Abstract
Background
Probiotics can alter the colonic microbiota and might improve bowel function.
Objectives
The aim of this study was to evaluate the effect of the consumption of yogurt, enriched with Bifidobacterium and Lactobacillus 4.8 × 1010 (CFU) on the symptoms of constipated pregnant women.
Materials and Methods
This triple-blind randomized controlled trial was conducted on 60 constipated pregnant women who were diagnosed by the ROME III criteria in Tabriz, Iran from December 2014 to July 2015. Participants were randomly put into two groups including the treatment and the control group through block randomization. The treatment group received 300 g of yogurt enriched with Bifidobacterium and Lactobacillus 4.8 × 1010 colony forming units (CFU) (n = 30) while the control group received conventional yogurt (n = 30) for 4 weeks. The defecation frequency, stool consistency, straining during defecation, sensation of anorectal obstruction, sensation of incomplete evacuation and manual manoeuvres to facilitate defecation were primary outcomes while the amount of defecation, stool colure, and quality of life were secondary outcomes.
Results
The frequency of defecation was increased from 2.1 (0.8) at baseline to 8.3 (4.4) in the probiotic yogurt group vs. 2.3 (0.7) at baseline to 8.1 (4.3) in the conventional yogurt group at the end of 4th week. These results were based on the repeated measure ANOVA test and there was no statistically significant difference between the two groups (mean difference: 0.1; Confidence Interval 95%: -1.4 to 1.7; P = 0.872). Constipation symptoms including straining, anorectal obstruction, manipulation to facilitate defecation, consistency of stool and color of stool were improved significantly (P < 0.05) in both groups. In addition, the amount of defecation was significantly increased in both groups (P < 0.05), while incomplete evacuation was significantly reduced in the treatment group (P = 0.01). There was no statistically significant difference between the groups in the mean scores of physical (P = 0.726) and mental (P = 0.678) aspects of quality of life after the intervention with the adjusting of baseline scores.
Conclusions
Consumption of 300 g/day probiotic and conventional yogurt can play a role in improving the symptoms of constipation during pregnancy.
Keywords: Constipation, Probiotic, Pregnancy
1. Background
Digestive tract disorders such as nausea and constipation are common during pregnancy while constipation is the second most common complaint in pregnant women (1). Constipation is a frequent clinical syndrome that occurs in approximately 2.6% to 24.8% in Asia (2) and 40% of pregnant women (1). Constipation reduces the quality of life and increases the burden for health care systems. The effect of it on the quality of life is comparable with patients who suffer from asthma, rheumatoid arthritis and psoriatic arthritis (3). Straining in patients with chronic constipation can injure the pudendal nerve and weaken the pelvic floor or impair the supportive function of the pelvic (4). Also, chronic constipation can result in potentially serious complications such as fecal impaction, incontinence, bowel damage, bleeding, hemorrhoids and anal fissure (5).
Patients with functional constipation are identified through the Rome III criteria. Constipation is diagnosed when 25% of bowel movements are associated with at least two symptoms and last for 3 months, such as straining, sensation of anorectal obstruction during defecation, sensation of incomplete evacuation, need for manipulation to facilitate defecation and less than three defecations per week (6).
The etiology of constipation is multifactorial and may occur when the colon absorbs too much water or the movements of bowel muscles are slowed which leads to hard and dry stool. Constipation may arise from inadequate fibers in diet, lack of physical activity, certain medications, irritable bowel syndrome (IBS), changes in living conditions such as pregnancy, age, travel, laxative abuse, dehydration and certain illnesses such as stroke and dementia. Also some drugs, such as analgesics especially narcotics, aluminum and calcium containing antacids, calcium channel blockers, Parkinson’s disease drugs, antispasmodics, some antidepressants, iron salts and diuretics can cause constipation (1, 7).
The prevalence of constipation in early pregnancy is influenced by hormonal factors and mechanical changes in the body associated with the progress of pregnancy. There is evidence suggesting that female sex hormones influence gastrointestinal motility in non-pregnant women (8). Progesterone and somatostatin may prevent the release of motilin, which is a peptide hormone that normally stimulates the GI motility (9). Relaxin is a polypeptide that normally prevents smooth muscle contractions during pregnancy. It also inhibits the activity of gastrointestinal tract’s smooth muscles (10). Both estrogen and progesterone increase renin secretion. Renin converts angiotensin to angiotensin-I which later turns into angiotensin-II, the latter leads to increased levels of aldosterone during pregnancy. Comprehensive studies on colon perfusion have shown that aldosterone increases water absorption during pregnancy, especially in the second trimester (1). Treatment for constipation depends on its severity and duration. The first line of therapy for constipation includes changes in diet, lifestyle, exercise and use of laxatives.
Constipation treatment during pregnancy needs special attention due to concerns for the safety of the mother and child (11). For example, anthraquinone laxatives such as danthron are associated with congenital malformations (3). Osmotic saline laxatives such as magnesium citrate and sodium phosphate can result in sodium and urinary retention in the mother’s body, while castor oil can cause premature uterine contractions. Mineral oils can theoretically affect the absorption of fat-soluble vitamins in the mother’s body. Some laxatives can result in infant diarrhea. Laxatives such as crownvetch should be used with caution in late pregnancy due to their secretion from breast milk. Therefore, constipation cannot be completely treated until the end of pregnancy (3, 9, 12).
Other therapeutic options and new methods to treat constipation is the consumption of probiotics and prebiotics (13). Probiotics can alter the colonic flora and might improve bowel function (14). Potential functions of probiotics include prevention and treatment of gastrointestinal disorders (15) and inflammatory of bowel disease (16). Probiotics are known as positive and friendly bacteria and include three very famous types of Lactobacillus, Bifidobacterium, and Saccharomyces. Probiotics have been added to various foods in recent decades due to their health effects on the human body. Some studies have shown the positive effects of probiotics-containing dairy products in the improvement of constipation in non-pregnant women. These clinical studies performed on fermented milk containing Bifidobacterium lactis DN-173010 showed an improvement and reduction in gastrointestinal motility time (17). The frequency of defecation after taking this product was significantly increased in patients with irritable bowel syndrome (IBS) (18).
It has been also shown that fermented milk containing Bifidobacterium lactis DN-173010 increases intestinal motility in healthy men and women with an increase in stool frequency (19).
In a literature search performed, only one uncontrolled pilot study has investigated the effectiveness of probiotics in the treatment of constipation in pregnant women (20) and there is no randomized clinical trial in this in regards to pregnant women. Therefore, considering the high prevalence of constipation during pregnancy and its serious complications as well as available evidence about the effects of probiotics on human digestive system, this controlled randomized clinical trial was performed on pregnant women in this field.
2. Objectives
The present research aimed to determine the impact of probiotics on constipation during pregnancy.
3. Materials and Methods
3.1. Study Design, Participants and Sampling
This study was a triple-blind randomized controlled trial (those involved in the sampling and data collection, analyzers and participants were unaware of the type of intervention received) with two parallel arms conducted on constipated women who were referred to health centers in Tabriz, Iran for receiving prenatal care from December 2014 to July 2015.
Inclusion criteria included healthy singleton pregnant women who suffered from constipation, being over 18 years of age, gestational age between 24 - 28 weeks, being literate and willing to participate in the study. Exclusion criteria included receiving any treatment in less than a week before the study, having mental retardation or metabolic disease (hypothyroidism), Hirschsprung disease, spinal anomalies, anorectal pathology, inflammatory of bowel disease, previous gastrointestinal surgery and use of fermented dairy products containing probiotics two weeks prior to the study.
Sampling started after obtaining permission from the ethics committee of research and technology deputy of Tabriz University of Medical Sciences. The research environments consisted of five public and governmental health centers and two governmental hospitals (Al-Zahra and Army) in Tabriz, Iran. The Al-Zahra hospital is a specialized referral center that provides services in fields of obstetrics and gynecology, oncology, neonatal and perinatology. This hospital has 150 beds and 6 operating rooms. The Army hospital is a general center that provides services in fields of internal, surgery, obstetrics and gynecology, neonatal and perinatology. This hospital has 100 beds and 4 operating rooms.
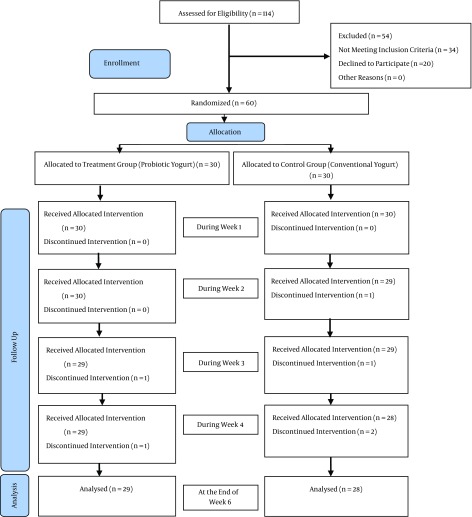
The researcher visited the research environments and reviewed all pregnant women in terms of eligibility criteria. In this study, 114 constipated pregnant women were selected through the convenience sampling method based on the Rome III diagnostic criteria and were assessed for the eligibility criteria; 54 women were excluded, 34 women had no eligibility criteria and 20 declined to participate in this study. Ultimately, 60 women participated in the study and after appropriate explanations about the type of study and method, informed written consent was obtained from them.
Participants filled out socio-demographic, constipation checklist (Rome III Criteria) and quality of life (SF-36) questionnaires before interventions. Clinical evaluations and side effects were investigated during the four weeks’ intervention and the sixth week after starting intervention. During intervention, doses of taking laxatives (Milk of Magnesia (MOM)), constipation checklist (Rome III Criteria) and side effects including digestive problems and other possible complications were weekly recorded by participants. In addition, participants recorded constipation checklists during the second week after the end of the intervention as well as fillinh out the quality of life questionnaire at the end of six weeks after the intervention. All of the observations and follow-ups for the participants were done by the corresponding author of this study.
3.2. Sample Size
Based on the data in a previous study conducted by Tateyama et al. (21), on women with constipation during pregnancy and by considering α = 0.05, Power = 90%, M1 = 6.8, M2 = 5.1 - 8.5, Sd1 = 1.8 and Sd2 = 1.8, the sample size was determined by 24 participants and by taking a %20 dropout rate, 30 participants from each group were considered. Then we included 60 constipated pregnant women for this trial.
3.3. Randomization
Pregnant women were assigned to two intervention groups (probiotic yogurt or conventional yogurt) through block randomization with block sizes of 4 and 6 with an allocation ratio of 1: 1 using a computerized random number tabulation. The allocation sequence was conducted by an individual uninvolved in sampling and data analysis. To conceal the allocation of probiotic and conventional yogurt, these were provided by Pegah company of Tabriz, Iran and were quite similar in appearance and taste with different codes. At the end of the study, data analysis was conducted based on these codes.
3.4. Intervention (Probiotic Treatment)
Both probiotic and conventional yogurts contained Lactobacillus bulgaricus and Streptococcus thermophilus. The probiotic yogurt was also enriched with Lactobacillus acidophilus (La-5) and Bifidobacterium lactic (Bb-12). Probiotic yogurts were sampled after production on day 1 (time of distribution) and microbiologically examined every week. Samples were refrigerated at 4°C, with consequent analyzing on day 7 of storage. Serial dilutions of yogurts were made with Ringer solutions.
The diluted samples were cultivated and counted using MRS-bile agar medium with applying pour plate method. Bile (Sigma chemical Co., St. Louis, MO) was added to the MRS agar (Merck, Darmstadt, Germany) in a concentration of 0.15% and was autoclaved at 121°C for 15 minutes. All the samples were incubated at 37°C for 72 hours in both aerobic and anaerobic conditions. Anaerobic state was made via the Gas Pak system (Merck). All of the tests were done in triplicate. Counts of L. acidophilus were done at aerobic situations and viable amounts of B. lactic were selectively achieved using the subtractive enumeration method (22, 23). Microbiological analyses of the probiotic yogurts showed that the average colony counts of L. acidophilus (La-5) and B. lactic (Bb-12) on day 1 was 3 × 108 CFU/g. yogurts a day 7 and 14 contained 3.33 × 107 CFU/g of L. acidophilus (La-5) and B. lactic (Bb-12). Both probiotic bacteria showed a steady survival rate during a 7-day storage time at the average rate of 1.6 × 108 CFU/g. The yogurt PH was 4.33 on day 1 and 4.22 on day 7 and the fat content was 2.5% comparable in both yogurt types. The probiotic and conventional yogurt containers were identical and had a similar taste and appearance. Expiration date of our probiotic yogurts was 21 days after manufacture date and because of this problem and the need to follow up on participants; the yogurts were produced weekly and distributed to the participants. Although both of the yogurts can be found in Iranian markets, the Pegah Dairy Industries Co. in Tabriz, Iran specially prepared these for this study.
Participants of the treatment group received 300 grams of yogurt containing 4.8 × 1010 colony forming units (CFU) of the probiotic strains, Bifidobacterium (Bb-12) and Lactobacillus acidophilus (La-5), three times a day for four weeks and participants of the control group received 300 grams of conventional yogurt three times a day for four weeks. The selection of resistant probiotic strains and adjusting the condition of production and storage for more survival rates can be useful methods for increasing viability of them (24-27) and some conditions such as high freezing and storage temperature or gastric acids and gut condition (28-30) can increase the viability of probiotics. All participants were asked to keep their yogurts refrigerated at 4°C in and to eat it every day three times a day by first drinking a glass of water and then eating the yogurt an hour before food. Each participant was also advised to continue their normal diet but to avoid any other probiotic products and laxatives supplements expect of MOM (Milk of magnesium). Follow-up was done during four weeks of intervention and two weeks after intervention by the corresponding author of this research.
3.5. Instrument
Socio-demographic questionnaire, constipation checklist and quality of life (QoL) questionnaire (SF-36) were used for data collection.
The socio-demographic questionnaire included items regarding age, occupation, economic status, education, husband’s education, body mass index (BMI) and Gravida that was completed before intervention. This questionnaire was developed by researchers and its content validity was confirmed by 10 faculty members from Tabriz University of Medical Sciences, Tabriz, Iran.
Constipation checklist (31) is based on the Rome III criterion that has been used in the several studies (13, 17-19) and it is a standard questionnaire for assessing constipation. This checklist included questions about frequency of bowel movements, status of defecation, straining during bowel movement, feeling of incomplete evacuation after bowel movement, sensation of anorectal obstruction, the need to manipulate the rectum to facilitate defecation, the amount of stool and stool color. This checklist was completed by participants during the four weeks’ intervention and sixth week after starting intervention.
The quality of life (SF-36) questionnaire (32) consists of 36 questions that evaluate individuals in terms of physical and mental health. It consists of eight subscales and each subscale includes 2 to 10 items. The subscales of this questionnaire include physical function, role limitations caused by physical health problems, role limitations caused by emotional problems, energy and fatigue, emotional well-being, social function, pain and general health. In addition, two general subscales specifically physical and mental health are obtained by integration of subscales. In this questionnaire, a lower score indicates lower quality of life and vice versa. The validity and reliability of the SF-36 has been confirmed by Montazeri et al. (2005) (26) while Cronbach’s alpha of the questionnaire was more than 0.7 (α = 0.805) and the ICC was 0.86. Participants completed this questionnaire before the sixth week and also at the end of the sixth week after the starting of intervention.
3.6. Data Analysis
Data was analyzed using the SPSS 21 software. To describe socio-demographic characteristics of participants, descriptive statistics (frequency, percentage, mean and standard deviation) were used. In order to investigate homogeneity of groups in terms of qualitative characteristics, chi-square and chi-square for trend tests were utilized and the independent t-test was used for quantitative characteristics. To compare the average frequency of bowel movements between two groups, independent t-test and repeated measure ANOVA test were used respectively before and after intervention with adjustment of the basic values. Friedman test and Mann-Whitney test were used respectively for intra-group comparison of the amount of stool, stool consistency, straining during defecation, feeling of incomplete evacuation after bowel movement, sensation of anorectal obstruction, the need to manipulate the rectum to facilitate defecation and stool color and for inter-group comparison of these outcomes. To compare quality of life between study groups, ANCOVA test was employed by controlling the basic score. A significance level of P < 0.05 was assumed. All analyses were conducted based on the intention to treat approach.
3.7. Ethical Consideration
Our study was a triple-blind, placebo-controlled randomized multicenter trial and was registered in the IRCT website under the code (IRCT2014070110324N18) and performed according to the ethical recommendations as well as approved by the ethical committee of the Tabriz University of Medical Sciences Iran (Code: 9343). All enrolled participants signed an informed consent.
4. Results
4.1. Patients
Among pregnant women receiving prenatal care at five health centers and two hospitals in Tabriz, Iran, 114 pregnant women with constipation were identified from the first of December 2014 to the 11 of July 2015. Thirty-four and twenty of them were excluded respectively due to the lack of inclusion criteria and unwillingness to participate in the study. 60 pregnant women participating in the study were randomly allocated into two 30-participant groups of intervention (probiotic yogurt) and control (conventional yogurt). There was one participant who dropped out in the probiotic group in the third week of the study due to travelling and subsequent lack of access. There were also two dropouts in the control group (conventional yogurt) in the second and fourth weeks of the study due to unwillingness to receive yogurt (Figure 1).
Figure 1. Flowchart of the Study.
The studied groups were similar in terms of socio-demographic characteristics including age, job, economic status, education, husband’s education, and body mass index (BMI) (Table 1). The mean standard deviation (SD) age of the participants was 28.6 years. Their income was relatively high in 76.6% of the participants. Approximately 90% of pregnant women were housewives, 45% had high a school education and 40% had normal BMI (18.5 - 24.9) in both group. Forty percent of the participants were primigravida. At baseline, the participants in both groups were almost identical in terms of constipation criteria (P = 0.637).
Table 1. Socio-Demographic Characteristics of the Participants in the Control and Intervention Groupsa.
| Parameter | Probiotic Group (N = 30) | Control Group (N = 30) | P-Value |
|---|---|---|---|
| Age, y, mean ± SD | 28.50 | 28.77 | 0.203b |
| Job | 0.728c | ||
| Housewife | 28 (93.3) | 26 (86.7) | |
| Employee | 2 (6.7) | 4 (13.3) | |
| Economic status | 0.288d | ||
| Favorable | 3 (10.0) | 3 (10.0) | |
| Partly favorable | 25 (83.3) | 21 (70.0) | |
| Unfavorable | 2 (6.7) | 6 (20.0) | |
| Education | 0.198d | ||
| Primary | 9 (30.0) | 5 (16.7) | |
| Secondary | 3 (10.0) | 3 (10.0) | |
| Diploma | 12 (40.0) | 15 (50) | |
| University | 6 (20.0) | 7 (23.3) | |
| Husband’s education | 0.146d | ||
| Primary | 4 (13.3) | 2 (6.7) | |
| Secondary | 5 (16.7) | 10 (33.3) | |
| Diploma | 14 (46.7) | 11 (36.7) | |
| University | 7 (23.3) | 7 (23.3) | |
| Body mass index, kg/m 2 , mean ± SD | 0.361d | ||
| ≤ 18.5 | - | - | |
| 18.5 to 24.9 | 12 (40.0) | 12 (40.0) | |
| 25 to 29.9 | 11 (36.7) | 12 (40.0) | |
| 30 ≤ | 7 (23.3) | 6 (20.0) | |
| Gravid e | 0.031d | ||
| First | 13 (43.3) | 11 (36.7) | |
| Second | 11 (36.7) | 14 (46.7) | |
| Third and more | 6 (20.0) | 5 (16.7) |
aValues are expressed as No. (%) unless otherwise indicated.
bIndependent t test.
cFisher exact test.
dChi-square test for trend.
eThe difference between the two groups was adjusted in the analysis.
4.2. Clinical Response
Clinical response to treatment was evaluated throughout the study period. Each participant kept a checklist for self-reporting daily record of constipation intensity. Participants were followed up weekly by researcher for providing the yogurt and responding to any issue regarding recording of data and to assess adverse events.
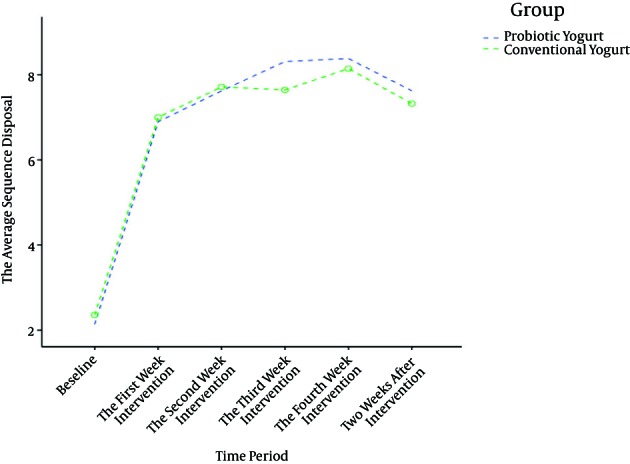
During the 4 weeks of intervention, the frequency of defecation was increased from 2.1 (0.8) at baseline to 8.3 (4.4) in the probiotic yogurt group vs. 2.3 (0.7) at baseline to 8.1 (4.3) in the conventional yogurt group at the end of 4th week that was based on the repeated measure ANOVA test, there was no statistically significant difference between the two groups (mean difference: 0.1; Confidence Interval 95%: -1.4 to 1.7; P = 0.872) (Table 2, Figure 2).
Table 2. Frequency of Defecation During Intervention in Study Groupsa.
| Variable | Probiotic Group (n = 30) | Control Group (n = 30) | Time Effect (P)b | Time and Group Effect (P)b |
|---|---|---|---|---|
| Frequency of defecation in baseline | 2.1 (0.8) | 2.3 (0.7) | < 0.001 | 0.914 |
| Frequency of defecation in week 1 | 6.9 (4.4) | 7.0 (4.1) | ||
| Frequency of defecation in week 2 | 7.6 (3.7) | 7.7 (3.9) | ||
| Frequency of defecation in week 3 | 8.3 (4.1) | 7.6 (4.0) | ||
| Frequency of defecation in week 4 | 8.3 (4.4) | 8.1 (4.3) | ||
| Frequency of defecation after study | 7.6 (4.4) | 7.3 (4.8) | ||
| Comparison between groups | AMD (CI 95%)c = 0.1, (-1.4 to 1.7), P = 0.872 | |||
aValues are expressed as mean ± SD.
bRepeated Measure ANOVA test.
cAdjusted Mean Difference (Confidence Interval 95%)
Figure 2. Trend in the Frequency of Defecation Before and During Intervention and 2 Weeks After Intervention According to Repeated Measurement Analysis.
Constipation symptoms including straining, anorectal obstruction, manipulation to facilitate defecation, consistency of stool and color of stool were improved significantly (P < 0.05) in both groups. In addition, the amount of defecation was significantly increased in both groups (P < 0.05), while incomplete evacuation was significantly reduced in the treatment group (P = 0.01) (Table 3).
Table 3. Comparison of Constipation Criteria Between Study Groupsa,b.
| Variable | Probiotic Group (n = 30) | Control Group (n = 30) | Comparison Between Groupsc (P-Value) | |
|---|---|---|---|---|
| The amount of stool in baseline | Great | 2 (6.7) | 2 (6.7) | 0.637 |
| The amount of stool in week 1 | Great | 3 (10.0) | 4 (13.3) | 0.487 |
| The amount of stool in week 2 | Great | 2 (6.7) | 2 (6.7) | 0.050 |
| The amount of stool in week 3 | Great | 5 (16.7) | 3 (10.0) | 0.444 |
| The amount of stool in week 4 | Great | 3 (10.0) | 3 (10.0) | 0.777 |
| The amount of stool after study | Great | 1 (3.3) | 1 (3.3) | 0.734 |
| Comparison within groups d | 0.002 | 0.042 | ||
| Stool consistency in baseline | Hard | 29 (96.7) | 29 (96.7) | 0.185 |
| Stool consistency in week 1 | Hard | 16 (53.3) | 13 (43.3) | 0.551 |
| Stool consistency in week 2 | Hard | 9 (30.0) | 10 (33.3) | 0.660 |
| Stool consistency in week 3 | Hard | 8 (26.7) | 11 (36.6) | 0.088 |
| Stool consistency in week 4 | Hard | 8 (26.7) | 7 (23.4) | 0.571 |
| Stool consistency after study | Hard | 4 (13.3) | 13 (43.4) | 0.003 |
| Comparison within groups d | 0.001 | 0.001 | ||
| Straining during defecation in baseline | Often | 20 (66.7) | 16 (53.3) | 0.330 |
| Straining during defecation in week 1 | Often | 13 (43.3) | 6 (20.0) | 0.035 |
| Straining during defecation in week 2 | Often | 11 (36.7) | 7 (23.3) | 0.123 |
| Straining during defecation in week 3 | Often | 7 (23.4) | 6 (20.0) | 0.802 |
| Straining during defecation in week 4 | Often | 8 (26.6) | 8 (26.7) | 0.559 |
| Straining during defecation after study | Often | 7 (23.3) | 10 (33.3) | 0.361 |
| Comparison within groups d | 0.001 | 0.001 | ||
| Sensation of incomplete evacuation after defecation in baseline | Often | 21 (70.0) | 12 (40.0) | 0.092 |
| Sensation of incomplete evacuation after defecation in week 1 | Often | 10 (33.3) | 9 (30.0) | 0.269 |
| Sensation of incomplete evacuation after defecation in week 2 | Often | 10 (33.3) | 9 (30.0) | 0.892 |
| Sensation of incomplete evacuation after defecation in week 3 | Often | 6 (20.0) | 8 (26.7) | 0.635 |
| Sensation of incomplete evacuation after defecation in week 4 | Often | 4 (13.3) | 7 (23.4) | 0.203 |
| Sensation of incomplete evacuation after defecation after study | Often | 8 (26.6) | 11 (36.7) | 0.450 |
| Comparison within groups d | 0.001 | 0.073 | ||
| Sensation of obstruction during defecation in baseline | Often | 11 (36.7) | 8 (26.6) | 0.628 |
| Sensation of obstruction during defecation in week 1 | Often | 8 (26.7) | 5 (16.7) | 0.091 |
| Sensation of obstruction during defecation in week 2 | Often | 8 (26.7) | 4 (13.3) | 0.063 |
| Sensation of obstruction during defecation in week 3 | Often | 4 (13.4) | 4 (13.3) | 0.069 |
| Sensation of obstruction during defecation in week 4 | Often | 3 (10.0) | 4 (13.4) | 0.408 |
| Sensation of obstruction during defecation after study | Often | 5 (16.7) | 11 (36.7) | 0.793 |
| Comparison within groups d | 0.003 | 0.001 | ||
| Manual manoeuvres to facilitate defecation in baseline | Often | 7 (23.3) | 3 (10.0) | 0.083 |
| Manual manoeuvres to facilitate defecation in week 1 | Often | 4 (13.4) | 1 (3.3) | 0.021 |
| Manual manoeuvres to facilitate defecation in week 2 | Often | 5 (16.7) | 2 (6.7) | 0.057 |
| Manual manoeuvres to facilitate defecation in week 3 | Often | 4 (13.4) | 2 (6.6) | 0.941 |
| Manual manoeuvres to facilitate defecation in week 4 | Often | 3 (10.0) | 2 (6.7) | 0.611 |
| Manual manoeuvres to facilitate defecation after study | Often | 7 (23.4) | 5 (16.7) | 0.882 |
| Comparison within groups d | 0.012 | 0.007 | ||
| Stool color in baseline | Black | 22 (73.3) | 10 (33.3) | 0.785 |
| Stool color in week 1 | Black | 10 (33.3) | 13 (43.3) | 0.686 |
| Stool color in week 2 | Black | 13 (43.3) | 12 (40.0) | 0.813 |
| Stool color in week 3 | Black | 11 (36.7) | 10 (33.3) | 0.757 |
| Stool color in week 4 | Black | 8 (26.7) | 8 (26.7) | 0.777 |
| Stool color in two weeks after intervention | Black | 7 (23.3) | 12 (40.0) | 0.181 |
| Comparison within groups d | 0.002 | 0.001 |
aValues are expressed as No. (%).
bThis tabulation indicates performance of intestines. 1, The amount of fecal evaluated based on values such as high, medium and low; 2, Fecal condition includes hard, soft and watery; 3, straining during defecation and sensation of incomplete evacuation after defecation, sensation of obstruction during defecation, manual maneuvers to facilitate defecation evaluated with criterias such as always, often, sometimes or never and stool color was evaluated with categories including black, brown, yellowish and yellow.
cMeasured by Mann-Whitney
dMeasured by Friedman
There was no statistically significant difference between the groups in the mean scores of physical (mean difference: 0.7; Confidence Interval 95%: -1.7 to 1.2; P = 0.726) and mental (mean difference: 1.2; Confidence Interval 95%: -4.6 to 7.0; P = 0.678) aspects of quality of life after the intervention with the adjustment of baseline scores (Table 4).
Table 4. Comparison of Quality of Life Components Scores in the Study Groupsa.
| Probiotic (n = 30) | Placebo (n = 30) | Pb | AMD (CI 95%)c | |||
|---|---|---|---|---|---|---|
| Baseline | After 6 Weeks | Baseline | After 6 Weeks | |||
| Mental health score (0-100) | 58.6 (13.4) | 60.1 (10.7) | 53.5 (14.3) | 55.7 (16.1) | 0.678 | 1.2 ( -4.5 to 7.0) |
| Physical health score (0-100) | 53.2 (11.9) | 51.4 (13.6) | 54.1 (11.1) | 47.8 (12.7) | 0.726 | 0.7 (-1.7 to 1.2) |
aValues are expressed as mean ± SD.
bANCOVA.
cAdjusted mean difference (95% Confidence Interval).
The frequency of defecation was increased from 2.1 at baseline to 7.6 in the probiotic yogurt group and from 2.3 at baseline to 7.3 in the conventional yogurt group at the end of the 6th week. There were no significant differences between the two groups amongst other criterias except stool consistency, which was an improvement in the probiotic yogurt group (P = 0.003).
Some participants in both groups reported nausea, bloating and diarrhea during the ingestion of either probiotic yogurt or conventional yogurt. The frequency of side events in the study groups was shown in Table 5.
Table 5. Frequency of Side Events in the Study Groupsa.
| Variable | Probiotic Group (N = 30) | Control Group (N = 30) |
|---|---|---|
| Nausea (week 1) | 5 (16.7) | 4 (13.3) |
| Nausea (week 2) | 6 (20.0) | 4 (13.3) |
| Nausea (week 3) | 2 (6.6) | 4 (13.3) |
| Nausea (week 4) | 5 (16.6) | 5 (16.6) |
| Bloating (week 1) | 12 (40.1) | 1 (3.3) |
| Bloating (week 2) | 9 (30.0) | 3 (10.0) |
| Bloating (week 3) | 6 (20.1) | 4 (13.3) |
| Bloating (week 4) | 4 (13.3) | 2 (6.7) |
| Diarrhea (week 1) | 3 (10.0) | 5 (16.7) |
| Diarrhea (week 2) | 4 (13.3) | 1 (3.3) |
| Diarrhea (week 3) | 4 (13.4) | 1 (3.3) |
| Diarrhea (week 4) | 4 (13.4) | 7 (23.3) |
aValues are expressed as No. (%).
5. Discussion
A significant increase was found in this study in the frequency of defecation in two groups of probiotic yogurt and conventional yogurt. In addition, the amount of stool, straining, sensation of anorectal obstruction and need for manipulation to facilitate defecation were significantly improved in both groups of treatment and control. However, sensation of incomplete evacuation after defecation was only decreased in the probiotic group.
Some studies reported clinical improvement and reduction of constipation symptoms in different people. Amongst these studies, an uncontrolled pilot study was performed in Amsterdam on 20 pregnant women with constipation, in which four grams per day of ecologic w relief containing (4 × 109 CFU) of the probiotic strains Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Bifidobacterium longum W108, Lactobacillus casei W79, Lactobacillus plantarum W62 and Lactobacillus rhamnosus W71 was administered for 4 weeks. The results showed an increase in frequency of bowel movements, reduced straining during defecation and sensation of incomplete evacuation after defecation (20). Additionally, in a clinical trial in Korea regarding elderly men and women with an average age of 77.1 years who reside in a nursing home and received supplements containing Lactobacillus acidophilus, Bifidobacterium longum and Pediococcus pentosaceus had symptoms of constipation which were reduced by increasing the frequency and amount of fecal defecation (33).
In a controlled clinical trial on 135 Chinese women between the age of 25 and 65 years, an increase in bowel movements and improvement in consistency and defecation status was reported after receiving fermented milk containing B. lactis DN-173010 with two classical yogurt ferments, S. thermophilus and L. bulgaricus (34). In a controlled clinical trial with participants of the age of 6 months old, 44 infants who received L. Reuteri for 8 weeks had an increase in bowel movement frequency (35).
Compared with previous studies, our study in terms of number of participants, gender, type of probiotics and duration of treatment were different. Due to mechanical and hormonal changes in pregnancy, the results of this study can be compared with previous studies which excluded pregnant women. In the studies mentioned above, it has been explained that short-term interventions with probiotics improved the symptoms of chronic constipation. However, it may be important to evaluate the outcomes after a longer duration of consumption of probiotics and whether or not these beneficial and special effects will be maintained after cessation of probiotics intake.
In studies, either no side effects were reported or the reported side effects were low and mild, including bloating, diarrhea and aggravation of constipation, nausea, epigastric pain and eczema. Side effects of our study were also nausea, bloating and diarrhea in both groups.
Given the effect of chronic constipation on the quality of life, the impact of probiotics on quality of life was also assessed in the present study and there was no difference between the groups in physical and mental aspects of the quality of life. However, a clinical study reported the beneficial effects of probiotics containing food on the quality of life in adults between the ages of 18 to 65 years (18). In other studies, after consumption of probiotics-containing yogurt by people with an average age of 61.8 years their mood was improved and this improvement was attributed to improvement in bowel function (36).
The strong points of this study was considering the principles of clinical trials including random allocation, allocation concealment, blinding and many more that prevented of selection and performance biases. Furthermore, weekly follow-ups of participants in order to ensure the correct use of intervention as well as answering questions and helping with problems raised by them was the other strong point. In addition, fortunately, our intervention was yogurt and because participants were aware of its benefits the acceptance of this type of intervention went well.
However, our study had some limitations: first, use of conventional yogurt by the control group was a limitation for the present study. Since yogurt is a fermented milk product containing Lactobacillus bulgaricus and Streptococcus thermophilus which is shown to have beneficial effects on human health and improvements of transit time of feces in the intestine (37) we found a higher rate of success than expected in the control group. However, we used yogurt in this study because it is a well-known food product as a probiotic carrier and most women were also willing to participate in a study in which known commercial products are used rather than substances in the form of medicines. Second, our participants were pregnant women who experienced pregnancy as a stressful period and did not want to aggravate it by participating in a scientific study. Third, in this study, due to iron supplementation in pregnant women and its impact on stool color, we could not see better changes in these criteria. By activation of bifidobacteria growth in the intestinal tract, the amount of short chain fatty acids increases, stool PH decreases and stool color changes to yellow (21). Fourth, since the performance of women declines during pregnancy both physically and emotionally, the lack of improvement in the quality of life cannot be definitely attributed to the lack of probiotics effect. Fifth, our follow-up was only for 2 weeks after the cessation of intervention and longer follow-ups should be put in place to assess the benefits of probiotic yogurt in general. Finally, since this study has been conducted on healthy pregnant women, therefore the results of present study cannot be generalized on pregnant women with a history of serious diseases.
5.1. Conclusion
In this study, bowel performance was improved after consumption of probiotic yogurt and conventional yogurt and no significant difference happened between the treatment and control groups. Given that constipation is caused by a combination of mechanical and hormonal factors during pregnancy, it is therefore recommended to include dairy products especially probiotics containing food products in their daily routine as a dietary supplement.
Acknowledgments
The authors appreciate research center of Tabriz University of Medical Sciences (Tabriz, Iran) for the financial support and Pegah Dairy Industries Co. (Tabriz, Iran) and SHAFA Co. (Tabriz, Iran) for supplying the probiotic and conventional yogurts and being study sponsors. We also want to give a special thanks to Mr. Jodeiri and Mr. Pourakrami and Mr. Souti for their valuable support in the field of laboratory studies and the patients who participated in this study.
Footnotes
Authors’ Contribution:Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis, administrative, technical and material support and study supervision by Mojgan Mirghafourvand and Kolsoum Shokri; study concept and design, critical revision of the manuscript for important intellectual content and study supervision by Aziz Homayouni Rad, Sakineh Mohammad Alizadeh Charandabi and Zahra Fardiazar.
Conflict of Interest:The authors declare no conflict of interest.
Funding/Support:This study was supported by the Research Center of Tabriz University of Medical Sciences (Tabriz, Iran).
References
- 1.Cullen G, O'Donoghue D. Constipation and pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):807–18. doi: 10.1016/j.bpg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Chu H, Zhong L, Li H, Zhang X, Zhang J, Hou X. Epidemiology characteristics of constipation for general population, pediatric population, and elderly population in china. Gastroenterol Res Pract. 2014;2014:532734. doi: 10.1155/2014/532734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu LW. Chronic constipation: current treatment options. Can J Gastroenterol. 2011;25 Suppl B:22B–8B. [PMC free article] [PubMed] [Google Scholar]
- 4.Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31(9):938–49. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- 5.Amselem C, Puigdollers A, Azpiroz F, Sala C, Videla S, Fernandez-Fraga X, et al. Constipation: a potential cause of pelvic floor damage? Neurogastroenterol Motil. 2010;22(2):150–3. doi: 10.1111/j.1365-2982.2009.01409.x. e48. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109 Suppl 1:S2–26. doi: 10.1038/ajg.2014.187. quiz S27. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20(10):2433–48. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trottier M, Erebara A, Bozzo P. Treating constipation during pregnancy. Can Fam Physician. 2012;58(8):836–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Pillitteri A. Maternal and child health nursing: Care of the childbearing and childrearing family. 6 ed. Lippincott williams and wilkins; 2010. [Google Scholar]
- 11.Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008;108(10):1716–31. doi: 10.1016/j.jada.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Blaker P, Wilkinson M. Chronic constipation: diagnosis and current treatment options. Prescriber. 2010;21(9):30–45. [Google Scholar]
- 13.Jayasimhan S, Yap NY, Roest Y, Rajandram R, Chin KF. Efficacy of microbial cell preparation in improving chronic constipation: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2013;32(6):928–34. doi: 10.1016/j.clnu.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Longo SA, Moore RC, Canzoneri BJ, Robichaux A. Gastrointestinal Conditions during Pregnancy. Clin Colon Rectal Surg. 2010;23(2):80–9. doi: 10.1055/s-0030-1254294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homayouni A. Letter to the editor. Food Chem. 2009;114:1073. [Google Scholar]
- 16.Singh VP, Sharma J, Babu S, Singla A, Rizwanulla. Role of probiotics in health and disease: a review. J Pak Med Assoc. 2013;63(2):253–7. [PubMed] [Google Scholar]
- 17.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29(1):104–14. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 18.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26(3):475–86. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 19.Bouvier M, Meance S, Bouley C, Berta J, Grimaud J. Effects of consumptionof a milk fermented by the probiotic strain bifidobacterium animalis DN-173 010 on colonic transit times in healthy humans. Bioscience Microflora. 2001;20(2):43–8. [Google Scholar]
- 20.de Milliano I, Tabbers MM, van der Post JA, Benninga MA. Is a multispecies probiotic mixture effective in constipation during pregnancy? 'A pilot study'. Nutr J. 2012;11:80. doi: 10.1186/1475-2891-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tateyama I, Hashii K, Johno I, Iino T, Hirai K, Suwa Y, et al. Effect of xylooligosaccharide intake on severe constipation in pregnant women. J Nutr Sci Vitaminol (Tokyo). 2005;51(6):445–8. doi: 10.3177/jnsv.51.445. [DOI] [PubMed] [Google Scholar]
- 22.Mortazavian AM, Ehsani MR, Sohrabvandi S, Reinheimer JA. MRS-bile agar: its suitability for the enumeration of mixed probiotic cultures in cultured dairy products. Milchwissenschaft. 2007;62(3):270–2. [Google Scholar]
- 23.Homayouni A, Ehsani MR, Azizi A, Razavi SH, Yarmand MS. Spectrophotometricaly evaluation of probiotic growth in liquid media. Asian Journal of Chemistry. 2008;20(3):2414–20. [Google Scholar]
- 24.Homayouni Rad A. Tabriz: Tabriz University of Medical Sciences; 2008. Therapeutical effects of functional probiotic, prebiotic and synbiotic foods. [Google Scholar]
- 25.Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. Journal of Dairy Science. 2014;(12):7386–93. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 26.Homayouni A, Payahoo L, Azizi A. Effects of probiotics on lipid profile: A review. American Journal of Food Technology. 2012;(5):251–65. doi: 10.3923/ajft.2012.251.265. [DOI] [Google Scholar]
- 27.Ejtahed H, Mohtadi Nia J, Homayouni Rad A, Niafar M, Asghari Jafarabadi M, Mofid V, et al. The effects of probiotic and conventional yoghurt on diabetes markers and insulin resistance in type 2 diabetic patients: A randomized controlled clinical trial. [in Persian] Iranian Journal of Endocrinology and Metabolism. 2011;13(1):112. [Google Scholar]
- 28.Homayouni A, Azizi A, Javadi M, Mahdipour S, Ejtahed H. Factors influencing probiotic survival in ice cream: A review. Int J Dairy Sci. 2012;7:1–10. doi: 10.3923/ijds.2012.1.10. [DOI] [Google Scholar]
- 29.Ferdousi R, Rouhi M, Mohammadi R, Mortazavian AM, Khosravi-Darani K, Rad AH. Evaluation of probiotic survivability in yogurt exposed to cold chain interruption. Iranian Journal of Pharmaceutical Research. 2013;(12):137–42. [PMC free article] [PubMed] [Google Scholar]
- 30.Homayoni Rad A, Mehrabany EV, Alipoor B, Mehrabany LV, Javadi M. Do probiotics act more efficiently in foods than in supplements? Nutrition. 2012;(28):733–6. doi: 10.1016/j.nut.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Collete V, Araujo C, Madruga S. Prevalência e fatores associados a constipacao intestinal: um estudo de base populacional em Pelotas, Rio Grande do Sul, Brasil, 2007. Cad saude publica. 2010;26(7):1391–402. doi: 10.1590/s0102-311x2010000700018. [DOI] [PubMed] [Google Scholar]
- 32.Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res. 2005;14(3):875–82. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 33.An HM, Baek EH, Jang S, Lee DK, Kim MJ, Kim JR, et al. Efficacy of Lactic Acid Bacteria (LAB) supplement in management of constipation among nursing home residents. Nutr J. 2010;9:5. doi: 10.1186/1475-2891-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang YX, He M, Hu G, Wei J, Pages P, Yang XH, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14(40):6237–43. doi: 10.3748/wjg.14.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coccorullo P, Strisciuglio C, Martinelli M, Miele E, Greco L, Staiano A. Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: a double-blind, randomized, placebo-controlled study. J Pediatr. 2010;157(4):598–602. doi: 10.1016/j.jpeds.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 36.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. European J Clin Nutr. 2007;61(3):355–61. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 37.Pelczar MJ. In: Microbiology. NR CK, editor. Mc Graw-Hill; 1986. [Google Scholar]