Abstract
Cre-driver mouse lines have been extensively used as genetic tools to target and manipulate genetically defined neuronal populations by expression of Cre recombinase under selected gene promoters. This approach has greatly advanced neuroscience but interpretations are hampered by the fact that most Cre-driver lines have not been thoroughly characterized. Thus, a phenotypic characterization is of major importance to reveal potential aberrant phenotypes prior to implementation and usage to selectively inactivate or induce transgene expression. Here, we present a biochemical and behavioural assessment of the dopaminergic system in hemizygous tyrosine hydroxylase (TH)-Cre mice in comparison to wild-type (WT) controls. Our data show that TH-Cre mice display preserved dopaminergic homeostasis with unaltered levels of TH and dopamine as well as unaffected dopamine turnover in striatum. TH-Cre mice also show preserved dopamine transporter expression and function supporting sustained dopaminergic transmission. In addition, TH-Cre mice demonstrate normal responses in basic behavioural paradigms related to dopaminergic signaling including locomotor activity, reward preference and anxiolytic behaviour. Our results suggest that TH-Cre mice represent a valid tool to study the dopamine system, though careful characterization must always be performed to prevent false interpretations following Cre-dependent transgene expression and manipulation of selected neuronal pathways.
Keywords: Cre-driver lines, striatum, tyrosine hydroxylase, phenotypic characterization
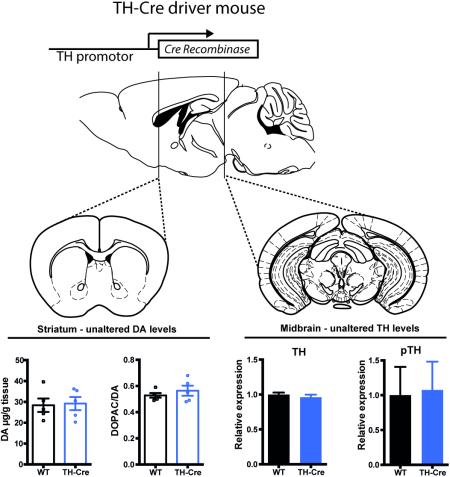
Graphical abstract
Phenotypic characterization of the TH-Cre mouse validates its use to target and study the dopamine system. Hemizygous TH-Cre mice display preserved dopaminergic homeostasis with unaltered levels of TH and dopamine as well as unaffected dopamine turnover in striatum. TH-Cre mice demonstrate normal responses in basic behavioural paradigms related to dopaminergic signaling (Sagittal and coronal brain sections in illustration were modified from Paxinos and Franklin, 2001).
Introduction
Tissue heterogeneity of the nervous system has always been a major obstacle for studying distinct neuronal subpopulations, but the advent of recent genetic tools exploiting specific promoters has enabled structure-function relationship studies in specific neuronal ensembles (Valjent et al., 2009). Expression of Cre-recombinase or fluorescent reporters under selected gene promoters has facilitated characterization of specific neuronal cell types (Lobo et al., 2006; Gerfen et al., 2013). With the Gene Expression Nervous System Atlas (GENSAT) project a plethora of bacterial artificial chromosome (BAC) transgenic mice was generated and has served as valuable tools to dissect cortical and basal ganglia pathways by targeting particular neuronal subtypes (Gong et al., 2003; Gerfen et al., 2013). Recently, implementation of Cre-driver lines, in combination with optogenetic or chemogenetic tools, has further allowed functional manipulation of genetically defined neuronal pathways in vivo, to assess behavioural effects of projection-specific manipulations (Tye & Deisseroth, 2012; Roth, 2016). Although the benefits of the Cre-driver lines are huge, and have greatly advanced studies of neuronal connectivity, one limitation is that the phenotypes of these mouse lines alone were not properly characterized before being implemented in neuroscience research (Kramer et al., 2011).
Dopamine (DA) is an essential neurotransmitter within the CNS modulating a wide array of physiological functions such as motor control, reward and neuroendocrine secretion. Imbalances in DA homeostasis are implicated in many neuropsychiatric diseases, such as addiction and depression (Tritsch & Sabatini, 2012). Accordingly, research into dopaminergic (DAergic) neurotransmission, including identification of DAergic projections and specific genetic manipulation within the DA system, are of major importance to enlighten mechanisms of pathologic phenotypes. Tyrosine hydroxylase (TH) is the rate-limiting enzyme in DA synthesis and has been considered a classical marker for identification of DAergic neurons. Although concerns have been raised recently regarding the use of a single gene marker to define the DAergic phenotype (Bjorklund & Dunnett, 2007; Apuschkin et al., 2015; Lammel et al., 2015; Stuber et al., 2015), targeting or identification of DAergic neurons in transgenic mice has so far relied on the promoter of either TH or the DA transporter (DAT) to drive specific expression of either a fluorescent reporter (Sawamoto et al., 2001; Donaldson et al., 2005; Jomphe et al., 2005; Kelly et al., 2006; Apuschkin et al., 2015) or Cre recombinase (Lindeberg et al., 2004; Savitt et al., 2005; Backman et al., 2006). Importantly though, Cre-driver lines might harbour aberrant phenotypes as a consequence of Cre-induced abnormalities. High expression levels of Cre recombinase have been associated with cellular toxicity leading to compromised neuronal development (Forni et al., 2006), significantly altered expression levels of endogenous proteins (Backman et al., 2006) and integration-mediated mutations or passenger-genes (Lusis et al., 2007), which all may cause aberrant phenotypes and confound the interpretation of observations (Gofflot et al., 2011).
Here, we investigate DAergic homeostasis and basal DA-related behaviour in hemizygous TH-Cre (TH-Cre) mice in comparison to wild-type (WT) controls in order to reveal potential aberrant phenotypes of the TH-Cre mice to correctly interpret research employing the mouse line.
Materials and Methods
Animals
TH-Cre mice (Jackson Laboratory, stock number: JAX:8601, strain name: B6.Cg-Tg(TH-Cre)1Tmd/J) generated as previously described (Savitt et al., 2005) were backcrossed with C57BL/6N mice for seven generations, and maintained in a hemizygous state. Hemizygous TH-Cre and WT mice were group-housed in a temperature-controlled room maintained on a 12:12 light:dark cycle (light on at 6 AM) and allowed access to food and water ad libitum, unless otherwise stated. Behavioral experiments were carried out in the inactive phase of the mice. Mice were sacrificed by cervical dislocation and the brains were rapidly dissected and processed as described for each experiment below. The guidelines of the Danish Animal Experimentation Inspectorate (permission number: 2012-15-2934-00279) was followed and experiments performed in a fully AAALAC accredited facility under the supervision of a local animal welfare committee. All efforts were made to minimize pain and discomfort as well as limiting the number of animals used.
Genotyping
Genotyping of TH-Cre was performed using the following primers: Cre-F (forward primer) 5- GCG GTC TGG CAG TAA AAA CTA TC-3; Cre-R (reverse primer) 5-GTG AAA CAG CAT TGC TGT CAC TT-3: Control-F (internal control forward primer) 5- CTA GGC CAC AGA ATT GAA AGA TCT -3; Control-R (internal control reverse primer) 5- GTA GGT GGA AAT TCT AGC ATC ATC C-3. The PCR was run with 1 cycle at 95 °C for 5 min, 35 cycles of 95 °C for 1 min, 60 °C for 1 min and 72 °C for 2 min followed by 1 cycle at 72 °C for 2 min. PCR product lengths are ~100 bp for the transgene and 324 bp for the internal positive control.
Immunoblotting
Midbrain and dorsal striatum from adult hemizygous TH-Cre mice and littermate controls (8-10 weeks old) were dissected from coronal slices using a brain matrix and a puncher. Striata were homogenized in lysis buffer (1 % Triton X-100, 0.1 % SDS, 1 mM EDTA, 50 mM NaCl, 20 mM Tris, pH 7.5) with added inhibitor cocktail of proteases (cOmplete Protease Inhibitor Cocktail, Roche Diagnostics GmbH, Mannheim, Germany) and phosphatases (Phosphatase Inhibitor Cocktail 3, Sigma, Darmstad, Germany). Tissue preparations were mechanically disrupted using a motor-driven pestle (800 rpm), and mixed by upside down rotation at 4 °C for at least 10 min. Homogenates were centrifuged at 16000 g for 10 min at 4 °C to remove debris. Lysates were prepared for immunoblotting following protein determination using a standard BCA™ Protein Assay kit (Thermo Scientific Pierce, Rockford, IL, USA). Equal amounts, corresponding to 9.2 μg and 16.7 μg protein of striatal and midbrain samples, respectively, were separated by SDS–polyacrylamide gel electrophoresis (any kD gels, BioRad, Hercules, CA, USA) and transferred to Immobilon-P membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked in PBS containing 0.05 % Tween-20 and 5 % dry milk and incubated overnight with antibodies against DAT (Millipore, MAB369, 1:1000), TH (Millipore, MAB318, 1:1000) or D1R (Sigma, D2944, 1:1000). For detection of pTH blocking was performed in PBS containing 0.05 % Tween-20 with 5 % polyvinylpyrrolidrone-40 (Sigma-Aldrich, Steinheim, Germany), and incubated overnight with antibody against pTH (Cell Signaling, Leiden, The Netherlands, 1:1000). Following incubation with HRP-conjugated anti-rat, -rabbit or -mouse (Thermo Scientific Pierce, Rockford, IL, USA, 1:2000) antibodies the blots were visualized by chemiluminescence (Amersham ECL-kit, GE Healthcare Life Sciences, Buckinghamshire, UK) using AlphaEase (Alpha Innotech, Miami, USA), and quantified. To verify equal protein loading, the membranes were re-probed with an HRP-conjugated antibody against β-actin (Sigma, Darmsted, Germany, 1:10000) to which protein levels of DAT, D1R, pTH and TH were normalized. Band intensities were quantified using ImageJ software (1.48 v, NIH, USA).
High-performance liquid chromatography analysis
Striatal lysates for high-performance liquid chromatography (HPLC) analysis were prepared from adult mice (10-12 weeks old). Mice were sacrificed and following rapid dissection of the brain, nucleus accumbens and dorsal striatum were punched out from coronal slices. Tissue samples were immediately frozen on dry ice and stored at −80 °C until homogenization in 500 μl perchloric acid 0.1 N. Following centrifugation at 14000 g for 30 min, 200 μl of the supernatant was filtered through a glass 0.22 μm filter (Avantec 13CP020AS, Frisenette ApS, Denmark). The samples were analysed using EC-HPLC methodology. The concentrations of monoamines and metabolites were determined by HPLC with electrochemical detection. The column was a Prodigy 3 μ ODS-3 C18 (DA 2 mm × 100 mm, particle size 3 μm, Phenomenex, YMC Europe, Schermbeck, Germany). The mobile phase (55 mM sodium acetate, 1 mM octanesulfonic acid, 0.1 mM Na2EDTA and 8 % Acetonitrile, pH of 3.2) was degassed with an on-line degasser. With a flow rate of 0.15 mL/min, 10 μl samples were injected. The electrochemical detection was accomplished using an amperometric detector (Antec Decade from Antec, Leiden, The Netherlands) with a glassy carbon electrode set at 0.8 V, with an Ag/AgCl reference electrode. The output was recorded and peak areas calculated by LC solution software (Shimadzu).
Synaptosomal dopamine uptake
Dorsal striata from adult hemizygous TH-Cre mice and littermate controls (8-10 weeks old) were dissected from coronal slices using a brain matrix and a puncher. Striata were weighed and homogenized in 1.5 mL ice-cold HEPES buffer (4 mM HEPES, 0.32 M sucrose, pH 7.4) using a motor-driven teflon pestle (800 rpm). Crude synaptosome fractions were obtained as previously described (Richards & Zahniser, 2009) and pellet was resuspended in homogenization buffer. Ten μl membrane suspension was incubated at 37 °C in 440 μl uptake buffer (25 mM HEPES pH 7.4, 120 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM L-ascorbic acid, 5 mM D-glucose, 1 μM pargyline, 100 nM desipramine and 10 nM cathechol-O-methyl-transferase inhibitor (RO-41-0960, R108 Sigma Aldrich, Germany)) with or without the presence of 500 μM cocaine used to assess non-specific uptake. DA (2, 5, 6-[3H]-DA (91.1 Ci/mmol, Perkin Elmer Life Sciences, Boston, MA, USA) in various final concentrations (0.031, 0.0625, 0.125, 0.25, 0.5 and 1.0 μM) was added for 5 min followed by addition of 1 mL ice-cold uptake buffer to arrest uptake. Synaptosomes were added to pre-rinsed glass microfiber filters (GF/C Whatman, GE Healthcare Life Sciences, Buckinghamshire, USA). Filters were then rinsed with 4 × 5 mL ice-cold uptake buffer and allowed to air-dry for an hour in scintillation tubes. HiSafe Scintillation fluid (Perkin Elmer) was added and filters were shaken vigorously for 1 h followed by counting of [3H]-DA in a MicroBeta™ (Wallac). A standard BCA™ Protein Assay kit (Thermo Scientific Pierce, Rockford, IL, USA) was used to determine protein concentrations of the synaptosomes for adjustments from counts per min to fmol/min/μg for analysis.
Phenotypic characterization
Adult male WT and hemizygous TH-Cre mice (8-11 weeks old) were used for behavioural experiments. All sessions were performed and analysed by a blinded-experimenter.
Habituation – basal locomotor activity
Mice were placed individually in white, square open field arenas (50 × 50 × 40 cm) for 120 min. Basal exploratory locomotor activity was recorded and analysed using the video-tracking software Ethovision (Noldus, Wageningen, The Netherlands).
Elevated plus maze test
Our elevated plus maze (EPM) setup is similar to that originally validated for mice (Lister, 1987), consisting of two open arms (30 cm × 5 cm × 0.25 cm) and two closed arms (30 cm × 5 cm × 15 cm) connected to a common central platform (5 cm × 5 cm) 70 cm above floor level. Mice were individually placed onto the maze's central platform facing a closed arm and allowed to explore the maze for 300 sec under dim lighting (5-9 lux at maze level). Measures recorded include: latency to first enter an open arm; number of entries into the open, closed and central arms; time spent in the open, closed and central arms; and number of stretch-attended postures (SAPs). A SAP is an example of a risk assessment behaviour in the closed arms, whereby a mouse displays an elongated body posture with its hind legs placed in the closed arms and its face and forelegs stretched into the open quadrant (Kaesermann, 1986). A mouse was recorded as being in the open areas when all four legs were passed into the open area. Mice were not placed back into their home cage until all cage mates had completed the EPM.
Reward Preference Test
The reward preference test (RPT) was carried out as previously described (Humby et al., 2005). Food-deprived mice, at 85-90 % of normal body weight, were exposed to either water or strawberry milk (Nestle, Copenhagen, Denmark) for 30 min over the course of 3 consecutive days. On each day, mice were placed into a novel, lidded cage (43 × 26 × 15 cm), in which two circular metal bowls (10 cm diameter × 1.5 cm high with maximum volume of 100 mL) were located at the cage's rear. On each day, one bowl was filled with water and the other with the reward substance, with bowls alternated daily in order to eliminate potential side preferences. Bowls were measured before and after each trial and the volume of each drink consumed during the test was calculated.
Statistical analysis
All between data groups were determined using unpaired t-tests when data showed Gaussian distribution. Otherwise, Mann-Whitney test was used. In the DA uptake experiments, Km and Vmax values were derived by fitting Michaelis-Menten kinetics to background subtracted data and analysed using non-parametric Mann-Whitney test. Statistical assessment of locomotor activity was performed using repeated measure two-way ANOVA. Outliers in behavioural experiments were removed after square root transformation had occurred, using a conventional inclusion criterion of group mean ± 1.96 times the standard deviation. There was a total of one outlier in the EPM, none in the RPT and habituation test. Statistical analysis was performed using GraphPad Prism software version 6 (GraphPad Software Inc., La Jolla, CA, USA). All values are expressed as mean ± SEM. Significance was set at P < 0.05.
Results
In order to reveal potential functional abnormalities that might accompany Cre expression in hemizygous TH-Cre mice, we investigated DAergic homeostasis and DA-related behaviour using biochemical and behavioural assays.
Intact dopamine homeostasis in striatum
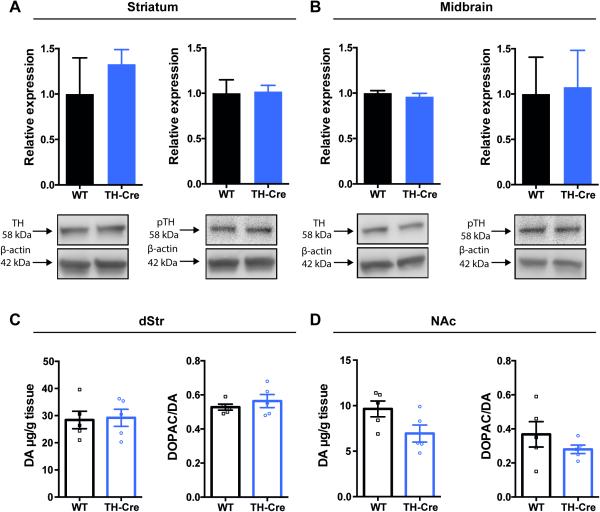
To investigate potential aberrations in DAergic homeostasis of TH-Cre mice, we investigated dorsal striatal and midbrain levels of TH, the rate-limiting enzyme in DA synthesis. Further, striatal levels of DA, its metabolites as well as other monoamines were investigated. Striatal and midbrain expression levels of TH were assessed by immunoblotting and showed no decrease in TH-Cre mice when compared to WT littermates in dorsal striatum (dStr) (Fig. 1A, left panel, P = 0.90, non-parametric Mann-Whitney test, n = 3) and in midbrain (Fig. 1B, left panel, P = 0.20, non-parametric Mann-Whitney test, n = 3). TH phosphorylation at serine residue 40 by protein kinase positively regulates catalytic activity of TH (Lindgren et al., 2000). Hence, to determine any consequences for DA synthesis in hemizygous TH-Cre mice, we also investigated striatal levels of the phosphorylated (at serine residue 40) form of TH (pTH) using immunoblotting analysis. No difference was found for pTH levels in hemizygous TH-Cre when compared to WT in dStr (Fig. 1A, right panel, P = 0.70; n = 3) or midbrain (Fig. 1B, right panel, P = 0.90; n = 3) when analyzed by non-parametric Mann-Whitney tests. Furthermore, high-performance liquid chromatography (HPLC) was used to assess the level of DA and other monoamines in striatal regions including dStr and nucleus accumbens (NAc). DA levels were found to be unaltered in hemizygous TH-Cre mice when compared to WT littermates in both dStr (Fig. 1C, left panel, TH-Cre: 29.2±3.2 μg/g tissue, WT: 28.4±3.2 μg/g tissue, unpaired t-test: t8 = 0.178, P = 0.86) and NAc (Fig. 1D, left panel, TH-Cre: 6.9±0.94 μg/g tissue, WT: 9.6±0.87 μg/g tissue, unpaired t-test: t8 = 2.12, P = 0.07). These levels are in accordance with previously published values for total striatal dopamine tissue levels (Jones et al., 1998; Van Dam et al., 2005). Besides DA, dihydroxyphenylacetic acid (DOPAC), 5-hydroxyindoleacetic acid (5-HIAA), 5-hydroxytryptamine (5-HT) and norepinephrine (NA), were analysed and revealed no differences between genotypes (Table 1). Also, DOPAC/DA ratios indicative of relative DA turnover were found to be unaffected in TH-Cre mice when compared to WT in both dStr (Fig. 1C, right panel, t8 = 0.85, P = 0.42) and NAc (Fig. 1D, right panel, t8 = 1.12, P = 0.30).
Figure 1. Unaltered levels of TH, pTH and DA in the nigrostriatal system of hemizygous TH-Cre mice.
Immunoblotting showed unaltered expression levels within striatum (A) and midbrain (B) of TH (left) and pTH (right) of hemizygous TH-Cre mice relative to wild-type (WT). A + B: Upper panels: densitometric analysis of immunoblots for WT and hemizygous TH-Cre (n = 3). TH and pTH expression levels were normalized to β-actin, no change observed. Lower panels: representative immunoblots for TH, pTH and β-actin. C + D: HPLC analysis showing unchanged levels of DA and DA turnover (DOPAC/DA ratio) in the striatal subcompartments dStr (C) and NAc (D) (n = 5). DA: dopamine, DOPAC: dihydroxyphenylacetic acid, dStr: dorsal striatum, NAc: nucleus accumbens, TH: tyrosine hydroxylase, pTH: phosphorylated TH
Table 1.
Levels (μg/g tissue) of dopamine and other monoamines/metabolites
| Dorsal striatum | Nucleus accumbens | |||
|---|---|---|---|---|
| WT | TH-Cre | WT | TH-Cre | |
| DOPAC | 15.2 ±2.30 | 16.5 ±2.00 | 3.5 ±0.80 | 1.9 ±0.30 |
| DA | 28.4 ±3.20 | 29.2 ±3.20 | 9.6 ±0.90 | 6.9 ±0.90 |
| 5-HT | 10.9 ±2.10 | 10.5 ±1.50 | 1.4 ±0.70 | 0.6 ±0.40 |
| HIAA | 1.7 ±0.40 | 2.2 ±0.30 | 0.6 ±0.10 | 0.8 ±0.20 |
| NA | 0.2 ±0.00 | 0.2 ±0.04 | 0.3 ±0.10 | 0.5 ±0.20 |
HPLC analysis of striatal areas including dorsal striatum (dStr) and nucleus accumbens (NAc) of hemizygous TH-Cre and littermate controls (n = 5). DOPAC; dihydroxyphenylacetic acid. 5-HIAA; 5-hydroxyindoleacetic acid, 5-HT; 5-hydroxytryptamine, NA; norepinephrine. Data are shown as mean +/− SEM.
Preserved dopamine transporter function and dopamine D1 receptor expression
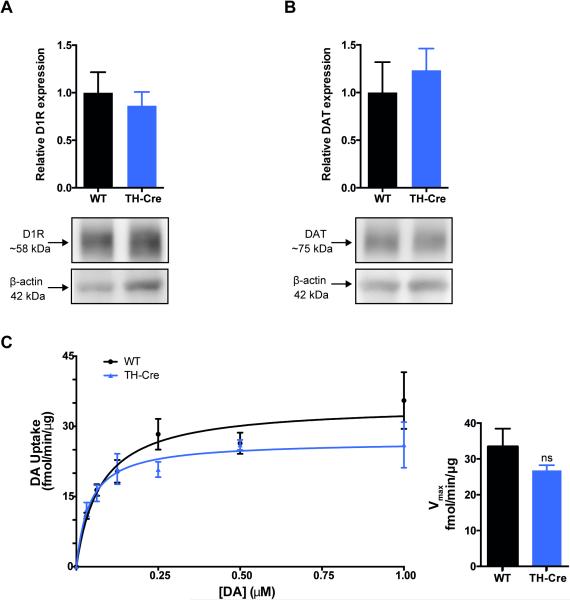
To further investigate DAergic homeostasis in hemizygous TH-Cre mice, we determined expression levels and uptake capacity of DAT, the major regulator of synaptic DA levels, as well as the expression levels of the postsynaptic DA D1-receptor (D1R). Immunoblotting of striatal lysates showed no alterations in expression levels of D1R in hemizygous TH-Cre when compared to WT controls (Fig. 2A, non-parametric Mann-Whitney; P = 0.70, n = 3). DAT is the primary determinant in controlling DAergic transmission by sequestering synaptic DA, thus DAT is fundamental for replenishing the intracellular pools of DA (Giros et al., 1996). Immunoblotting showed that striatal DAT expression is unaltered in hemizygous TH-Cre mice when compared to WT controls (Fig. 2B, non-parametric Mann-Whitney; P = 0.40, n = 3). Furthermore, [3H]-DA uptake in striatal synaptosomes revealed intact levels of functional DAT in striatum of hemizygous TH-Cre mice shown by unaltered DA uptake capacity when compared to WT (Fig. 2C) (Vmax for TH-Cre = 26.8 ± 1.5 fmol/min/μg, n = 3; Vmax for WT = 33.4 ± 5.0 fmol/min/μg, n = 4; unpaired t-test, P = 0.3) with no difference in apparent affinity (Km for TH-Cre = 42.9 ± 7.5 nM, n = 3; Km for WT = 41.2 ± 10.5 nM, n = 4; unpaired t-test, P = 0.9).
Figure 2. Dopamine D1-receptor and dopamine transporter protein expression is unaltered in striatum of hemizygous TH-Cre mice concomitant with unaltered dopamine uptake.
Immunoblotting showed unaltered protein levels of both D1R (A) and DAT (B) in dorsal striatum of hemizygous TH-Cre mice when compared to WT. Upper panels: densitometric analysis of immunoblots for TH-Cre and WT (n = 3). D1R and DAT expression levels were normalized to β-actin. A + B, lower panels: representative immunoblots for D1R, DAT and β-actin. C: Saturation curve for DA uptake in striatal synaptosomes from hemizygous TH-Cre mice (blue triangles, n = 3) and WT littermates (black circles, n = 4) of triplicate determinations. There was no significant genotype difference in the maximal dopamine uptake capacity (Vmax) (Vmax WT = 33.4 ± 5.0 fmol/min/ug protein; Vmax TH-Cre = 26.8 ± 1.5 fmol/min/ug protein, P = 0 .33). D1R: D1-receptor, DA: dopamine, DAT: dopamine transporter, WT: wild-type
TH-Cre mice do not display differences in basal behavioural paradigms
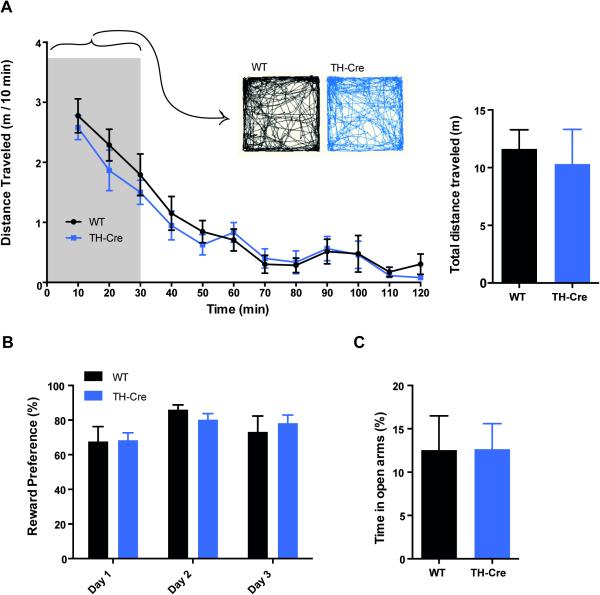
To assess potential behavioural aberrations in TH-Cre mice, basal behaviours influenced by DA signalling, including basal locomotor activity, reward preference test and elevated plus maze were studied (Nelson et al., 2012). Locomotor activity during initial exploration and habituation was assessed in an open field in TH-Cre and WT littermate controls (Fig. 3A). We found that basal locomotor activity throughout the session of 120 min was unaffected by genotype (factor genotype: F(1,13) = 0.40, P = 0.54 and factor genotype × time: F(11,143) = 0.44, P = 0.94). Both groups showed an equal and significant reduction in activity over time (factor time: F(11,143) = 37.59, P<0.0001) as analyzed by a two-way repeated-measure ANOVA. Furthermore, we observed no difference in the initial exploration patterns, as shown by representative activity tracks from TH-Cre and WT in the arena during the initial 30 min of the habituation to the open field.
Figure 3. Unaltered dopamine-related behaviours in hemizygous TH-Cre mice.
(A) Open field assessment of basal locomotor activity in TH-Cre (blue) and WT (black) mice. The mice were placed in an open field and video-traced for 120 min calculating the distance travelled in 10 min bins (left) and summarized with total distance travelled throughout the 120 min session (right). Distance travelled was unaffected by genotype (P = 0.54, n = 7-8) and both genotypes showed an equal and significant reduction in activity over time (P<0.0001) as analyzed by a two-way repeated-measure ANOVA. Representative tracks of activity within the initial 30 min of the session demonstrate similar exploration patterns between genotypes. B: There was no significant difference between genotypes in reward-based motivation in the RPT (P = 0.57, n = 9-13). C: Elevated Plus Maze test assessing the anxiolytic-like profile of the mice by the time spend in the open arm, showed no significant difference between genotypes (P = 0.98, n = 9-12).
The reward preference test (RPT) revealed no significant difference between genotypes in the percentage preference for a reward-associated substance over water (Fig. 3B, unpaired t-test; t19 = 0.58, P = 0.57, n = 9-13). Furthermore, as pharmacologic manipulations of DA signalling via D1- and D2-R has been shown to modulate behaviour in the elevated plus maze (EPM) (Rodgers et al., 1994), we tested the TH-Cre mice in the EPM to detect possible adaptations affecting DA D1-/D2-R signalling. In the EPM, TH-Cre mice did not differ in percentage time spent in open arms (Fig. 3C, t18 = 0.026, P = 0.98, n = 9-12). Furthermore, the EPM showed no difference in latency to first enter open arms (t19 = 0.14, P = 0.89, n = 9-13) or number of entries into the open arms (t19 = 0.60, P = 0.55, n = 9-13), nor did they vary in their number of attempted stretch-attended postures (SAPs) (t19 = 0.34, P = 0.74, n = 9-13) (data not shown).
Discussion
Cre-driver lines are extensively used to inactivate or induce expression of a protein of interest in a cell-specific manner and thereby target distinct neuronal populations. Identification and targeting of DA neurons have relied on the DAT or TH-promoter, but the lack of phenotypic characterization of these Cre-driver lines may hamper interpretation of the obtained results. Our study demonstrates preserved DA homeostasis with unaltered levels of TH and DA concomitant with unchanged behaviour in hemizygous TH-Cre, which validate the DA system of this TH-Cre strain.
Biochemical and behavioural assessment validate the DA system of hemizygous TH-Cre mice
Our investigation of hemizygous TH-Cre mice was focused on DA homeostasis and basal DA-related behaviour as potential aberrant phenotypes accompanying the expression of Cre under the TH-promoter. Cre-driver lines have previously been reported to influence expression levels of endogenous proteins. Knock-in mice expressing Cre under control of the DAT promoter has shown a gene-dose dependent reduction of DAT levels, although immunohistochemistry showed that DAT expression and Cre activity were localized to the same neurons in ventral midbrain. Homozygous mutants showed significant loss of DAT expression compared to WT controls (47 %) and this was furthermore associated with elevated levels of prodynorphin suggesting altered DAergic neurotransmission. Authors proposed that insertion of IRES-Cre sequence in these mice interferes with DAT expression (Backman et al., 2006). In contrast, a knock-in mouse expressing Cre under the 3'untranslated region of TH by use of IRES showed successful Cre expression to catecholaminergic neurons without altering TH-levels (Lindeberg et al., 2004). Here, we show that also a present commonly used hemizygous TH-Cre mice were characterized by unaltered expression of TH as well as phosphorylation of TH at serine position-40 in midbrain and dStr (Fig. 1A + B). In accordance, no adaptive changes to sustain DA transmission were observed reflected by unchanged DA levels as well as DA turnover in striatum of TH-Cre mice (Fig. 1C + D). Furthermore, DAergic neurotransmission seems to be unaffected in hemizygous TH-Cre mice showing unaltered levels of the postsynaptic D1R (Fig. 2A) and the presynaptic DAT (Fig. 2B) showing preserved DA uptake in striatal synaptosomes (Fig. 2C). Importantly though, 5-HT levels in the striatal compartment in our study is different from previous investigations but this discrepancy can be explained by experimental differences. Firstly, we have dissected two parts of the striatum including NAc and dStr which might account for the difference compared to previous reports analyzing entire striatum (Jones et al., 1998; Van Dam et al., 2005). Entire striatal measurements would have shown lower 5-HT levels since our levels in NAc are one order of magnitude lower compared to dStr. Secondly, we have analysed our striatal tissue soon after mice sacrifice which might be different from procedures in (Jones et al., 1998; Van Dam et al., 2005). We have experimental data suggesting that 5-HT is rapidly degraded even below −80°C storage (unpublished observations). For this reason, we carry out the experimental procedure soon after tissue dissection to avoid potential degradation that might compromise the results.
Phenotypic characterization of BAC transgenic lines has revealed aberrant behavioural traits attributed to transgene-induced biochemical alterations. For instance, elevated DA D2-receptor (D2R) mRNA and protein levels were observed in homozygous D2R-eGFP, accompanied by locomotor hyperactivity, impaired locomotor response to cocaine and behavioural sensitization when compared to WT controls (Kramer et al., 2011). Hyperlocomotion was also demonstrated in hemizygous D2R-eGFP mice (Ade et al., 2011). Contrary, Nelson and colleagues showed that hemizygous D2R-eGFP and D1R-eGFP mice were indistinguishable from WT littermates when investigating open field behaviour, acute response to cocaine, locomotor sensitization, active avoidance learning and conditioned place preference (Nelson et al., 2012). Thus, it is evident that transgenic mouse lines with expression of Cre or fluorescent reporters should be thoroughly characterized before being used as tools in neuroscience.
Our characterization of the commonly used TH-Cre strain available from Jacksons Laboratory (Savitt et al., 2005) revealed no biochemical alterations of DA homeostasis (Fig. 1 + 2). Accordingly, phenotypic behavioural assessment showed that TH-Cre is indistinguishable from WT littermates (Fig. 3). Basal locomotor activity was assessed in an open field with no difference between TH-Cre and WT controls (Fig. 3A) in relation to initial novelty exploration or habituation to the arena. Further DA-related behavioural paradigms were assessed (Fig. 3B + C); the RPT was used to determine motivation for a reward-associated substance (Humby et al., 2005) and the EPM used as a conflict-based anxiety model by assessing rodents’ aversion to open spaces (Lister, 1987). This basal behavioural investigation of the TH-Cre line revealed no differences between hemizygous TH-Cre and WT controls, which supports a functional DAergic system of the TH-Cre.
Our data further suggest that the hemizygous TH-Cre mouse strain does not suffer from Cre-induced toxicity as previously observed for different Cre-driver mouse lines (Forni et al., 2006). Neither does this TH-Cre mouse seem to be affected by Cre-induced abnormalities in targeted neurons. The TH-Cre did not display critical endogenous differences in DAergic homeostasis or neurotransmission, affecting behaviour as reported for other transgenic strains targeting neurons of the DA system (Backman et al., 2006; Kramer et al., 2011). However, it is unknown from our studies whether application of the TH-Cre line to target the expression of adeno-associated virus (AAV)-induced genes (such as optogenetic or chemogenetic proteins) could affect the neurons and possibly infer with DA homeostasis.
Considerations when using the TH-Cre to study the DA system
A recent immunohistochemical investigation of the TH-Cre revealed substantial ectopic expression of Cre recombinase in non-DAergic neurons in ventral midbrain that potentially might confound midbrain circuitry analysis (Lammel et al., 2015). It was suggested that different Cre-driver lines target overlapping but not identical ventral tegmental area cell populations (Stuber et al., 2015). Indeed, this should be taken into account when using the TH-Cre mouse since it clearly emphasizes cellular heterogeneity of the ventral midbrain, supporting previous observations (Lammel et al., 2008; Apuschkin et al., 2015). The ventral midbrain is composed of different cell types that are genetically diverse and have been implicated in depression-related behaviour, reward and aversion as well as learning and motivation (Lammel et al., 2012; Tye & Deisseroth, 2012; Chaudhury et al., 2013; Stamatakis et al., 2013; Ilango et al., 2014).
Transgenic mouse lines targeting midbrain DAergic cell populations have been utilized to confer selectivity of transgenes to distinct cell populations but the degree of ectopic labelling has been a recent concern using these reporter lines. This hemizygous TH-Cre line, which is indistinguishable from WT in the tested parameters, is suitable for studying the DA system as long as one is aware of the possible Cre expression in non-canonical DA neurons when interpreting the results. The emerging view that neurons in ventral midbrain represent heterogeneous cell populations has brought to attention that the definition of a DAergic phenotype is complex and has implications for using single gene Cre-driver lines to study particular neuronal pathways. The development of an expanding transgenic and viral toolbox in recent years offers great variety of approaches for cell- and projection-specific targeting to limit undesired off-target effects. Projection-oriented targeting has been achieved by the canine adenovirus (CAV)-Cre approach (Carter et al., 2013; Boender et al., 2014; Gompf et al., 2015), suitable for targeting more isolated cell populations. Considering the heterogeneity of VTA DA neurons, an intersectional viral-genetic approach to target neuronal populations based on cell type and projection pattern (Schwarz et al., 2015) might be required to fully decipher the complexity and role of the different DA neurons.
In general, Cre-driver mouse lines should be carefully characterized, not only regarding selectivity and penetrance of transgene expression but also in terms of phenotypic abnormalities. There is no doubt that Cre-driver mouse lines represent valid tools to dissect neuronal circuitry in a heterogeneous nervous system, but implementation of TH-Cre mice for the study of the DA system must take into consideration the complex DA heterogeneity to make plausible conjectures and to avoid misinterpretations. Careful validation of Cre-driver lines accompanied by the expanding toolbox of AAV and CAV will allow well-designed experiments to decipher more complex neural processes.
Acknowledgements
This work was supported by the UCPH 2016 Program of Excellence (U.G., A.R., K.J.), the Lundbeck Foundation (M.R., C.F.) the Lundbeck Foundation Center for Biomembranes in Nanomedicine (U.G.), the National Institute of Health Grants P01 DA 12408 (U.G.), the Danish Council for independent Research – Medical Sciences (U.G.) and the Psychiatric Center Copenhagen Research foundation (D.D.) TH-Cre mice were kindly provided by professor Birgitte Holst at University of Copenhagen.
Abbreviations
- AAALAC
The Association for Assessment and Accreditation of Laboratory Animal Care International
- AAV
adeno-associated virus
- BAC
bacterial artificial chromosome
- CAV
canine adenovirus
- Cre
cre recombinase
- D1R
D1-receptor
- D2R
D2-receptor
- DA
dopamine
- DAergic
dopaminergic
- DAT
dopamine transporter
- DOPAC
dihydroxyphenylacetic acid
- dStr
dorsal striatum
- eGFP
enhanced green fluorescent protein
- EPM
elevated plus maze
- GENSAT
gene expression nervous system atlas
- HPLC
high-performance liquid chromatography
- IRES
internal ribosome entry site
- KI
knock-in
- NAc
nucleus accumbens
- NA
norepinephrine
- rpm
rounds per minute
- RPT
reward preference test
- SDS
sodium-dodecyl-sulphate
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- WT
wild-type
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
5-hydroxytryptamine
References
- Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons. Front Syst Neurosci. 2011;5:32. doi: 10.3389/fnsys.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apuschkin M, Stilling S, Rahbek-Clemmensen T, Sorensen G, Fortin G, Herborg Hansen F, Eriksen J, Trudeau LE, Egerod K, Gether U, Rickhag M. A novel dopamine transporter transgenic mouse line for identification and purification of midbrain dopaminergic neurons reveals midbrain heterogeneity. Eur J Neurosci. 2015;42:2438–2454. doi: 10.1111/ejn.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MC, van der Plasse G, Adan RA. Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS One. 2014;9:e95392. doi: 10.1371/journal.pone.0095392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AE, Marshall CE, Yang M, Suon S, Iacovitti L. Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture. Mol Cell Neurosci. 2005;30:601–610. [PubMed] [Google Scholar]
- Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastru W, Sala V, Betz UA, Muzzi P, Martinuzzi D, Vercelli AE, Kageyama R, Ponzetto C. High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci. 2006;26:9593–9602. doi: 10.1523/JNEUROSCI.2815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gofflot F, Wendling O, Chartoire N, Birling MC, Warot X, Auwerx J. Characterization and Validation of Cre-Driver Mouse Lines. Current protocols in mouse biology. 2011;1:1–15. doi: 10.1002/9780470942390.mo100103. [DOI] [PubMed] [Google Scholar]
- Gompf HS, Budygin EA, Fuller PM, Bass CE. Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Frontiers in behavioral neuroscience. 2015;9:152. doi: 10.3389/fnbeh.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson L, Dawson G. Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr Protoc Neurosci. 2005 doi: 10.1002/0471142301.ns0805hs31. Chapter 8, Unit 8 5H. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C, Bourque MJ, Fortin GD, St-Gelais F, Okano H, Kobayashi K, Trudeau LE. Use of TH-EGFP transgenic mice as a source of identified dopaminergic neurons for physiological studies in postnatal cell culture. J Neurosci Methods. 2005;146:1–12. doi: 10.1016/j.jneumeth.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaesermann HP. Stretched attend posture, a non-social form of ambivalence, is sensitive to a conflict-reducing drug action. Psychopharmacology (Berl) 1986;89:31–37. doi: 10.1007/BF00175185. [DOI] [PubMed] [Google Scholar]
- Kelly BB, Hedlund E, Kim C, Ishiguro H, Isacson O, Chikaraishi DM, Kim KS, Feng G. A tyrosine hydroxylase-yellow fluorescent protein knock-in reporter system labeling dopaminergic neurons reveals potential regulatory role for the first intron of the rodent tyrosine hydroxylase gene. Neuroscience. 2006;142:343–354. doi: 10.1016/j.neuroscience.2006.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock J, Goldstein M, Hokfelt T, Fisone G. Regulation of tyrosine hydroxylase activity and phosphorylation at Ser(19) and Ser(40) via activation of glutamate NMDA receptors in rat striatum. J Neurochem. 2000;74:2470–2477. doi: 10.1046/j.1471-4159.2000.0742470.x. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, Yu J, Wang SS. The problem of passenger genes in transgenic mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2100–2103. doi: 10.1161/ATVBAHA.107.147918. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Hang GB, Grueter BA, Pascoli V, Luscher C, Malenka RC, Kreitzer AC. A comparison of striatal-dependent behaviors in wild-type and hemizygous Drd1a and Drd2 BAC transgenic mice. J Neurosci. 2012;32:9119–9123. doi: 10.1523/JNEUROSCI.0224-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Nikulina EM, Cole JC. Dopamine D1 and D2 receptor ligands modulate the behaviour of mice in the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:985–995. doi: 10.1016/0091-3057(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci. 2005;25:6721–6728. doi: 10.1523/JNEUROSCI.0760-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Stamatakis AM, Kantak PA. Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry. Neuron. 2015;85:439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Van Dam D, Marescau B, Cremers T, Mulder J, Engelborghs S, De Deyn PP. Regional distribution of biogenic amines, amino acids and cholinergic markers in the CNS of the C57BL/6 strain. Amino acids. 2005;28:377–387. doi: 10.1007/s00726-005-0208-7. [DOI] [PubMed] [Google Scholar]