Abstract
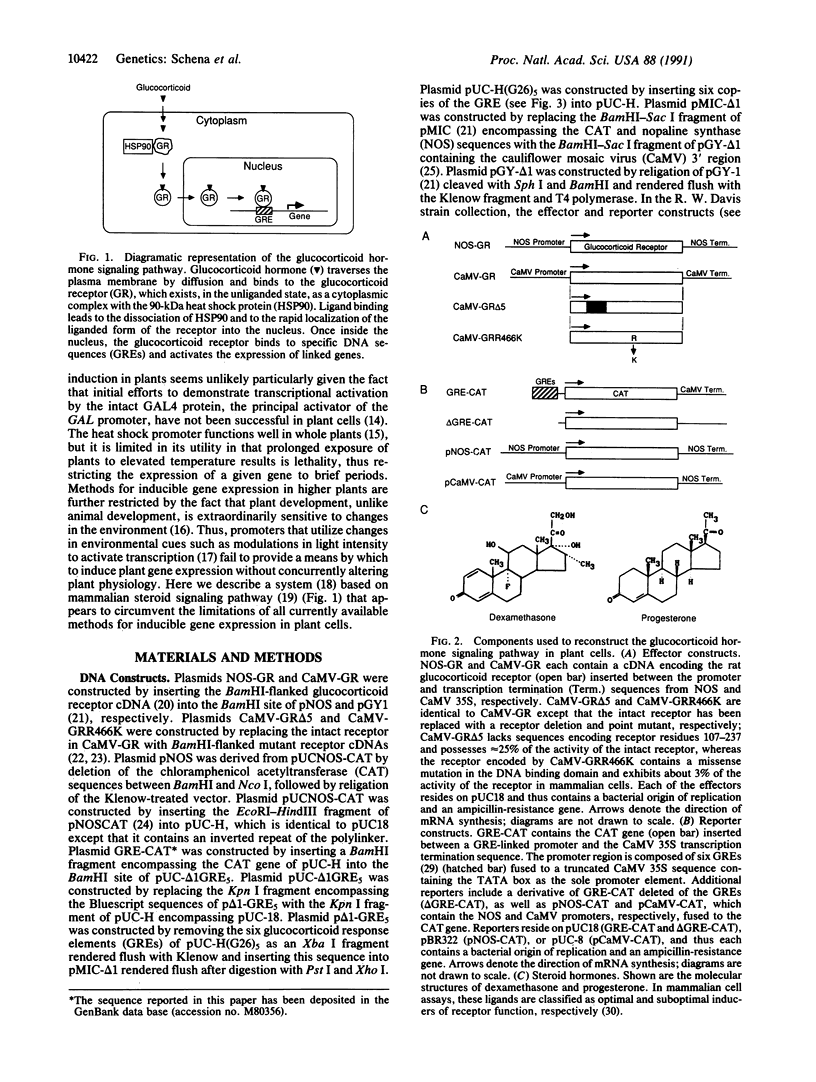
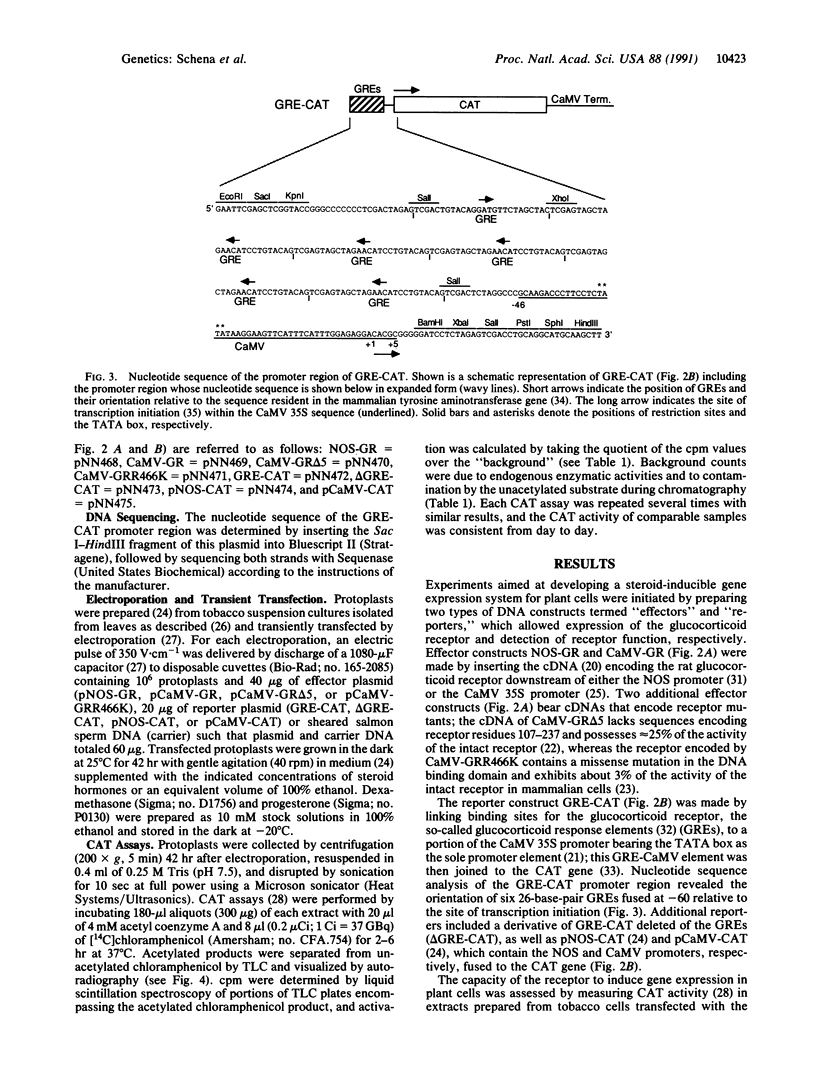
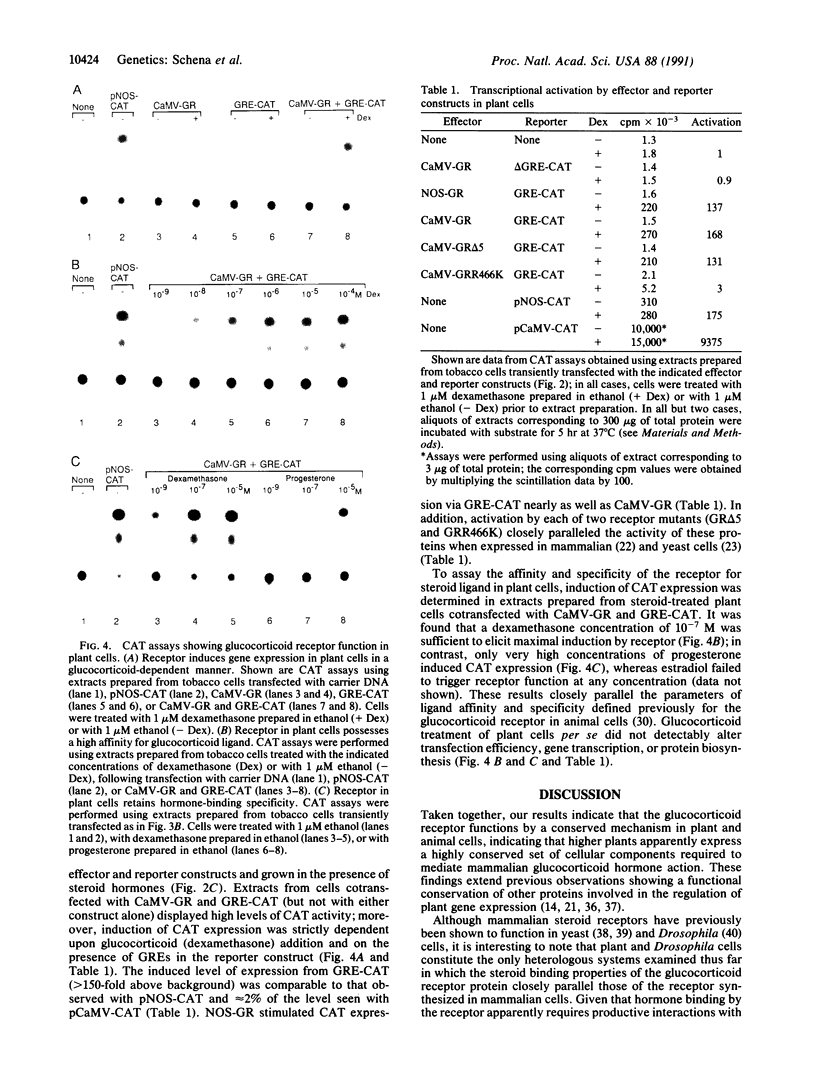
Promoters that allow the selective induction of gene expression in vivo constitute an important methodology in eukaryotic organisms such as yeast and the fruit fly, but to date no such system has been described for higher plants. Given the fact that mammalian steroid receptors can function as hormone-dependent inducers of gene expression in heterologous systems, the feasibility of using mammalian steroid hormones as selective inducers of plant gene expression was investigated. Here it is shown that the glucocorticoid receptor expressed in plant cells is capable of activating a test gene linked to glucocorticoid response elements, providing the transfected plant cells are treated with glucocorticoid hormone. Nanomolar concentrations of glucocorticoids are sufficient to induce gene expression more than 150-fold, without causing detectable alterations in the physiology of the cultured plant cells. These findings indicate that glucocorticoid induction of steroid-responsive promoters should provide a general method for regulating gene expression in plant cells and imply that such a system might ultimately function in whole plants such as Arabidopsis thaliana.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Puffs, genes, and hormones revisited. Cell. 1990 Apr 6;61(1):1–3. doi: 10.1016/0092-8674(90)90205-s. [DOI] [PubMed] [Google Scholar]
- Auricchio F. Phosphorylation of steroid receptors. J Steroid Biochem. 1989 Apr;32(4):613–622. doi: 10.1016/0022-4731(89)90397-x. [DOI] [PubMed] [Google Scholar]
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Davis R. W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990 Feb 9;60(3):357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Yoshinaga S. K., Vanderbilt J. N., Yamamoto K. R. In vitro transcription enhancement by purified derivatives of the glucocorticoid receptor. Science. 1989 Jul 21;245(4915):298–301. doi: 10.1126/science.2473529. [DOI] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa T., Obonai K., Segawa T., Nogi Y. The enzymes of the galactose cluster in Saccharomyces cerevisiae. II. Purification and characterization of uridine diphosphoglucose 4-epimerase. J Biol Chem. 1980 Apr 10;255(7):2705–2707. [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A., Hoffmann A., Horikoshi M., Roeder R. G., Chua N. H. Arabidopsis thaliana contains two genes for TFIID. Nature. 1990 Jul 26;346(6282):390–394. doi: 10.1038/346390a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Yocum R. R., Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilson P., de Froidmont D., Lejour C., Hirai S., Jacquemin J. M., Yaniv M. Fos and Jun oncogenes transactivate chimeric or native promoters containing AP1/GCN4 binding sites in plant cells. Plant Cell. 1990 Jul;2(7):651–658. doi: 10.1105/tpc.2.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T., Richards K., Geneviève-Lebeurier Cauliflower mosaic virus on its way to becoming a useful plant vector. Curr Top Microbiol Immunol. 1982;96:194–236. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987 Dec;51(4):458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Ma J., Przibilla E., Hu J., Bogorad L., Ptashne M. Yeast activators stimulate plant gene expression. Nature. 1988 Aug 18;334(6183):631–633. doi: 10.1038/334631a0. [DOI] [PubMed] [Google Scholar]
- Medford J. I., Horgan R., El-Sawi Z., Klee H. J. Alterations of Endogenous Cytokinins in Transgenic Plants Using a Chimeric Isopentenyl Transferase Gene. Plant Cell. 1989 Apr;1(4):403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Schena M., Freedman L. P., Yamamoto K. R. Mutations in the glucocorticoid receptor zinc finger region that distinguish interdigitated DNA binding and transcriptional enhancement activities. Genes Dev. 1989 Oct;3(10):1590–1601. doi: 10.1101/gad.3.10.1590. [DOI] [PubMed] [Google Scholar]
- Schena M., Picard D., Yamamoto K. R. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. The organization and transcription of the galactose gene cluster of Saccharomyces. J Mol Biol. 1981 Oct 25;152(2):285–315. doi: 10.1016/0022-2836(81)90244-8. [DOI] [PubMed] [Google Scholar]
- Struhl G. Near-reciprocal phenotypes caused by inactivation or indiscriminate expression of the Drosophila segmentation gene ftz. Nature. 1985 Dec 19;318(6047):677–680. doi: 10.1038/318677a0. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E., Veit B., Sinha N., Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991 Mar 21;350(6315):241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S. K., Yamamoto K. R. Signaling and regulation by a mammalian glucocorticoid receptor in Drosophila cells. Mol Endocrinol. 1991 Jun;5(6):844–853. doi: 10.1210/mend-5-6-844. [DOI] [PubMed] [Google Scholar]






