Abstract
Background:
Previous studies suggested a relationship between aspirin use and mortality reduction. The mechanism for the effect of aspirin on cancer outcomes remains unclear. The aim of this study was to evaluate aspirin use and survival in patients with gastrointestinal tract cancer.
Methods:
Patients with gastrointestinal tract cancer diagnosed between 1998 and 2011 were included. The population-based Eindhoven Cancer Registry was linked to drug-dispensing data from the PHARMO Database Network. The association between aspirin use after diagnosis and overall survival was analysed using Cox regression models.
Results:
In total, 13 715 patients were diagnosed with gastrointestinal cancer. A total of 1008 patients were identified as aspirin users, and 8278 patients were identified as nonusers. The adjusted hazard ratio for aspirin users vs nonusers was 0.52 (95% CI 0.44–0.63). A significant association between aspirin use and survival was observed for patients with oesophageal, hepatobiliary and colorectal cancer.
Conclusions:
Post-diagnosis use of aspirin in patients with gastrointestinal tract malignancies is associated with increased survival in cancers with different sites of origin and biology. This adds weight to the hypothesis that the anti-cancer effects of aspirin are not tumour-site specific and may be modulated through the tumour micro-environment.
Keywords: gastrointestinal cancer, aspirin, epidemiology, drug repurposing
The incidence of cancer is increasing, particularly in low-and medium-resource countries; by the end of 2015 there were an estimated 15.2 million new cases globally with a predicted increase to 21.6 million by 2030 (Sullivan et al, 2015). The cost of health care is also increasing, and there is a real need for reasonably priced, widely available therapeutics to improve cancer outcomes. Although, the US Food and Drug Administration has approved a higher percentage of oncology drugs since 2008, many of these are expensive targeted agents with approvals based on surrogate end points, and infrequently improve overall survival (Kim and Prasad, 2015). Aspirin (acetylsalicylic acid) was originally synthesised and used as an analgesic in 1897, with the anti-platelet functions of low-dose aspirin subsequently discovered in the early 1970s. This latter discovery led to many large randomised controlled trials delineating the role of aspirin in the treatment and prevention of vascular disease. Retrospective long-term analyses of cancer outcomes in these randomised trials have revealed two interesting phenomena. Firstly, there was a 24% reduction in cancer incidence in patients allocated to aspirin, and this effect was seen across tumour types but was most marked in tumours arising from the gastrointestinal tract. Secondly, if cancers did develop they were less likely to have metastasised at presentation or subsequently if the patient received aspirin (Rothwell et al, 2011, 2012; Algra and Rothwell, 2012; Cook et al, 2013).
Much of the work to date relating to aspirin and cancer has focussed on colorectal cancer. In particular there have been several epidemiological studies showing a reduction in cancer mortality and improved overall survival for patients taking aspirin after a diagnosis of colorectal cancer (Chia et al, 2012; Jacobs et al, 2012; Paleari et al, 2015; Coyle et al, 2016; Elwood et al, 2016). This has led to several ongoing adjuvant studies in colorectal cancer; the Add-Aspirin trial (Langley and Rothwell, 2014), Adjuvant Aspirin for Colon Cancer (NCT02467582), the ALASCCA trial (NCT02647099), the ASCOLT trial (NCT00565708) and the Aspirin trial (NCT02301286). In addition, two other randomised controlled trials have focussed on primary prevention, and after long-term follow-up showed a beneficial effect on primary prevention in both hereditary and sporadic colorectal cancer (Burn et al, 2011; Cook et al, 2013). Also, a meta-analysis of four other randomised controlled trials showed an absolute risk reduction of 6.7% for the recurrence of adenoma's in patients with a history of these lesions (Cole et al, 2009).
The mechanism(s) underlying the beneficial effects of aspirin on cancer outcomes remains unclear. Several different potential biomarkers have been investigated, but due to the multiple potential cellular pathways and conflicting results of previous studies, the mechanism of action remains unknown; although platelets may have a central role (Langley et al, 2011). The aim of this study was to provide epidemiological evidence and further mechanistic insights on the potential beneficial effects of aspirin use after diagnosis of cancer that arises from any part of the gastrointestinal tract. Because many studies have tried to differentiate effects of aspirin use both before and after diagnosis, an additional analysis was performed including the patients that use aspirin both pre- and post diagnosis.
Materials and methods
Study population
Data from the Eindhoven Cancer Registry was used to identify patients diagnosed with cancer of the gastrointestinal tract between January 1998 and December 2011 in the south of the Netherlands. This area is served by 10 hospitals, covers a demographic region of approximately 1.5 million Dutch citizens and is part of the nationwide Netherlands Comprehensive Cancer Organisation. The Eindhoven Cancer Registry is linked to the municipal population registry, which records the vital status (alive/dead) of all inhabitants. Patients are informed about the registration and registered except patients who objected to be registered. The Netherlands Cancer Registry is obliged to work according to the law about protection of privacy data; informed consent of the patients for this specific study was not applicable. Patient selection and data cleaning was performed by the Eindhoven Cancer Registry. Follow-up for this project was until 31 December 2012.
The PHARMO Database Network is a population-based network which combines data from different health-care settings in the Netherlands. For this study the out-patient pharmacy database was used, which contains drug-dispensing records from all community pharmacies. Drugs are coded using the Anatomical Therapeutic Chemical classification (www.whocc.no/atc_ddd_index) and the records include information on the type of product, date prescribed, dose and regimen, quantity and route of administration. The PHARMO database was linked to the Eindhoven Cancer Registry and thus allows drug use by cancer patients to be analysed (van Herk-Sukel et al, 2010). From this linked database, prescriptions for aspirin (only the ones that were actually dispensed) were selected.
Definition of users and nonusers
For this study, patients older than 18 years who used aspirin after a diagnosis of a gastrointestinal cancer were selected. The gastrointestinal tumours were coded according to the International Classification of Disease 10 [ICD-10] C15-C26. This comprises cancer from the following sites: oesophagus; stomach; small intestine; colon; recto-sigmoid and rectum; anus; liver and intra hepatic bile ducts; gallbladder and extra hepatic bile ducts; pancreas; and a group ‘gastrointestinal tumours not otherwise specified (nos)'.
Patients who used aspirin before diagnosis were excluded from the analyses. Aspirin users (ATC codes: B01AC06, B01AC08, B01AC56, N02BA01, N02BA15, N02BA51, N02BA65) were defined as those prescribed aspirin for at least 30 days. Nonusers were defined as patients who received for less than 30 days or never used aspirin. Time after diagnoses was defined in periods of use and no use by analysing each single prescription during follow-up. Periods of less than 14 days in between two prescriptions were considered consecutive. Follow-up started 14 days after diagnosis because there was no information about in-hospital use of medication. Immortal time bias is avoided by analysing prescriptions as a time-varying covariate, in periods of use and no use (Stricker and Stijnen, 2010; Suissa and Azoulay, 2012).
Statistical analysis
Information from the ECR-contained information about the presence or absence of the following comorbidities at cancer diagnosis: lung disease; cardiovascular disease; diabetes and disorders of the gastrointestinal tract; urinary tract; nervous system; musculoskeletal system; and a group of other comorbidities. Comorbidity was analysed as 0, 1 or ⩾2 comorbidities. A χ2-test was used to assess baseline characteristics for categorical values.
Survival analysis were performed with the Simon–Makuch method, an alternative for Kaplan–Meier and with the ability to process time-varying covariates in survival curves (Simon and Makuch, 1984). A Cox proportional hazards model was used with aspirin use as a time-varying covariate, as described by Stricker and Stijnen (2010). Schoenfeld residuals were tested to verify the assumption of proportional hazards. Follow-up duration (survival) was recorded in months from diagnosis (t=0). Multivariable survival models were built with the following covariates: age at diagnosis (continuous), sex, stage of cancer (categorical), number of comorbidities (0, 1 or ⩾2), treatment (surgery yes/no, radiotherapy yes/no and chemotherapy yes/no). Missing/unknown values were included in the multivariable model as missing indicator. Analysis were performed using Stata statistical software version 12 (StataCorp, 2011). Statistical tests were two-sided and considered significant at the P<0.05 level.
Relative survival rates were used to take into account the risk of dying from causes other than the disease of interest. The excess mortality reflects the difference between the overall survival of patients and the survival that would be expected in the absence of cancer. The excess mortality was calculated as the ratio of the observed (all-cause) survival proportion to the expected survival proportion (Dickman and Adami, 2006). National life tables were used to estimate background mortality (expected survival) according to sex, year of age and incidence year. Relative excess risks were estimated using a flexible parametric model, implemented in the Stata command stpm2 (Lambert and Royston, 2009).
Different parts of the gastrointestinal tract were analysed separately if there were as at least 10 aspirin users (therefore small bowel, anal cancer and gastrointestinal tumours NOS were not considered separately). Histological subtypes (adenocarcinoma and squamous cell carcinomas) were also analysed separately in groups with at least 10 aspirin users. Statistical interaction for this subgroup was tested by including an interaction term in the model of aspirin use and histological subtype and significance was assessed using the Wald test. A sensitivity analysis was performed by repeating the analysis and excluding patients with stage IV disease and separately repeating the analysis and excluding the first year of follow-up from the analysis. The main analysis and all subgroup analysis were pre-planned.
Pre- and post-diagnosis use of aspirin
For the analysis in patients that use both aspirin before and after diagnosis, the groups were selected with the same method as described in the ‘definition of users and nonusers' heading. The only difference was that patients who started aspirin use before diagnosis and continued this after diagnosis were selected for the group of users of aspirin. Patients using aspirin only after diagnosis were excluded for this analysis. The statistical analysis was also equal to the analysis described above, where aspirin use was analysed as time-varying covariate, and the same factors were used for the multivariable analysis.
Results
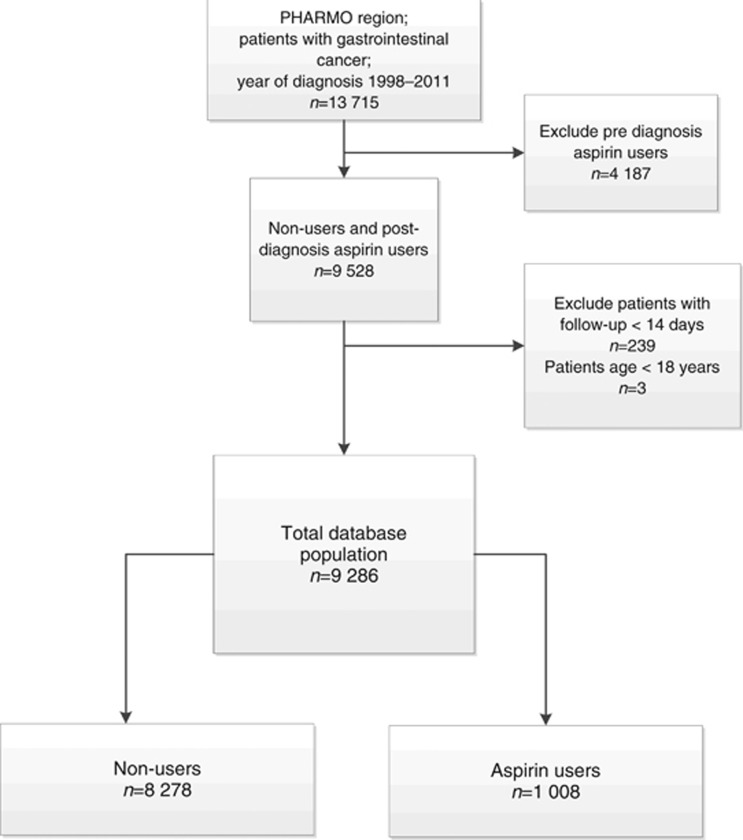
In total 13 715 patients were identified with a cancer of the gastrointestinal tract diagnosed between January 1998 and December 2011. The following were excluded from the analysis (CONSORT diagram Figure 1): 4187 patients who were using aspirin before diagnosis, 239 patients with follow-up of less than 14 days and three patients below the age of 18 years. Thus, 9286 patients were included in the survival analysis, of which 8278 patients (person years: 4375) did not use aspirin and 1008 (person years: 2150) used aspirin after diagnosis. In total, 5138 events (deaths) were recorded. Table 1 shows the characteristics of this population.
Figure 1.
Flowchart of patients selected for analysis.
Table 1. Characteristics of the cohort.
| All patients | % | No aspirin use | % | Aspirin post diagnosis | % | P-value | |
|---|---|---|---|---|---|---|---|
| Total | 9286 | 100 | 8278 | 89 | 1008 | 11 | |
| Location tumour | |||||||
| Oesophageal cancer | 946 | 10.19 | 886 | 10.7 | 60 | 5.95 | |
| Gastric cancer | 750 | 8.08 | 700 | 8.46 | 50 | 4.96 | |
| Small intestine cancer | 97 | 1.04 | 88 | 1.06 | 9 | 0.89 | |
| Colon cancer | 3977 | 42.83 | 3434 | 41.48 | 543 | 53.9 | <0.001 |
| Rectal cancer | 2358 | 25.39 | 2069 | 24.99 | 289 | 28.7 | |
| Anal cancer | 67 | 0.72 | 60 | 0.72 | 7 | 0.69 | |
| Hepatobiliary cancer | 385 | 4.15 | 360 | 4.35 | 25 | 2.5 | |
| Pancreatic cancer | 692 | 7.45 | 667 | 8.06 | 25 | 2.47 | |
| Cancer of the gastrointestinal tract nos | 14 | 0.15 | 14 | 0.17 | 0 | 0.0 | |
| Sex | |||||||
| Male | 5140 | 55.35 | 4517 | 54.6 | 623 | 61.8 | <0.001 |
| Female | 4146 | 44.65 | 3761 | 45.43 | 385 | 38.19 | |
| Age mean (s.d.). Median (IQR) | 67.1 (11) | 68 (60–75) | 66.7 (12) | 68 (59–76) | 67.7 (9.9) | 69 (61–74) | |
| 18–60 Years | 2420 | 26.06 | 2219 | 26.8 | 201 | 19.9 | |
| 60–69 Years | 2763 | 29.75 | 2437 | 29.4 | 326 | 32.3 | <0.001 |
| 70–79 years | 2831 | 30.49 | 2464 | 29.8 | 367 | 36.4 | |
| 80 Years and older | 1272 | 13.7 | 1158 | 14 | 114 | 11.3 | |
| Stage | |||||||
| 0 | 204 | 2.2 | 176 | 2.13 | 28 | 2.78 | |
| I | 1496 | 16.11 | 1258 | 15.2 | 238 | 23.56 | |
| II | 2222 | 23.93 | 1900 | 22.95 | 322 | 31.94 | <0.001 |
| III | 2058 | 22.16 | 1788 | 21.6 | 270 | 26.8 | |
| IV | 2249 | 24.22 | 2162 | 26.12 | 87 | 8.63 | |
| Unknown | 1057 | 11.4 | 994 | 12.01 | 63 | 6.25 | |
| Surgery | |||||||
| No | 2693 | 29 | 2603 | 31.44 | 90 | 8.93 | <0.001 |
| Yes | 6593 | 71 | 5675 | 68.56 | 918 | 91.07 | |
| Chemotherapy | |||||||
| No | 6544 | 70.47 | 5798 | 70.04 | 746 | 74.01 | 0.009 |
| Yes | 2742 | 29.53 | 2480 | 29.96 | 262 | 25.99 | |
| Radiotherapy | |||||||
| No | 7042 | 75.83 | 6291 | 76 | 751 | 74.5 | 0.3 |
| Yes | 2244 | 24.17 | 1987 | 24 | 257 | 25.5 | |
| Comorbidities | |||||||
| None | 3383 | 36.43 | 3056 | 36.92 | 327 | 32.44 | 0.05 |
| One | 2664 | 28.69 | 2359 | 28.5 | 305 | 30.26 | |
| Two or more | 2295 | 24.71 | 2027 | 24.49 | 268 | 26.63 | |
| Unknown | 944 | 10.17 | 836 | 10.1 | 108 | 10.69 | |
| Morphology | |||||||
| Adenocarcinoma | 8343 | 89.84 | 7378 | 89.13 | 965 | 95.73 | <0.001 |
| Squamous cell carcinoma | 298 | 3.21 | 280 | 3.38 | 18 | 1.79 | |
| Epithelial | 140 | 1.51 | 135 | 1.63 | 5 | 0.5 | |
| Gastrointestinal Stromal tumour | 58 | 0.62 | 50 | 0.6 | 8 | 0.79 | |
| Other (not specified) | 447 | 4.81 | 435 | 5.25 | 12 | 1.19 | |
| Months survival, median (IQR) | 48 | (15.4–95.4) | 24 | (7.5–58.6) | 89.4 | (54.8–132.6) |
Abbreviations: IQR=interquartile range; nos=not otherwise specified; s.d.=standard deviation.
The majority of patients were diagnosed with colon cancer (43%), rectal cancer (25%) and oesophageal cancer (10%). Median age at diagnosis was 68 years (interquartile range (IQR) 59–76) in the aspirin group and 69 (IQR 61–74) in the nonusers group. Aspirin users were less often female and more frequently diagnosed with stages I and II disease compared with nonusers. In the nonusers group, 26% of patients had stage IV disease compared with 9% in the aspirin users group. Median survival for all patients was 48 months.
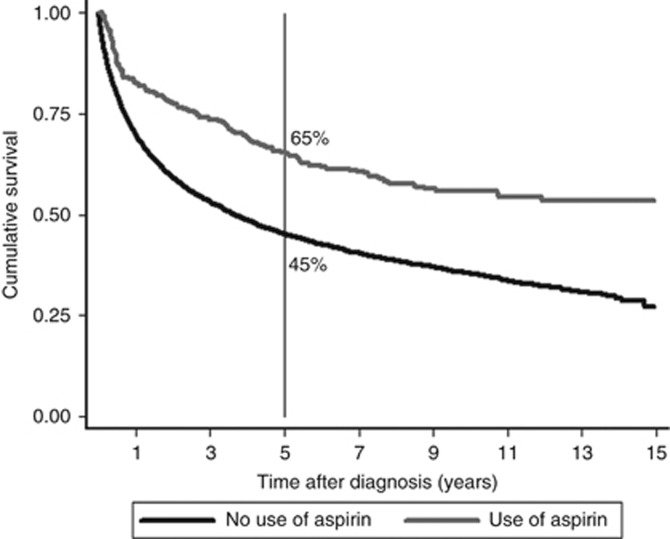
Figure 2 shows survival curves for users of aspirin after diagnosis vs nonusers. In the group of aspirin users, 65% (95% CI 59–71%) of patients was alive after five years, in contrast to nonusers, where 45% (95% CI 44–46%) of patients was alive after five years.
Figure 2.
Survival comparison for aspirin users vs nonusers with Simon–Makuch method.
A Cox proportional hazard model was used with use of aspirin as a time-varying covariate. The proportional hazard assumption was fulfilled. For all patients with gastrointestinal cancer, aspirin use was associated with a significant reduction in overall mortality, hazard ratio (HR) 0.57 (95% confidence interval (CI) 0.48–0.69) (Table 2). Adjusted for age at diagnosis, sex, stage of cancer, number of comorbidities, treatment (surgery, radiotherapy, and chemotherapy) the multivariable HR was 0.52 (95% CI 0.44–0.63).
Table 2. Time-dependent survival analysis (overall survival) stratified according to tumour type.
|
Overall survival |
Relative survival |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. at risk | No. events | Crude hazard ratio | 95% CI | P-value | Adjusted hazard ratioa | 95% CI | P-value | Crude relative excess risk | 95% CI | Adjusted relative excess riska | 95% CI | |
|
Aspirin users vs nonusers (n=9286) | ||||||||||||
| Nonusers | 8278 | 4776 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin Users | 1008 | 362 | 0.57 | 0.48–0.69 | <0.001 | 0.52 | 0.44–0.63 | <0.001 | 0.41 | 0.30–0.56 | 0.44 | 0.33–0.58 |
|
Aspirin users vs nonusers per tumour type | ||||||||||||
| Oesophageal cancer (n=946) | ||||||||||||
| Nonusers | 886 | 722 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin Users | 60 | 30 | 0.42 | 0.22–0.81 | 0.01 | 0.45 | 0.23–0.88 | 0.02 | 0.34 | 0.15–0.77 | 0.31 | 0.14–0.70 |
| Gastric cancer (n=750) | ||||||||||||
| Nonusers | 700 | 555 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin Users | 50 | 30 | 0.75 | 0.41–1.36 | 0.34 | 0.87 | 0.47–1.61 | 0.66 | 0.50 | 0.19–1.29 | 0.70 | 0.29–1.70 |
| Pancreatic cancer (n=692) | ||||||||||||
| Nonusers | 667 | 631 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin users | 25 | 21 | 1.06 | 0.58–1.93 | 0.84 | 0.81 | 0.44–1.48 | 0.49 | 1.03 | 0.56–1.89 | 0.77 | 0.42–1.41 |
| Hepatobiliary cancer (n=385) | ||||||||||||
| Nonusers | 360 | 311 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin Users | 25 | 10 | 0.39 | 0.16–0.95 | 0.04 | 0.34 | 0.14–0.84 | 0.02 | 0.34 | 0.18–1.06 | 0.35 | 0.13–0.95 |
| Colon cancer (n=3977) | ||||||||||||
| Nonusers | 3434 | 1587 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin Users | 543 | 178 | 0.62 | 0.48–0.80 | <0.001 | 0.56 | 0.43–0.72 | <0.001 | 0.41 | 0.23–0.72 | 0.44 | 0.27–0.72 |
| Rectal cancer (n=2358) | ||||||||||||
| Nonusers | 2069 | 891 | 1 (Reference) | 1 (Reference) | ||||||||
| Aspirin Users | 289 | 85 | 0.51 | 0.33–0.77 | 0.001 | 0.41 | 0.27–0.63 | <0.001 | 0.26 | 0.09–0.78 | 0.25 | 0.09–0.68 |
Abbreviation: CI, confidence interval.
Adjusted for stage, sex, age at diagnosis, surgery, radiotherapy, chemotherapy and comorbidities.
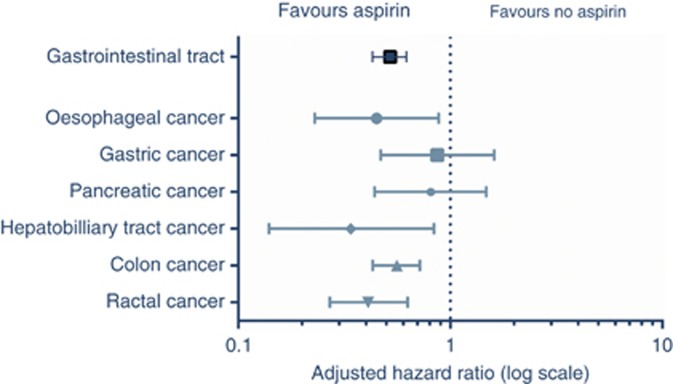
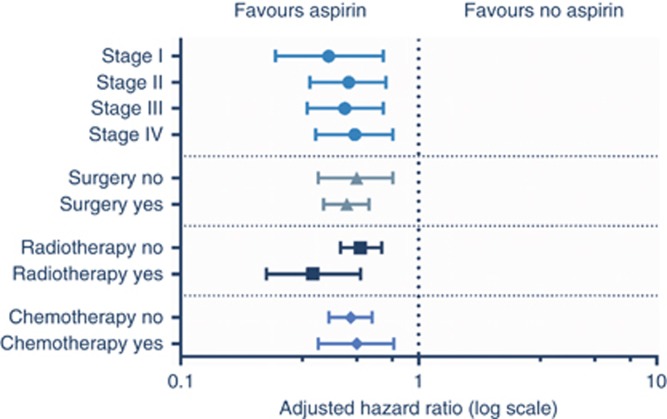
Stratification according to tumour type is shown in Table 2 and Figure 3. In patients with oesophageal, colon, rectal, and hepatobiliary tract cancer a significant association was found between the use of aspirin after diagnosis and overall survival. For patients with gastric and pancreatic cancer using aspirin, there was no statistically significant increase in survival. A survival benefit with aspirin was seen regardless of the stage of cancer at presentation and after all primary treatment modalities including chemotherapy, radiotherapy or surgery (Figure 4).
Figure 3.
Overall survival analysis for aspirin users vs nonusers stratified according to tumour type.
Figure 4.
Forest plot of adjusted overall survival analysis in patients with gastrointestinal malignancies grouped according to aspirin users vs nonusers and stratified for stage and treatment.
Table 2 additionally shows the relative survival estimates which are a good estimation of the cancer-specific survival (Sarfati et al, 2010). Equal to the overall survival rates, the observed relative excess risks were significant in patients with oesophageal cancer, hepatobiliary cancer, colon and rectal cancer.
Squamous cell cancers accounted for 3% of the total cohort of which, 81% (n=242) arose from the oesophagus and 18% (n=53) were anal cancers. Patients with adenocarcinoma of the oesophagus who used aspirin had an adjusted HR 0.24 (95% CI 0.10–0.59) for overall survival, while those with a squamous cell carcinoma of the oesophagus had a HR for overall survival of 1.02 (95% CI 0.37–2.83) for aspirin users compared with nonusers. The test for heterogeneity of the effect of aspirin in patients with oesophageal squamous cell carcinoma vs patients with oesophageal adenocarcinoma was significant (P for interaction=0.01).
In 72% of prescriptions, 2435 in total, the dose was reported. Of all prescribed dosages, 98% were 100 mg daily or lower. It was therefore not possible to analyse a dose–effect relationship, because only 31 prescriptions were for higher-dose aspirin.
The sensitivity analysis with the exclusion of the first year follow-up showed a similar effect, with an unadjusted HR of 0.56 (95% C.I. 0.45–0.69) and adjusted HR 0.49 (0.39–0.61). The sensitivity analysis or stages I–III showed an adjusted HR 0.49 (95% CI 0.39–0.61), consistent with the stratified analysis by stage in Figure 4.
The analysis in the patients that use aspirin both pre- and post diagnosis can be found in Table 3. Supplementary Table 1 and Supplementary Figure 1 show the PRISMA flowchart for this cohort and the patient characteristics.
Table 3. Time-dependent survival analysis (overall survival) stratified according to tumour type with prediagnosis aspirin users.
|
Overall survival |
||||||||
|---|---|---|---|---|---|---|---|---|
| No. at risk | No. events | Crude hazard ratio | 95% CI | P-value | Adjusted hazard ratioa | 95% CI | P-value | |
|
Aspirin users vs nonusers (n=12 109) | ||||||||
| Nonusers | 8366 | 4913 | 1 (Reference) | 1 (Reference) | ||||
| Pre and post-diagnosis aspirin usersb | 2736 | 1647 | 0.69 | 0.64–0.75 | <0.001 | 0.61 | 0.57–0.66 | <0.001 |
|
Aspirin users vs nonusers per tumour type | ||||||||
| Oesophageal cancer (n=1180) | ||||||||
| Nonusers | 894 | 741 | 1 (Reference) | 1 (Reference) | ||||
| Aspirin Usersb | 286 | 229 | 0.64 | 0.52–0.79 | <0.001 | 0.61 | 0.49–0.76 | <0.001 |
| Gastric cancer (n=933) | ||||||||
| Nonusers | 714 | 574 | 1 (Reference) | 1 (Reference) | ||||
| Aspirin Usersb | 219 | 184 | 0.90 | 0.72–1.13 | 0.37 | 0.85 | 0.67–1.07 | 0.17 |
| Pancreatic cancer (n=876) | ||||||||
| Nonusers | 681 | 648 | 1 (Reference) | 1 (Reference) | ||||
| Aspirin Usersb | 195 | 183 | 0.68 | 0.54–0.84 | <0.001 | 0.67 | 0.53–0.84 | 0.001 |
| Hepatobiliary cancer (n=477) | ||||||||
| Nonusers | 364 | 317 | 1 (Reference) | 1 (Reference) | ||||
| Aspirin Usersb | 113 | 101 | 0.81 | 0.61–1.08 | 0.16 | 0.69 | 0.51–0.93 | 0.02 |
| Colon cancer (n=4730) | ||||||||
| Nonusers | 3469 | 1642 | 1 (Reference) | 1 (Reference) | ||||
| Aspirin Usersb | 1261 | 612 | 0.67 | 0.59–0.76 | <0.001 | 0.55 | 0.48–0.63 | <0.001 |
| Rectal cancer (n=2687) | ||||||||
| Nonusers | 2080 | 910 | 1 (Reference) | 1 (Reference) | ||||
| Aspirin Usersb | 607 | 306 | 0.78 | 0.65–0.94 | 0.008 | 0.63 | 0.52–0.75 | <0.001 |
Abbreviation: CI=confidence interval.
Adjusted for stage, sex, age at diagnosis, surgery, radiotherapy, chemotherapy and comorbidities.
Only patients using aspirin both pre- and post diagnosis were analysed.
Discussion
Aspirin use after diagnosis of a gastrointestinal malignancy is associated with significantly lower mortality rates and this effect remains after adjusting for potential cofounders. It was most marked for tumours arising from the oesophagus, colon, rectum and hepatobilliary tract. This large cohort study of almost 9300 patients is the first observational cohort study evaluating the association of aspirin and survival in various gastrointestinal malignancies. The statistically significant effect on survival seen in patients with tumours of the oesophagus, colon and rectum is consistent with data from other published studies (Chia et al, 2012; Jacobs et al, 2012; Macfarlane et al, 2015; Coyle et al, 2016; Elwood et al, 2016). The effect in the tumour types was also present in patients that used aspirin both pre- and post diagnosis.
In a recent prospective cohort study, Cao et al (2016) found that the reduced overall reduced cancer risk associated with the use of aspirin was primarily owing to gastrointestinal tract cancers. In addition, in a meta-analysis of randomised trials evaluating aspirin for the prevention of cardiovascular disease, Rothwell et al (2011) showed a reduced risk of cancer-specific death with aspirin (HR of 0.79 (95% CI 0.68–0.92)) in all types of cancer. Stratified for tumour location, the largest benefit was found in patients with gastrointestinal tumours, with no significant heterogeneity between different gastrointestinal cancers. Consistent with our study they also showed that patients with adenocarcinomas were most likely to benefit from aspirin HR 0.70 (95% CI 0.54–0.91). However, in contrast, patients in our study only started aspirin after diagnosis of cancer, which is most relevant when considering recommendations for subsequent management after a cancer diagnosis. In our study, 11% of patients started using aspirin after diagnosis, which is also consistent with previous studies in cancer patients (McCowan et al, 2013).
A strength of our study is that the data is derived from linked cancer registry and pharmacy data, eliminating both recall and information bias. Although we cannot verify that patients actually ingested the aspirin, the prescriptions registered by the PHARMO institute are actually handed out to the patients by the pharmacy and this therefore adds weight to the definition of user. In addition, immortal time bias and misclassification of exposure in follow-up is avoided by the use of a Cox proportional hazards model with time-varying covariate (Suissa and Azoulay, 2012). With this technique, accurate risk estimates are provided as each individual prescription is analysed (Stricker and Stijnen, 2010). Moreover, the exclusion of patients already using aspirin before diagnosis and the determination of patient characteristics at diagnosis (t=0) mimics the use of aspirin as adjuvant therapy. In our study, patients are identified at diagnosis but before they are exposed to the treatment of interest and differentiated into groups of users and nonusers. This ‘new-user design' eliminates important biases associated with observational studies (Ray, 2003). In addition, it has been suggested that for measuring the side effects of drugs, which the effect of aspirin on cancer could theoretically be considered, observational data could in some cases be considered non-inferior to results from randomised controlled trials (Vandenbroucke, 2004). Our study has limitations. First, since exposure to aspirin depends on a clinician's decision to prescribe aspirin to a certain patient, it is prone to confounding by selective prescribing. For instance, oncologists may withhold aspirin treatment (as secondary prevention for cardiovascular disease) in patients diagnosed with incurable (stage IV) cancer because of the poor prognosis. Thus patients with a particularly poor prognosis may end up in the non-user group. This reverse causation was addressed by the pre-planned sensitivity analysis excluding the first year of follow-up, which restricted the study population to patients alive at one year after diagnosis. By introducing this one year exposure lag, any undiagnosed recurrence at baseline or early recurrence would have been likely to become apparent and therefore baseline prognosis between the two groups is believed to be more similar (Chubak et al, 2013). Second, proven cardiovascular disease is the main indication for low-dose aspirin in The Netherlands. This could imply that patients prescribed aspirin have a worse life expectancy at baseline because of lifestyle factors and risks associated with both cardiovascular disease and cancer development. Considering the absence of information on cancer-specific survival and cause of death in our study, hypothetically part of the overall survival gain we observed could be explained by the prevention of cardiovascular mortality. However, in a large meta-analysis of individual participant data, the reduction in vascular-specific mortality from aspirin was only 9%, HR 0.91 (Baigent et al, 2009). Therefore a reduction in cardiovascular mortality could only partly explain the reduction in mortality we observed. Several of the studies evaluating the effect of aspirin use after a diagnosis of colorectal cancer have shown a significant reduction in colorectal cancer-specific mortality (Chia et al, 2012; Coyle et al, 2016). Third, over the counter aspirin use was not included. However, prescription data can give valid estimations of association even though available over the counter (Yood et al, 2007). No data were available to adjust for lifestyle factors, health-related behaviour and mutational status. Lastly, Table 1 shows that the groups aspirin users and nonusers are different with respect to baseline characteristics. This is to a large extent the result of the size of the cohort. After adjustment for these factors the association between aspirin use and survival remained significant. Nevertheless, the confounding by indication as described remains, and therefore randomised controlled trials remain inevitable before aspirin can be used as regular anti-cancer treatment.
The mechanism responsible for the effect of aspirin on cancer remains unknown. Aspirin (acetylsalicylic acid) reduces prostanoid generation by irreversible inhibition of platelet COX-1 (cyclooxygenase-1) and COX-2 isozymes. Activated platelets release several growth factors which impact on tumour progression and metastasis (Bruno et al, 2012). Maximum platelet inactivation by COX-1 is thought to be obtained by low-dose aspirin (75–100 mg daily) and over 95% of platelet activity is inhibited for up to 24 h (Patrignani et al, 2014). A number of potential biomarkers have been identified as predictors of response to aspirin in terms of cancer outcomes. Chan et al (2009) reported that the effect of high-dose (325 mg) aspirin after a colorectal cancer diagnosis was predominantly in patients with COX-2 (also called PTGS2) overexpression. However, to achieve constant inhibition of COX in tissues, the administered daily dose of aspirin would have to be higher than 2000 mg (Thun et al, 2012). In some studies mutations in phosphatidylinositol 3-kinase (PIK3CA) have been associated with aspirin response; however, in a previous study we did not find this association but showed that the effect of aspirin was associated with tumours that expressed human leukocyte antigen (HLA) class1 molecules (Reimers et al, 2014; Paleari et al, 2015).
Our observation that aspirin use is similarly associated with good prognosis in various tumour types with clearly different biology makes a nonspecific mode of action plausible. It is possible that aspirin executes its effect by inhibiting platelet aggregation around circulating epithelial tumour cells, irrespective of organ site which then facilitates immune clearance. The coming years will hopefully provide answers. Several randomised clinical trials have commenced in the past years (NCT02647099 (Langley and Rothwell, 2014), NCT02467582, NCT02301286, NCT00565708). Many of these trials are united in the ‘Aspirin Trialist Collaborative Group' and will pool results regarding clinical outcome and expression of biomarkers.
Conclusion
Aspirin use after diagnosis of gastrointestinal malignancies is associated with improved overall survival. This observation makes a nonspecific mode of action for aspirin on cancer plausible. These results offer direction towards future studies, both in terms of new randomised controlled trials as well as further studies to identify biomarkers that predict response to aspirin.
Acknowledgments
MPPVH-S is an employee of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related health-care authorities and several pharmaceutical companies. However, this study was not financially supported by a pharmaceutical company. R.E.L. has received financial support through grants from Cancer Research UK and the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) as Chief Investigator of the Add-Aspirin Trial, has received compensation from Bayer and Aspirin Foundation for service on scientific advisory boards, and has received a supply of aspirin and placebo from Bayer Pharmaceuticals AG for the Add-Aspirin Trial.
Author contributions
MAF contributed to study design, data preparation, data analysis and interpretation, prepared the first draft of the report, contributed to subsequent versions and the final report. EB contributed to study design, data preparation, data analysis and interpretation, contributed to the first draft of the report and drafting of the final report. REL and WKC contributed to data interpretation, reviewed the first draft of the report, contributed to subsequent drafts and the final report. HP contributed to study design, data preparation, data analysis and interpretation and contributed to the drafting of the final report. MPPVH-S, VEPPL, HHH, BB, CJHVDV and JEAP contributed to data interpretation and drafting of the final report. GJL initiated the study, contributed to data analysis and interpretation, contributed to the first draft of the report, subsequent versions and the final version. G.J.L. had full access to all the data in the study and the final responsibility for the decision to submit the article for publication.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Algra AM, Rothwell PM (2012) Effects of regular aspirin on long-term qcancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 13(5): 518–527. [DOI] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373(9678): 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A, Dovizio M, Tacconelli S, Patrignani P (2012) Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract. Best Pract Res Clin Gastroenterol 26(4): e1–e13. [DOI] [PubMed] [Google Scholar]
- Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard ML, Dunlop MG, Ho JW, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJ, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT (2011) Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378(9809): 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, Spiegelman D, Fuchs CS, Giovannucci EL, Chan AT (2016) Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol 2(6): 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS (2009) Aspirin use and survival after diagnosis of colorectal cancer. JAMA 302(6): 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia WK, Ali R, Toh HC (2012) Aspirin as adjuvant therapy for colorectal cancer—reinterpreting paradigms. Nat Rev Clin Oncol 9(10): 561–570. [DOI] [PubMed] [Google Scholar]
- Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS (2013) Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst 105(19): 1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101(4): 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE (2013) Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med 159(2): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C, Cafferty FH, Langley RE (2016) Aspirin and colorectal cancer prevention and treatment: is it for everyone? Curr Colorectal Cancer Rep 12: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman PW, Adami HO (2006) Interpreting trends in cancer patient survival. J Intern Med 260(2): 103–117. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, Kelson M, Dolwani S (2016) Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One 11(4): e0152402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EJ, Newton CC, Gapstur SM, Thun MJ (2012) Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst 104(16): 1208–1217. [DOI] [PubMed] [Google Scholar]
- Kim C, Prasad V (2015) Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 Years of US Food and Drug Administration approvals. JAMA Intern Med 175(12): 1992–1994. [DOI] [PubMed] [Google Scholar]
- Lambert PC, Royston P (2009) Further development of flexible parametric models for survival analysis. Stata J 9(2): 265. [Google Scholar]
- Langley RE, Burdett S, Tierney JF, Cafferty F, Parmar MK, Venning G (2011) Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br J Cancer 105(8): 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RE, Rothwell PM (2014) Aspirin in gastrointestinal oncology: new data on an old friend. Curr Opin Oncol 26(4): 441–447. [DOI] [PubMed] [Google Scholar]
- Macfarlane TV, Murchie P, Watson MC (2015) Aspirin and other non-steroidal anti-inflammatory drug prescriptions and survival after the diagnosis of head and neck and oesophageal cancer. Cancer Epidemiol 39(6): 1015–1022. [DOI] [PubMed] [Google Scholar]
- McCowan C, Munro AJ, Donnan PT, Steele RJ (2013) Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer 49(5): 1049–1057. [DOI] [PubMed] [Google Scholar]
- Paleari L, Puntoni M, Clavarezza M, DeCensi M, Cuzick J, DeCensi A (2015) PIK3CA mutation, aspirin use after diagnosis and survival of colorectal cancer. a systematic review and meta-analysis of epidemiological studies. Clin Oncol (R Coll Radiol) 28(5): 317–326. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Tacconelli S, Piazuelo E, Di Francesco L, Dovizio M, Sostres C, Marcantoni E, Guillem-Llobat P, Del Boccio P, Zucchelli M, Patrono C, Lanas A (2014) Reappraisal of the clinical pharmacology of low-dose aspirin by comparing novel direct and traditional indirect biomarkers of drug action. J Thromb Haemost 12(8): 1320–1330. [DOI] [PubMed] [Google Scholar]
- Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158(9): 915–920. [DOI] [PubMed] [Google Scholar]
- Reimers MS, Bastiaannet E, Langley RE, van ER, van Vlierberghe RL, Lemmens VE, van Herk-Sukel MP, van WT, Fodde R, Kuppen PJ, Morreau H, van de Velde CJ, Liefers GJ (2014) Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med 174(5): 732–739. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377(9759): 31–41. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW (2012) Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 379(9826): 1602–1612. [DOI] [PubMed] [Google Scholar]
- Sarfati D, Blakely T, Pearce N (2010) Measuring cancer survival in populations: relative survival vs cancer-specific survival. Int J Epidemiol 39(2): 598–610. [DOI] [PubMed] [Google Scholar]
- Simon R, Makuch RW (1984) A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med 3(1): 35–44. [DOI] [PubMed] [Google Scholar]
- StataCorp (2011) Stata Statistical Software: Release 12. College Station, TX, USA: StataCorp LP.
- Stricker BH, Stijnen T (2010) Analysis of individual drug use as a time-varying determinant of exposure in prospective population-based cohort studies. Eur J Epidemiol 25(4): 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S, Azoulay L (2012) Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35(12): 2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, Balch C, Brennan MF, Dare A, D'Cruz A, Eggermont AMM, Fleming K, Gueye SM, Hagander L, Herrera CA, Holmer H, Ilbawi AM, Jarnheimer A, Ji J-f, Kingham TP, Liberman J, Leather AJM, Meara JG, Mukhopadhyay S, Murthy SS, Omar S, Parham GP, Pramesh CS, Riviello R, Rodin D, Santini L, Shrikhande SV, Shrime M, Thomas R, Tsunoda AT, van de Velde C, Veronesi U, Vijaykumar DK, Watters D, Wang S, Wu Y-L, Zeiton M, Purushotham A (2015) Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 16(11): 1193–1224. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Jacobs EJ, Patrono C (2012) The role of aspirin in cancer prevention. Nat Rev Clin Oncol 9(5): 259–267. [DOI] [PubMed] [Google Scholar]
- van Herk-Sukel MP, van de Poll-Franse LV, Lemmens VE, Vreugdenhil G, Pruijt JF, Coebergh JW, Herings RM (2010) New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer 46(2): 395–404. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP (2004) When are observational studies as credible as randomised trials? Lancet 363(9422): 1728–1731. [DOI] [PubMed] [Google Scholar]
- Yood MU, Campbell UB, Rothman KJ, Jick SS, Lang J, Wells KE, Jick H, Johnson CC (2007) Using prescription claims data for drugs available over-the-counter (OTC). Pharmacoepidemiol Drug Saf 16(9): 961–968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.