Abstract
C4-dicarboxylates are important molecules for the human pathogen C.jejuni, as they are used as carbon and electron acceptor molecules, as sugars cannot be utilized by this microaerophilic organism. Based on the genome analysis, C. jejuni may possess five different C4–dicarboxylate transporters: DctA, DcuA, DcuB, and two homologs of DcuC. Here, we investigated the regulation and function of various C4–dicarboxylate transporters in C. jejuni. Transcription of the dctA and dcuC homologs is constitutive, while dcuA and dcuB are both directly regulated by the two-component RacR/RacS system in response to limited oxygen availability and the presence of nitrate. The DctA transporter is the only C4-dicarboxylate transporter to allow C. jejuni to grow on C4-carbon sources such as aspartate, fumarate, and succinate at high oxygen levels (10% O2) and is indispensable for the uptake of succinate from the medium under these conditions. Both DcuA and DcuB can sequester aspartate from the medium under low-oxygen conditions (0.3% O2). However, under these conditions, DcuB is the only transporter to secrete succinate to the environment. Under low-oxygen conditions, nitrate prevents the secretion of succinate to the environment and was able to overrule the phenotype of the C4-transporter mutants, indicating that the activity of the aspartate–fumarate–succinate pathway in C. jejuni is strongly reduced by the addition of nitrate in the medium.
Keywords: Campylobacter jejuni, C4-dicarboxylates transporters, DctA, Dcu, gene regulation, metabolism, RacRS
Introduction
Bacteria utilize C4-dicarboxylates such as fumarate, succinate, malate, and aspartate when sugars or related compounds are not available (Janausch et al., 2002). C4-dicarboxylates serve as carbon and energy source and are oxidized to CO2 in the citric acid cycle under aerobic conditions. Under anaerobic conditions fumarate, malate, and aspartate are taken up into the cell. Malate and aspartate are reduced to fumarate, which is used as electron acceptor in the fumarate respiration pathway where it is converted to succinate. Succinate cannot be further metabolized by most bacteria due to the lack of a functional citric acid cycle under these conditions and is excreted.
The microaerophilic Gram-negative bacterium Campylobacter jejuni is the most common cause of food-borne bacterial gastroenteritis worldwide. Despite the medical and public health importance of Campylobacter infection, it is remarkable that C. jejuni is one of the least understood enteropathogens. C. jejuni possesses a highly branched electron transport chain, which allows both aerobic and anaerobic respiration (Kelly, 2008). Most C. jejuni strains cannot utilize sugars (Parkhill et al., 2000; Pearson et al., 2007; Stahl et al., 2011) and it seems that selected amino acids and C4-dicarboxylates act as primary energy source (Guccione et al., 2008; Zientz et al., 1999). It remains largely unknown how the transport and regulation of C4-dicarboxylates occurs in C. jejuni.
Five C4-dicarboxylate carriers, DctA, DcuAB, DcuC, CitT, and DctPQM, are known to transport C4-dicarboxylates from the periplasm across the inner membrane into bacteria (Janausch et al., 2002). In Escherichia coli DctA is a C4-dicarboxylate/H+ or Na+ cation symporter that catalyses the uptake of C4-dicarboxylates during aerobic growth. During anaerobiosis the transcription of the dctA gene is strongly repressed by the two-component ArcBA system. Due to the cAMP-CRP complex glucose can also prevent the transcription of the dctA gene. DcuAB and DcuC have similar functions as they catalyse the exchange, uptake and efflux of C4-dicarboxylates under anaerobic growth conditions (Zientz et al., 1999). DcuB and DcuC are the main transporters for succinate efflux during anaerobic growth (Zientz et al., 1999). While the dcuA gene is expressed constitutively, both DcuB and DcuC are activated by the O2-dependent regulator FNR. Furthermore, DcuB is repressed by nitrate due to the two-component NarXL regulatory system and activated by the two-component DcuSR system in response to presence of fumarate (Overton et al., 2006). CitT is a citrate:succinate antiporter, which is regulated by the two-component CitAB system in response to citrate (Scheu et al., 2012). Finally, the three proteins DctPQM in Rhodobacter capsulatus form a C4-dicarboxylate transporter which is in Pseudomonas aeruginosa dependent on the two-component dctSR system (Forward et al., 1997; Valentini et al., 2011).
C. jejuni contains all the enzymes for a complete oxidative TCA cycle, central to a flexible energy metabolism. Campylobacter possess only the C4-dicarboxylate carriers, DctA and DcuAB, some strains also contain one or two proteins similar to DcuC (Hofreuter et al., 2006). Like in other bacteria under oxygen-limited conditions, the transcription of the C. jejuni dcuA and dcuB genes is upregulated and under these conditions the antiporters are able to transport aspartate and fumarate (Woodall et al., 2005; Guccione et al., 2008). In contrast, all the transcription factors known to regulate the C4-dicarboxylate transporters carriers in other bacteria are lacking in C. jejuni.
Recently, we have shown that the two-component RacR/RacS system of C. jejuni directly represses the operon aspA-dcuA-cj0089 under oxygen-limited conditions in the presence of nitrate (van der Stel et al., 2015). In this work, we investigated the function and regulation of all C4-dicarboxylate carriers in C. jejuni.
Materials and methods
Bacterial strains, media, and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. C. jejuni was routinely cultured under microaerophilic conditions (5% O2, 10% CO2, 75% N2) on Blood Agar Base No. 2 (BA) medium containing 5% horse blood or in Heart Infusion broth (HI; Oxoid). Kanamycin (25 μg ml−1) and/or chloramphenicol (15 μg ml−1) and/or spectinomycin (30 μg ml−1) were added when appropriate. E. coli strains were routinely grown at 37°C in Luria–Bertani (LB) broth or on LB agar plates supplemented with ampicillin (50 μg ml−1), kanamycin (30 μg ml−1) or chloramphenicol (34 μg ml−1).
Table 1.
Bacterial strains and plasmids used in this study.
| Strains or plasmids | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| STRAINS | ||
| C. jejuni 81116 | wildtype | Palmer et al., 1983 |
| dctA | 81116 derivative dctA::Cm | This study |
| dcuA | 81116 derivative dcuA::Cm | This study |
| dcuB | 81116 derivative dcuB::Km | This study |
| dcuC | 81116 derivative dcuC::SpeC | This study |
| dcuC2 | 81116 derivative dcuC2::Cm | This study |
| dcuAB | 81116 derivative dcuA::Cm dcuB::Km | This study |
| dcuABC | 81116 derivative dcuA::Cm dcuB::Km, dcuC::SpeC | This study |
| E. coli PC2955 | relA1 Φ80dlacZΔM15 phoA8 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 luxS glnV44 | NCCB |
| PLASMIDS | ||
| pJet 1.2 blunt | ApR PCR cloning vector, Ampr | Fermentas, thermoscientific |
| pAV35 | ApR CmR pBluescript II SK containing Campylobacter coli CmR cassette | van Vliet et al., 1998 |
| pMW2 | ApR KmR pBluescript KS containing C. jejuni Kmr cassette | Wösten et al., 2010 |
| pZW2 | ApR SpecR E. coli-C. jejuni shuttle vector | Zhou et al., 2012 |
| pNBspec | ApR SpecR pAV35 containing SpecR cassette | This study |
| pJetdctA | ApR; 6.2 kb pJet containing dctA region | This study |
| pJetdcuA | ApR; 7.5 kb pJet containing dcuA region | This study |
| pJetdcuB | ApR; 6.3 kb pJet containing dcuB region | This study |
| pJetdcuC | ApR; 6.6 kb pJet containing dcuC region | This study |
| pJetdcuC2 | ||
| pJetdcuC2 | ApR; 8.7 kb pJet containing dcuC2 region | This study |
| pJetdctA::Cm | ApR CmR pJet containing dctA gene on a 2009 bp fragment | This study |
| pJetdcuA::Cm | ApR CmR; 5.9 kb, dcuA replaced by CmR | This study |
| pJetdcuB::Km | ApR KmR; 6.5 kb, dcuB replaced by KmR | This study |
| pJetdcuC::Spec | ApR SpecR; 6.2 kb, dcuC replaced by SpecR | This study |
| pJetdcuC2::Cm | ApR CmR; 8.5 Kb, dcuC2 replaced by CmR | This study |
Construction of a dcuA, dcuB, dcuC, dcuC2 or dctA mutant
To disrupt the dcuA, dcuB, dcuC, dcuC2, or dctA genes, the genes as well as ~1 kb of the flanking regions were first amplified by PCR using the primer pairs dcuA-F/dcuA-R, dcuB-F/dcuB-R, dcuC-F/dcuC-R, dcuC-2F/dcuC-2R, or dctA-F/dctA-R, respectively. Primers are listed in Table 2. The ~3 kb PCR fragments were ligated into pJET1.2/blunt Cloning Vector resulting into the plasmids pJETdcuA, pJETdcuB, pJETdcuC, pJETdcuC2, and pJETdctA. Inverse PCR was performed on the plasmids pJETdcuA, pJETdcuB, pJETdcuC, and pJETdctA using the primers sets dcuABamHI F/dcuABamHI R, dcuBBamHI F/dcuBBamHI R, dcuCBamHI F/dcuCBamHI R, or dctABamHI F/dctABamHI R, respectively, to delete the dcuA, dcuB, dcuC, and dctA genes present in pJET and to introduce a BamHI restriction site. The pJETdcuA and pJETdctA inverse PCR fragment were ligated to a BamHI fragment containing a chloramphenicol resistance gene of pAV35 resulting in the knock-out constructs pJETdcuA::Cm and pJETdctA::Cm. The pJETdcuB and pJETdcuC inverse PCR fragment were ligated to a BamHI fragment containing the kanamycin resistance gene of pMW2 or spectomycin resistance gene of pNBspec resulting into the knock-out constructs pJETdcuB::Km and pJETdcuC::speC, respectively. Plasmid pNBspec is a pAV35 derivate containing the spectomycin resistance gene of pZW2 (Zhou et al., 2012). pNBspec was constructed by amplifying pAV35 with the primers RBSCATrev and CATstop and the spectomycin resistance gene of pZW2 with the primers RBSspec and Specstop. In a second PCR these two PCR fragments were connected and after self-ligating of the PCR product pNBspec was obtained. To disrupt the dcuC2 gene, plasmid pJETdcuC2 was digested with XmaJI and ligated to an XbaI fragment containing the pAV35 chloramphenicol resistance gene, resulting in the pJETdcuC2::Cm knock-out construct. To mutate the dcuA, dcuB, dcuC, dcuC2, and dctA genes, the knock-out constructs pJETdcuA::Cm, pJETdcuB::km, pJETdcuC::speC, pjetDcuC2::Cm, and pJETdctA::Cm were introduced by natural transformation in C. jejuni 81116. Homologous recombinations resulting in double cross-over events were verified by PCR.
Table 2.
Primers used in this study.
| Primer | DNA sequence (5′-3′) |
|---|---|
| MUTANTS | |
| dcuA-F | AGTCAAAGTACTAATGATGC |
| dcuB-R | TCAAGAGCTGCAACAGGACC |
| dcuA-BamHI F | AGGATCCTTACTTGCAAAATTATCATTATATCC |
| dcuA-BamHI R | AGGATCCGGCTTTGTATTAGCTCCTATGCTTATT |
| dcuB-F | TTAGATCCTGTAGGAGTGGG |
| dcuB-BamHI R | AGGATCCCTCACTAAGGCTTGTTAAAAAGTCC |
| dcuB-BamHI F | AGGATCCATTTTATTGCTATGGCAGCGGGTTAT |
| dcuC-F | TAAAATATTGATAATTCTTGTGAGT |
| dcuC-R | GCCTTTATTATCGGCGAAATTTGCA |
| dcuC-BamHI F | AGGATCCAAATAGCAGTATCTTTAAATAATAAATA |
| dcuC-BamHI R | AGGATCCGCTAAAAGTGTAGAACATATCATAAA |
| dcuC-2F | TAAAATATCACCCATATCTCCAATTTT |
| dcuC-2R | TATCCACCCCAGCTAGCACCAATTGA |
| dctA-F | CATAAATTTACCCCTTTTTATTGAAA |
| dctA-R | CCCTTTTTTATTTTAATTATACACTTA |
| dctA-BamHI F | TGGATCCTTGCCATCTGGGATAAACAAATTGAT |
| dctA-BamHI R | TGGATCCAGCAATGGCTAATTCTTTATCTATATA |
| SPEC CASSETTE | |
| RBSCATrev | TTATCCTCCGTAAATTCCGATTTGTTG |
| CATstop | TAAAATCCCAGTTTGTCGCACTG |
| RBSspec | CGGAATTTACGGAGGATAAATGATGAATAGTTATGAAGTAAC |
| Specstop | GCGACAAACTGGGATTTTAAGCAAAACCTTTTATTTTTTGTTGAAGG |
| RT-PCR | |
| dctARtaq | GTGTAACTGGATCAGGTTTTATAGTGCTT |
| dctAFtaq | GCTAAGGTTGCATTTGCTTCTGAA |
| dcuBFtaq | TGGACTTATGCTGTAATGCTTCTTTTA |
| dcuBRtaq | GCCAAAGGAACAAAAGCTGAA |
| aspAFtaq81116 | TTTGTTAGAGCTTTGGCTAGAGTAAAAA |
| aspARtaq81116 | CGCTTTAATAATCGCATCTTGGA |
| gyrAFtaq81116 | ACGACTTACACGACCGATTTCA |
| gyrARtaq81116 | ATGCTCTTTGCAGTAACCAAAAAA |
| dcuCFtaq | CACTTGGTGGAGTTAATATCCTTGCT |
| dcuCRtaq | AAAGAATTCCAAACCATGCAAAA |
| dcuC2Ftaq | TGCTTGCAGTTTGTGCTTTCTT |
| dcuC2Rtaq | TTGGGATAAGTGGAGCAAAGCT |
| dcuAFtaq | GTTTCGGCACTTTTTGTGCTT |
| dcuARtaq | CCAATTCTAGTCGTTCCTGTATCATC |
| EMSA | |
| dcuBR | ATTTGGATTGCAAATTGCCCT |
| dcuBF | AAAATTCTATCAATCTATCAAACC |
| dcuCR | TAAGTATATAATAAGCAACGACAATT |
| dcuCF | TTTCAGTAACCAAACTATACATATT |
| dctAF | GCGGTTTTCTTTCAGCTAAAGTTTGA |
| dctAR | GCAAATAATGAGAAATTTTGTAACATT |
| dcuC2R | GCTGAGTATGGACCAATGGCCTTTG |
| dcuC2F | TACTCTTTATACTTTAAAACATTTCTT |
| aspAF | AGCTTGCAAAAATATATTAATTT |
| aspAR | TAATAAACCTCATCAGAGATTTC |
RNA isolation
RNA was extracted from C. jejuni wild-type grown in HI with or without 25 mM serine, aspartate, fumarate or succinate under 10 or 0.3% oxygen concentration at logarithmic (10 h) or stationary phase (20 h). RNA was also extracted from the wild-type, the racR mutant and the racR complemented strain grown in HI with 50 mM of NaNO3 under 0.3% oxygen concentration until late logarithmic (log) phase (16 h) using the RNA-Bee™ kit (Tel-Test, Inc.). RNA samples were treated with RNAse-free DNAse I (Fermentas) according to the manufacturer's manual.
Real-time RT-PCR
Real-time RT-PCR analysis was performed as previously described (Wösten et al., 2004). Primers used in this assay are listed in Table 2. The calculated threshold cycle (Ct) for each gene amplification was normalized to the Ct of the gyrA gene amplified from the corresponding sample before calculating fold change using the arithmetic formula 2−ΔΔCt (Schmittgen, 2001). Each sample was examined in four replicates and was repeated with at least two independent preparations of RNA. Standard deviations were calculated and are displayed as error bars.
Electrophoretic mobility shift assay (EMSA)
Recombinant His-tag labeled RacR was isolated and EMSA were performed as described before (van der Stel et al., 2015). The promoter regions upstream of the genes aspA, dcuB, dcuC, dcuC2, and dctA were amplified by PCR using the primer pairs listed in Table 2 and C. jejuni 81116 genomic DNA as template. Radioactive labeled PCR products, ~25 pmol, were incubated with 0 or 50 pmol of recombinant RacR and 25 pmol RacScyto (van der Stel et al., 2015) for 20 min at RT in binding buffer containing 20 mM Tris, pH 7.4, 5 mM MgCl2, 50 mM KCl, 2 mM ATP, 50 μg/ml bovine serum albumin, 10 μg/ml poly-(dI-dC), and 10% glycerol. For competition assays RacR was pre-incubated for 15 min with 10 times excess of unlabelled PCR fragment. Samples were run on 6% non-denaturing Tris-glycine polyacrylamide gels at 4°C. After electrophoresis, gels were dried and autoradiographed.
Growth experiments
Growth curves were generated under microaerobic conditions (10% O2, 10% CO2, 70% N2, 10% H2) or under oxygen-limited conditions (0.3% O2, 10% CO2, 79% N2, 10% H2) at 42°C in a “honeycomb” 10 × 10 well micro-plate using a Bioscreen C MRB (Oy Growth Curves Ab Ltd) computer-controlled incubator placed in the anaerobic chamber (Coy labs, Michigan, United States). Campylobacter precultures grown for 6 h in HI were diluted to OD600nm of 0.01 in fresh HI media containing 25 mM fumarate, 25 mM aspartate, 25 mM serine, or 25 mM succinate with or without 25 mM nitrate. The optical density at 600 nm was measured every 15 min. The experiments were repeated at least three times in duplicate.
High-performance liquid chromatography (HPLC)-MS-MS analysis
During the growth experiments of C. jejuni at 10% O2, culture samples (50 μl) were taken at 4, 8, 16, and 24 h, under oxygen-limited conditions (0.3% O2) samples were taken at 6, 12, 24, and 36 h. The culture samples were centrifuged at 14,000 rpm for 5 min. The supernatants were diluted in milli-Q water and adjusted to pH 2.4 with formic acid and injected on a Synergi 4u Fusion-RP (150 × 2.0 mm, particle size of 4 μm) analytical column (Phenomenex, Utrecht, NL). Elution was performed isocratically with milli-Q (adjusted to pH 2.4 with formic-acid): acetonitrile (9:1 [v/v]) at a flow rate of 0.3 ml/min, and the column effluent was introduced by an atmospheric pressure chemical ionization (APCI) interface, in negative mode with an ionization current of −1 μA and a source temperature of 350°C, into a 2000 QTRAP mass spectrometer (Sciex, Toronto, ON). For maximal sensitivity and for linearity of the response, the mass spectrometer was operated in multiple-reaction monitoring (MRM) mode at unit mass resolution. Peaks were identified by comparison of retention time and mass spectrum with authentic standards. Ion transitions monitored were m/z 115.0/71.0 (fumarate), 117.0/73.0 (succinate), 132.1/88.0 (aspartic acid), and 104.0/74.0 (serine) at collision energies of −12, −15, −20, and −18V, respectively. Simultaneously the four molecules were monitored by single-ion monitoring (SIM). Data were analyzed with Analyst software version 1.6.1 (Applied Biosystems).
Statistical analysis
Prism software (GraphPad, San Diego, CA) was used for statistical analysis. Data was expressed as mean ± SD. Results were analyzed by two tailed paired t-test, p < 0.05 was considered statistically significant.
Results
Genome locations of the C. jejuni C4-dicarboxylate transporters
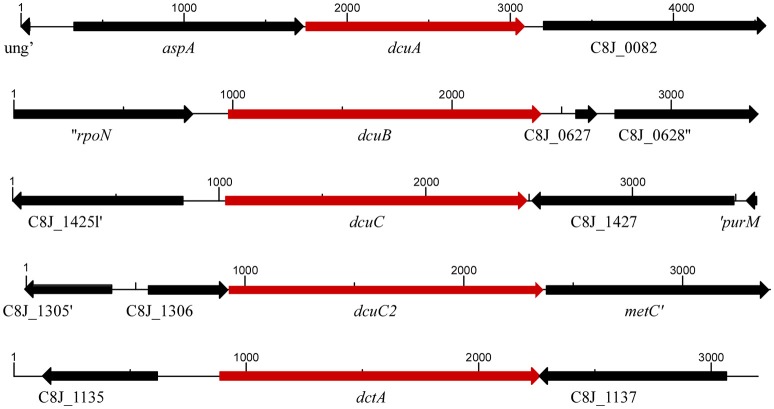
Genome analysis revealed that C. jejuni possesses up to five putative C4-dicarboxylate transporters, DctA, DcuA, DcuB, and some strains contain one or two homologs of the DcuC C4-dicarboxylate transporter. C. jejuni 81116 possess five C4-dicarboxylate transporters, which are all located at a different locus in the genome (Figure 1). The genes dcuB, dcuC, and dctA are not co-transcribed with other genes located in a single-gene operon, while dcuA is located in one operon together with the aspA gene and the dcuC2 gene is located in one operon with C8J_1306 and the metC genes.
Figure 1.
Organization of the C4-dicarboxylate transporters in the genome of C. jejuni 81116. The size and orientation of the genes in C4 transporters regions are indicated.
Transcription regulation of the C. jejuni C4-dicarboxylate transporters
In a number of bacterial species, the C4-dicarboxylate transporters are regulated by oxygen, the available C4-dicarbolytes and/or by growth phase. To investigate whether this also applies for the C4-dicarboxylate transporters in C. jejuni we performed real-time RT PCR. We first used mRNA isolated from the wild-type 81116 strain grown at 10 or 0.3% oxygen (Figure 2A). Only the transcripts of the dcuA and dcuB genes showed a minor increase of three- to four-fold under oxygen limited conditions. Addition of serine or the C4-carbon sources, aspartate, succinate or fumarate to the culture medium of the wild-type grown until the logarithmic or stationary phase (data not shown) at 0.3 or 10% oxygen (data not shown) did not influence the transcription of the C4-dicarboxylate transporters (Figure 2B). Finally, we tested whether the growth phase (by comparing logarithmic vs. stationary phase) influences the transcription of the C4-dicarboxylate genes (Figure 2C). Only the transcription of the dcuA gene was regulated by growth phase as a 15-fold higher transcript level was observed at the logarithmic phase compared to the stationary phase. These results indicate that the regulation of C4-dicarboxylate transporters in other bacteria cannot be extrapolated to that of C. jejuni.
Figure 2.
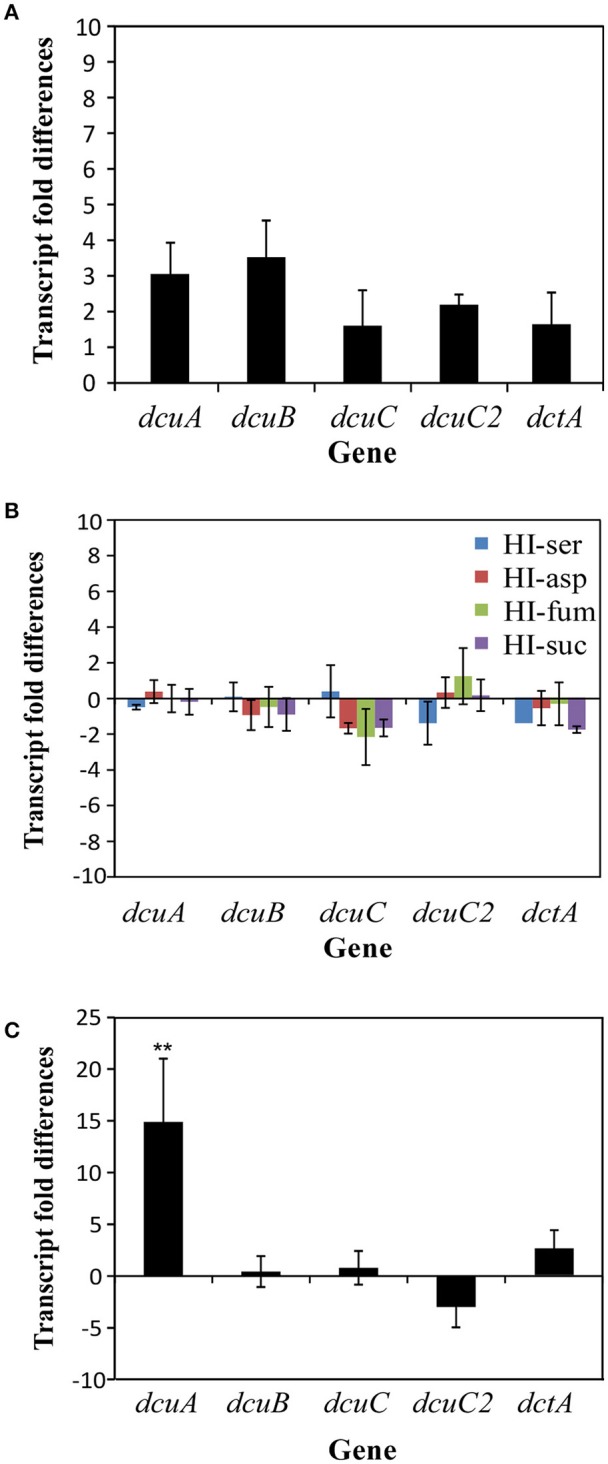
Influence of oxygen, carbon sources, and growth phase on the transcription of the C4-dicarboxylate transporter genes as measured by real-time RT-PCR. (A) Transcript fold difference of the dcuA, dcuB, dcuC, dcuC2, and dctA genes in logarithmic phase grown wild-type C. jejuni (10 h) at 10% O2 compared to 16 h at 0.3% O2. (B) The effect of adding various carbon sources to the HI culture medium on the transcription of the C4-dicarboxylate genes. Hereto total RNA was isolated from wild-type grown in HI or HI with 25 mM serine, aspartate, fumarate, or succinate for 10 h. (C) Influence of the growth phase on the transcription of the C4-dicarboxylate genes as estimated for wild-type bacteria by real-time RT-PCR. Total RNA was extracted from logarithmic (10 h), or stationary (20 h) phase cultures. Fold change relative to the transcription levels was calculated using the arithmetic formula (2−ΔΔCt). The gyrA gene was used as normalization gene. Data of four independent experiments with two independent preparations of RNA are presented as mean values ± standard deviation, **P < 0.01.
dcuA and dcuB are directly regulated by racR
We previously showed that the two-component regulator RacR regulates the dcuA transporter gene. As dcuC and dcuC2 were not present on the used microarrays slides we performed real-time RT PCR using RNA isolated of the wild-type and RacR mutant grown under RacR inducing conditions (0.3% O2 and 25 mM nitrate). Transcription of dcuC, dcuC2, and dctA genes was not affected by mutation of racR, however like the proven RacR dependent genes dcuA and aspA, a decrease of the dcuB transcription was observed in the racR mutant (Figure 3A).
Figure 3.
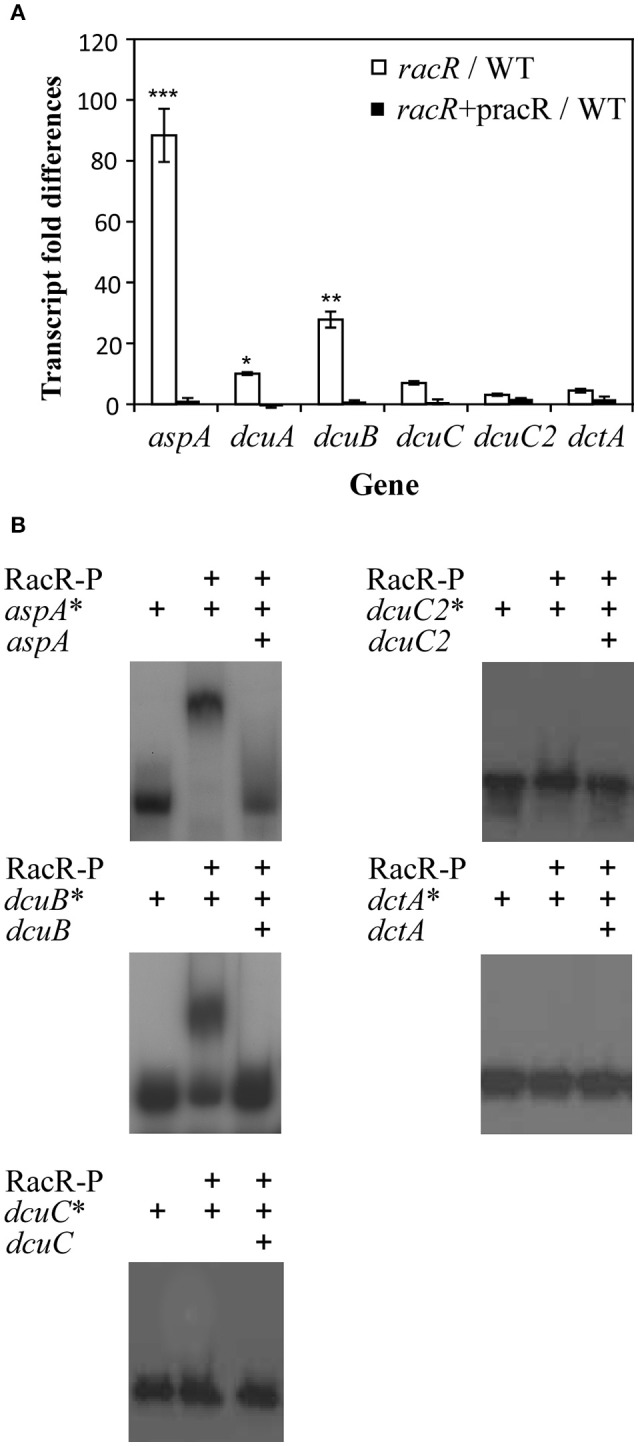
RacR directly regulates the dcuA and dcuB promoters. (A) Real-time RT-PCR data of the dcuA, dcuB, dcuC, dcuC2, and dctA genes in wild-type C. jejuni compared to the RacR mutant or the complemented RacR mutant strain. Data of four independent experiments with two independent preparations of RNA are presented as mean ds ± standard deviation, *P < 0.05, **P < 0.01, ***P < 0.001. (B) Electrophoretic mobility shift assays with the aspA, dcuB, dcuC, dcuC2, and dctA promoter regions labeled with [γ-32P]ATP and phosphorylated RacR protein. Radioactive labeled promoter regions are marked with *. RacR was phosphorylated by RacScyto in the presence of ATP. The specificity of the protein-DNA interaction was determined by the addition of a 10-fold excess of unlabelled promoter region DNA.
To investigate whether RacR directly regulates the dcuB promoter we performed electrophoretic mobility shift assays (EMSA). As previously shown, phosphorylated RacR was able to bind to the 32P-labeled aspA-dcuA promoter region, however RacR also binds to the dcuB promoter (Figure 3B). The band shift disappeared when unlabelled DNA corresponding to the dcuB promoter region was added in excess. As expected from the real-time RT PCR results, the dcuC, dcuC2, and dctA promoter regions are not recognized by the RacR protein. These results show that the dcuA as well as dcuB are directly regulated by the two-component system regulator RacR.
Dcta is active at elevated oxygen levels
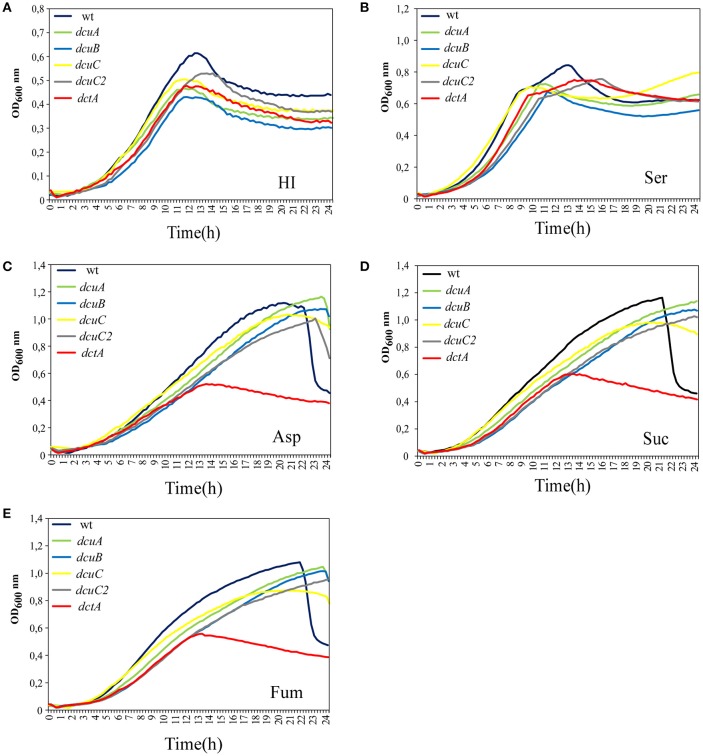
To phenotypically address the function of the C4-dicarboxylate transporter in C. jejuni we disrupted the dctA, dcuA, dcuB, dcuC, and dcuC2 genes by substituting large parts of the genes with an antibiotic resistance cassette. Although the transcription of C4-dicarboxylate transporter in C. jejuni is not regulated by oxygen (Figure 2A), we investigated whether oxygen has an influence of the activity of these transporters. Hereto growth curves in the presence of 10% O2 were generated of the wild-type and the dctA, dcuA, dcuB, dcuC, and dcuC2 mutants in HI or with the addition of 25 mM serine, aspartate, succinate, or fumarate. No clear growth differences between the strains were observed when they were growing in HI or HI with 25 mM serine (Figures 4A,B). However, all strains reached a higher OD when serine was added to the medium, suggesting that the available serine or carbon is a limited compound in HI. When one of the C4-carbon sources, aspartate, succinate, or fumarate were added to the HI medium, the maximum final OD of all strains except for the dctA mutant, was also higher compared to HI alone (Figures 4C–E), indicating that carbon availability in HI is a limiting growth factor. The dctA mutant reached a similar maximum final OD in HI as in HI with additional aspartate, succinate or fumarate indicating that the dctA mutant is unable to utilize or to take up these carbon sources from the medium. Based on these results we conclude that the DctA transporter is the only C4-dicarboxylate transporter needed to allow C. jejuni to growth on C4-carbon sources at high oxygen levels.
Figure 4.
DctA is the only C4-carboxylate transporter necessary at high oxygen conditions (10% O2). Growth curves of C. jejuni wild-type or the dcuA, dcuB, dcuC, dcuC2, and dctA mutants were generated in HI (A), or HI with the addition of 25 mM serine (B), aspartate (C), succinate (D), or fumarate (E) under microaerobic conditions (10% O2, 10% CO2, 70% N2, and 10% H2) at 42°C. The optical density at 600 nm was measured every 15 min. The experiments were repeated three times in duplicate.
Main function of dctA is the uptake of succinate from the medium at elevated oxygen levels
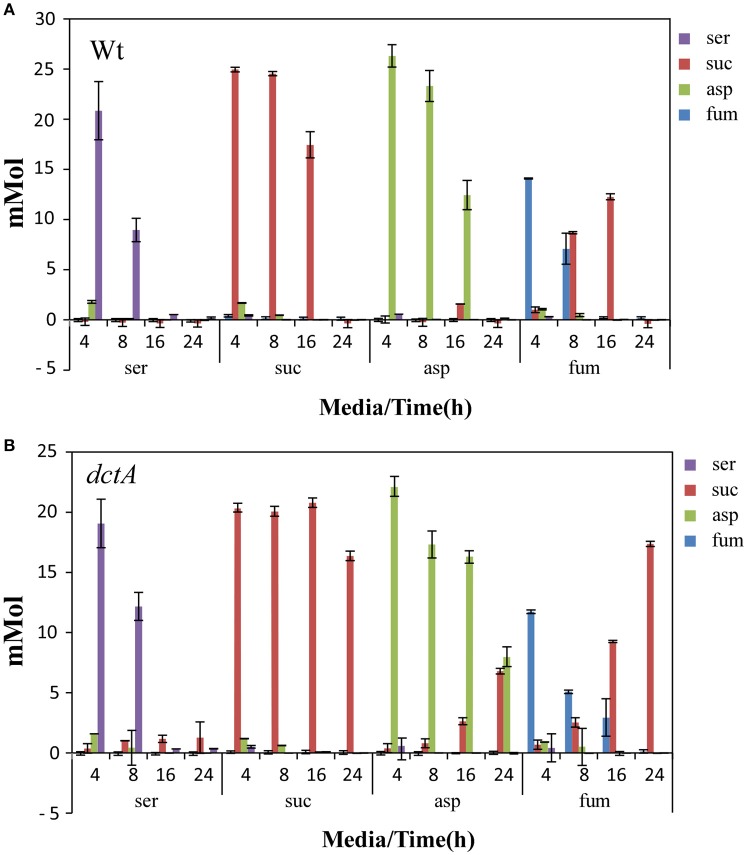
To investigate why the dctA mutant is unable to use aspartate, fumarate, and succinate at 10% O2, we measured concentrations of serine, aspartate, succinate, and fumarate in the culture supernatants of the wild-type and C4-dicarboxylate transporter mutants at 4, 8, 16, and 24 h (Figure 5A). Without the addition of extra C4-dicarboxylate compounds HI medium contains no detectable fumarate, 0.4 mM succinate, 1.0 mM aspartate, and 1.2 mM serine. All added carbon sources, serine, succinate, aspartate, and fumarate were completely utilized by the wild-type bacteria within 24 h growth at 10% O2, however serine and fumarate were removed earlier from the media than succinate and aspartate. Fumarate was converted to succinate and then secreted to the medium. Once the fumarate was completely used the secreted succinate was taken up again and utilized by C. jejuni (Figure 5A) Similar results were obtained for the dcuA, dcuB, dcuC, and dcuC2 mutants (data not shown). A different result was obtained for the dctA mutant. The dctA mutant was still able to take up serine, to convert fumarate to succinate and, although reduced, to take up aspartate (Figure 5B), but was unable to take up succinate from the media and accumulated in the supernatant of media containing excess serine, aspartate, or fumarate. These results indicate that the DctA transporter of C. jejuni is involved in the uptake of aspartate and indispensable is for the uptake of succinate from the media at elevated oxygen conditions.
Figure 5.
DctA transports succinate into the cell. HPLC-MS-MS analysis of the culture supernatants of wild-type (A) and dctA mutant (B) taken after 4, 8, 16, or 24 h of growth under 10% O2 with 25 mM serine, aspartate, fumarate, or succinate. Data represent the mean and standard error of three independent experiments.
Redundancy of C4-dicarboxylate transporter function under oxygen-limited conditions
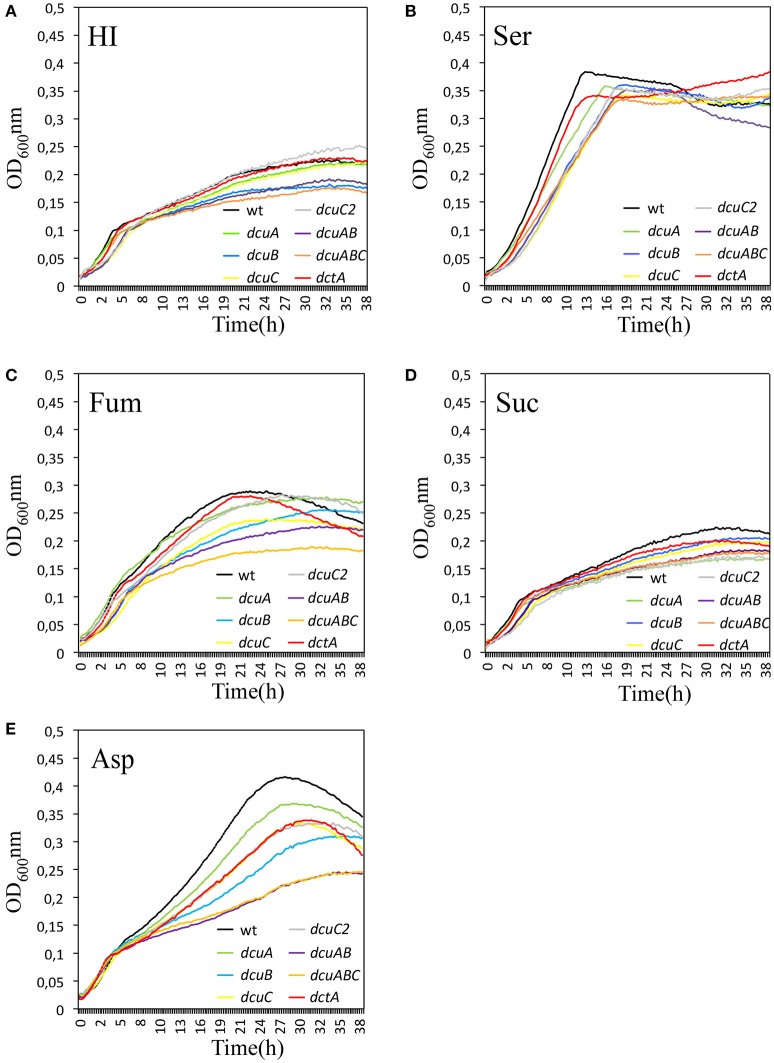
To investigate which of the C4-dicarboxylate transporters are active under oxygen-limited conditions, we generated growth curves of the wild-type, the dctA, dcuA, dcuB, dcuC, and dcuC2 mutants as well as a double dcuA/dcuB and a triple dcuA/dcuB/dcuC mutant in HI or HI with the addition of 25 mM serine, aspartate, succinate, or fumarate (Figures 6A–E). The maximum optical densities reached under these oxygen-limited conditions were all lower compared to the growth curves obtained at 10% O2. No obvious growth differences were observed between the strains when they were grown in HI, HI + serine, or HI + succinate. The maximum OD of all strains was higher in HI + serine compared to HI alone, indicating that serine is utilized by C. jejuni under these conditions (Figure 6B). In contrast, succinate is not utilized under the oxygen-limited conditions as the growth curves generated in HI + succinate were similar as in HI. Growth defects were observed when fumarate or aspartate was added to the HI medium (Figures 6C,E). All single mutants grew less compared to the wild-type in HI + aspartate, especially the dcuB mutant. To investigate whether the C4-carboxylate transporter can replace each other function, we also tested double and triple mutants. A more severe growth defect in HI + aspartate compared to the dcuB mutant was observed for the dcuA/dcuB mutant but not for a dctA/dcuA (data not shown). A similar dcuA/dcuB growth defect was seen for the dcuA/dcuB/dcuC triple mutant, suggesting that dcuC is not involved in the utilization of aspartate. A small reduction in growth yield was observed for the single mutants dcuC and dcuB in HI + fumarate. The growth defect was more obvious in the triple mutant dcuA/dcuB/dcuC, suggesting that both DcuB and DcuC are involved in the utilization of fumarate.
Figure 6.
DcuA and DcuB are the main C4-dicarboxylate transporters under oxygen-limited conditions. Growth curves were generated of C. jejuni wild-type, the single mutants dcuA, dcuB, dcuC, dcuC2, and dctA, the double mutant dcuA/B and the triple mutant dcuA/B/C under oxygen-limited conditions (0.3% O2). Strains were growing in HI (A), or HI with the addition of 25 mM serine (B), fumarate (C), succinate (D), or aspartate (E) under oxygen-limited conditions (0.3% O2, 10% CO2, 79% N2, 10% H2) at 42°C. The optical density at 600 nm was measured every 15 min. The experiments were repeated three times in duplicate.
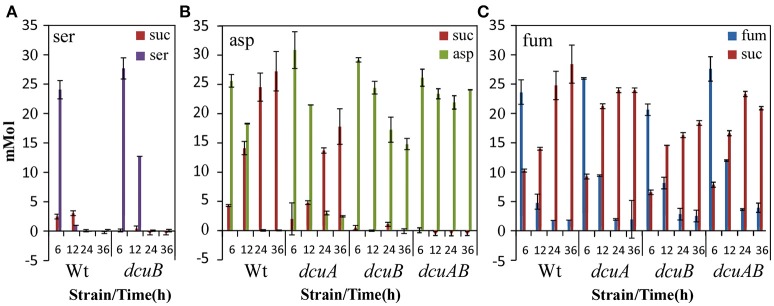
Secretion of succinate is dependent on a functional dcuB under limited oxygen conditions
To investigate the role of various active C4-transporters under oxygen-limited conditions we measured at time points 6, 12, 24, and 36 h the serine, aspartate, succinate, and fumarate concentration in the culture supernatants of the wild-type and C4-transporters mutants (Figure 7). Serine was used within 12 h by the wild-type and within 24 h by the dcuB mutant, the slowest grower in HI + serine (Figure 7A). When the strains were grown on aspartate they secreted succinate to the medium, except for the strains which were also mutated in the dcuB gene (Figure 7B). No succinate could be measured in the supernatant of the dcuB mutants showing that DcuB under oxygen-limited conditions transports succinate into the environment. Both the dcuA and dcuB mutants were able to utilize aspartate, however when both genes were mutated no decrease in the aspartate concentration in the medium was observed, indicating that both genes are needed to sequester aspartate from the medium. Growing the strains in excess fumarate revealed that not dcuC, but both dcuA and dcuB might be involved in the uptake of fumarate, as at 12 h the supernatant of these single mutants contain more fumarate than the supernatant of the wild-type (Figure 7C). The supernatant of all strains tested grown in excess fumarate contained succinate. However, the supernatant of the dcuB mutant strains contained less succinate, indicating that Campylobacter possesses a dcu-independent fumarate–succinate reductase.
Figure 7.
DcuB secretes succinate into the environment under oxygen-limited conditions. HPLC-MS-MS data of the supernatants of wild-type, dcuA, dcuB, or dcuAB mutant cultures taken after 6, 12, 24, or 36 h of growth at 0.3% O2 with 25 mM serine (A), aspartate (B), or fumarate (C). Data represent the mean and standard error of three independent experiments.
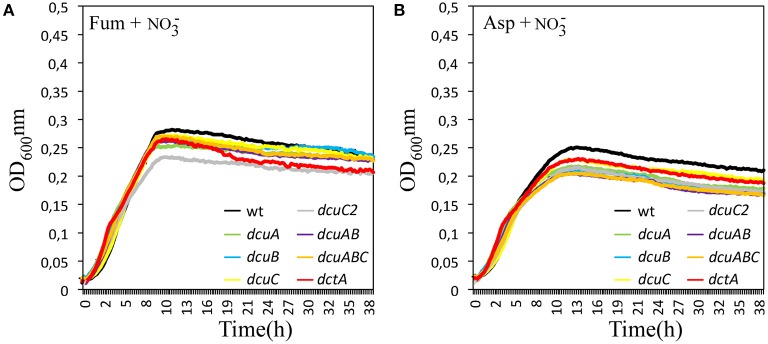
The availability of nitrate under oxygen-limited conditions reduces the role of the C4-dicarboxylate transporters
We showed that RacR directly regulates by inhibition, the dcuA and dcuB genes under limited oxygen and high nitrate conditions. To address the role of the dcu genes under these conditions, we performed growth curves in HI under oxygen-limited conditions with nitrate and aspartate or fumarate (Figure 8). The growth rate as well as the maximum optical densities of the wild-type and the mutants were similar under these conditions, suggesting that the C4-dicarboxylate transporters are less active under these conditions.
Figure 8.
Addition of nitrate under limited oxygen conditions nullifies the C4-dicarboxylate mutant phenotype. Growth curves were generated of C. jejuni wild-type, the single mutants dcuA, dcuB, dcuC, dcuC2, and dctA, the double mutant dcuA/B and the triple mutant dcuA/B/C in HI with 25 mM nitrate and 25 mM fumarate (A) or aspartate (B) at 42°C under oxygen-limited conditions. The optical density at 600 nm was measured every 15 min. The experiments were repeated three times in duplicate.
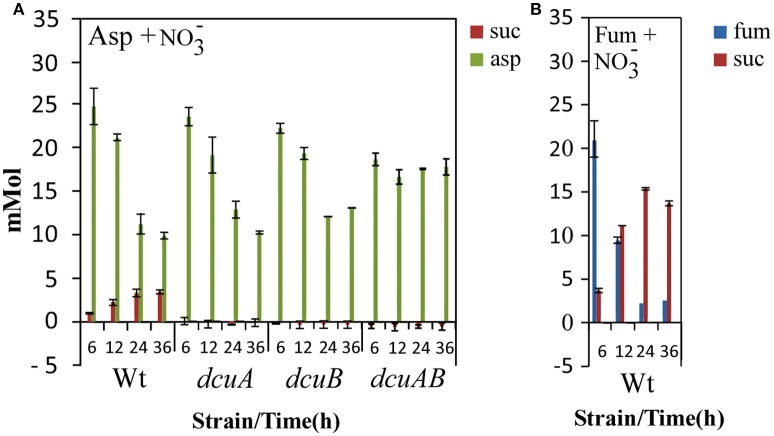
Nitrate prevents the secretion of succinate under oxygen-limited conditions
To further address the role of the C4-dicarboxylate transporters under RacR inducing conditions, we measured the aspartate, fumarate, and succinate concentrations in the supernatant of the wild-type and the C4-dicarboxylate mutants at 6, 12, 24, and 36 h. The C4-carbon content in the supernatants of the strains growing under oxygen-limited conditions with nitrate were much more similar compared to the strains grown under oxygen-limited conditions (Figure 9). The aspartate uptake in the wild-type, dcuA and dcuB mutants under these conditions were similar, however only half of the aspartate in the supernatant of these cultures was utilized (Figure 9A). These results are in accordance with the growth curves and show that the C4-transporters are less active under these conditions. No reduction of the aspartate concentration was seen in the supernatant of the dcuAB mutant, confirming that both DcuA and DcuB are needed to take up aspartate from the medium. The amount of succinate produced by the wild-type under these conditions was nine-fold lower than under oxygen-limited conditions, similar results were seen for the dctA and dcuC2 mutants. In all other mutants no succinate could be detected in the supernatant. These results show that the activity like the transcription (Figure 3A) of aspartate–fumarate–succinate pathway under oxygen-limited condition is strongly reduced when nitrate is available. In the wild-type the utilization of fumarate in the presence of nitrate was similar as without nitrate, however the amount of secreted succinate was two-fold lower when nitrate was present (Figure 9B). Similar results were obtained for all other mutants (data not shown), indicating that Campylobacter possesses a dcu nitrate independent fumarate–succinate reductase.
Figure 9.
Nitrate prevents the secretion of succinate to the environment. HPLC-MS-MS of the supernatants of wild-type, dcuA, dcuB, or dcuAB mutant cultures taken after 6, 12, 24, or 36 h of growth under 0.3% O2 with 25 mM nitrate and 25 mM aspartate (A) or 25 mM nitrate and 25 mN fumarate (B). Data represent the mean and standard error of three independent experiments.
Discussion
Dependent on the oxygen concentration, C4-dicarboxylate transporters have been shown to play an important role in the transport of C4-carbon sources such as fumarate, succinate, and aspartate. These carbon sources are even more important for the microaerophilic bacterium C. jejuni as most strains cannot ferment nor oxidize carbohydrates. C. jejuni cannot grow under aerobic or strictly anaerobic oxygen conditions therefore we have attempted to obtain a more complete understanding of the function and regulation of the C4-dicarboxylate transporters in C. jejuni.
Like in other bacteria the C4-dicarboxylate transporter genes of C. jejuni are dispersed over the genome (Figure 1). As they all are transcribed from different promoters, they might be regulated differently, as is seen in E. coli, where several transcription factors are involved in the regulation of the C4-dicarboxylate transporters in response to oxygen, C4-dicarboxylate compounds and growth phase (Janausch et al., 2002). Here we showed by real-time RT-qPCR that the oxygen concentration had only a minor but not significant effect on the transcription of the dcuA and dcuB genes in strain 81116, and that the addition of C4-dicarboxylate compounds in the complex media HI had no influence on the transcription of the C4-dicarboxylate transporter genes (Figure 2). Similar results were obtained in another C. jejuni strain 11168 (Woodall et al., 2005). Growth phase of the culture influenced only the transcription of the dcuA gene. Based on these results no transcription factor of C. jejuni has obtained a similar function as the E. coli transcription factors regulating the C4-dicarboxylates, such as FNR, ArcAB, DcuRS, or CRP (Janausch et al., 2002). Apparently, the transcription regulation in response to oxygen, C4-dicarboxylates and growth phase as seen in E. coli is not important for the function of the genes in C. jejuni.
We have shown that both the dcuA and dcuB genes are repressed and directly regulated by the RacRS system in response to low oxygen and nitrate. The dcuA is in most bacteria constitutively expressed and so far C. jejuni is the only organism in which both genes are regulated in a similar manner. The phenotypes observed for the dcuA and dcuB mutants under oxygen-limited conditions (Figure 6) were restored by the addition of nitrate to the medium (Figure 8), clearly showing that these genes are not needed under these conditions. Regulation of the C4-dicarboxylate genes by nitrate is not uncommon as the dcuB gene in E. coli is repressed by the two-component NarXL system (Overton et al., 2006). The use of nitrate as electron acceptor (Em nitrate/nitrite + 430 mV) is preferred over fumarate (Em fumarate/succinate + 30 mV) enabling fumarate to be used as carbon source instead of electron acceptor under oxygen-limited conditions. This explains the observed reduced secretion of succinate depicted in Figure 9.
The secretion of succinate in C. jejuni appeared to be mediated solely by the DcuB C4-transporter (Figure 7). In E. coli, the DcuB and DcuC C4-transporters, both regulated by FNR, can act synergistically to secrete succinate under anaerobic conditions (Golby et al., 1999). So far, we were unable to address the function of the two DcuC homologs in C. jejuni 81116 which are not co-regulated with dcuB and are absent in other C. jejuni strains (Parkhill et al., 2000; Hofreuter et al., 2006). Both DcuA and DcuB are involved in the uptake of aspartate as well as fumarate, confirming the data of Guccione et al. (2008). By regulating both DcuA and DcuB, the RacRS system completely controls the fumarate respiration in response to limited oxygen availability and the presence of nitrate.
Succinate is taken up by the DctA transporter and used as carbon source by C. jejuni when oxygen is not scarce (Figure 5). The DctA transporter is also needed to allow C. jejuni to use aspartate and fumarate as carbon source, indicating that the dctA mutant is unable to use or to take up these carbon sources from the medium. In E. coli and Bacillus subtilis, DctA also mediates the uptake of succinate as well as fumarate and aspartate under aerobic conditions (Davies et al., 1999; Asai et al., 2000; Janausch et al., 2002). However, no difference was observed between the C. jejuni wild-type and dctA mutant in the uptake of fumarate, suggesting that C. jejuni DctA is not involved in the uptake of fumarate. When fumarate or aspartate are present in the media, a large amount of energy rich succinate accumulated in the media which could not be re-used by the dctA mutant explaining the reduced growth of dctA mutant under these conditions. Beside the regulation it also appears that the function of C4-dicarboxylate genes differs in C. jejuni.
In our work, we highlighted the regulation and function of various C4-dicarboxylate genes in the microaerophilic bacterium C. jejuni. The DctA transporter is responsible for the uptake of succinate under high oxygen levels. The dcuA and dcuB genes are the only C4-dicarboxylate-regulated genes and are dependent on the two-component RacRS system in response to low O2 and high nitrate concentrations. DcuB is the only C4-dicarboxylate/succinate antiporter in C. jejuni which secretes succinate when oxygen levels are low, but is not necessary when nitrate is available.
Author contributions
MW designed experiments, wrote the article, and performed growth experiments. JVP wrote the article, CVDL performed the High-Performance Liquid Chromatography analysis, and LVD performed all other experiments.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Asai K., Baik S. H., Kasahara Y., Moriya S., Ogasawara N. (2000). Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology 146(Pt 2), 263–271. 10.1099/00221287-146-2-263 [DOI] [PubMed] [Google Scholar]
- Davies S. J., Golby P., Omrani D., Broad S. A., Harrington V. L., Guest J. R., et al. (1999). Inactivation and regulation of the aerobic C(4)-dicarboxylate transport (dctA) gene of Escherichia coli. J. Bacteriol. 181, 5624–5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forward J. A., Behrendt M. C., Wyborn N. R., Cross R., Kelly D. J. (1997). TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQM genes of Rhodobacter capsulatus and by homologs in diverse gram-negative bacteria. J. Bacteriol. 179, 5482–5493. 10.1128/jb.179.17.5482-5493.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby P., Davies S., Kelly D. J., Guest J. R., Andrews S. C. (1999). Identification and characterization of a two-component sensor-kinase and response-regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli. J. Bacteriol. 181, 1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione E., Leon-Kempis Mdel R., Pearson B. M., Hitchin E., Mulholland F., van Diemen P. M., et al. (2008). Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol. Microbiol. 69, 77–93. 10.1111/j.1365-2958.2008.06263.x [DOI] [PubMed] [Google Scholar]
- Hofreuter D., Tsai J., Watson R. O., Novik V., Altman B., Benitez M., et al. (2006). Unique features of a highly pathogenic Campylobacter jejuni strain. Infect. Immun. 74, 4694–4707. 10.1128/IAI.00210-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janausch I. G., Zientz E., Tran Q. H., Kroger A. (2002). Unden, G. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553, 39–56. 10.1016/S0005-2728(01)00233-X [DOI] [PubMed] [Google Scholar]
- Kelly D. J. (2008). Complexity and versatility in the metabolism and physiology of Campylobacter jejuni, in Campylobacter, 3rd Edn., eds Nachamkin I., Szymanski C., Blaser M. (Washington, DC: American Society for Microbiology Press; ), 41–61. [Google Scholar]
- Overton T. W., Griffiths L., Patel M. D., Hobman J. L., Penn C. W., Cole J. A., et al. (2006). Microarray analysis of gene regulation by oxygen, nitrate, nitrite, FNR, NarL and NarP during anaerobic growth of Escherichia coli: new insights into microbial physiology. Biochem. Soc. Trans. 34, 104–107. 10.1042/BST0340104 [DOI] [PubMed] [Google Scholar]
- Palmer S. R., Gully P. R., White J. M., Pearson A. D., Suckling W. G., Jones D. M., et al. (1983). Water-borne outbreak of campylobacter gastroenteritis. Lancet 1, 287–290. 10.1016/S0140-6736(83)91698-7 [DOI] [PubMed] [Google Scholar]
- Parkhill J., Wren B. W., Mungall K., Ketley J. M., Churcher C., Basham D., et al. (2000). The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403, 665–668. 10.1038/35001088 [DOI] [PubMed] [Google Scholar]
- Pearson B. M., Gaskin D. J., Segers R. P., Wells J. M., Nuijten P. J., van Vliet A. (2007). H. the complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J. Bacteriol. 189, 8402–8403. 10.1128/JB.01404-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu P. D., Witan J., Rauschmeier M., Graf S., Liao Y. F., Ebert-Jung A., et al. (2012). CitA/CitB two-component system regulating citrate fermentation in Escherichia coli and its relation to the DcuS/DcuR system in vivo. J. Bacteriol. 194, 636–645. 10.1128/JB.06345-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D. (2001). Real-time quantitative PCR. Methods 25, 383–385. 10.1006/meth.2001.1260 [DOI] [PubMed] [Google Scholar]
- Stahl M., Friis L. M., Nothaft H., Liu X., Li J., Szymanski C. M., et al. (2011). L-fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc. Natl. Acad. Sci. U.S.A. 108, 7194–7199. 10.1073/pnas.1014125108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini M., Storelli N., Lapouge K. (2011). Identification of C(4)-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. J. Bacteriol. 193, 4307–4316. 10.1128/JB.05074-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stel A. X., van Mourik A., Heijmen-van Dijk L., Parker C. T., Kelly D. J., van de Lest C. H., et al. (2015). The Campylobacter jejuni RacRS system regulates fumarate utilization in a low oxygen environment. Environ. Microbiol. 17, 1049–1064. 10.1111/1462-2920.12476 [DOI] [PubMed] [Google Scholar]
- van Vliet A. H., Wooldridge K. G., Ketley J. M. (1998). Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 180, 5291–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall C. A., Jones M. A., Barrow P. A., Hinds J., Marsden G. L., Kelly D. J., et al. (2005). Campylobacter jejuni gene expression in the chick cecum: evidence for adaptation to a low-oxygen environment. Infect. Immun. 73, 5278–5285. 10.1128/IAI.73.8.5278-5285.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten M. M., van Dijk L., Veenendaal A. K., de Zoete M. R., Bleumink-Pluijm N. M., van Putten J. P. (2010). Temperature-dependent FlgM/FliA complex formation regulates Campylobacter jejuni flagella length. Mol. Microbiol. 75, 1577–1591. 10.1111/j.1365-2958.2010.07079.x [DOI] [PubMed] [Google Scholar]
- Wösten M. M., Wagenaar J. A., van Putten J. P. (2004). The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J. Biol. Chem. 279, 16214–16222. 10.1074/jbc.M400357200 [DOI] [PubMed] [Google Scholar]
- Zhou W., Wang Y., Lin J. (2012). Functional cloning and characterization of antibiotic resistance genes from the chicken gut microbiome. Appl. Environ. Microbiol. 78, 3028–3032. 10.1128/AEM.06920-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientz E., Janausch I. G., Six S., Unden G. (1999). Functioning of DcuC as the C4-dicarboxylate carrier during glucose fermentation by Escherichia coli. J. Bacteriol. 181, 3716–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]