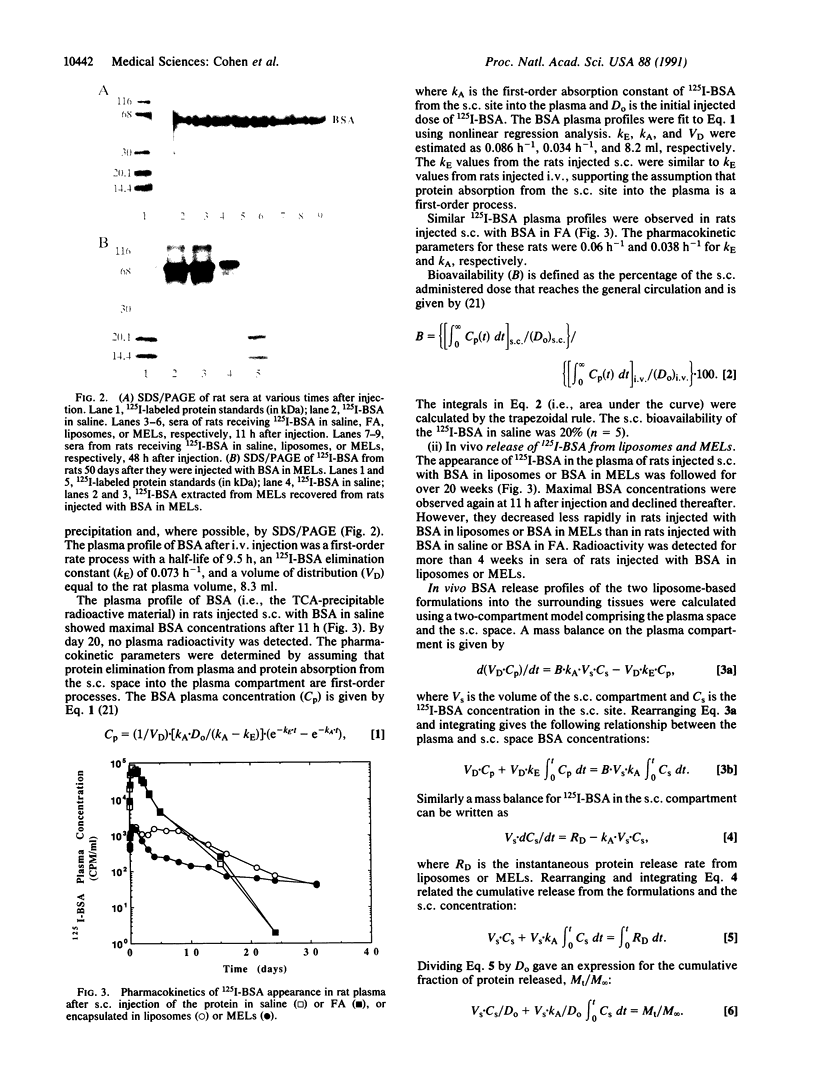
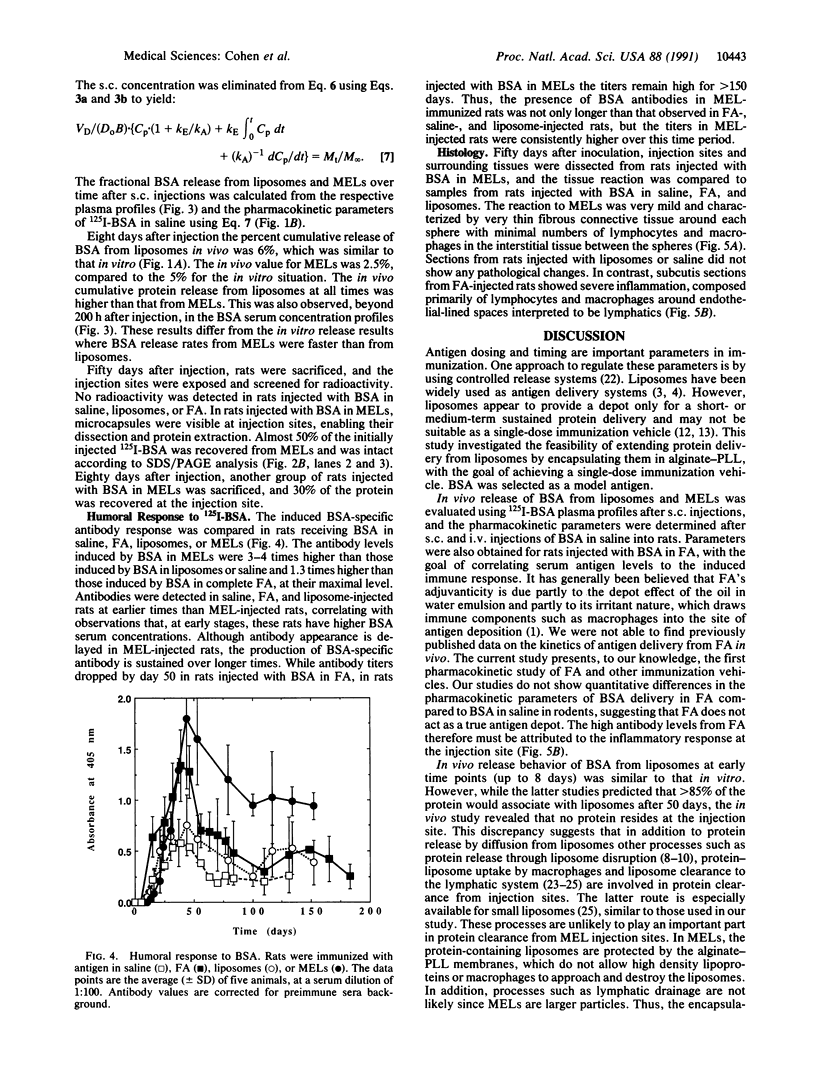
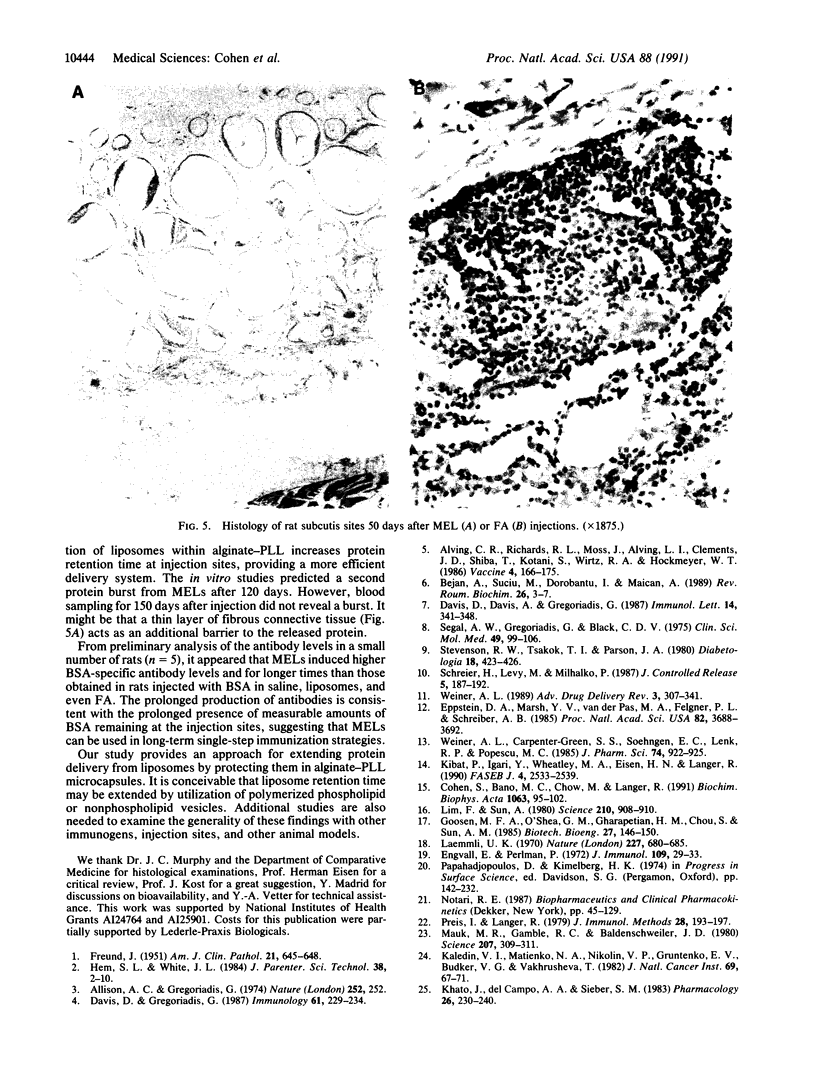
Abstract
The feasibility of creating a s.c. depot for sustained protein delivery with the goal of enhancing antigen immunogenicity was investigated. The depot was designed as antigen-laden liposomes of hydrogenated egg phosphatidylcholine and cholesterol (1:1 molar ratio) encapsulated in alginate-poly(L-lysine) microcapsules and evaluated using iodinated bovine serum albumin (BSA) as a model antigen. The in vivo release behavior of the liposomes and microencapsulated liposomes (MELs) was evaluated from the BSA serum concentration profiles after s.c. injection into rats and the pharmacokinetic parameters of 125I-labeled BSA appearance after s.c. or i.v. injections of BSA in saline. Maximal BSA concentrations were detected 11 h after s.c. injection in all rats. The BSA serum concentrations decreased rapidly in rats injected with BSA in saline or Freund's adjuvant and less rapidly in rats injected with BSA in liposomes or MELs. Four to 5 weeks after injection, BSA-associated radioactivity was detected only in sera of rats injected with BSA in liposomes or MELs. Fifty days after injection, 50% of the originally injected BSA was recovered form the s.c. sites of rats injected with BSA in MELs; no radioactivity was recovered from the other three groups of rats. The antigen-reactive antibody levels induced in rats immunized with BSA in MELs were 2- to 3-fold higher than those obtained in rats immunized with BSA in liposomes, saline, or Freund's adjuvant. More significantly, high antibody levels were maintained for more than 150 days after a single injection of BSA in MELs, suggesting that MELs can serve as a long-term single-dose immunization vehicle.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Richards R. L., Moss J., Alving L. I., Clements J. D., Shiba T., Kotani S., Wirtz R. A., Hockmeyer W. T. Effectiveness of liposomes as potential carriers of vaccines: applications to cholera toxin and human malaria sporozoite antigen. Vaccine. 1986 Sep;4(3):166–172. doi: 10.1016/0264-410x(86)90005-8. [DOI] [PubMed] [Google Scholar]
- Cohen S., Bañ M. C., Chow M., Langer R. Lipid-alginate interactions render changes in phospholipid bilayer permeability. Biochim Biophys Acta. 1991 Mar 18;1063(1):95–102. doi: 10.1016/0005-2736(91)90358-f. [DOI] [PubMed] [Google Scholar]
- Davis D., Davies A., Gregoriadis G. Liposomes as adjuvants with immunopurified tetanus toxoid: the immune response. Immunol Lett. 1987 Apr;14(4):341–348. doi: 10.1016/0165-2478(87)90016-2. [DOI] [PubMed] [Google Scholar]
- Davis D., Gregoriadis G. Liposomes as adjuvants with immunopurified tetanus toxoid: influence of liposomal characteristics. Immunology. 1987 Jun;61(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Eppstein D. A., Marsh Y. V., van der Pas M., Felgner P. L., Schreiber A. B. Biological activity of liposome-encapsulated murine interferon gamma is mediated by a cell membrane receptor. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3688–3692. doi: 10.1073/pnas.82.11.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND J. The effect of paraffin oil and mycobacteria on antibody formation and sensitization; a review. Am J Clin Pathol. 1951 Jul;21(7):645–656. doi: 10.1093/ajcp/21.7.645. [DOI] [PubMed] [Google Scholar]
- Hem S. L., White J. L. Characterization of aluminum hydroxide for use as an adjuvant in parenteral vaccines. J Parenter Sci Technol. 1984 Jan-Feb;38(1):2–10. [PubMed] [Google Scholar]
- Kaledin V. I., Matienko N. A., Nikolin V. P., Gruntenko Y. V., Budker V. G., Vakhrusheva T. E. Subcutaneously injected radiolabeled liposomes: transport to the lymph nodes in mice. J Natl Cancer Inst. 1982 Jul;69(1):67–71. [PubMed] [Google Scholar]
- Khato J., del Campo A. A., Sieber S. M. Carrier activity of sonicated small liposomes containing melphalan to regional lymph nodes of rats. Pharmacology. 1983;26(4):230–240. doi: 10.1159/000137806. [DOI] [PubMed] [Google Scholar]
- Kibat P. G., Igari Y., Wheatley M. A., Eisen H. N., Langer R. Enzymatically activated microencapsulated liposomes can provide pulsatile drug release. FASEB J. 1990 May;4(8):2533–2539. doi: 10.1096/fasebj.4.8.2110539. [DOI] [PubMed] [Google Scholar]
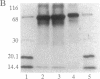
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim F., Sun A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980 Nov 21;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Gamble R. C., Baldeschwieler J. D. Vesicle targeting: timed release and specificity for leukocytes in mice by subcutaneous injection. Science. 1980 Jan 18;207(4428):309–311. doi: 10.1126/science.7350660. [DOI] [PubMed] [Google Scholar]
- Preis I., Langer R. S. A single-step immunization by sustained antigen release. J Immunol Methods. 1979;28(1-2):193–197. doi: 10.1016/0022-1759(79)90341-7. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Gregoriadis G., Black C. D. Liposomes as vehicles for the local release of drugs. Clin Sci Mol Med. 1975 Aug;49(2):99–106. doi: 10.1042/cs0490099. [DOI] [PubMed] [Google Scholar]
- Stevenson R. W., Tsakok T. I., Parsons J. A. Matched glucose responses to insulin administered subcutaneously and intravenously. Evidence for subcutaneous inactivation of insulin. Diabetologia. 1980 May;18(5):423–426. doi: 10.1007/BF00276825. [DOI] [PubMed] [Google Scholar]
- Weiner A. L., Carpenter-Green S. S., Soehngen E. C., Lenk R. P., Popescu M. C. Liposome-collagen gel matrix: a novel sustained drug delivery system. J Pharm Sci. 1985 Sep;74(9):922–925. doi: 10.1002/jps.2600740903. [DOI] [PubMed] [Google Scholar]