Abstract
PURPOSE: To investigate the prognostic value of preoperative intratumoral 18F-FDG uptake heterogeneity (IFH) derived from positron emission tomography (PET)/computed tomography (CT) in patients with endometrioid endometrial cancer. METHODS: We retrospectively evaluated clinicopathological data from patients with pathologically proven endometrioid endometrial cancer who had undergone 18F-FDG PET/CT scans before surgery. Patients were divided into two groups according to their IFH. The main outcome measure was disease-free survival (DFS). RESULTS: Between January 2010 and January 2015, data from 72 patients were available for analysis. The median duration of DFS was 23 months (range, 6 to 57 months), and 4 (5.6%) patients experienced recurrence. There were significant differences in tumor size, IFH, and DFS between patients with and without recurrence. In regression analysis, high IFH value [P = .007, hazard ratio (HR) 2.545, 95% confidence interval (CI) 1.468-8.674] was the only independent risk factor for recurrence. The Kaplan-Meier survival graphs showed that DFS significantly differed in groups categorized based on IFH (P < .001, log-rank test). CONCLUSIONS: Preoperative IFH measured by 18F-FDG PET/CT was associated with recurrence of endometrioid endometrial cancer. The finding supports evidence that FDG-based heterogeneity can be a novel and useful predictor of endometrioid endometrial cancer recurrence.
Introduction
The incidence of endometrial cancer is rapidly increasing worldwide, with the highest disease burden reported in developed countries. In the United States, endometrial cancer has been the most common gynecologic malignancy with more than 60,000 newly diagnosed cases projected for 2016 [1], and there will be a doubling in the number of women diagnosed with endometrial cancer by the year 2030 to 122,000 cases per year [2]. Such trend is global, and the age-standardized incidence of endometrial cancer has been doubled in South Korea [3], [4]. However, endometrial cancer has been understudied and remains an underfunded field of research.
18F-FDG positron emission tomography (PET)/computed tomography (CT) combines morphologic and physiologic techniques and is the preferred imaging method especially in clinical oncology [5], and previous studies have suggested beneficial role of preoperative 18F-FDG PET/CT in endometrial cancer [6], [7], [8], [9]. 18F-FDG uptake in tissue is a useful indicator of tumor metabolism, and the maximum standardized uptake value (SUVmax) reflects the highest metabolic activity within the tumor. There has been increasing interest in assessing the tumor heterogeneity and, specifically, intratumoral 18F-FDG uptake heterogeneity (IFH) [10]. The association between IFH and prognosis has been reported in several malignancies [10], [11], [12], [13]. Several physiological processes including glucose metabolism, necrosis, vascularization, and angiogenesis were regarded as having correlation with heterogeneous distribution of 18F-FDG PET activity in the same tumor [14], [15]. Although there were several studies on the clinical role of IFH in predicting prognosis in various cancers [16], [17], [18], there is no study on the 18F-FDG heterogeneity in endometrial cancer.
The objective of this study was to investigate the prognostic value of preoperative intratumoral IFH in patients with endometrioid endometrial cancer. For this purpose, we investigated the relationship between the IFH and various clinical and PET/CT parameters.
Materials and Methods
Patients
We retrospectively reviewed all consecutive patients with histologically biopsy proven endometrioid endometrial cancer who underwent preoperative 18F-FDG PET/CT imaging between January 2010 and January 2015. The diagnoses were established through preoperative endometrial biopsy, and stage was assessed according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 criteria for surgical staging. All clinicopathological and imaging data from patients were collected and reviewed. Patients were required to have undergone both preoperative integrated 18F-FDG PET/CT imaging in the 2 weeks prior to surgery. Patients were excluded from analysis if 1) they had another malignant disease; 2) they had nonendometrioid endometrial cancer; 3) they had a follow-up duration <6 months; 4) they received a primary treatment other than surgery, such as neoadjuvant chemotherapy or preoperative radiation; 5) they had received scan at outside institution; or 6) their scan had no sign of FDG uptake abnormality. After treatment, all patients were clinically and radiologically followed up. The study protocol was approved by the institutional review board, and informed consent was waived due to its retrospective design.
Demographic and clinical characteristics and survival data were obtained from the patients' medical records and institutional tumor records. Tumor histology, grade, and size were obtained from the surgical pathology report.
PET/CT Technique
Patients were examined using an integrated Biograph PET/CT scanner (Siemens Medical Solutions, USA). Requested minimum fasting time for 18F-FDG PET/CT imaging was 6 hours, and diuretics were not used for preparation. Fasting blood sugar level was checked using a commercially provided portable glucometer (Accu-Chek; Roche, Indianapolis, IN). Approximately 0.14 mCi/kg body weight of FDG was administered intravenously 1 hour prior to imaging. After voiding, CT was performed before PET, and the resulting data were used to generate an attenuation correction map for PET. The following parameters were used for CT: 80 mA, 120 kV, 5-mm section thickness, 0.5 second per rotation, and reconstruction onto a 512 × 512 matrix. Each PET scan was acquired from skull base to proximal thigh in three-dimensional row action maximum likelihood algorithm mode with 4 iterations, 8 subsets, and 4.8-mm full-width half-maximum reconstruction onto a 512 × 512 matrix. A total of 7 to 9 bed positions were examined for PET acquisition, with 2.5 min/bed position.
Image Analysis
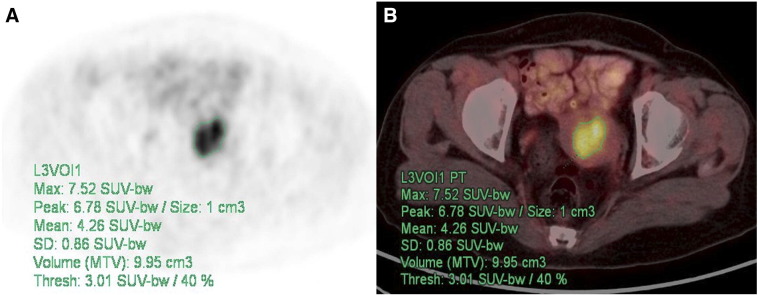
18F-FDG PET/CT scans were reviewed by two experienced nuclear physicians, and intensity values were converted to SUVs. Findings were recorded by consensus, and the nuclear physicians were masked to all other clinical and imaging information. The contour around the target lesions inside the boundaries was automatically produced, and the voxels presenting a threshold of 40% SUVmax in the volume of interest within the contouring margin were incorporated to define the precise tumor volumes (Figure 1).
Figure 1.
Measurement of IFH. PET (A) and PET/CT (B) for IFH measurement using an SUV-based automated contouring program in a 64-year-old female patient. Transaxial PET or PET/CT images show hypermetabolic lesion with heterogeneous distribution of FDG inside uterine cavity.
The SUVmax and average SUV (SUVavg) were then quantitatively used to determine 18F-FDG avidity. SUV was defined as the concentration of 18F-FDG divided by the injected dose, corrected for the body weight of the patient and radioactive decay at scanning time (SUV = activity concentration/[injected dose/body weight]).
Intratumoral FDG Heterogeneity Analysis
In the current analysis, coefficient of variation (CV) was chosen to calculate tumor heterogeneity. CV is one of the representative parameters of global level that has been reported to predict therapy response and prognosis in several cancers [19]. CV was defined as the standard deviation (SD) of SUVs divided by the SUVavg within the automatically delineated tumor volume, expressed as CV = SD of SUVs/SUVavg.
Clinical Endpoints and Follow-Up
To determine recurrence or death and other clinical characteristics, serial data were collected from medical records. Surveillance after treatment was performed according to the institutional protocol: every 2 months for 12 months, then every 3 months for 18 months followed by every 6 months for 36 months. Gynecological examination and test for serum CA-125 were performed at every visit, and imaging checkup using CT or magnetic resonance imaging was performed every 6 months. 18F-FDG PET/CT was considered when recurrence was suspected during surveillance.
Disease-free survival (DFS) was defined as the time from the date of surgery to date of the first finding on clinical or imaging examination that suggested local, regional, or distant disease recurrence. Recurrent tumor and distant metastasis were diagnosed based on either a positive biopsy or unequivocal clinical or radiographic evidence of progression.
Statistical Analysis
We tried to determine the prognostic value of preoperative 18F-FDG PET/CT parameters for DFS. Kaplan-Meier estimates and the log-rank test were done to assess the equality of the survival functions across variables in the DFS analysis. The Cox proportional hazard model was used to evaluate prognostic variables, and hazard ratio (HR) with 95% confidence interval (95% CI) was presented. A P value < .05 was considered statistically significant. All analyses were performed using SPSS software for Windows (version 19.0; IBM SPSS, Somers, NY).
Results
Patient Characteristics
During the study period, 217 patients at our institution were newly diagnosed with endometrioid endometrial cancer, and 125 of these patients underwent PET/CT prior to primary treatment. However, scans from 53 patients were unavailable for SUV measurement: scans from 34 patients were unavailable for analysis with current workstation because their scans were performed at outside institutions, and 19 scans had no sign of FDG uptake abnormality. Eventually, scans from 72 of these patients (median age, 55 years; range, 28-76 years) were eligible for IFH analysis. The median follow-up for surviving patients was 23 months (range, 6-57 months). Patient clinicopathological findings and preoperative PET/CT findings are summarized in Table 1.
Table 1.
Clinicopathological Characteristics of Patients Who Underwent PET/CT before Operation for Endometrioid Endometrial Cancer (n = 72)
| Characteristic | Patients | % |
|---|---|---|
| Age, median (range) | 55 (28-76) | |
| Median DFS, months (range) | 23 (6-57) | |
| FIGO stage | ||
| I | 49 | 68.1 |
| II | 3 | 4.2 |
| III | 16 | 22.2 |
| IV | 4 | 5.6 |
| Tumor grade | ||
| 1 | 33 | 45.8 |
| 2 | 28 | 38.9 |
| 3 | 11 | 15.3 |
| Recurrence | 5 | 6.9 |
| Median tumor size, cm (range) | 3.5 (1-12) | |
| Median SUVmax (range) | 11.96 (2.73-36.10) | |
| Median IFH (range) | 0.2434 (0.17-0.29) | |
| Recurrence | 4 | 5.6 |
Measurement of Cutoff Value for Intratumoral Heterogeneity
The receiver-operating characteristic (ROC) curves were used to analyze the IFH in relation to DFS. IFH at an SUV threshold of 40% was used for ROC analysis, and area under the curve (AUC) of the ROC curve of IFH was 0.886 (P = .010, 95% CI 0.751-1.000), and 0.2651 was determined as the optimal threshold (Figure 2). The sensitivity at this cutoff value was 0.750, and the specificity was 0.897.
Figure 2.
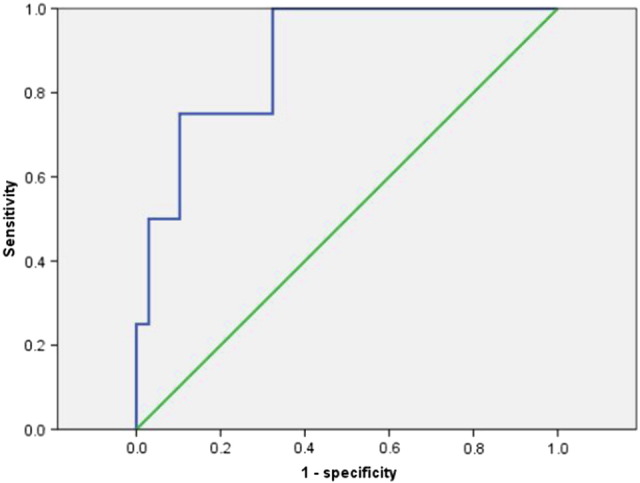
ROC curve analysis for determination of the cutoff value of IFH for predicting recurrence in patients with endometrial cancer. Area under the ROC curve of IFH was 0.886 (P = .010, 95% CI 0.751-1.000), and 0.2651 was determined as the optimal threshold.
Prediction of Recurrence
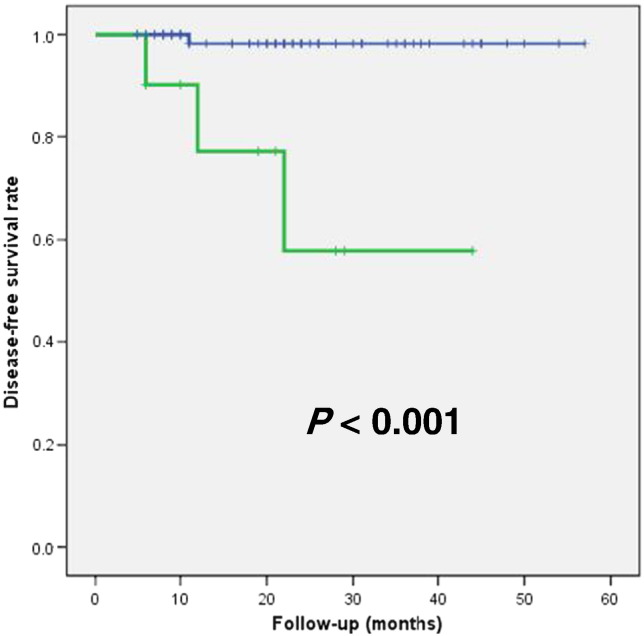
Tables 2 and 3 summarize the results of univariate and multivariate regression analyses of prognostic factors for DFS, respectively. Univariate regression analysis revealed that high IFH value (P = .012, HR 9.608, 95% CI 1.182-7.809) was associated with recurrence (Table 2). Large tumor size (P = .053, HR 1.356, 95% CI 0.996-1.845) and advanced FIGO stage (P = .071, HR 8.018, 95% CI 0.834-77.124) showed borderline significance. In multivariate regression analysis, high IFH value (P = .007, HR 2.545, 95% CI 1.468-8.674) was the only independent risk factor for recurrence (Table 3). Kaplan-Meier survival graphs in Figure 3 depict that DFS significantly differed in groups categorized based on threshold IFH (P < .001, log-rank test).
Table 2.
Univariate Regression Analyses of Prognostic Factors for DFS in Patients with Endometrial Cancer
| Variables | Test for Progression-Free Survival | HR | 95% CI | P |
|---|---|---|---|---|
| Age (years) | 0.978 | 0.893-1.071 | .634 | |
| FIGO stage | III, IV vs I, II | 8.018 | 0.834-77.124 | .071 |
| Tumor grade | 3 vs 1, 2 | 0.032 | 0.000-598.471 | .494 |
| Tumor size | 1.356 | 0.996-1.845 | .053 | |
| Deep myometrial invasion | Present vs absent | 1.580 | 0.222-11.224 | .648 |
| LVSI | Present vs absent | 2.791 | 0.393-19.836 | .305 |
| LN metastasis | Present vs absent | 1.627 | 0.168-15.714 | .674 |
| SUVmax | 1.023 | 0.919-1.139 | .677 | |
| IFH | 9.608 | 1.182-7.809 | .012 |
Table 3.
Multivariate Regression Analyses of Prognostic Factors DFS in Patients with Endometrial Cancer
| Variables | Test for Progression-Free Survival | HR | 95% CI | P |
|---|---|---|---|---|
| FIGO stage | III, IV vs I, II | 5.375 | 0.317-91.266 | .244 |
| Tumor size | 2.084 | 0.901-4.816 | .086 | |
| IFH | 2.545 | 1.468-8.674 | .007 |
Figure 3.
Kaplan-Meier survival graph shows significantly different DFS between the groups categorized by intratumoral FDG uptake heterogeneity below (blue line) and above (green line) the optimal threshold (0.2651) (P < .001, log-rank test).
Summary of Patients with Recurrence
Table 4 shows the clinicopathological characteristics of four patients who experienced recurrence after treatment for endometrioid endometrial cancer. Two patients had FIGO stage III disease, one had stage II, and another had IVB, respectively. DFS ranged from 6 to 22 months, and the SUVmax ranged from 8.95 to 20.42. Patients with stage IVB had the lowest IFH value, whereas patients with stage II had the highest IFH value (range, 0.2521-0.2873).
Table 4.
Clinicopathological Characteristics of Patients Who Experienced Recurrence after Treatment for Endometrioid Endometrial Cancer (n = 4)
| Age (Year) | FIGO Stage | Tumor Grade | DFS (Months) | SUVmax | IFH | |
|---|---|---|---|---|---|---|
| Patient 1 | 58 | II | 2 | 6 | 12.08 | 0.2873 |
| Patient 2 | 60 | IIIA | 3 | 22 | 8.95 | 0.2732 |
| Patient 3 | 38 | IIIC | 3 | 12 | 20.42 | 0.2658 |
| Patient 4 | 45 | IVB | 2 | 11 | 19.52 | 0.2512 |
Differences between Recurrent and Nonrecurrent Groups
Table 5 summarizes the clinicopathological and 18F-FDG PET/CT imaging–derived characteristics of patients with and without recurrence. There were significant differences in tumor size (P = .004), IFH (P = .018), and DFS (P = .022) between patients with and without recurrence.
Table 5.
Clinicopathological and PET/CT-Derived Characteristics of Patients without and with Recurrence (n = 72)
| Variable | Without Recurrence (n = 68) |
With Recurrence (n = 4) |
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (year) | 53.412 | 10.315 | 50.250 | 10.532 | .596 |
| Tumor size | 4.282 | 2.624 | 7.325 | 1.118 | .004 |
| SUVmax | 13.660 | 8.762 | 15.243 | 5.618 | .627 |
| IFH | 0.238 | 0.024 | 0.269 | 0.015 | .018 |
| DFS (months) | 25.559 | 12.691 | 12.750 | 6.702 | .022 |
Discussion
The principal finding of the current study was that preoperative IFH measured by 18F-FDG PET/CT was significantly associated with recurrence in endometrioid endometrial cancer. To the best of our knowledge, this is the first report investigating the prognostic value of preoperative IFH in endometrioid endometrial cancer. The result depicts that 18F-FDG–based heterogeneity can be a useful and potential predictor of patient recurrence before treatment in patients with endometrioid endometrial cancer.
Despite improved understanding of the pathogenesis of endometrial cancer, the prognosis for those with recurrent or metastatic disease, the options are few, and the prognosis is poor [20]. In this view, identification of patients with a poor prognosis who may benefit from aggressive surveillance and treatment is very important.
18F-FDG uptake is heterogeneous throughout a tumor, and there are some factors known to contribute to IFH such as necrosis [21], cellular proliferation [22], blood flow [23], microvessel density [24], and hypoxia [25], [26]. As the tumoral uptake of a radiopharmaceutical tracer is heterogeneously distributed in the tumor, it may be useful to quantify and follow up IFH throughout treatment period.
The risk of recurrence increased significantly with increased IFH. This result highlights the importance of the metabolic complexity of tumors and the possibility that IFH can be a novel and powerful prognostic biomarker of endometrial cancer. Although the clinical efficacy and benefit of more intensive surveillance strategy in patients with high IFH are not established, earlier detection of residual or metastatic lesions might influence the patients' outcome.
In the current study, we advocated the use of SUV-based automated contouring program to provide a reference for volume delineation, and voxels presenting a threshold of 40% SUVmax in the volume of interest within the contouring margin were incorporated to define the tumor volumes. Tumor delineation influences heterogeneity matrix, and we evaluated a threshold of 40% and a fixed isocontour at SUVs over 2.5, 3.0, 3.5, or 4.0. After comparison using ROC curve analysis, a threshold of 40% of SUVmax was chosen because it showed the highest value of the AUC in this study.
In the multivariate regression analyses, we included “nonsignificant borderline” parameters at univariate analysis because these are well-established risk factors of recurrence [27], [28]. Although not included in the FIGO or TNM classifications, histological grade, the patient's age, tumor size, and lymphovascular space invasion (LVSI) have been identified as prognostic factors. Of interest, only the high IFH value (P = .007) was revealed as an independent risk factor of recurrence. Previous studies have underlined the high accuracy of 18F-FDG PET/CT in detection of myometrial and cervical invasion and lymph node (LN) metastatic disease. However, although its prognostic value has been shown for advanced-stage endometrial cancer, its clinical use in preoperative staging in early-stage disease remains controversial [29], [30]. In the current study, 68.1% of patients were FIGO stage I, and less than 30% of patients were in advanced stage, and such stage distribution might have influenced the result. A larger study is warranted to further investigate the association between these parameters and prognosis.
Limitations to our study include relatively small group size and short follow-up. It was a retrospective study with a small number of patients and a short follow-up period without overall survival analysis. Also, there were only four patients with recurrence. Therefore, interpretation of the current study should be confined to short-term outcome focused on recurrence. The results in this study may not be generalizable to all patients with endometrial cancer because not all scans were available for IFH measurement and only measurable scans were included in the analysis. Also, this study may have spectrum bias because recruited patients had been selected as those with FDG PET/CT, and the subjects in this study may not represent the intended-use population. However, the current study is the first to demonstrate the prognostic value of preoperative IFH in patients with endometrioid endometrial cancer and suggests the clinical relevance of metabolic parameters. Second, histological confirmation of tumor heterogeneity or comparison with IFH observed in this study was not performed. Therefore, the extent and difference of histological features could not be investigated. Third, IFH applied in this study as a method to quantify heterogeneity might lose some information concerning spatial relationships within the tumor and might be confounded by high noise level. Partial volume effect can also cause distributions of measured intensities to appear more heterogeneous and may underestimate the SUV measure. In this aspect, we assumed that IFH may be less influenced by partial volume effect and can be a better candidate for prognostic parameter
Clinical implications of this study include possible stratification of endometrioid endometrial cancer recurrence before treatment. IFH helps identifying patients with an increased risk for disease recurrence, and these patients may benefit from an intensive therapy and follow-up strategy. The direct influence on prognosis using IFH needs to be investigated and verified in future studies.
The results of this study confirm the association between preoperative tumor 18F-FDG uptake heterogeneity and a high tumor recurrence in endometrioid endometrial cancer. Current intuitive method showed statistically significant predictive capability for recurrence. Consequently, assessment of IFH may be included in routine preoperative 18F-FDG PET/CT scans to identify those with a high risk of recurrence in patients with endometrioid endometrial cancer.
Compliance with Ethical Standards
Disclosure of Potential Conflict of Interest
Funding: none.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Research Involving Human Participants and/or Animals
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of retrospective study, formal consent is not required.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46(2):124–130. doi: 10.4143/crt.2014.46.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, Kang S, Kim JW, Kim JY, Park SY. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013;24(4):298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2(9):683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 6.Antonsen SL, Loft A, Fisker R, Nielsen AL, Andersen ES, Høgdall E, Tabor A, Jochumsen K, Fagö-Olsen CL, Asmussen J. SUVmax of 18FDG PET/CT as a predictor of high-risk endometrial cancer patients. Gynecol Oncol. 2013;129(2):298–303. doi: 10.1016/j.ygyno.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Chung HH, Lee I, Kim HS, Kim JW, Park NH, Song YS, Cheon GJ. Prognostic value of preoperative metabolic tumor volume measured by (1)(8)F-FDG PET/CT and MRI in patients with endometrial cancer. Gynecol Oncol. 2013;130(3):446–451. doi: 10.1016/j.ygyno.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Ghooshkhanei H, Treglia G, Sabouri G, Davoodi R, Sadeghi R. Risk stratification and prognosis determination using (18)F-FDG PET imaging in endometrial cancer patients: a systematic review and meta-analysis. Gynecol Oncol. 2014;132(3):669–676. doi: 10.1016/j.ygyno.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima K, Suenaga Y, Ueno Y, Maeda T, Ebina Y, Yamada H, Okunaga T, Kubo K, Sofue K, Kanda T. Preoperative risk stratification using metabolic parameters of (18)F-FDG PET/CT in patients with endometrial cancer. Eur J Nucl Med Mol Imaging. 2015;42(8):1268–1275. doi: 10.1007/s00259-015-3037-2. [DOI] [PubMed] [Google Scholar]
- 10.Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, Corcos L, Visvikis D. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–378. doi: 10.2967/jnumed.110.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, Chaudhari S, Yang D, Schmitt M, Laforest R. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009;42(6):1162–1171. doi: 10.1016/j.patcog.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, Marsden P, Ahmad S, Landau D. Are pretreatment 18F-FDG PET tumor textural features in non–small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54(1):19–26. doi: 10.2967/jnumed.112.107375. [DOI] [PubMed] [Google Scholar]
- 13.Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14(16):5236–5241. doi: 10.1158/1078-0432.CCR-07-5252. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38(6):987–991. doi: 10.1007/s00259-011-1787-z. [DOI] [PubMed] [Google Scholar]
- 15.Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, Humm JL. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62(2):545–553. doi: 10.1016/j.ijrobp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Hatt M, Majdoub M, Vallières M, Tixier F, Le Rest CC, Groheux D, Hindié E, Martineau A, Pradier O, Hustinx R. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56(1):38–44. doi: 10.2967/jnumed.114.144055. [DOI] [PubMed] [Google Scholar]
- 17.Lee M, Lee H, Cheon GJ, Kim HS, Chung HH, Kim JW, Park NH, Song YS. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in patients with epithelial ovarian cancer. Eur Radiol. 2017;27(1):16–23. doi: 10.1007/s00330-016-4368-5. [DOI] [PubMed] [Google Scholar]
- 18.Tixier F, Groves AM, Goh V, Hatt M, Ingrand P, Le Rest CC, Visvikis D. Correlation of intra-tumor 18F-FDG uptake heterogeneity indices with perfusion CT derived parameters in colorectal cancer. PLoS One. 2014;9(6):e99567. doi: 10.1371/journal.pone.0099567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, Rosenberg R, Becker K, Astner ST, Henninger M. Textural parameters of tumor heterogeneity in 18F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med. 2014;55(6):891–897. doi: 10.2967/jnumed.113.127340. jnumed.113.127340 [pii] [DOI] [PubMed] [Google Scholar]
- 20.McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother's cancer. Cancer. 2016 doi: 10.1002/cncr.30094. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen M, Horsman MR, Cumming P, Munk OL, Keiding S. Effect of intratumoral heterogeneity in oxygenation status on FMISO PET, autoradiography, and electrode Po2 measurements in murine tumors. Int J Radiat Oncol Biol Phys. 2005;62(3):854–861. doi: 10.1016/j.ijrobp.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, Nathrath W, Schwaiger M. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42(1):9–16. [PubMed] [Google Scholar]
- 23.Zasadny KR, Tatsumi M, Wahl RL. FDG metabolism and uptake versus blood flow in women with untreated primary breast cancers. Eur J Nucl Med Mol Imaging. 2003;30(2):274–280. doi: 10.1007/s00259-002-1022-z. [DOI] [PubMed] [Google Scholar]
- 24.Tateishi U, Nishihara H, Tsukamoto E, Morikawa T, Tamaki N, Miyasaka K. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr. 2002;26(2):185–190. doi: 10.1097/00004728-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S, Kuge Y, Mochizuki T, Takahashi T, Nakada K, Sato M, Takei T, Tamaki N. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46(4):675–682. [PubMed] [Google Scholar]
- 26.van Baardwijk A, Bosmans G, van Suylen RJ, van Kroonenburgh M, Hochstenbag M, Geskes G, Lambin P, De Ruysscher D. Correlation of intra-tumour heterogeneity on 18F-FDG PET with pathologic features in non–small cell lung cancer: a feasibility study. Radiother Oncol. 2008;87(1):55–58. doi: 10.1016/j.radonc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Vance S, Yechieli R, Cogan C, Hanna R, Munkarah A, Elshaikh MA. The prognostic significance of age in surgically staged patients with type II endometrial carcinoma. Gynecol Oncol. 2012;126(1):16–19. doi: 10.1016/j.ygyno.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu FY, Chao A, Lai CH, Chou HH, Yen TC. Metabolic tumor volume by 18F-FDG PET/CT is prognostic for stage IVB endometrial carcinoma. Gynecol Oncol. 2012;125(3):566–571. doi: 10.1016/j.ygyno.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Lai CH, Lin G, Yen TC, Liu FY. Molecular imaging in the management of gynecologic malignancies. Gynecol Oncol. 2014;135(1):156–162. doi: 10.1016/j.ygyno.2014.07.092. [DOI] [PubMed] [Google Scholar]