Abstract
Introduction:
The phantom limb pain (PLP) and phantom limb sensation (PLS) are very common among amputated cancer patients, and they lead to considerable morbidity. In spite of this, there is a lack of epidemiological data of this phenomenon among the Asian population. This study was done to provide the data from Indian population.
Methods:
The prevalence of PLP, stump pain (SP), and PLS was prospectively analyzed from the amputated cancer patients over a period of 2 years in Dr. B.R.A. Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi. The risk factors and the impact of phantom phenomenon on patients were also noted.
Results:
The prevalence of PLP was 41% at 3 and 12 months and 45.3% at 6 months, whereas that of SP and PLS was 14.4% and 71.2% at 3 months, 18.75% and 37.1% at 6 months, 15.8% and 32.4% at 12 months, respectively. There was higher prevalence of PLP and PLS among the patients with history of preamputation pain, smoking with proximal level of amputation, receiving general anesthesia, receiving intravenous (IV) opioid postoperative analgesia, and developing neuroma or infection.
Conclusion:
The prevalence of PLP and PLS was higher among the cancer amputees as compared to SP, and a few risk factors responsible for their higher prevalence were found in our study. The PLP and PLS lead to considerable morbidity in terms of sleep disturbance and depression.
Keywords: Amputation, cancer, neuropathic pain, phantom limb pain, phantom limb phenomenon, phantom limb sensation, stump pain
INTRODUCTION
Phantom limb phenomenon was first described by a French military surgeon Ambroise Pare.[1] The phantom phenomenon consists of three distinct elements:
Phantom limb pain (PLP): Painful sensations that are being referred to the amputated limb
Phantom limb sensation (PLS): Sensations other than pain that are being referred to the amputated limb
Stump pain (SP): Pain localized to the amputated stump.
The PLP has an incidence of 49%–88%, with more recent studies showing a higher incidence.[2] The differences in the incidence may be due to the differences in the study design, population studied, methodology used, definitions used for PLP, different study periods and time of assessment, etc., Most of the study data are from the Caucasian populations, with only a few studies being from Asian and African population.[3,4,5]
The mechanisms for phantom phenomenon are complex and involve various elements in the somatic pain generators, peripheral nervous system, spinal cord, and brain.[6,7,8,9,10,11,12,13] The phantom limb pain episodes vary in intensity, frequency, duration, location, character, etc.[3,14,15,16,17,18,19] It may be aggravated and relieved by a variety of factors.[15,16,17,19,20,21,22,23] A strong relationship between phantom limb pain and preoperative pain has been shown in a few studies. Similarly, a protective role of regional anesthesia has also been emphasized.[24]
We undertook this prospective, observational study among cancer amputees at a tertiary care cancer center to quantify the prevalence of PLP, SP, and PLS. As far as we are aware, this is the first attempt to quantify the prevalence in Indian patient population. In addition, their association with various risk factors, to characterize the PLP, SP, and PLS, and to assess their impact on patients quality of life or functionality.
METHODS
This was a prospective, observational study carried out over a period of 2 years from November 2009 to November 2011 at Dr. B.R.A. Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India, after obtaining approval from the Institutional Ethical Committee. After obtaining informed consent, 160 patients over the age of 18 years undergoing amputation were consecutively enrolled into the study. Patients suffering from multiple malignancy, metastatic cancer, diabetes mellitus, psychiatric illness, neurological disorder were excluded from the analysis.
Definitions
Phantom limb pain (PLP) was defined as painful sensation arising out of the amputated limb
Nonpainful sensation arising out of amputated limb was referred to as PLS
SP was defined as the pain arising out of amputated stump.
Mechanisms of PLP and phantom limb sensation
The mechanism responsible for PLP and PLS is still debatable, however a lot of theories had been given. Following amputation, there may be formation of neuroma showing abnormal spontaneous activity, and on mechanical and chemical stimulation, which is thought to be due to upregulation of sodium channels. Furthermore, other factors though to have an influence on the PLP are decreased threshold for PLP, increased c-fiber activity, inverse relationship between pressure pain threshold and phantom limb pain intensity, abnormal activity of dorsal root ganglion, and so on.[12,13] Sympathetic nervous system also plays a role in maintaining PLP.[10,11] Further, there is spinal plasticity, i.e., increase in the excitability of spinal neurons, more accessibility of Aδ- and c-fibers to other pathways.[9] N-methyl-D-aspartate receptor systems are also believed to have a role in “wind-up” phenomenon seen in PLP.[4] Furthermore, spinal and cerebral reorganization occurs and there is a relationship between degree of reorganization and pain.[6,7]
Data collection
Suitable patients were assessed by an independent physician who was not a part of the team involved in the study.
Patients were assessed preoperatively, intraoperatively, and postoperatively at 3, 6, and 12 months.
Preoperatively, the subjects were assessed for presence of preoperative limb pain, for their concerns regarding the disease and surgical concerns regarding amputation using a 5-point Likert scale as 1 = strongly agree, 2 = agree, 3 = neutral, 4 = disagree, and 5 = strongly disagree. Scores 1 and 2 are taken as a positive response of having disease and surgical concerns. Pain was assessed before surgery and after surgery by means of an 11-point numerical rating scale (NRS); 0 for no pain and 10 for worst imaginable pain. The subjects were also explained about the potential for future development of PLP, SP, and PLS and were requested to report them.
Perioperatively, development of postoperative pain, use of regional analgesia, postoperative epidural analgesia, and development of infection, hematoma, and neuroma were noted.
Postoperatively, PLP, SP, and PLS were assessed by means of a questionnaire. The intensity of PLP and SP was evaluated using the 11-point NRS. The frequency, duration, character, aggravating and relieving factors, analgesic requirement, and other treatment factors were also noted.
The risk factors thought to be associated with PLP, SP, and PLS were also studied, such as age, sex, preoperative pain, postoperative infections, seroma production, radiotherapy, chemotherapy, and site and level of amputation. The patients were assessed for depression, sleep disturbance, and anxiolytic intake. They were also assessed for the use of prosthesis. The implications of PLP, SP, and PLS on functional state of the patient were also assessed using the Karnofsky performance status score.
The statistical analysis
The statistical analysis was performed using Statistical Package for the Social Sciences SPSS (version 16, SPSS Inc., Chicago, Illinois, USA) for Windows. The variables were expressed as mean and standard deviation. The association between PLP, SP, and PLS with the various risk factors was analyzed by Chi-square test or Fisher's exact test. The results were later verified by Mann–Whitney U-test due to skewness of the data and smaller number of patients experiencing PLP, SP, and PLS. A Bonferroni–Holm analysis was also performed at the end as there were multiple comparisons for PLP and PLS. For PLP, SP, and PLS, relative risks with odds ratios (ORs) with 95% confidence interval were calculated. Level of significance was set at P < 0.05. Regression analysis is performed for each risk factor to analyze the risk for development of PLP, SP, and PLS.
RESULTS
In our study, 160 patients were recruited and 139 patients completed the study. Three patients had died during the follow-up, four patients developed metastasis to other organs, three patients opted to move out of the study, four patients had developed pain at other sites, and seven patients were lost in follow-up.
Demographic factors
Age
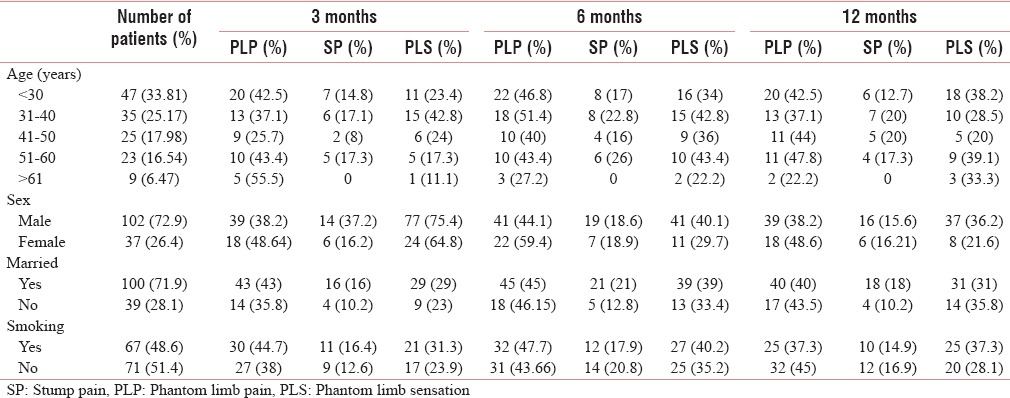
The mean age of the patients was 38.23 ± 1.54 years The prevalence of PLP, SP and PLS among various age groups are well described in Table 1.
Table 1.
Various demographic factors and the prevalence of phantom limb pain, sensation, and stump in relation to the demographic factors
Sex
More than two-third of the patients were male (71.9%) [Table 1]. The risk for development of PLP and SP was low whereas for PLS was higher in males [Tables 2–4].
Table 2.
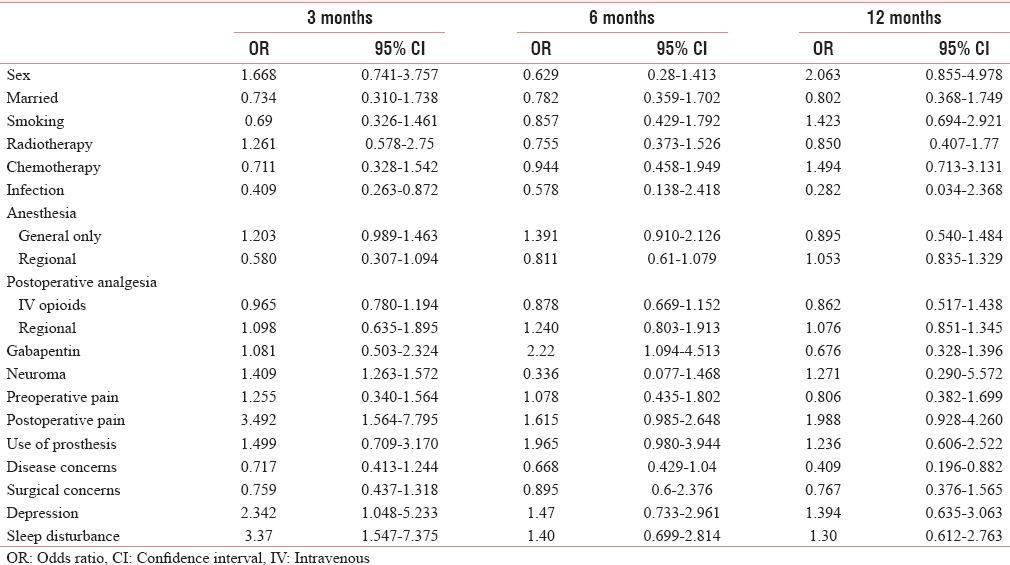
Odds ratio for phantom limb pain
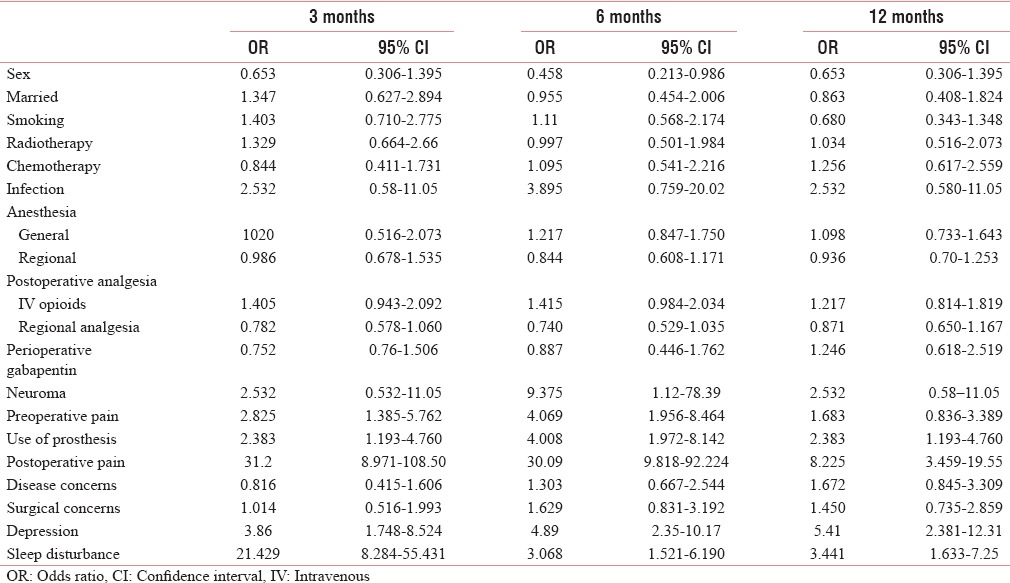
Table 4.
Odds ratio of various factors for phantom limb sensations
Smoking
Smoking was present in almost equal number of patients (48.6% were smoking and 51.4 were nonsmoking) [Table 1]. The risk for PLP with smoking was higher at 3 and 6 months, for SP was high at 3 months, and for development of PLS was high at 12 months [Tables 2–4].
Marriage
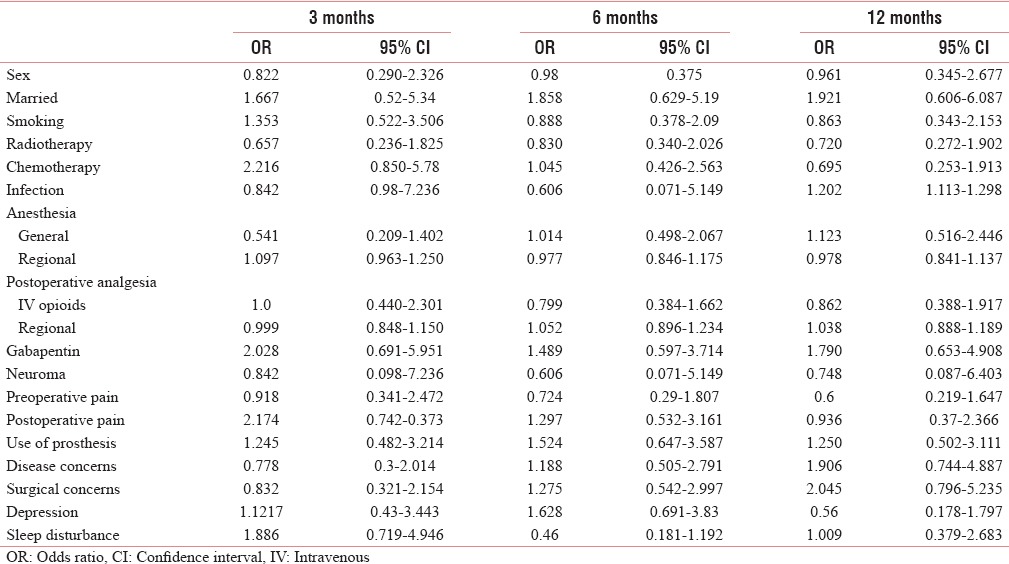
In our study, 71.9% of patients were married. Among the married patients, the risk of development of PLP was high at 3 months and of that of SP was higher at throughout the period of study [Tables 2 and 3].
Table 3.
Odds ratio of various factors for stump pain
Level of amputation
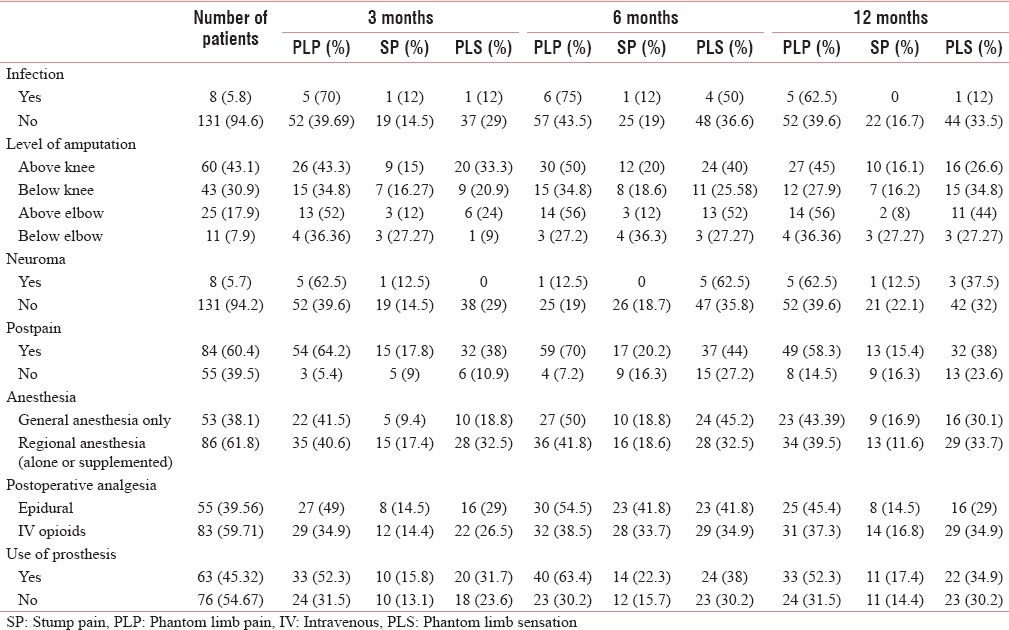
In our study, most of the amputations were at the level of above knee (43.1%), followed by below knee (34.88%), above elbow (17.9%), and below elbow (7.9%). The proximal level of amputation had a higher prevalence of PLP than distal level of amputation [Table 5].
Table 5.
Various intra-and post-operative fact and the prevalence of phantom limb pain, stump pain and phantom limb sensation in relation to the intra-and post-operative factors
Preoperative factors
Preoperative pain
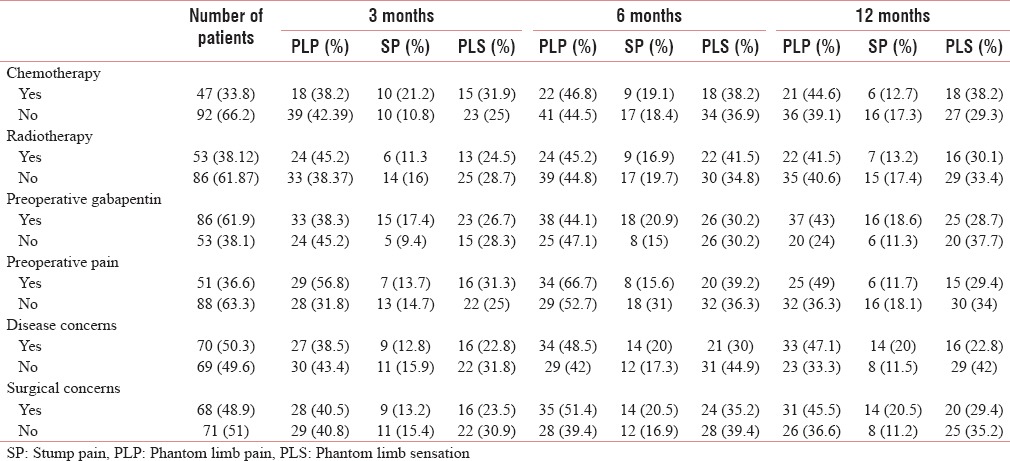
Preoperative pain has been found in 36.67% patients. There was higher prevalence of PLP and PLS among the patients with preoperative pain, with a higher risk of the development of both with preoperative pain [Tables 1, 2 and 4], whereas there were low prevalence and low risk of development of SP with preoperative pain [Tables 6 and 3].
Table 6.
Various preoperative factors and the prevalence of phantom limb pain, sensation, and stump in relation to the preoperative factors
Preoperative gabapentin
Preoperatively, 61.9% of patients had received gabapentin. The use of preoperative gabapentin did not have any significant impact on the prevalence of PLP and PLS, however there was a decrease in prevalence and risk of development of SP in those patients who had used it [Tables 6 and 3].
Disease and surgical concerns
Positive disease and surgical concerns were present in almost half of the patients, 50.35% and 48.92%, respectively. The prevalence and risk of developing PLP and SP with disease concern were high at 6 and 12 months, whereas the prevalence and risk for PLS were low [Tables 6 and 2–4].
With surgical concerns, the prevalence and risk of developing PLP and SP were higher at 6 and 12 months, on the other hand it did not have any significant impact on PLS [Tables 1 and 2–4].
Treatment-related factors
Chemotherapy
Chemotherapy was given to only 33.8% of patients. The prevalence and risk of developing PLP and PLS were higher at the end of the study, whereas the prevalence and risk of developing SP were high at the early part of the study [Tables 6 and 2–4].
Radiotherapy
Radiotherapy was given to 53% of patients. The prevalence and the risk of developing PLP were higher with radiotherapy at only in the early part of the study, whereas it has no impact on SP [Tables 6 and 2–4].
Intraoperative factors
Anesthetic technique
The general anesthesia alone was given in 53 patients (38.1%) and regional anesthesia (alone or supplemented to general anesthesia) was given in 86 patients (61.8%). With general anesthesia, the prevalence and risk of developing of PLP were high and that of SP were low, whereas the risk of developing PLS was high till 6 months [Tables 6 and 2–4].
Postoperative factors
Postoperative analgesia
Postoperative analgesia was provided with intravenous (IV) opioid in 83 (59.71%) patients, whereas 55 (39.5%) patients received regional analgesia. In patients receiving IV opioids, the prevalence of PLP and PLS was found to be high [Table 6]. The OR for developing PLP was higher with IV opioid as compared to those who received regional anesthesia [Table 2].
Neuroma
Neuroma occurred in about 8 (5.75%) patients. The prevalence and risks of developing PLP with neuroma were high. With neuroma, the prevalence and risks of developing PLS were high throughout the study period whereas that of SP were low [Tables 2–5].
Infection
Infection occurred in only eight patients. The prevalence and risk of developing PLP with infection were high whereas that of SP and PLS were low [Tables 2–5].
Postoperative severe pain
Postoperative severe pain (NRS >6) had occurred in 84 (60.43%) patients. The prevalence and the risk of developing PLP, SP, and PLS were higher with severe postoperative pain [Tables 2–5].
Use of prosthesis
The prosthesis was used by 63 (45.32%) patients and it had resulted in higher prevalence and risks of developing PLP, SP, and PLS [Tables 2–5].
Phantom limb pain
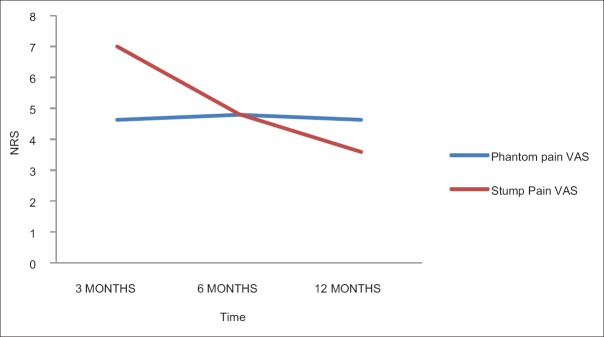
The prevalence and average intensity of the PLP remained almost same in the study [Table 7 and Figures 1 and 2].
Table 7.
The prevalence of phantom limb pain, stump pain, phantom limb sensation, depression, sleep disturbance along with visual analog scale of phantom limb pain, stump pain at 3, 6, and 12 months
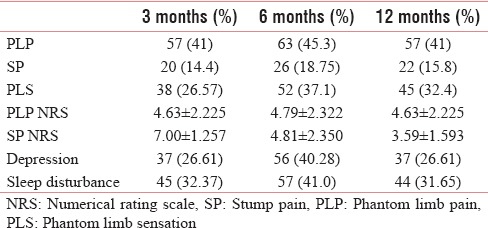
Figure 1.
The pain intensity in numerical rating scale of phantom and stump pain with time.
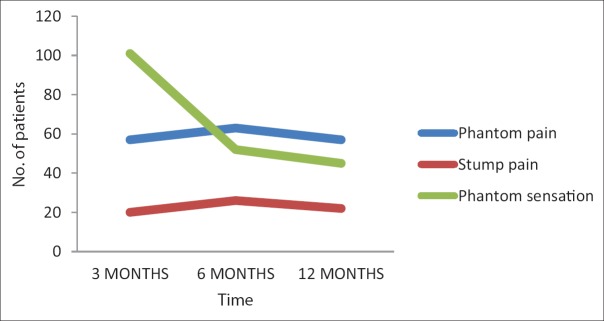
Figure 2.
The number of patients with phantom pain, stump pain and phantom sensation with time.
The time of onset, resolution, and increase and decrease of intensity of pain intensity are described in Table 8.
Table 8.
The onset, resolution, and increase and decrease in pain intensity of phantom limb pain with time

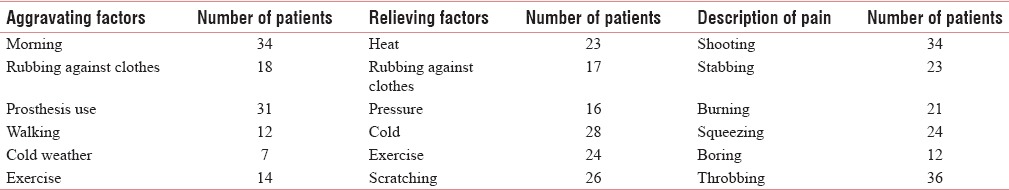
The PLP was aggravated and relieved by various factors which are well described in Table 9.
Table 9.
Characteristics factors for phantom limb pain
The frequency and duration of PLP are well described in Tables 10 and 11.
Table 10.
Frequency of phantom limb pain, stump pain, and phantom limb sensation during the various period
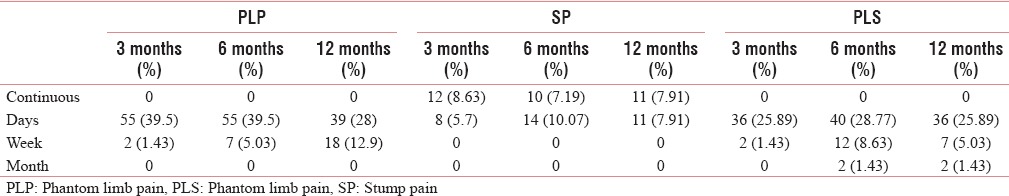
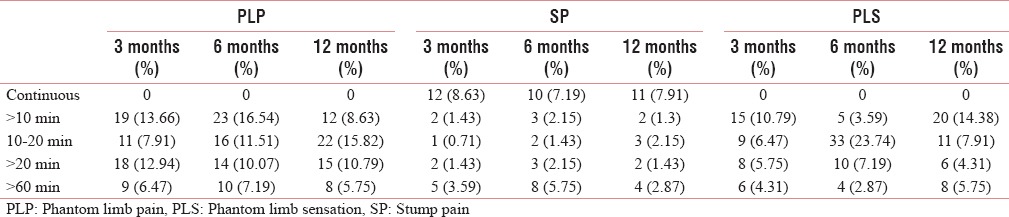
Table 11.
The duration of phantom limb pain, stump pain, and phantom limb sensation during the various period
In our study, only 23 patients had PLP in whole limb, but in them, there was gradual decrease in the area of the limb in about 18 patients and most of them felt that there was limb shortening.
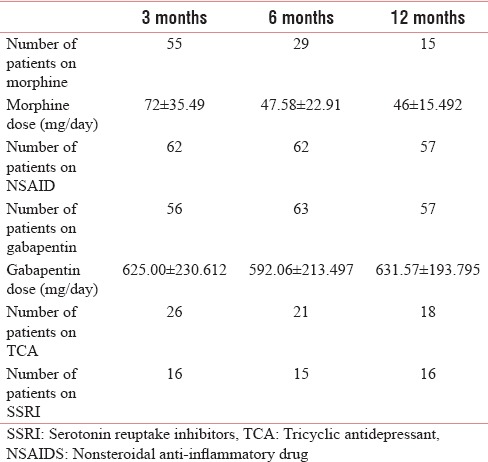
In our study, 56, 63, and 57 patients were taking anticonvulsants such as gabapentin, with nearly 40%–50% relief of pain. While about 55, 29, and 15 patients were on strong opioid at 3, 6, and 12 months with nearly 30%–40% relief (of them, in 14 patients in whom the opioid were started within a week got the maximum benefit of 60%–70%, while in rest of patients in whom it was started late got moderate benefit). The daily opioid dose of in oral morphine equivalent had decreased from 72 ± 35.49 mg/day to 47.58 ± 13.66mg/day and 46 ± 15.492 mg/day at 6 and 12 months, respectively. Tramadol was used in 21, 20, and 18 patients at 3, 6, and 12 months, with pain relief up to 30%–40%. The nonsteroidal anti-inflammatory drugs were taken by 62, 62, and 57 patients at 3, 6, and 12 months, respectively. In three patients with neuroma, stump revision was done [Table 12].
Table 12.
Number of patients on medications and their average dose
PLP with stump pain and phantom limb sensation
Among the patients with PLP, SP was present in 10, 12, and 8 patients, while PLS was present in 27 (47.36%), 33, and 23 patients at 3, 6, and 12 months, respectively. The OR for developing SP and PLS with PLP was high.
Stump pain
The prevalence of SP remained low and constant throughout the study period [Table 1 and Figure 2]. There was significant decrease in pain intensity (NRS) with time [Table 1 and Figure 1]. There were neuropathic features such as allodynia and hyperalgesia. The characteristics, frequency, and duration of SP are well described in Tables 10–12.
Phantom limb sensation
The prevalence, frequency, and duration are well described in Tables 1, 10, and 11 [Figure 2].
In about 61.7% patients, the PLS occurred in the distal part of the limb, while in about 17.3% patients, it occurred near the proximal part, and in the rest of the patients, it occurred in the whole limb.
PLS was observed in 47.3% and 52.3% patients with PLP, at 3, 12, and 6 months and the risk of developing PLS was very high among the patients with PLP.
The PLS may be kinetic (25 patients), kinesthetic (49 patients), or superadded sensation (12 patients). At the end of 12 months, about 32.37% of patients felt that gradually their sensation of limbs has become proximal (telescoping).
Psychological factors
Depression and sleep disturbance
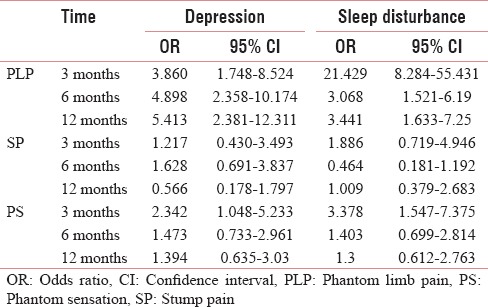
The prevalence and risk of developing PLP, SP, and PLS with depression are high. The sleep disturbance leads to higher prevalence of PLP and PLS, where the prevalence and risk of developing SP are low [Tables 13 and 14]. The risk of developing PLP and PLS with depression is found to be low [Tables 3, 4, 7].
Table 13.
The relationship between depression and sleep disturbance with phantom limb pain, stump pain, and phantom sensation
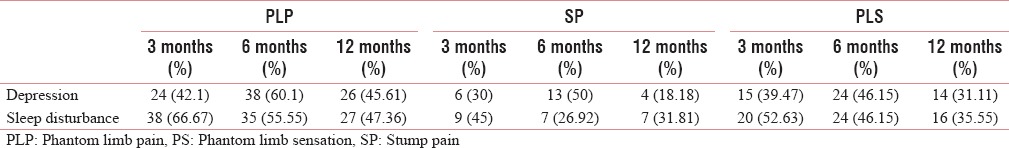
Table 14.
Risk of developing depression and sleep disturbance with phantom limb pain, stump pain, and phantom sensation
Performance status
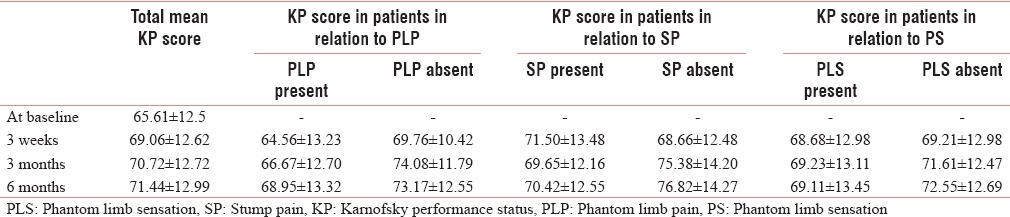
The performance status improves in cancer amputees over a period of 1 year and it is significantly worse in patients with both PLP and SP (P < 0.05) [Table 15].
Table 15.
Performance status (Karnofsky performance status score) at baseline and postoperative period and with the presence of phantom limb pain, stump pain, and phantom limb sensation
DISCUSSION
Prevalence of PLP
In our study, we had found a higher prevalence of PLP among the amputated cancer patients. Our prevalence was 41% at 3 and 12 months and 45.3% at 6 months. The few other studies done in cancer patients had also found higher prevalence of PLP in the range of 46.7%–76%.[25,26,27]
The previous studies have also shown very higher incidences (49%–88%) [Table 16].[2] However, in two recent studies done in Asian population in Singapore and African traumatic amputees had showed lower incidence of 25% and 32.5%, respectively.[3,4] There was PLP in 68% of the amputees (19 out of 28) in landmine accident survivors in Cambodia and Kurdistan.[5]
Table 16.
The incidence of phantom limb pain as found in various earlier studies
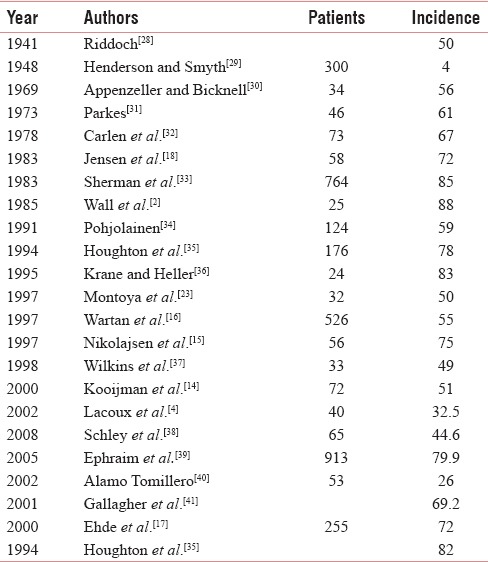
In our study, the prevalence remained almost static throughout the period of study, which was similar to earlier prospective studies.[15,16] Some earlier studies also found that the PLP improves with time and even disappears.[19,42]
There was increase in PLP in about 18 and 12 patients at 6 and 12 months. Interestingly, one previous case report found that the increase in PLP was consistent with local cancer recurrence.[43] However, in our study, we did not recorded the cancer stage at the time of increase in PLP.
Onset of PLP
In our study, PLP started as early as the next postoperative day in 33.34% of patients and within 1 week in 35% of patients. Various previous studies have also found early onset on PLP within a few days after surgery in most of the patients.[15,18,31,32,36]
Frequency of PLP
PLP was constant in nature in about six patients, whereas in rest of the patients, it occurred either as a few episodes per day or per week. Kooijman et al. also found that nine out of 37 PLP had constant PLP, whereas other had intermittent PLP occurring as a few episodes per day or week.[14] In an Asian study in Singapore, the PLP was constant in 8% and intermittent daily in 42% of patients.[3] Jensen et al. also found that PLP was constant in 47% of patients and intermittent daily in 35% of patients.[19] Two previous studies by Wartan et al. and Nikolajsen et al. also reported similar findings.[15,16,18]
Character of PLP and stump pain
The PLP and SP were found to be sharp, tingling, shooting, stabbing, throbbing, and aching in character; most of them have more than two types of pain, which is similar to previous studies by Ehde et al. and Jensen et al [Tables 9 and 17].[17,18]
Table 17.
The characteristics of stump pain

Aggravating and relieving factors
The PLP and SP were aggravated or relieved by various factors such as in the morning, by rubbing against clothes, by wearing prosthesis, exercise, walking, cold weather, application of heat, and pressure and scratching. Earlier studies have also found that rest, heat, a firm pressure applied to the stump, use of prosthesis and distraction, changes in weather, chronic problems with the prosthesis, mental stress, fatigue, intestinal and back problems, acute stump problems lead to increase in PLP.[15,16,17,19,20,21,22,23] Weiss et al. found that prosthesis use had decreased the development of PLP.[44]
Preoperative factors
There was high prevalence of PLP among the patients with preoperative pain, which was similar to earlier studies.[28,35,45] Interestingly, a few other studies also showed contradicting results.[2,14,29] The PLP was similar in character to preamputation pain in nearly more than 30% of patients.[19,46]
Patient with postoperative complications as in infection, neuromas have a higher risk of developing PLP throughout the period of the study, whereas these complications resulted in increased risk of development of SP and PLS occasionally. There was a strong association between neuroma and PLP at 3 and 12 months, not at the 6 months. However, in an earlier study by Lacoux et al., they did not find any association between them.[4]
Level of amputation and PLP
There was increased prevalence of PLP among the patients with proximal level of amputations as compared to the distal ones. Previous studies had shown a very high prevalence of 68%–88% with hemipelvectomy and 40%–88% with hip disarticulation.[2,47]
Anesthesia and PLP
The risk of developing PLP and SP was greater with general anesthesia. Risk of developing PLS was higher with general anesthesia till 6 months, and after that, risk was greater with regional anesthesia. Interestingly in a recent study, Sahin et al. also found that anesthetic technique had no effect in the development of PLP, SP, and PLS.[24] In addition, a few other previous studies could not come find any relationship between PLP and epidural analgesia.[48,49,50,51]
Duration of PLP
The duration of PLP was <1 h in most of the patients (about 85% throughout the study period). However, in one earlier study by Sherman et al., they found that the duration of PLP was <1 h in only 20% of patients.[19]
Prevalence of stump pain
The prevalence of SP was lower and remained almost static (14.4%, 18.75%, and 15.8% at 3, 6, and 12 months, respectively) whereas previous studies have reported a higher prevalence between 32% and 93% [Table 18].[16,39,41,52,53,54,55] Interestingly, in one African study, the traumatic amputees had incidence of up to 100%.[4] However, another two recent study showed little higher incidence of SP of up to 39% and 61.5% [Table 18].[3,38] With the SP, there was higher occurrence of neuropathic features such as allodynia and hyperalgesia in our study, which was similar to previous studies.[14,15,18,21]
Table 18.
Epidemiology of stump pain and phantom limb sensation among various previous studies
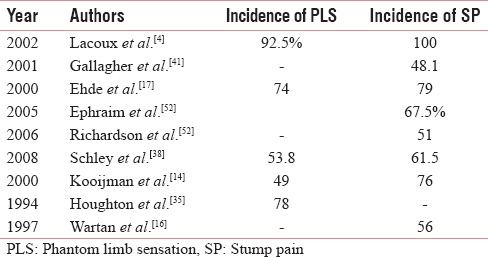
Prevalence of phantom limb sensation
PLS was very high at 3 months with a prevalence of 71.2%, which gradually decreased to 37.1% and 32.4% at 6 and 12 months, respectively. Previous studies have reported a prevalence of 63%–90% [Table 18].[3,21] The PLS was found to be very high among the African traumatic amputees (92.5%) and another Asian study had also reported a high incidence of 63% [Table 18].[3,4] Another recent study reported a similar incidence of 53.8% [Table 18].[38]
In our study, there was no change in the intensity of the PLP with time, whereas the intensity has decreased significantly for SP after 3 months.
Relationship of PLP with stump pain and phantom limb sensation
There was higher risk of developing SP and PLS among the patients with PLP, which is similar to earlier study by Sherman et al. and Sin et al.[3,19]
Phantom limb sensation and telescoping
The PLS in the distal part of the limb gradually approached the residual limb with time (telescoping) in 45 (32.37%) patients, which was also present in 11 (35%) and 10 (25%) patients in a previous Asian and African study.[3,4] Telescoping phenomenon was also reported by previous studies.[14,15,16,18,21]
PLP and sex differences
Although a few earlier studies had shown that PLP was more common in women, our study and a few other studies had shown no sex differences in PLP.[39,50,51,56] In most of earlier, the sample size was very small to capture any potential difference of the occurrence of PLP among the two genders. It was well known that both man and woman perceive and express pain in different ways, which may be due to difference in body size, neural organization, humoral responses, social expectations, psychological differences, and use of different coping mechanism for pain.[57,58]
Postoperative pain
There was higher prevalence of PLP and PLS among the patients with severe postoperative pain, with lower prevalence of SP.
PLP and psychological factors
In our study, there was higher prevalence of depression among the patients with PLP. In another study, Lacoux et al. also found a higher incidence (62%) of low mood among amputees with PLP.[4] There was also a moderate prevalence of depression among the patients with PLS (31%–46%), which is similar to the study of Lacoux et al.[4] There was also higher prevalence of sleep disturbance among the patients with PLP, and among the patients with depression and sleep disturbance, there was high chances of development of PLP and PLS.
PLP and performance status
The performance status was found to be low and it had improved with time in amputated cancer patients. The presence of PLP and SP leads to considerable lowering of performance status of the amputated patients which is a new finding in our study.
Limitations of the study are as follows.
Our study design being an observational cohort study
Noninterventional type of the study
Only pharmacological treatment given for the phantom limb phenomena was analyzed
For analysis of prevalence of phantom phenomenon, longer follow-up may be needed
Preventive measures are not analyzed in this study
Impact of phantom phenomenon on the quality of life was not analyzed
The study was done among the cancer patients, and the role other factors in phantom phenomenon among the traumatic amputees, in gangrene, etc., may be different
In addition, no imaging studies such as functional MRI were not done.
CONCLUSION
The prevalence of PLP and PLS was found to be higher among the amputated cancer patients, whereas prevalence of SP was found to be lower. The PLP and PLS were found to be higher among the patients with preamputation pain, smoking, chemotherapy, receiving general anesthesia, receiving IV opioid postoperative analgesia, proximal level of amputation, patients developing neuroma, infection and severe immediate postoperative pain. The presence of PLP also increases the risk of developing SP and PLS. Further, PLP and PLS had led to increased incidence of sleep disturbance and depression among the amputees.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Keil G. So-called initial description of phantom pain by Ambroise Paré. Fortschr Med. 1990;108:62–6. [PubMed] [Google Scholar]
- 2.Wall R, Novotny-Joseph P, Macnamara TE. Does preamputation pain influence phantom limb pain in cancer patients? South Med J. 1985;78:34–6. doi: 10.1097/00007611-198501000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sin EI, Thong SY, Poon KH. Incidence of phantom limb phenomena after lower limb amputations in a Singapore tertiary hospital. Singapore Med J. 2013;54:75–81. doi: 10.11622/smedj.2013028. [DOI] [PubMed] [Google Scholar]
- 4.Lacoux PA, Crombie IK, Macrae WA. Pain in traumatic upper limb amputees in Sierra Leone. Pain. 2002;99:309–12. doi: 10.1016/s0304-3959(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 5.Husum H, Resell K, Vorren G, Heng YV, Murad M, Gilbert M, et al. Chronic pain in land mine accident survivors in Cambodia and Kurdistan. Soc Sci Med. 2002;55:1813–6. doi: 10.1016/s0277-9536(01)00315-x. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 7.Flor H, Elbert T, Mühlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp Brain Res. 1998;119:205–12. doi: 10.1007/s002210050334. [DOI] [PubMed] [Google Scholar]
- 8.Doubell TP, Mannion RJ, Woolf CJ. The dorsal horn: State-dependent sensory processing, plasticity and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of Pain. 4th ed. Edinburgh: Churchill Livingstone; 1999. pp. 165–81. [Google Scholar]
- 9.Nikolajsen L, Hansen CL, Nielsen J, Keller J, Arendt-Nielsen L, Jensen TS. The effect of ketamine on phantom pain: A central neuropathic disorder maintained by peripheral input. Pain. 1996;67:69–77. doi: 10.1016/0304-3959(96)03080-1. [DOI] [PubMed] [Google Scholar]
- 10.Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain response to perineuromal injection of normal saline, epinephrine, and lidocaine in humans. Pain. 1992;49:9–12. doi: 10.1016/0304-3959(92)90181-A. [DOI] [PubMed] [Google Scholar]
- 11.Sherman RA, Bruno GM. Concurrent variation of burning phantom limb and stump pain with near surface blood flow in the stump. Orthopedics. 1987;10:1395–402. doi: 10.3928/0147-7447-19871001-09. [DOI] [PubMed] [Google Scholar]
- 12.Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–8. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- 13.Nikolajsen L, Ilkjaer S, Jensen TS. Relationship between mechanical sensitivity and postamputation pain: A prospective study. Eur J Pain. 2000;4:327–34. doi: 10.1053/eujp.2000.0194. [DOI] [PubMed] [Google Scholar]
- 14.Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: An epidemiological study. Pain. 2000;87:33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 15.Nikolajsen L, Ilkjaer S, Krøner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997;72:393–405. doi: 10.1016/s0304-3959(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 16.Wartan SW, Hamann W, Wedley JR, McColl I. Phantom pain and sensation among British veteran amputees. Br J Anaesth. 1997;78:652–9. doi: 10.1093/bja/78.6.652. [DOI] [PubMed] [Google Scholar]
- 17.Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81:1039–44. doi: 10.1053/apmr.2000.7583. [DOI] [PubMed] [Google Scholar]
- 18.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983;17:243–56. doi: 10.1016/0304-3959(83)90097-0. [DOI] [PubMed] [Google Scholar]
- 19.Sherman RA, Sherman CJ, Parker L. Chronic phantom and stump pain among American veterans: Results of a survey. Pain. 1984;18:83–95. doi: 10.1016/0304-3959(84)90128-3. [DOI] [PubMed] [Google Scholar]
- 20.Sherman RA, Sherman CJ, Gall NG. A survey of current phantom limb pain treatment in the United States. Pain. 1980;8:85–99. doi: 10.1016/0304-3959(80)90092-5. [DOI] [PubMed] [Google Scholar]
- 21.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: Incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985;21:267–78. doi: 10.1016/0304-3959(85)90090-9. [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Melzack R. Pain 'memories' in phantom limbs: Review and clinical observations. Pain. 1990;43:319–36. doi: 10.1016/0304-3959(90)90029-D. [DOI] [PubMed] [Google Scholar]
- 23.Montoya P, Larbig W, Grulke N, Flor H, Taub E, Birbaumer N. The relationship of phantom limb pain to other phantom limb phenomenon in upper limb amputees. Pain. 1997;87:7–17. doi: 10.1016/s0304-3959(97)00004-3. [DOI] [PubMed] [Google Scholar]
- 24.Sahin SH, Colak A, Arar C, Tutunculer E, Sut N, Yilmaz B, et al. A retrospective trial comparing the effects of different anesthetic techniques on phantom pain after lower limb amputation. Curr Ther Res Clin Exp. 2011;72:127–37. doi: 10.1016/j.curtheres.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgoyne LL, Billups CA, Jirón JL, Jr, Kaddoum RN, Wright BB, Bikhazi GB, et al. Phantom limb pain in young cancer-related amputees: Recent experience at St. Jude Children's Research Hospital. Clin J Pain. 2012;28:222–5. doi: 10.1097/AJP.0b013e318227ce7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith J, Thompson JM. Phantom limb pain and chemotherapy in pediatric amputees. Mayo Clin Proc. 1995;70:357–64. doi: 10.4065/70.4.357. [DOI] [PubMed] [Google Scholar]
- 27.Mishra S, Bhatnagar S, Gupta D, Diwedi A. Incidence and management of phantom limb pain according to World Health Organization analgesic ladder in amputees of malignant origin. Am J Hosp Palliat Care. 2008;24:455–62. doi: 10.1177/1049909107304558. [DOI] [PubMed] [Google Scholar]
- 28.Riddoch G. Phantom limbs and body shape. Brain. 1941;64:197–222. [Google Scholar]
- 29.Henderson WR, Smyth GE. Phantom limbs. J Neurol Neurosurg Psychiatry. 1948;11:88–112. doi: 10.1136/jnnp.11.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appenzeller O, Bicknell JM. Effects of nervous system lesions on phantom experience in amputees. Neurology. 1969;19:141–6. doi: 10.1212/wnl.19.2.141. [DOI] [PubMed] [Google Scholar]
- 31.Parkes CM. Factors determining the persistence of phantom pain in the amputee. J Psychosom Res. 1973;17:97–108. doi: 10.1016/0022-3999(73)90010-x. [DOI] [PubMed] [Google Scholar]
- 32.Carlen PL, Wall PD, Nadvorna H, Steinbach T. Phantom limbs and related phenomena in recent traumatic amputations. Neurology. 1978;28:211–7. doi: 10.1212/wnl.28.3.211. [DOI] [PubMed] [Google Scholar]
- 33.Sherman RA, Sherman CJ. Prevalence and characteristics of chronic phantom limb pain among American veterans. Results of a trial survey. Am J Phys Med. 1983;62:227–38. [PubMed] [Google Scholar]
- 34.Pohjolainen T. A clinical evaluation of stumps in lower limb amputees. Prosthet Orthot Int. 1991;15:178–84. doi: 10.3109/03093649109164285. [DOI] [PubMed] [Google Scholar]
- 35.Houghton AD, Nicholls G, Houghton AL, Saadah E, McColl L. Phantom pain: Natural history and association with rehabilitation. Ann R Coll Surg Engl. 1994;76:22–5. [PMC free article] [PubMed] [Google Scholar]
- 36.Krane EJ, Heller LB. The prevalence of phantom sensation and pain in pediatric amputees. J Pain Symptom Manage. 1995;10:21–9. doi: 10.1016/0885-3924(94)00062-P. [DOI] [PubMed] [Google Scholar]
- 37.Wilkins KL, McGrath PJ, Finley GA, Katz J. Phantom limb sensations and phantom limb pain in child and adolescent amputees. Pain. 1998;78:7–12. doi: 10.1016/S0304-3959(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 38.Schley MT, Wilms P, Toepfner S, Schaller HP, Schmelz M, Konrad CJ, et al. Painful and nonpainful phantom and stump sensations in acute traumatic amputees. J Trauma. 2008;65:858–64. doi: 10.1097/TA.0b013e31812eed9e. [DOI] [PubMed] [Google Scholar]
- 39.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–9. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Alamo Tomillero F, Rodríguez de la Torre R, Caba Barrientos F, Hachero Torrejón A, Echevarría Moreno M, García García A, et al. Prospective study of prevalence and risk factors for painful phantom limb in the immediate postoperative period of patients undergoing amputation for chronic arterial ischemia. Rev Esp Anestesiol Reanim. 2002;49:295–301. [PubMed] [Google Scholar]
- 41.Gallagher P, Allen D, Maclachlan M. Phantom limb pain and residual limb pain following lower limb amputation: A descriptive analysis. Disabil Rehabil. 2001;23:522–30. doi: 10.1080/09638280010029859. [DOI] [PubMed] [Google Scholar]
- 42.Hord AH, Shannon C. Phantom pain. In: Raj PP, editor. Practical Management of Pain. 3rd ed. Philadelphia: Mosby, Inc; 2000. pp. 212–22. [Google Scholar]
- 43.Sugarbaker PH, Weiss CM, Davidson DD, Roth YF. Increasing phantom limb pain as a symptom of cancer recurrence. Cancer. 1984;54:373–5. doi: 10.1002/1097-0142(19840715)54:2<373::aid-cncr2820540234>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Weiss T, Miltner WH, Adler T, Brückner L, Taub E. Decrease in phantom limb pain associated with prosthesis-induced increased use of an amputation stump in humans. Neurosci Lett. 1999;272:131–4. doi: 10.1016/s0304-3940(99)00595-9. [DOI] [PubMed] [Google Scholar]
- 45.Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: A survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186–94. doi: 10.1080/03093640108726601. [DOI] [PubMed] [Google Scholar]
- 46.Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP. Phantom pain and risk factors: A multivariate analysis. J Pain Symptom Manage. 2002;24:578–85. doi: 10.1016/s0885-3924(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 47.Bailey AA, Moersch FP. Phantom limb. Can Med Assoc J. 1941;45:37–42. [PMC free article] [PubMed] [Google Scholar]
- 48.Bach S, Noreng MF, Tjéllden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain. 1988;33:297–301. doi: 10.1016/0304-3959(88)90288-6. [DOI] [PubMed] [Google Scholar]
- 49.Jahangiri M, Jayatunga AP, Bradley JW, Dark CH. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaine. Ann R Coll Surg Engl. 1994;76:324–6. [PMC free article] [PubMed] [Google Scholar]
- 50.Nikolajsen L, Ilkjaer S, Christensen JH, Krøner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet. 1997;350:1353–7. doi: 10.1016/S0140-6736(97)06315-0. [DOI] [PubMed] [Google Scholar]
- 51.Lambert AW, Dashfield AK, Cosgrove C, Wilkins DC, Walker AJ, Ashley S. Randomized prospective study comparing preoperative epidural and intraoperative perineural analgesia for the prevention of postoperative stump and phantom limb pain following major amputation. Reg Anesth Pain Med. 2001;26:316–21. doi: 10.1053/rapm.2001.23934. [DOI] [PubMed] [Google Scholar]
- 52.Richardson C, Glenn S, Nurmikko T, Horgan M. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin J Pain. 2006;22:353–8. doi: 10.1097/01.ajp.0000177793.01415.bd. [DOI] [PubMed] [Google Scholar]
- 53.Smith DG, Ehde DM, Legro MW, Reiber GE, del Aguila M, Boone DA. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;361:29–38. doi: 10.1097/00003086-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Ong BY, Arneja A, Ong EW. Effects of anesthesia on pain after lower-limb amputation. J Clin Anesth. 2006;18:600–4. doi: 10.1016/j.jclinane.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Hanley MA, Jensen MP, Smith DG, Ehde DM, Edwards WT, Robinson LR. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain. 2007;8:102–9. doi: 10.1016/j.jpain.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Bosmans JC, Geertzen JH, Post WJ, van der Schans CP, Dijkstra PU. Factors associated with phantom limb pain: A 31/2-year prospective study. Clin Rehabil. 2010;24:444–53. doi: 10.1177/0269215509360645. [DOI] [PubMed] [Google Scholar]
- 57.Derbyshire SW. Gender, pain and the brain. Pain Clin Updates. 2008;16:1–4. [Google Scholar]
- 58.Keogh E, Denford S. Sex differences in perceptions of pain coping strategy usage. Eur J Pain. 2009;13:629–34. doi: 10.1016/j.ejpain.2008.07.002. [DOI] [PubMed] [Google Scholar]