björkhem I, Pikuleva IA. The antifungal drug voriconazole is an efficient inhibitor of brain cholesterol 24S-hydroxylase in vitro and in vivo. J Lipid Res. 2010;51:318–323. doi: 10.1194/jlr.M900174-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mast N, Charvet C, Pikuleva IA, Stout CD. Structural basis of drug binding to CYP46A1, an enzyme that controls cholesterol turnover in the brain. J Biol Chem. 2010;285:31783–31795. doi: 10.1074/jbc.M110.143313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mast N, Zheng W, Stout CD, Pikuleva IA. Binding of a cyano- and fluoro-containing drug bicalutamide to cytochrome P450 46A1: Unusual features and spectral response. J Biol Chem. 2013;288:4613–4624. doi: 10.1074/jbc.M112.438754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mast N, Li Y, Linger M, Clark M, Wiseman J, Pikuleva IA. Pharmacologic stimulation of cytochrome P450 46A1 and cerebral cholesterol turnover in mice. J Biol Chem. 2014;289:3529–3538. doi: 10.1074/jbc.M113.532846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson KW, Mast N, Hudgens JW, Lin JB, Turko IV, Pikuleva IA. Mapping of the allosteric site in cholesterol hydroxylase CYP46A1 for efavirenz, a drug that stimulates enzyme activity. J Biol Chem. 2016;291:11876–11886. doi: 10.1074/jbc.M116.723577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolt HM, Bolt M, Kappus H. Interaction of rifampicin treatment with pharmacokinetics and metabolism of ethinyloestradiol in man. Acta Endocrinol. 1977;85:189–197. doi: 10.1530/acta.0.0850189. [DOI] [PubMed] [Google Scholar]
- 50.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 51.Guengerich FP. Inhibition of drug metabolizing enzymes: Molecular and biochemical aspects. In: Woolf TF, editor. Handbook of Drug Metabolism. Marcel Dekker; New York: 1999. pp. 203–227. [Google Scholar]
- 52.VandenBrink BM, Foti RS, Rock DA, Wienkers LC, Wahlstrom JL. Prediction of CYP2D6 drug interactions from in vitro data: Evidence for substrate-dependent inhibition. Drug Metab Dispos. 2012;40:47–53. doi: 10.1124/dmd.111.041210. [DOI] [PubMed] [Google Scholar]
- 53.Correia MA, Hollenberg PF. Inhibition of cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 4. Springer; New York: 2015. pp. 177–259. [Google Scholar]
- 54.Wattenberg LW. Inhibition of carcinogenesis by minor dietary constituents. Cancer Res. 1992;52:2085s–2091s. [PubMed] [Google Scholar]
- 55.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swanson HI, Njar VC, Yu Z, Castro DJ, Gonzalez FJ, Williams DE, Huang Y, Kong AN, Doloff JC, Ma J, Waxman DJ, Scott EE. Targeting drug-metabolizing enzymes for effective chemoprevention and chemotherapy. Drug Metab Dispos. 2010;38:539–544. doi: 10.1124/dmd.109.031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopkins NE, Foroozesh MK, Alworth WL. Suicide inhibitors of cytochrome P450 1A1 and P450 2B1. Biochem Pharmacol. 1992;44:787–796. doi: 10.1016/0006-2952(92)90417-h. [DOI] [PubMed] [Google Scholar]
- 58.Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–1056. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- 59.Langouet S, Furge LL, Kerriguy N, Nakamura K, Guillouzo A, Guengerich FP. Inhibition of human cytochrome P450 enzymes by 1,2-dithiole-3-thione, oltipraz and its derivatives, and sulforaphane. Chem Res Toxicol. 2000;13:245–252. doi: 10.1021/tx990189w. [DOI] [PubMed] [Google Scholar]
- 60.Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, Jacobson LP, Munoz A, Helzlsouer KJ, Groopman JD, Kensler TW. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 61.Yoshigae Y, Sridar C, Kent UM, Hollenberg PF. The inactivation of human CYP2E1 by phenethyl isothiocyanate, a naturally occurring chemopreventive agent, and its oxidative bioactivation. Drug Metab Dispos. 2013;41:858–869. doi: 10.1124/dmd.112.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Weymarn LB, Chun JA, Hollenberg PF. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: Potential for chemoprevention in smokers. Carcinogenesis. 2006;27:782–790. doi: 10.1093/carcin/bgi301. [DOI] [PubMed] [Google Scholar]
- 63.Shimada T, Murayama N, Tanaka K, Takenaka S, Guengerich FP, Yamazaki H, Komori M. Spectral modification and catalytic inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2A6, and 2A13 by four chemopreventive organoselenium compounds. Chem Res Toxicol. 2011;24:1327–1337. doi: 10.1021/tx200218u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yano JK, Denton TT, Cerny MA, Zhang X, Johnson EF, Cashman JR. Synthetic inhibitors of cytochrome P-450 2A6: Inhibitory activity, difference spectra, mechanism of inhibition, and protein cocrystallization. J Med Chem. 2006;49:6987–7001. doi: 10.1021/jm060519r. [DOI] [PubMed] [Google Scholar]
- 65.Chun YJ, Kim S, Kim D, Lee SK, Guengerich FP. A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res. 2001;61:8164–8170. [PubMed] [Google Scholar]
- 66.Edgar B, Bailey DG, Bergstrand R, Johnsson G, Lurje L. Formulation dependent interaction between felodipine and grapefruit juice. Clin Pharmacol Ther. 1990;47:181. [Google Scholar]
- 67.Lin HL, Kent UM, Hollenberg PF. The grapefruit juice effect is not limited to cytochrome P450 (P450) 3A4: Evidence for bergamottin-dependent inactivation, heme destruction, and covalent binding to protein in P450s 2B6 and 3A5. J Pharmacol Exp Ther. 2005;313:154–164. doi: 10.1124/jpet.104.079608. [DOI] [PubMed] [Google Scholar]
- 68.Federspiel JD, Codreanu SG, Goyal S, Albertolle ME, Lowe E, Teague J, Wong H, Guengerich FP, Liebler DC. Specificity of protein covalent modification by the electrophilic proteasome inhibitor carfilzomib in human cells. Mol Cell Proteomics. 2016 doi: 10.1074/mcp.M116.059709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papadopoulos KP, Siegel DS, Vesole DH, Lee P, Rosen ST, Zojwalla N, Holahan JR, Lee S, Wang Z, Badros A. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33:732–739. doi: 10.1200/JCO.2013.52.3522. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Yang J, Kirk C, Fang Y, Alsina M, Badros A, Papadopoulos K, Wong A, Woo T, Bomba D, Li J, Infante JR. Clinical pharmacokinetics, metabolism, and drug-drug interaction of carfilzomib. Drug Metab Dispos. 2013;41:230–237. doi: 10.1124/dmd.112.047662. [DOI] [PubMed] [Google Scholar]
- 71.Temple TE, Liddle GW. Inhibitors of adrenal steroid biosynthesis. Annu Rev Pharmacol. 1970;10:199–218. doi: 10.1146/annurev.pa.10.040170.001215. [DOI] [PubMed] [Google Scholar]
- 72.Raven PW, Hinson JP. Transport, action, and metabolism of adrenal hormones and pathology and pharmacology of adrenal gland. In: Harvey PW, editor. The Adrenal in Toxicology: Target Organ and Modulator of Toxicity. Taylor and Francis; London: 1996. pp. 53–79. [Google Scholar]
- 73.Johansson MK, Sanderson JT, Lund BO. Effects of 3-MeSO2-DDE and some CYP inhibitors on glucocorticoid steroidogenesis in the H295R human adrenocortical carcinoma cell line. Toxicol In Vitro. 2002;16:113–121. doi: 10.1016/s0887-2333(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 74.Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. New Engl J Med. 1984;310:1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 75.Hinson JP, Raven PW. Effects of endocrine-disrupting chemicals on adrenal function. Best Practice Res Clinical Endocrinology Metab. 2006;20:111–120. doi: 10.1016/j.beem.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Engelhardt D, Weber MM, Miksch T, Abedinpour F, Jaspers C. The influence of ketoconazole on human adrenal steroidogenesis: incubation studies with tissue slices. Clin Endocrinol. 1991;35:163–168. doi: 10.1111/j.1365-2265.1991.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 77.Martz F, Straw JA. Metabolism and covalent binding of 1-(o-chlorophenyl)-1-(p-chlorophenyl)-2,2-dichloroethane (o, p′, -DDD). Correlation between adrenocorticolytic activity and metabolic activation by adrenocortical mitochondria. Drug Metab Dispos. 1980;8:127–130. [PubMed] [Google Scholar]
- 78.Cai W, Counsell RE, Djanegara T, Schteingart DE, Sinsheimer JE, Wotring LL. Metabolic activation and binding of mitotane in adrenal cortex homogenates. J Pharm Sci. 1995;84:134–138. doi: 10.1002/jps.2600840203. [DOI] [PubMed] [Google Scholar]
- 79.Johansson M, Larsson C, Bergman A, Lund BO. Structure-activity relationship for inhibition of CYP11B1-dependent glucocorticoid synthesis in Y1 cells by aryl methyl sulfones. Pharmacol Toxicol. 1998;83:225–230. doi: 10.1111/j.1600-0773.1998.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 80.Lindhe O, Lund BO, Bergman A, Brandt I. Irreversible binding and adrenocorticolytic activity of the DDT metabolite 3-methylsulfonyl-DDE examined in tissue-slice culture. Environ Health Perspect. 2001;109:105–110. doi: 10.1289/ehp.109-1240628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu X, Latendresse JR, Muskhelishvili L, Blaydes BS, Delclos KB. Dietary modulation of 7,12-dimethylbenz[a]anthracene (DMBA)-induced adrenal toxicity in female Sprague-Dawley rats. Food Chem Toxicol. 2005;43:765–774. doi: 10.1016/j.fct.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Hallberg E. Metabolism and toxicity of xenobiotics in the adrenal cortex, with particular reference to 7,12-dimethylbenz(a)anthracene. J Biochem Toxicol. 1990;5:71–90. doi: 10.1002/jbt.2570050202. [DOI] [PubMed] [Google Scholar]
- 83.Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol. 2011;125:13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang LP. Abiraterone acetate: in metastatic castration-resistant prostate cancer. Drugs. 2011;71:2067–2077. doi: 10.2165/11208080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 85.DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaku T, Hitaka T, Ojida A, Matsunaga N, Adachi M, Tanaka T, Hara T, Yamaoka M, Kusaka M, Okuda T, Asahi S, Furuya S, Tasaka A. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19:6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 87.Toren PJ, Kim S, Pham S, Mangalji A, Adomat H, Guns ES, Zoubeidi A, Moore W, Gleave ME. Anticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancer. Mol Cancer Ther. 14:59–69. doi: 10.1158/1535-7163.MCT-14-0521. [DOI] [PubMed] [Google Scholar]
- 88.Tagashira H, Kominami S, Takemori S. Kinetic studies of cytochrome P45017α,lyase dependent androstenedione formation from progesterone. Biochemistry. 1995;34:10939–10945. doi: 10.1021/bi00034a028. [DOI] [PubMed] [Google Scholar]
- 89.Pallan PS, Nagy LD, Lei L, Gonzalez E, Kramlinger VM, Azumaya CM, Wawrzak Z, Waterman MR, Guengerich FP, Egli M. Structural and kinetic basis of steroid 17α,20-lyase activity in teleost fish cytochrome P450 17A1 and its absence in cytochrome P450 17A2. J Biol Chem. 2015;290:3248–3268. doi: 10.1074/jbc.M114.627265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaur P, Chamberlin AR, Poulos TL, Sevrioukova IF. Structure-based inhibitor design for evaluation of a CYP3A4 pharmacophore model. J Med Chem. 2016;59:4210–4220. doi: 10.1021/acs.jmedchem.5b01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hargrove TY, Friggeri L, Wawrzak Z, Sivakumaran S, Yazlovitskaya Heibert SW, Guengerich FP, Waterman MR, Lepesheva GI. Probing human cytochrome P450 sterol 14α-demethylase (CYP51) as a target for anticancer chemotherapy: Towards structure-aided drug design. J Lipid Res. 2016;57 doi: 10.1194/jlr.M069229. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kempf DJ, Marsh KC, Kumar G, Rodrigues AD, Denissen JF, McDonald E, Kukulka MJ, Hsu A, Granneman GR, Baroldi PA, Sun E, Pizzuti D, Plattner JJ, Norbeck DW, Leonard JM. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sevrioukova IF, Poulos TL. Pyridine-substituted desoxyritonavir is a more potent inhibitor of cytochrome P450 3A4 than ritonavir. J Med Chem. 2013;56:3733–3741. doi: 10.1021/jm400288z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jakoby WB. Detoxication enzymes. In: Jakoby WB, editor. Enzymatic Basis of Detoxication. Vol. 1. Academic Press; New York: 1980. pp. 1–6. [Google Scholar]
- 95.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 96.Guengerich FP, Martin MV, Beaune PH, Kremers P, Wolff T, Waxman DJ. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1986;261:5051–5060. [PubMed] [Google Scholar]
- 97.Yasuda K, Lan LB, Sanglard D, Furuya K, Schuetz JD, Schuetz EG. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J Pharmacol Exp Ther. 2002;303:323–332. doi: 10.1124/jpet.102.037549. [DOI] [PubMed] [Google Scholar]
- 98.Schuetz EG, Furuya KN, Schuetz JD. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther. 1995;275:1011–1018. [PubMed] [Google Scholar]
- 99.Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P450 3A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996;49:311–318. [PubMed] [Google Scholar]
- 100.Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y. Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008;73:1474–1483. doi: 10.1124/mol.107.041459. [DOI] [PubMed] [Google Scholar]
- 101.Jugsuwadee P, Vore ME. Efflux transporters. In: Guengerich FP, McQueen CA, editors. Biotransformation. 2. Elsevier; Osford, UK: 2010. pp. 557–601. Vol. 4 of Comprehensive Toxicology. [Google Scholar]
- 102.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinogen. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 103.Cummins CL, Jacobsen W, Benet LZ. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;300:1036–1045. doi: 10.1124/jpet.300.3.1036. [DOI] [PubMed] [Google Scholar]
- 104.Blomme EA, Will Y. Toxicology strategies for drug discovery: Present and future. Chem Res Toxciol. 2016;29:473–504. doi: 10.1021/acs.chemrestox.5b00407. [DOI] [PubMed] [Google Scholar]