Abstract
Introduction
Pulmonary hypertension (PH) is a potentially life-threatening condition characterised by elevated pulmonary artery pressure. Early stage PH patients are often asymptomatic. Disease progression is associated with impairment of right ventricular function and progressive dyspnoea. Current guidelines recommend exercise training (grade IIa, level B). However, many questions remain regarding the mechanisms of improvement, intensity of supervision and optimal frequency, duration and intensity of exercise. This study will assess the effect of an outpatient rehabilitation programme on haemodynamics and cardiac right ventricular function in patients with pulmonary arterial hypertension (PAH), a subgroup of PH.
Methods and analysis
This randomised controlled trial involves both a major urban tertiary and smaller regional hospital in New South Wales, Australia. The intervention will compare an outpatient rehabilitation programme with a control group (home exercise programme). Participants will be stable on oral PAH-specific therapy. The primary outcome measure will be right ventricular ejection fraction measured by cardiac MRI. Secondary outcomes will include haemodynamics measured by right heart catheterisation, endurance, functional capacity, health-related quality of life questionnaires and biomarkers of cardiac function and inflammation.
Ethics approval and dissemination
Ethical approval has been granted by St Vincent's Hospital, Sydney (HREC/14/SVH/341). Results of this study will be disseminated through presentation at scientific conferences and in scientific journals.
Trial registration number
ACTRN12615001041549; pre-results.
Keywords: Pulmonary hypertension, outpatient exercise training, cardiac magnetic resonance imaging, haemodynamics
Strengths and limitations of this study.
To the best of our knowledge, this is the first clinical trial evaluating the effect of an outpatient rehabilitation programme (OP REH) on right ventricular ejection fraction measured by cardiac MRI.
We will also be measuring the effect of an OP REH on haemodynamic parameters as assessed by right heart catheterisation.
The nature of the OP REH reflects ‘real-world’ conditions generalisable to routine clinical practice (1 hour, twice weekly, for 12 weeks).
Although participants are not blinded to their allocated treatment group, all study personnel are blinded.
The short follow-up period for this study (6 months) is a limitation; however, we were limited by budget constraints.
Introduction
Pulmonary hypertension (PH) is a condition characterised by elevated pulmonary arterial pressure (PAP), and is associated with fatigue, syncope, angina and progressive exertional dyspnoea. It is defined as a mean resting pulmonary arterial pressure (mPAP) of ≥25 mm Hg, measured by right heart catheterisation (RHC).1 PH is characterised by vascular remodelling, with a consequent increase in PAP, resulting in increased right ventricular afterload.2 3 The ability of the right ventricle to adapt to the increased right-sided afterload is key to prognosis.3 4 Patients with PH generally have a poor prognosis5 in particular patients with pulmonary arterial hypertension (PAH). Median survival of patients with PAH is between 2 and 3 years.6 Underlying aetiology is an important prognostic factor, with scleroderma-related PAH (a subtype of PAH) having a worse prognosis.7–9
PAH is a precapillary subtype of PH, with additional haemodynamic criteria of pulmonary artery wedge pressure ≤15 mm Hg and pulmonary vascular resistance (PVR) >3 Wood units.10 The focus of this study is patients with PAH.
Exercise has not previously been recommended in patients with PAH, particularly in patients with more advanced disease, due to safety concerns.11 However, exercise training has been shown to be safe in patients with PAH,12 and current European guidelines recommend exercise training and rehabilitation in experienced centres for patients with PAH who are clinically stable, on optimal pharmacological treatment (grade IIa, level B).1
The first major study involving patients with PH (patients with PAH and chronic thromboembolic PH) showed that exercise was beneficial. Thirty participants were randomised to either exercise training or a control group (education on healthy nutrition, muscular relaxation).13 The exercise training group underwent an intensive 3-week inpatient programme of a minimum of 2 hours/day of exercise (strength and endurance training, and respiratory muscle exercises) and education (healthy nutrition, muscle relaxation). This was followed by a 12-week home exercise programme (HEP). The exercise training group had a marked improvement in 6 min walk test (6MWT) distance compared with the control group, with mean between-group difference of 111 m (95% CI 65 to 139; p<0.001). Quality of life (QOL), measured by the Short Form-36 (SF-36),14 also improved in the exercise training group.
Seventeen more studies examining the effect of exercise training on PH have been published up to June 2016, since the above landmark study in 2006. These studies are generally small in size, and are of varying quality (only six randomised controlled trials (RCTs)).15 Several studies12 16–22 using the Mereles et al13 inpatient protocol (described previously) reported improvement in a number of outcome measures, including 6MWT and QOL. The largest RCT of these studies (n=87), recently published by Ehlken et al20 has further added to the current body of knowledge by showing that exercise training using the Mereles et al inpatient protocol improved haemodynamics.
However, it is difficult to replicate the intensity of this inpatient exercise programme in routine clinical practice due to limited resources and personnel. In the Australian context, the Mereles et al protocol is not currently widely available due to cost constraints. Cardiac and pulmonary rehabilitation programmes in Australia are usually run by public hospital outpatient services or community settings, two to three times per week at minimal or no cost to patients.23 24
A more achievable programme of exercising two to three times per week for 1 hour per session may still have beneficial effects on walk distance,25–30 peak oxygen consumption,26 QOL, self-reported symptoms,27 fatigue and activity levels.28 We have therefore designed this study with ‘real-world’ conditions in mind to ensure broad generalisability. Given anxiety and depression are important factors associated with dyspnoea,31 we have chosen to include a structured psychology intervention as part of the outpatient rehabilitation programme (OP REH) to address this.
Hypotheses
We hypothesise that an OP REH (involving endurance training, respiratory muscle training (RMT) and structured psychology intervention) will have beneficial effects on cardiac remodelling (reflected in an improvement in right ventricular ejection fraction (RVEF)), haemodynamics, strength and endurance, and QOL. We hypothesise that these benefits will be due to improved right-sided cardiac function as determined by an improvement in RVEF.
The key difference of this study compared with previous studies is the inclusion of cardiac MRI (cMRI) and haemodynamics, as well as other secondary outcome measures (as listed below) that will help elucidate the mechanism of improvement of exercise training. If secondary outcome measures increase despite no improvement in RVEF, this would suggest that other mechanisms are responsible for the improvement in exercise capacity.
Objectives
To assess whether the ‘real-world’ frequency of OP REH available in Australia (ie, 1 hour, twice weekly, for 12 weeks) has a beneficial effect on:
Cardiac function (RVEF and volume as assessed by cMRI);
Haemodynamics, measured by RHC, the gold standard for assessing PAH;10
Dominant hand grip strength (DHGS) and exercise capacity (6MWT);
Function and QOL (measured by questionnaires: the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), Depression and Anxiety Severity Scale (DASS21), Lawton's Instrumental Activities of Daily Living (IADL);
Serum biomarkers of cardiac function and inflammation (N terminal pro b-type natriuretic peptide (NT-proBNP), interleukin (IL-6));
Lung function (vital capacity).
Methods and analysis
Study design
The study is designed as a dual-centre, investigator-blinded clinical trial to compare the efficacy of an OP REH versus a HEP. Participants will be randomised in a 1:1 ratio. The exercise intervention will occur for 12 weeks.
Study population
Study participants will be identified by cardiologists and rheumatologists during the course of routine care at St Vincent's Hospital, Sydney, Australia, and from the Coffs Harbour PAH Clinic, an outpatient clinic affiliated with the Coffs Harbour Health Campus (regional hospital). Participants must fulfil all inclusion criteria (table 1). Exclusion criteria are outlined in table 2. We have excluded WHO Class IV participants, due to safety concerns regarding exercising these participants in an outpatient setting.
Table 1.
Inclusion criteria
| Criteria | Characteristics of eligible participants |
|
|---|---|---|
| 1. | Male and female patients aged ≥18 years | |
| 2. | PAH diagnosed by RHC (at any time prior to baseline) with the following parameters:32 | |
| i. | Resting mPAP≥25 mm Hg, and | |
| ii. | Resting PCWP or LVEDP≤15 mm Hg | |
| 3. | Stable PAH-specific medication in the preceding 3 months | |
| 4. | Female participants of childbearing potential must have a negative pregnancy test and agree to use contraception for study duration. | |
LVEDP, left ventricular end diastolic pressure; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterisation.
Table 2.
Exclusion criteria for the ExPAH study
| Criteria | Characteristics of ineligible participants |
|---|---|
| 1. |
General exclusion criteria:
|
| 2. |
PAH-specific:
|
| 3. |
Presence of significant cardiac disease, including:
|
| 4. |
Patients unable to undergo MRI:
|
CCF, congestive cardiac failure; eGFR, estimated glomerular filtration rate; PAH, pulmonary arterial hypertension.
Recruitment, randomisation and allocation concealment
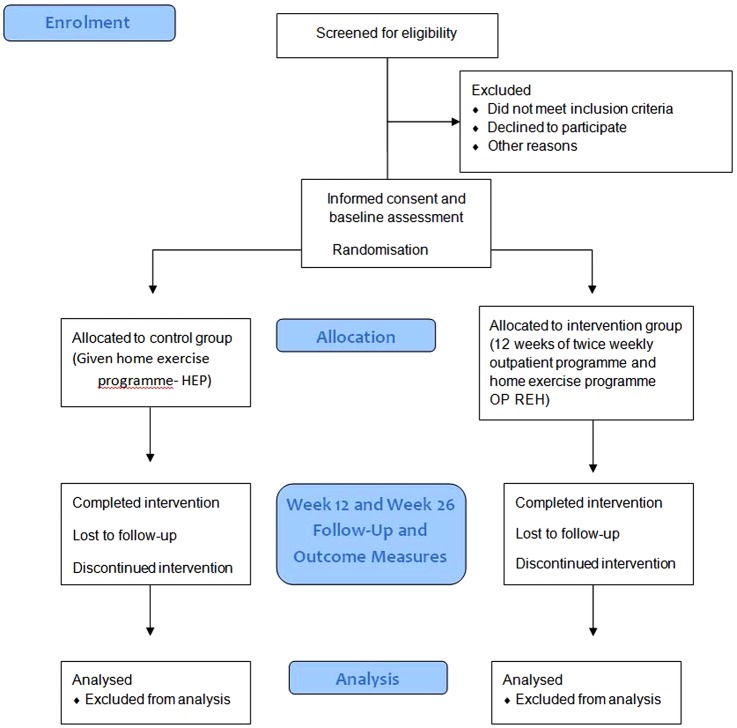
The ExPAH study has been designed in line with the Consolidated Standards of Reporting Trials (CONSORT) statement.33 The study protocol is outlined in figure 1.
Figure 1.
ExPAH protocol: enrolment, allocation, follow-up and analysis. Participants are screened for eligibility. If eligible, once informed consent obtained, participants are then randomised to control group (home exercise) or intervention group (outpatient rehabilitation exercise programme). Participants are reviewed at weeks 12 and 26; participants who have not completed the required attendance (>70%) are recorded. Analysis of results will be both by intention-to-treat and per protocol methods. OP REH, outpatient rehabilitation programme.
All eligible participants will have the aims, methods and potential adverse events explained to them by the study investigators prior to signing informed consent. Recruitment will occur until sample size requirements are met (anticipated duration of recruitment 1–2 years).
Randomisation will occur after screening (see below) and will be carried out using a minimisation scheme to achieve balance by research site and presence of connective tissue disease-associated PAH, as this particular cohort of patients with PAH has a worse prognosis7 as mentioned previously. Randomisation will be carried out by an independent biostatistician using a customised programme for minimisation (QMinim).34 The participant will be allocated to either control (HEP) or the intervention (OP REH). Administrative staff not involved with the project will inform participants via mail regarding their allocation group.
Participants will not be blinded to the intervention. However, assessments and data analysis will be carried out by blinded investigators.
Outcome measures
Cardiac MRI
cMRI was chosen as it is the ‘gold standard’ for assessing right ventricular structure and function.35 cMRI studies will be performed using a single 1.5 Tesla (T) MR system (Philips) and 3T (Siemens Healthcare, Germany). A complete stack of short axis images will be obtained during breath-hold and cardiac gating for cine, and late gadolinium enhancement (LGE) imaging. LGE imaging will be performed as previously described36 using a T1-weighted phase-sensitive inversion recovery sequence ∼8 min after intravenous administration of a contrast agent (Gadoterate meglumine–Gd-DOTA, Dotarem, Guerbet LLC, France; 0.15 mmol/kg body weight). Imaging parameters will be the same as previously published.37 All cMRIs will be analysed using CMR42 software (Canada), as previously described,38 by the investigators blinded to participant randomisation group.
Right heart catheterisation
Standard haemodynamic parameters measured by RHC10 were chosen as a secondary outcome measure based on the known impairments in PAH and previous noted improvements with inpatient rehabilitation in the cardiac index, mPAP and PVR.20 RHC will be performed according to a standard protocol10 by trained cardiologists who are blinded to the participant's randomisation group.
Frailty and exercise capacity
As skeletal muscle weakness is a known complication of PH,39–43 DHGS will also be included as a secondary end point. In addition, decreased hand grip strength (HGS) has been noted to be associated with increased all-cause mortality.44 DHGS will be measured by a physiotherapist blinded to the participant's randomisation group (using a Jamar Hydraulic Hand Dynamometer, Sammons Preston, Canada).
Exercise capacity, as measured by 6MWT, has been widely used as an outcome measure in trials of exercise training and pharmacological management of PAH. This study will assess whether the increase in 6MWT seen with previous trials45 46 will be able to be reproduced with an outpatient programme. Exercise capacity will be measured by a trained assessor (who is blinded to the study randomisation). All participants will be assessed undertaking two supervised 6MWTs separated by a rest of at least 30 min, in accordance with European Respiratory Society/American Thoracic Society technical standards.47
Questionnaires of function and QOL
Mental health disorders are very common, with ∼35% of patients with PAH suffering depression and anxiety.48 Depression and anxiety in patients with PAH is associated with reduced QOL.49 While reduction in health-related QOL is well documented in PAH, the focus of these studies have been on physical function, mental health and social isolation,50 with less emphasis on activities of daily living. We have therefore included a short questionnaire of daily activities (Lawton's IADL) which looks at other household tasks/roles.51 All study participants will complete three questionnaires at baseline, 12 and 26 weeks:
Blood test markers of cardiac function and inflammation
N-terminal fragment (NT-proBNP) and IL-6 were included as outcome measures for their prognostic value in PH.56 57 NT-proBNP is the inactive N-terminal component produced when the ventricles secrete a prohormone, pro-BNP that is cleaved into an active form (BNP) and an inactive fragment (NT-proBNP).58 NT-proBNP is a clinically useful and minimally invasive test that correlates well with survival. NT-proBNP values >1400 to 1700 were associated with a poorer prognosis.59 IL-6 is a marker of inflammation that adds further prognostic information in patients with PAH.57 Blood samples will be collected at baseline, weeks 12 and 26 follow-up visits in serum separator tubes for analysis.
Lung function
Respiratory muscle weakness is a known association with PAH.60 61 A previous study of inspiratory muscle training (IMT) in patients with PAH has demonstrated significant improvements in respiratory muscle strength, functional capacity, and dyspnoea and fatigue perception.30 Vital capacity is useful for monitoring respiratory muscle weakness.62 Therefore, all participants will undergo vital capacity testing to ascertain whether OP REH improves respiratory muscle weakness. We did not include direct measures of respiratory muscle strength (maximum inspiratory pressure and maximum expiratory pressure) due to financial constraints and safety concerns in this population.
Assessment
All participants will undergo the following assessments:
The medical screening visit
Participants will have eligibility reviewed, demographics (age, gender, aetiology of PAH), WHO Functional PH Class, medical history, physical examination and informed consent (if eligible) documented. Eligible participants will undergo cMRI, RHC, lung function tests (vital capacity) and venepuncture for cardiac markers (NT-proBNP and IL-6). Candidates who fail to meet inclusion criteria, or fulfil exclusion criteria will have the reason for study ineligibility documented.
Baseline visit
Participants will return for the baseline visit to review results. Participants will also have a separate visit with a trained assessor (who is blinded to the study randomisation) to undergo two supervised 6MWTs separated by a rest of at least 30 min, in accordance with international guidelines.47 DHGS will be recorded, and participants will complete QOL and function questionnaires as described previously. Participants will then be randomised as outlined above.
Visit 1 (week 12–14)
Participants will undergo medical review including physical examination at completion of the intervention period after week 12. Adverse events will be recorded, and any clinical episodes (change in PAH-specific medication or hospitalisation secondary to PAH) will be documented. Participants will undertake two supervised 6MWTs by a blinded assessor, have DHGS measured and complete the three study questionnaires, as before. Participants will also undergo cMRI, RHC, lung function tests and venepuncture for cardiac markers (NT-proBNP and IL-6).
Visit 2 (week 26)
Participants will undergo medical review, including physical examination. Any adverse events will be recorded, and any clinical episodes (change in PAH-specific medication or hospitalisation secondary to PAH) will be documented. Participants will undertake two supervised 6MWTs by a blinded assessor as before. Participants will undergo venepuncture for serum cardiac and inflammatory biomarkers. Participants will have assessment of DHGS and complete the three QOL questionnaires as in visit 1.
Interventions
Exercise:
Intervention (OP REH)
All participants randomised to this group will undertake physiotherapist-supervised, outpatient hospital based, group exercise training for 1 hour/week, twice weekly, for 12 weeks, with the following components:
Endurance training
Twenty minutes of lower limb endurance training by walking or cycling. Participants will start at a speed of 60% of the speed of their 6MWT. If a participant chooses to cycle instead of walk, then intensity will be based on an initial level of light-to-somewhat hard exertion using the Borg Rating of Perceived Exertion (RPE)63 at a level of 12–13).
If participants are unable to complete 20 min of lower limb endurance training, they will increase duration by 5 min each time until able to complete 20 min total. During the exercise training class, participants' heart rate (HR) will be monitored via portable HR and oxygen saturations machine. HR will be kept <120 bpm, as per current recommendations.64 Participants' oxygen saturations (O2 sats) will be kept >88% on room air, as per recent international guidelines.65
Progression of training will occur by increasing the treadmill gradient by 1% on each visit, or increase speed by 5% each week, as tolerated, maintaining an intensity of 12–13 on Borg RPE, if HR and O2 sats are able to be maintained within the range as stated above.
Respiratory muscle training
During the exercise training group sessions, all participants will undergo RMT taught by a physiotherapist. Training will involve education at the first intervention visit regarding breathing techniques to manage symptoms (pursed-lip breathing and diaphragmatic breathing). All subsequent sessions will include 10 min of supervised IMT using individual IMT devices (threshold IMT positive inspiratory pressure device, Phillips). Ideally, maximal inspiratory pressure (MIP) should be measured by formal lung function tests. However, these tests are expensive, and may be difficult for some patients to undertake. Even without formal MIP, IMT training can be prescribed, using the Borg RPE at a target of RPE 12–13.66 The benefit from IMT is seen at levels as low as 30% of MIP.67 Training at a Borg RPE of 12–13 correlates with ∼40% of MIP.66
Strength training
To achieve benefit, strength training needs to occur at repetitions of 50–80% of the one repetition maximum (1RM), at least two to three times per week.65 If the 1RM is difficult to obtain, we will alternatively use a weight that the participant can only lift 10 times.23 Strength training of the upper limbs and lower limbs will occur twice weekly at the supervised training sessions for a total of 20 min. Initial intensity will be based on the 1RM, and increased by 10% of load once the participant is able to perform two sets of 10 repetitions on two consecutive training sessions.68 Upper limb exercises will include dumbbell row, seated chest press and lateral arm raise.
Lower limb strength training exercises will include step ups and sit to stands.
HEP at week 3
Participants will be given a HEP, consisting of instructions to walk 3 days/week for 30 min total per day, in addition to attending the twice weekly classes with the physiotherapist. Participants will also be provided with their own IMT device as described above, and given written instructions on undertaking IMT training for 30 min per day for 5 days/week. Participants who desaturate to <88% on room air (RA) while exercising (as determined during their exercise class) will be given a modified HEP of interval training. This will consist of advice to walk for 1 min then 1 min of rest, initially for 15–20 min for the first four sessions, then increasing to 45–60 min (including rest time).69 All participants will be advised to cease their exercise programme until review by their physiotherapist if they become symptomatic (feel unwell, dizziness, light headedness, dyspnoea).
Control group (HEP)
Participants randomised to the HEP will be given written material recommending a walking programme for 5 days/week for 30 min total per day. This can be broken up into smaller sessions per day to make it more achievable (eg, walking for 10 min, three times per day). Advice will be provided on how to stay motivated, as well as a diary to record exercise undertaken.
Psychology intervention
Intervention group (OP REH)
The OP REH group will also have standardised individual counselling support for three sessions (20 min sessions) via telephone by the study psychologist during the 12-week intervention. This will be structured as follows:
Session 1: education on how to manage hyperventilation and anxiety during breathlessness.70
Session 2: structured cognitive–behavioural therapy to manage dyspnoea. This will involve education and challenging of catastrophic thoughts.71
Session 3: skills training. This will involve education and practice in progressive muscle relaxation.72 Participants will be given written instructions (as well as verbal instructions) regarding how to do this.
Control group
The control group will not receive any psychology input as part of the study protocol.
Adherence
The intervention group will have attendance at each exercise session recorded by the treating physiotherapist. The intervention and the control groups will both be given a diary to record their home activity level. Participants who have completed >70% of sessions (including self-documented HEP) will be deemed to have completed the programme.
Withdrawal criteria
Participants may voluntarily withdraw from the study at any time, or if they experience a serious adverse event (SAE) that is associated with an exercise session (during or up to 24 hours postintervention). An SAE is defined as an untoward medical occurrence that results in any of the following: death, a life-threatening event, inpatient hospitalisation, persistent or significant disability/incapacity, and a condition requiring medical or surgical intervention. Other adverse events (defined as any unfavourable and unintended sign, symptom or disease temporally associated with any of the investigations or the intervention), such as failure to progress according to protocol, syncope and presyncope will also be recorded. Participants may also be withdrawn if the designated blinded medical assessor at the participant's site deems it unsafe for them to continue with the study.
Data collection, confidentiality, storage and archiving of study documents
All personnel involved in data collection will be blinded. Study documents will be de-identified and stored for 15 years, as per protocol for non-clinical trial notification (CTN) interventional studies.73 Data will be stored electronically, on double password-protected computers. Data on paper (the Clinical Research File) will be stored in a locked, secure office. The principal investigators will have access to the final trial data set.
Analysis of outcome measures
The primary outcome measure is the absolute change (increase) in RVEF from baseline to visit 1, assessed by cMRI.
Secondary outcome measures include:
Absolute change in other parameters on cMRI from baseline to visit 1. These parameters include stroke volume, right ventricular end systolic volume, right ventricular end diastolic volume, right ventricular mass, left ventricular end systolic volume, left ventricular end diastolic volume and left ventricular mass, cross-sectional septum thickness at the level of the papillary muscle.
Absolute change in haemodynamics measured by RHC from baseline to visit 1, including PVR, cardiac output, mean right atrial pressure, mPAP and systemic vascular resistance.
Absolute change in measures of strength and exercise capacity from baseline to visit 1 and 2, including 6MWT, muscle strength testing (DHGS).
Measures of function and QOL scores at baseline, visit 1 and 2, including the CAMPHOR, Lawton's IADL and DASS21.
Blood test markers of cardiac function and inflammation (NT-proBNP and IL-6).
Lung function tests (vital capacity).
Statistical considerations
Sample size calculation
Power calculations are based on comparison of randomised arms achieving the primary outcome of an increase in RVEF. We consider a 5% absolute excess increase in RVEF, measured by cMRI, for the intervention group compared with the control group as the desired clinically important effect to be detected.74 This increase corresponds to at least a 14% improved outcome for the intervention group, in conjunction with no improvement in the control group within a population of patients with an average RVEF of 35%.75
Although measurement of RVEF by cMRI has high reproducibility,76 we will minimise bias by conducting analyses of cMRI by the same investigator.75 76 In our power calculation, we have incorporated 6% SD for both SD of changes compared with baseline and measurement variability at the time of follow-up, reported by Addetia et al.75 Using two-sided normal test with a significance level of 5% based on clinical equivalent design77 and potential 10% drop off, 26 patients in each arm are required to detect a 5% excess increased RVEF associated with intervention versus control group with a power of 80%. Our power calculation is comparable to other published papers calculating sample size required for cMRI and RVEF trials.62 76
Analysis populations
The primary and secondary end points A–D will be assessed in the intention-to-treat study population, defined as all participants who are randomised according to their randomised arm. In this analysis, participants missing their primary end point data for any reason will be imputed as failures for binary end points, and will have their previous observation carried forward for continuous end points. The primary end point will be analysed as per protocol analysis (this excludes participant data from the time at which they cease their randomised intervention). Secondary end points E–G will be assessed in a per protocol population, using available data.
Results analysis
Results will be analysed using STATA V.13 (StataCorp), SAS (Cary, North Carolina, USA) or R V.3.2.2 (R Core Team, Austria). Primary outcome and participant continuous characteristics will be compared between two randomised arms using a t-test or Wilcoxon test, as appropriate. Binary end points and categorical characteristics will be compared using a χ2 or Fisher's exact test as appropriate. In case of differences in baseline characteristics across arms, adjusted characteristics effects on outcomes and their changes over time points will be evaluated using generalised linear models or non-parametric equivalents. Adherence to intervention will be described based on data in the participant diary. Participants will be deemed to have completed the intervention if their total (attendance and self-recorded home diary) adherence is >70%. For QOL questionnaire components with categorical cut-offs (the DASS21) the proportion of participants in each category at each visit will be compared by randomised arm. As the CAMPHOR and Lawton's IADL are ordinal scales, ordinal-based tests and regression modelling or non-parametric equivalents will be used.
Discussion
To the best of our knowledge, the ExPAH study will be the first RCT to measure both haemodynamics and cMRI parameters. It is hypothesised that the data obtained will define reverse cardiac remodelling (defined by an increase in RVEF) as a mechanism that is important in the improvement observed in patients with PAH following exercise training.
Secondary outcome measures will also measure the effect of an OP REH on HGS and exercise capacity, QOL, depression, stress and anxiety, cardiac biomarkers and vital capacity. While these outcomes are all of clinical relevance, such a comprehensive data set requires significant time commitment by participants. This may limit recruitment, adherence and attendance.
Pragmatic approach
There was some debate regarding which exercise components should be included. However, we were determined to design an exercise intervention with broad generalisability within a ‘real-world’ setting of limited therapy time and resources.
The results of this study will assist in guiding clinical practice regarding the type of exercise, duration and frequency that is of benefit in patients with PAH.
Acknowledgments
The authors wish to thank Professor Stephen Kerr (Kirby Institute, New South Wales, Australia) for his assistance with the Statistics section.
Footnotes
Contributors: KSWC conceived the study, coordinated its design and drafted the manuscript. SGF and EK had significant input into the study protocol. PKKW, CSM, SGF and EK read and were involved in critical appraisal and revision of the manuscript. CH provided MRI expertise. HA provided statistical expertise. All authors approved the final manuscript prior to submission.
Funding: KSWC has received grants from the Mid-North Coast Local Health District and the Royal Australasian College of Physicians for this study. KSWC and EK have received an unrestricted educational grant from Actelion. Actelion have reviewed the manuscript. However, final content and design is exclusively retained by the authors.
Competing interests: None declared.
Ethics approval: St Vincent's Hospital Research and Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Galiè N, Humbert M, Vachiery JL et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 2.Howard LS. Prognostic factors in pulmonary arterial hypertension: assessing the course of the disease. Eur Respir Rev 2011;20:236–42. 10.1183/09059180.00006711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peacock AJ, Vonk Noordegraaf A. Cardiac magnetic resonance imaging in pulmonary arterial hypertension. Eur Respir Rev 2013;22:526–34. 10.1183/09059180.00006313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13–18. 10.1097/00019501-200502000-00003 [DOI] [PubMed] [Google Scholar]
- 5.Corciova FC, Arsenescu-Georgescu C. Prognostic factors in pulmonary hypertension. Maedica (Buchar) 2012;7:30–7. [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Archer SL, Badesch DB et al. . ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573–619. 10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 7.Stupi AM, Steen VD, Owens GR et al. . Pulmonary hypertension in the CREST syndrome variant of systemic sclerosis. Arthritis Rheum 1986;29:515–24. 10.1002/art.1780290409 [DOI] [PubMed] [Google Scholar]
- 8.Hachulla E, Gressin V, Guillevin L et al. . Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005;52:3792–800. 10.1002/art.21433 [DOI] [PubMed] [Google Scholar]
- 9.Steen V, Medsger TA Jr. Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum 2003;48:516–22. 10.1002/art.10775 [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, Bogaard HJ, Condliffe R et al. . Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62(Suppl 25):D42–50. 10.1016/j.jacc.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 11.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet 1998;352:719–25. 10.1016/S0140-6736(98)02111-4 [DOI] [PubMed] [Google Scholar]
- 12.Grunig E, Ehlken N, Ghofrani A et al. . Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 2011;81:394–401. 10.1159/000322475 [DOI] [PubMed] [Google Scholar]
- 13.Mereles D, Ehlken N, Kreuscher S et al. . Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114:1482–9. 10.1161/CIRCULATIONAHA.106.618397 [DOI] [PubMed] [Google Scholar]
- 14.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 15.Chia KS, Wong PK, Faux S et al. . The benefit of exercise training in pulmonary hypertension: a clinical review. Intern Med J 2016. doi:10.1111/imj.13159 [Epub ahead of print 24 Jun 2016]. 10.1111/imj.13159 [DOI] [PubMed] [Google Scholar]
- 16.Grunig E, Lichtblau M, Ehlken N et al. . Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012;40:84–92. 10.1183/09031936.00123711 [DOI] [PubMed] [Google Scholar]
- 17.Grunig E, Maier F, Ehlken N et al. . Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 2012;14:R148 10.1186/ar3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagel C, Prange F, Guth S et al. . Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PLoS ONE 2012;7:e41603 10.1371/journal.pone.0041603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker-Grunig T, Klose H, Ehlken N et al. . Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. Int J Cardiol 2013;168:375–81. 10.1016/j.ijcard.2012.09.036 [DOI] [PubMed] [Google Scholar]
- 20.Ehlken N, Lichtblau M, Klose H et al. . Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J 2016;37:35–44. 10.1093/eurheartj/ehv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley S, Fink C, Risse F et al. . Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol 2013;23:324–31. 10.1007/s00330-012-2606-z [DOI] [PubMed] [Google Scholar]
- 22.Kabitz HJ, Bremer HC, Schwoerer A et al. . The combination of exercise and respiratory training improves respiratory muscle function in pulmonary hypertension. Lung 2014;192:321–8. 10.1007/s00408-013-9542-9 [DOI] [PubMed] [Google Scholar]
- 23.Alison Jea. The Pulmonary Rehabilitation Toolkit on behalf of the Australian Lung Foundation (2009) 2009. http://www.pulmonaryrehab.com.au.
- 24.Heart Foundation A. Recommended framework for cardiac rehabilitation ‘04 2004. (http://www.heartfoundation.org.au/SiteCollectionDocuments/Recommended-framework.pdf).
- 25.Mainguy V, Maltais F, Saey D et al. . Effects of a rehabilitation program on skeletal muscle function in idiopathic pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 2010;30:319–23. 10.1097/HCR.0b013e3181d6f962 [DOI] [PubMed] [Google Scholar]
- 26.Fox BD, Kassirer M, Weiss I et al. . Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. Jf Cardiac Failure 2011;17:196–200. 10.1016/j.cardfail.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 27.Chan L, Chin LM, Kennedy M et al. . Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013;143:333–43. 10.1378/chest.12-0993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein AA, Chin LM, Keyser RE et al. . Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med 2013;107:778–84. 10.1016/j.rmed.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki T, Terada J, Tanabe N et al. . Home-based pulmonary rehabilitation in patients with inoperable or residual chronic thromboembolic pulmonary hypertension: a preliminary study. Respir Investig 2014;52:357–64. 10.1016/j.resinv.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Saglam M, Arikan H, Vardar-Yagli N et al. . Inspiratory muscle training in pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 2015;35:198–206. 10.1097/HCR.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 31.Neuman A, Gunnbjörnsdottir M, Tunsäter A et al. . Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respir Med 2006;100:1843–9. 10.1016/j.rmed.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 32.Galiè N, Hoeper MM, Humbert M et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–537. 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 33.Schulz KF, Altman DG, Moher D et al. . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:834–40. 10.1016/j.jclinepi.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 34.Saghaei MaS S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials . J Biomed Sci Eng 2011;4:734–9. 10.4236/jbise.2011.411090:734-39 [DOI] [Google Scholar]
- 35.Vonk-Noordegraaf A, Souza R. Cardiac magnetic resonance imaging: what can it add to our knowledge of the right ventricle in pulmonary arterial hypertension? Am J Cardiol 2012;110(Suppl 6):25s–31s. 10.1016/j.amjcard.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 36.Kellman P, Arai AE, McVeigh ER et al. . Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med 2002;47:372–83. 10.1002/mrm.10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holloway CJ, Ntusi N, Suttie J et al. . Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013;128: 814–22. 10.1161/CIRCULATIONAHA.113.001719 [DOI] [PubMed] [Google Scholar]
- 38.Holloway CJ, Montgomery HE, Murray AJ et al. . Cardiac response to hypobaric hypoxia: persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J 2011;25:792–6. 10.1096/fj.10-172999 [DOI] [PubMed] [Google Scholar]
- 39.Panagiotou M, Peacock AJ, Johnson MK. Respiratory and limb muscle dysfunction in pulmonary arterial hypertension: a role for exercise training? Pulm Circ 2015;5:424–34. 10.1086/682431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batt J, Ahmed SS, Correa J et al. . Skeletal muscle dysfunction in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2014;50:74–86. 10.1165/rcmb.2012-0506OC [DOI] [PubMed] [Google Scholar]
- 41.Breda AP, Pereira de Albuquerque AL, Jardim C et al. . Skeletal muscle abnormalities in pulmonary arterial hypertension. PLoS ONE 2014;9:e114101 10.1371/journal.pone.0114101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimopoulos S, Tzanis G, Manetos C et al. . Peripheral muscle microcirculatory alterations in patients with pulmonary arterial hypertension: a pilot study. Respir Care 2013;58:2134–41. 10.4187/respcare.02113 [DOI] [PubMed] [Google Scholar]
- 43.Mainguy V, Maltais F, Saey D et al. . Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 2010;65:113–17. 10.1136/thx.2009.117168 [DOI] [PubMed] [Google Scholar]
- 44.Leong DP, Teo KK, Rangarajan S et al. . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266–73. 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 45.Pandey A, Garg S, Khunger M et al. . Efficacy and safety of exercise training in chronic pulmonary hypertension: systematic review and meta-analysis. Circ Heart Fail 2015;8:1032–43. 10.1161/CIRCHEARTFAILURE.115.002130 [DOI] [PubMed] [Google Scholar]
- 46.Buys R, Avila A, Cornelissen VA. Exercise training improves physical fitness in patients with pulmonary arterial hypertension: a systematic review and meta-analysis of controlled trials. BMC Pulm Med 2015;15:40 10.1186/s12890-015-0031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland AE, Spruit MA, Troosters T et al. . An official European respiratory society/American thoracic society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–46. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 48.Löwe B, Gräfe K, Ufer C et al. . Anxiety and depression in patients with pulmonary hypertension. Psychosom Med 2004;66:831–6. 10.1097/01.psy.0000145593.37594.39 [DOI] [PubMed] [Google Scholar]
- 49.Harzheim D, Klose H, Pinado FP et al. . Anxiety and depression disorders in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir Res 2013;14:104 10.1186/1465-9921-14-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shafazand S, Goldstein MK, Doyle RL et al. . Health-related quality of life in patients with pulmonary arterial hypertension. Chest 2004;126:1452–9. 10.1378/chest.126.5.1452 [DOI] [PubMed] [Google Scholar]
- 51.Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs 2008;108:52–62; quiz 62-3 10.1097/01.NAJ.0000314810.46029.74 [DOI] [PubMed] [Google Scholar]
- 52.McKenna SP, Doughty N, Meads DM et al. . The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health-related quality of life and quality of life for patients with pulmonary hypertension. Qual Life Res 2006;15:103–15. 10.1007/s11136-005-3513-4 [DOI] [PubMed] [Google Scholar]
- 53.Ganderton L, Jenkins S, McKenna SP et al. . Validation of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) for the Australian and New Zealand population. Respirology 2011;16:1235–40. 10.1111/j.1440-1843.2011.02030.x [DOI] [PubMed] [Google Scholar]
- 54.Graf C. The Lawton Instrumental Activities of Daily Living (IADL) Scale. Medsurg Nurs 2009;18:315–16. [PubMed] [Google Scholar]
- 55.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol 2005;44(Pt 2):227–39. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- 56.Nagaya N, Nishikimi T, Uematsu M et al. . Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation 2000;102:865–70. 10.1161/01.CIR.102.8.865 [DOI] [PubMed] [Google Scholar]
- 57.Heresi GA, Aytekin M, Hammel JP et al. . Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J 2014;43:912–14. 10.1183/09031936.00164713 [DOI] [PubMed] [Google Scholar]
- 58.Souza R, Jardim C, Julio Cesar Fernandes C et al. . NT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir Med 2007;101:69–75. 10.1016/j.rmed.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 59.Fijalkowska A, Kurzyna M, Torbicki A et al. . Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest 2006;129:1313–21. 10.1378/chest.129.5.1313 [DOI] [PubMed] [Google Scholar]
- 60.de Man FS, van Hees HW, Handoko ML et al. . Diaphragm muscle fiber weakness in pulmonary hypertension. Am J Respir Crit Care Med 2011;183:1411–18. 10.1164/rccm.201003-0354OC [DOI] [PubMed] [Google Scholar]
- 61.Kabitz HJ, Schwoerer A, Bremer HC et al. . Impairment of respiratory muscle function in pulmonary hypertension. Clin Sci 2008;114:165–71. 10.1042/CS20070238 [DOI] [PubMed] [Google Scholar]
- 62.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518–624. 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 63.Scherr J, Wolfarth B, Christle JW et al. . Associations between Borg's rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol 2013;113:147–55. 10.1007/s00421-012-2421-x [DOI] [PubMed] [Google Scholar]
- 64.Holland AE, Wadell K, Spruit MA. How to adapt the pulmonary rehabilitation programme to patients with chronic respiratory disease other than COPD. Eur Respir Rev 2013;22:577–86. 10.1183/09059180.00005613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spruit MA, Singh SJ, Garvey C et al. . An official American Thoracic Society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 66.Hill K, Cecins NM, Eastwood PR et al. . Inspiratory muscle training for patients with chronic obstructive pulmonary disease: a practical guide for clinicians. Arch Phys Med Rehabil 2010;91:1466–70. 10.1016/j.apmr.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 67.Cahalin LP, Arena RA. Breathing exercises and inspiratory muscle training in heart failure. Heart Fail Clin 2015;11:149–72. 10.1016/j.hfc.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 68.Kraemer WJ, Adams K, Cafarelli E et al. . American college of sports medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2002;34:364–80. [DOI] [PubMed] [Google Scholar]
- 69.Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev 2013;22:178–86. 10.1183/09059180.00000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CRUFAD CRUFAD, St Vincent's Hospital Sydney, Australia. Anxiety and Panic Disorder. Patient Treatment Manual 2010. https://crufad.org/images/stories/pdf/manuals/crufad_Panicmanual.pdf (accessed 14 Mar 2016).
- 71.Sage N, Sowden M, Chorlton E et al. . CBT for chronic illness and palliative care: a workbook and toolkit. England: John Wiley and Sons, 2008. [Google Scholar]
- 72.Jacobsen E. Progressive relaxation. Chicago, IL, USA: University of Chicago Press, 1938. [Google Scholar]
- 73.NHMRC. National Statement on Ethical Conduct in Human Research (2007)—Updated March 2014 2007.
- 74.Kawut SM, Horn EM, Berekashvili KK et al. . New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005;95:199–203. 10.1016/j.amjcard.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 75.Addetia K, Bhave NM, Tabit CE et al. . Sample size and cost analysis for pulmonary arterial hypertension drug trials using various imaging modalities to assess right ventricular size and function end points. Circ Cardiovasc Imaging 2014;7:115–24. 10.1161/CIRCIMAGING.113.000932 [DOI] [PubMed] [Google Scholar]
- 76.Bradlow WM, Hughes ML, Keenan NG et al. . Measuring the heart in pulmonary arterial hypertension (PAH): implications for trial study size. J Magn Reson Imaging 2010;31:117–24. 10.1002/jmri.22011 [DOI] [PubMed] [Google Scholar]
- 77.Zhong B. How to calculate sample size in randomized controlled trial? J Thorac Dis 2009;1:51–4. [PMC free article] [PubMed] [Google Scholar]