Abstract
Pembrolizumab is an approved first-line systemic therapy for unresectable metastatic melanoma. Despite the achievement of complete and durable responses in a small subgroup of patients, it is standard practice that pembrolizumab therapy continues beyond complete response. Nevertheless, the incidence of immune-related toxicities gradually increases with continuing pembrolizumab therapy. We report a case highlighting the occurrence of serious induced immune-related adverse events, which were attributed to pembrolizumab in a patient with metastatic melanoma who obtained a complete response (CR) after receiving pembrolizumab for a total of 6.5 months. Although mild pembrolizumab-related toxicity persists, the patient remains disease-free 5.5 months after discontinuation of pembrolizumab. Accordingly, we believe that cessation of pembrolizumab should be considered in patients who achieve a CR because of the ongoing risk of toxicity with extended pembrolizumab administration.
Background
Pembrolizumab is an antiprogrammed death-1 (PD1) inhibitory monoclonal antibody immunotherapy approved for treatment of metastatic melanoma. Pembrolizumab mediates its antitumour effect by unleashing latent tumour-specific T-cell responses.1 2 Partial response (PR) is the typical tumour response pattern observed after first-line pembrolizumab therapy, but complete responses (CRs), which are durable, occur in 15% of patients.3 However, chronic PD-1 blockade may be marked by immune dysregulation, which can manifest in a diverse range of organ-related toxicities.1 4 The incidence of these immune-related adverse events (irAEs) tends to increase slowly with continued exposure to anti-PD1 therapy. Not all of the irAEs are self-limiting or reversible despite temporary suspension or permanent cessation of anti-PD1 therapy.1 2 Nevertheless, with the exception of vitiligo, there are insufficient data to indicate whether the occurrence of certain irAEs correlates with increased likelihood of tumour response to anti-PD1 therapy. Similarly, it is not known whether patients who achieve a CR with anti-PD1 therapy can stop therapy and thus, avoid potential and cumulative risks of irAEs associated with prolonged drug exposure.
Case presentation
We report the case of a 66-year-old man who lived in a rural area. He was diagnosed with stage 3 cutaneous melanoma of the right (R) leg in December 2012. A completion (R) groin dissection was performed in January 2013 after a positive sentinel lymph node biopsy. In August 2014, after clinical (R) groin recurrence, fluorodeoxyglucose-positron emission tomography CT (FDG-PET/CT) scan also demonstrated locoregional FDG-avid disease of (R) external iliac, (R) pelvic side wall lymph nodes and a 2 cm (R) mid-thigh nodule. A (R) pelvic dissection revealed that three of seven lymph nodes were involved; the largest was 80 mm and extending into surrounding fat. Intraoperative bleeding limited resection of the remaining pelvic disease and the (R) thigh lesion was also not removed. This disease was Q61K NRAS-mutant but V600 BRAF mutation-negative. His comorbidities included treated hypertension, hypercholesterolaemia, type 2 diabetes mellitus and depression.
Given that he had unresectable stage 4 pTxN2bM1a melanoma, pembrolizumab 2 mg/kg intravenous Q3W was started on 9 September 2015. After two cycles (23 October 2015), he developed grade 2 pruritic, erythematous rash involving his upper chest and back, forearms and back of legs bilaterally. Hydrocortisone 1% cream and an oral antihistamine were started and the rash improved to grade 1 by cycle 5 (3 December 2015). Later, areas of rash were replaced by vitiligo (figure 1). At cycle 8 pembrolizumab (5 February 2016), the patient reported unintentional weight gain of 4 kg over 3 weeks associated with intolerance to cold. He demonstrated primary hypothyroidism with raised thyroid-stimulating hormone (38 mlU/L; reference range (RR): 0.5–4.0 mlU/L) and reduced free T4 (<5pM; RR: 10–25 pM). Thyroxine 100 µg daily was started gradually normalising his thyroid function.
Figure 1.
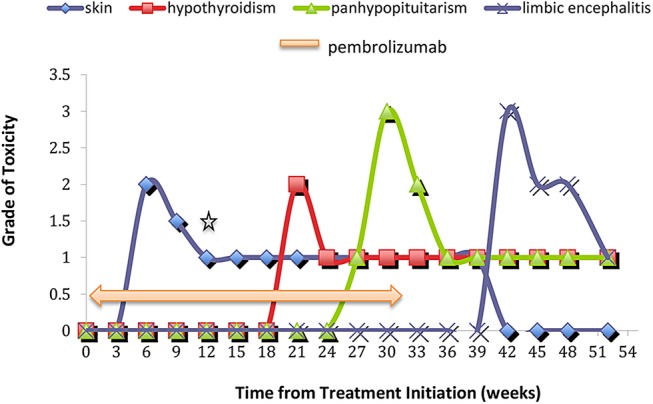
Schematic diagram indicating severity and time course of immune-related adverse events with pembrolizumab *Reduction of skin toxicity to grade 1 indicates ongoing vitiligo.
By cycle 10 (17 March 2016), the patient's (R) thigh nodule was no longer palpable but he reported of intolerance to heat, daily hot flushes, sweats and feelings of faintness without postural hypotension. Pregabalin 75 mg daily, which was started a few weeks previously for painful diabetic neuropathy was found responsible for this and was stopped. Nonetheless, his symptoms persisted and his Eastern Cooperative Oncology Group performance status declined from 1 to 3 with increasing lethargy and nausea. Consequently, cycle 11 pembrolizumab was withheld. Concurrent laboratory test results diagnosed panhypopituitarism and the patient was hospitalised. He had low serum values for cortisol (18 nmM; RR: <133–540 nM at 09:00 hours), adrenocorticotropic hormone (<10 ng/L; RR: 10–60 ng/L), potassium (3.0 mMl RR: 3.5–4.0 mM) and free testosterone (2.5 nM; RR: 8–30 nM). MRI of his brain did not show evidence of hypophysitis or intracranial metastatic disease (figure 2). Hormone replacement therapy was started by using hydrocortisone 20 mg mane and 10 mg nocte, testosterone patch 2.5 mg/24 hours, and ongoing thyroxine at 100 µg daily. CT restaging at the same time provided radiological confirmation of the clinical CR. Given the absence of measurable disease clinically and radiologically, together with the accumulation of several treatable pembrolizumab-related toxicities, the decision was made after discussion with the patient to discontinue treatment with pembrolizumab but to continue clinical and radiological surveillance of his disease.
Figure 2.
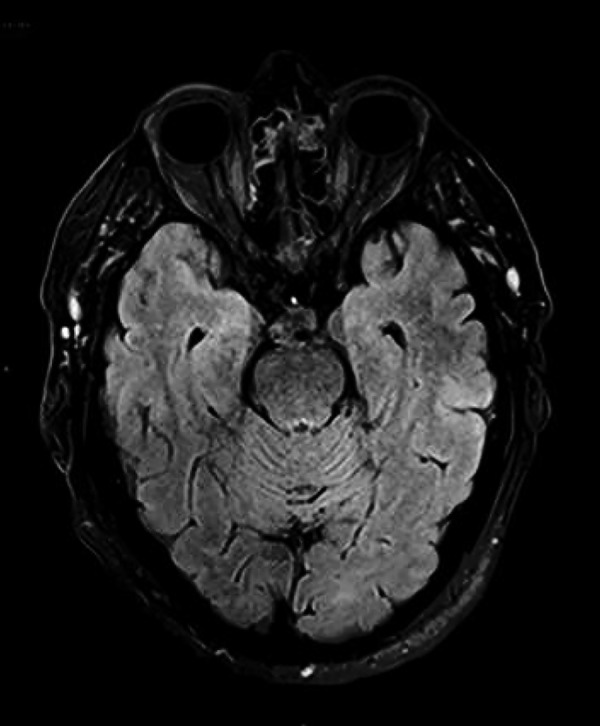
Baseline MRI brain FLAIR imaging showing no convincing abnormality in the mesial temporal lobes. FLAIR, fluid-attenuated inversion recovery.
By 28 April 2016, 7.5 months after starting pembrolizumab, the patient and family members reported mild short-term memory loss associated with increasing daily occurrences of multiple unprovoked panic attacks. In consultation with a clinical psychologist, the patient was given deep breathing exercises, acceptance and commitment therapy and cognitive-behavioural therapy to help manage these episodes. By 20 June 2016, all of his symptoms, in particular his memory impairment, had worsened. There was new onset of confusion and wandering behaviour, which prevented him from undertaking activities of daily living. Again, he was hospitalised for further investigation. His Addenbrooke's Cognitive Examination (ACE-R) score was reduced at 84/100. A septic screen and cerebrospinal fluid (CSF) test were negative for infection and multiple sclerosis as causes of his new symptoms. The paraneoplastic antibodies, anti-Hu, anti-Ri and anti-Yo were not detected in his CSF, but immunofluorescence examination of his CSF detected an anti-CASPR2 antibody.
MRI brain performed on 22 June 2016 showed increased T2 signal involving bilateral medial temporal lobes without leptomeningeal involvement and suggested a diagnosis of pembrolizumab-related limbic encephalitis (figure 3). The patient was given a 3-day course of intravenous methylprednisolone 1 g daily followed by oral prednisolone at 50 mg daily for 7 days then a slow taper of 5 mg every week. EEG showed background slow waves (6–7Hz) and intermittent δ slowing. Although the EEG was not typical for epileptiform activity, sodium valproate 500 mg twice daily was started because the patient's frequent ‘acute panic attacks’ were thought to relate to complex partial seizures.
Figure 3.
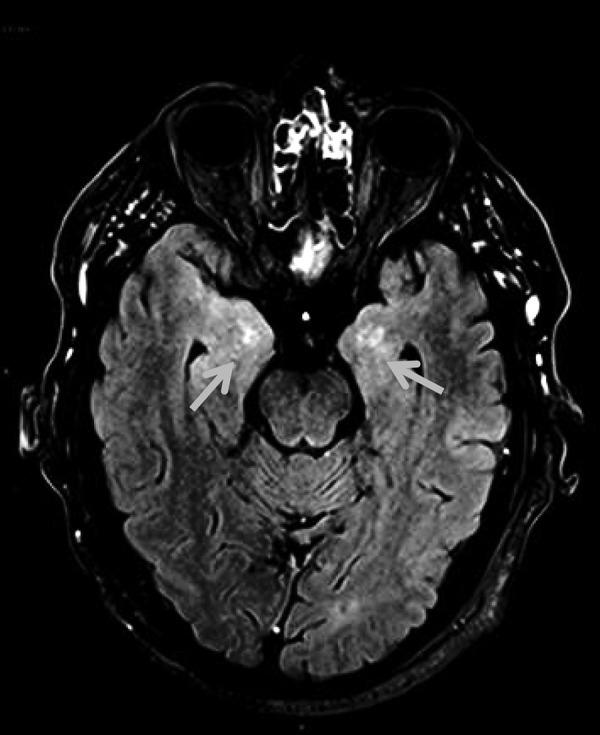
MRI brain postcontrast FLAIR imaging showing asymmetric bilateral abnormal high signal in the mesial temporal lobes with abnormal parenchymal enhancement. FLAIR, fluid-attenuated inversion recovery.
Outcome and follow-up
On 27 July 2016, some improvement was observed in his MRI brain with less T2 signal involving the left temporal lobe in addition to an overall reduction in cerebral swelling (figure 4). On 2 September 2016, at patient's latest follow-up visit, he reported a reduced number of ‘panic attacks’ as well as the resolution of his previous disorientation and wandering behaviour. However, he still required supervised accommodation and has continuing short-term cognitive impairment with a repeat ACE-R score of 88/100. The patient remains in clinical and radiological disease remission from melanoma 5.5 months after ceasing pembrolizumab.
Figure 4.
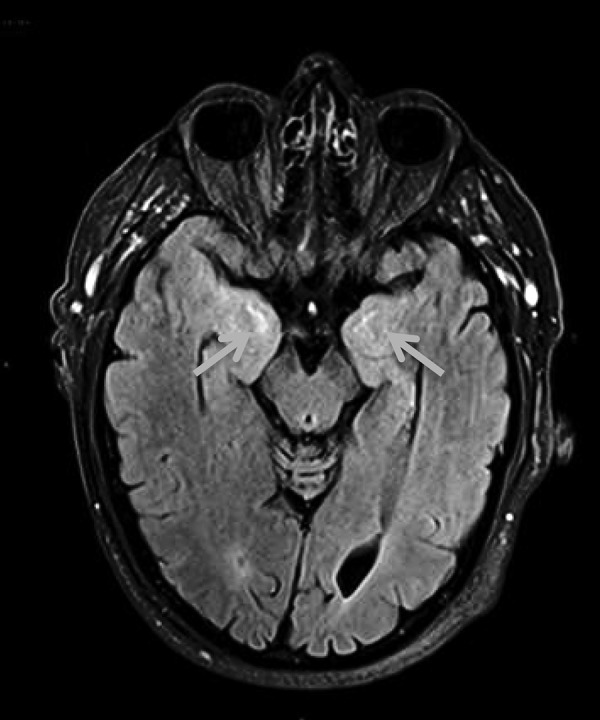
Follow-up postcontrast MRI brain FLAIR imaging showing persistent abnormal high signal in the bilateral mesial temporal lobes. FLAIR, fluid-attenuated inversion recovery.
Discussion
Pivotal phase 3 trials of pembrolizumab have demonstrated its superior efficacy in both treatment-naïve and patients with ipilimumab-refractory metastatic melanoma.5 6 Most irAEs related to PD1 blockade appear during the first 6 months of treatment as illustrated with our case.7 As we report, dermatological irAEs are the earliest to arise, typically by cycle 2 pembrolizumab. Later, gastrointestinal and hepatic irAEs occur between cycles 3 and 6 although these were not encountered in our patient. Instead, we observed endocrine irAEs, which tend to occur after cycle 4, with thyroid dysfunction arising earlier than other endocrinopathies such as hypophysitis. The timing of neurological events such as limbic encephalitis, which occur in fewer than 1% of pembrolizumab-treated patients is less certain because of their rarity.2 7 8
Interestingly, at the time of his limbic encephalitis diagnosis, our patient showed a CSF antibody specific for contactin-associated protein-like 2 (CASPR2). CASPR2 is a transmembrane protein found in the juxtaparanodes of myelinated axons and can be complexed with voltage-gated potassium channels to maintain electrical functioning of neurons.9 Anti-CASPR2 antibodies in CSF have been associated with rare cases of autoimmune (non-paraneoplastic) limbic encephalitis.10 11
As we demonstrate, some irAEs such as skin manifestations are self-limiting but others, particularly endocrine and severe neurological irAEs (grade 3 and above), may not resolve completely and become permanent. Although extending pembrolizumab beyond 6 months may result in cumulative irAEs in some individuals, the data from recent clinical studies of anti-PD1 therapy in metastatic melanoma are not conclusive. For example, in the Keynote-006 study, a 2.9% increase in grade 3 and higher irAEs in patients who were treated with extended pembrolizumab therapy was found.12 In contrast, long-term follow-up data from both the Keynote-001 study and studies of nivolumab have not indicated additional detrimental effect with prolonged anti-PD1 therapy.3 13 Furthermore, no predictive markers exist that would identify patients most at risk of cumulative irAEs.
We discontinued pembrolizumab therapy in our patient because symptomatic hypophysitis developed in the presence of a CR. The CR rates with pembrolizumab monotherapy in the treatment-naïve and second-line settings are 15% and 12–13%, respectively.3 5 Our case of severe irAEs occurring in a pembrolizumab-treated patient who had achieved a CR raises the question of whether discontinuing anti-PD1 therapy at or soon after CR or deep PR may lessen the occurrence of severe irAEs while preserving the clinical benefit of a likely ongoing and durable tumour response.2 14 Clinical trial designs using randomised discontinuation are being pursued internationally to address this kind of question. They include randomisation to continuation of anti-PD1 therapy versus therapy cessation with anti-PD1 rechallenge at disease progression, after either maximal tumour response has been achieved or a fixed period of anti-PD1 therapy (such as 12 months) has elapsed.
In the KEYNOTE-001 study of pembrolizumab, 64% of patients (n=60) discontinued pembrolizumab after CR and 97% of these patients remained disease free on active surveillance.3 These data suggest that patients in CR should be counselled about the risk–benefit profile of continuation pembrolizumab. In the patients who achieve maximal clinical benefit from CR, the decision to continue pembrolizumab may lack equipoise in the face of an ongoing risk of further irAEs. In addition, the financial costs associated with both continuation pembrolizumab and the management of any further irAEs should be borne in mind.
Data are emerging on potential clinical biomarkers of treatment response with pembrolizumab. Our patient experienced vitiligo as he developed CR. In a retrospective study of 83 advanced patients with melanoma treated with pembrolizumab, any cutaneous irAEs including pruritus, erythematous macular/papular rash and hypopigmentation were associated with a longer progression-free survival.15 Similarly, in a prospective observational study of 67 patients, a higher objective response rate to pembrolizumab was observed among patients with vitiligo (71% vs 28%).16 It is unknown whether other organ-specific irAEs are also associated with improved clinical benefit after pembrolizumab therapy.
Other laboratory parameters may also be useful to predict clinical responses to pembrolizumab therapy. For example, studies of tumour mutation burden using DNA sequencing methods indicate that a high tumour mutational load is associated with a better response to PD-1 immune checkpoint inhibition than a low mutational load.17–19 Pretreatment density of CD8+ T-cells density is associated with better treatment response to pembrolizumab.20 Despite the promise of these biomarkers, the data are not sufficiently robust or mature for routine clinical use.
In conclusion, in spite of pembrolizumab's effectiveness as metastatic melanoma therapy, its clinical benefit in some patients may be ‘purchased’ at the cost of multiple irAEs, some of which are cumulative and permanent in nature. Consequently, we recommend a heightened awareness of irAEs, which can develop unexpectedly over time. In patients who achieved a CR, discontinuation of anti-PD1 therapy should be considered but ideally patients should be enrolled in prospective clinical trials to gather information about the risk/benefit profile of this approach.
Learning points.
Pembrolizumab, an inhibitory antiprogrammed death-1-specific monoclonal antibody, can elicit a diverse range of clinically significant immune-related adverse events (irAEs).
Some irAEs are not reversible, and there is the risk of accumulated toxicity in some patients on extended pembrolizumab therapy.
Early data suggest that dermatological irAEs, in particular, vitiligo/hypopigmentation are associated with improved treatment response and longer progression-free survival.
In discussion with the patient, discontinuation of pembrolizumab should be considered after a complete response is obtained.
Footnotes
Contributors: AH-CH contributed by writing, proof reading and finalising of manuscript SF was involved in image processing, proof reading and finalising of manuscript. MPB participated in writing, proof reading and finalising of manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dsyimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559–74. 10.1093/annonc/mdv623 [DOI] [PubMed] [Google Scholar]
- 2.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4:560–75. 10.3978/j.issn.2218-6751.2015.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Ribas AQ, Hamid O et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 2016;34 (suppl; abstr 9503).
- 4.Okazaki T, Chikuma S, Iwai Y et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013;14:1212–18. 10.1038/ni.2762 [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 6.Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 2015;6:3479–92. 10.18632/oncotarget.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salam S, Lavin T, Turan A. Case report: limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep 2016;2016:doi:10.1136/bcr-2016-215012 10.1136/bcr-2016-215012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bien CG. Contactin-associated protein-like 2 antibodies: tackling the issue of syndrome diversity. JAMA Neurol 2016;73:1058–9. 10.1001/jamaneurol.2016.1739 [DOI] [PubMed] [Google Scholar]
- 10.Joubert B, Saint-Martin M, Noraz N et al. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol 2016;73:1115–24. 10.1001/jamaneurol.2016.1585 [DOI] [PubMed] [Google Scholar]
- 11.Lancaster E, Martinez-hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011;77:179–89. 10.1212/WNL.0b013e318224afde [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival analysis of KEYNOTE-006. J Clin Oncol 2016;34(Suppl; abstr 9504). [Google Scholar]
- 13.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 14.Luke JJ, Ott PA. PD-1 pathway inhibitors. The next generation of immunotherapy for advanced melanoma. Oncotarget 2015;6:3479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanlorenzo M, Vujic I, Daud A et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151:1206–12. 10.1001/jamadermatol.2015.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua C, Boussemart L, Mateus C et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152:45–51. 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- 17.Le DT, Uram JN, Wang H et al. PD-1 blockade in tumours with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campesato LF, Barroso-Sousa R, Jimenez L et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget 2015;6:34221–7. 10.18632/oncotarget.5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumeh PC, Harview CL, Yearley JH et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]


