Abstract
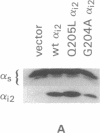
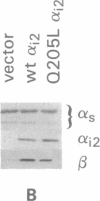
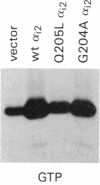
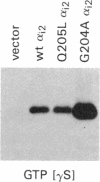
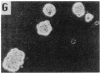
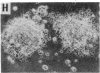
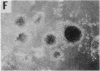
Previous studies have demonstrated that mutations of highly conserved residues in the alpha subunit of Gs (alpha s) can inhibit either the intrinsic GTPase activity (glutamine-227 to leucine, Q227L) or the ability of the protein to be activated by GTP (glycine-226 to alanine, G226A). We stably transfected NIH 3T3 cells with cDNAs encoding Gi2 alpha subunit (alpha i2) containing either wild-type sequence or the homologous mutations Q205L and G204A. High expression of wild-type alpha i2, Q205L alpha i2, and G204A alpha i2 was confirmed in transfected cells by immunoblot analysis. The overexpression of all three alpha i2 proteins was accompanied by an increase in beta-subunit expression. Q205L alpha i2 was a poor substrate for ADP-ribosylation by pertussis toxin as compared with wild-type alpha i2. Expression of Q205L alpha i2 markedly decreased forskolin- or cholera toxin-stimulated intracellular cAMP levels in intact cells, confirming the constitutively activated state of the protein. In contrast, G204A alpha i2 increased intracellular cAMP and was resistant to guanosine 5'-[gamma-thio]triphosphate-induced inhibition of ADP-ribosylation by pertussis toxin, as expected for an inactive alpha i2. Transfection of wild-type, Q205L, or G204A alpha i2 cDNA did not induce focus formation of NIH 3T3 cells. However, overexpression of Q205L alpha i2 induced a decreased serum requirement, a reduced doubling time, and an 8- to 10-fold increase in [3H]thymidine incorporation. Q205L alpha i2 cells formed small colonies in soft agar, demonstrating some degree of anchorage-independent proliferation. Expression of G204A alpha i2 slowed the growth of NIH 3T3 cells. We conclude that alpha i2 plays an important role in regulation of fibroblast growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Bloch D. B., Bonventre J. V., Neer E. J., Seidman J. G. The G protein alpha o subunit alters morphology, growth kinetics, and phospholipid metabolism of somatic cells. Mol Cell Biol. 1989 Dec;9(12):5434–5439. doi: 10.1128/mcb.9.12.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Talbot N., Barbacid M. C127 cells resistant to transformation by tyrosine protein kinase oncogenes. Cell Growth Differ. 1990 Jan;1(1):9–15. [PubMed] [Google Scholar]
- Falloon J., Malech H., Milligan G., Unson C., Kahn R., Goldsmith P., Spiegel A. Detection of the major pertussis toxin substrate of human leukocytes with antisera raised against synthetic peptides. FEBS Lett. 1986 Dec 15;209(2):352–356. doi: 10.1016/0014-5793(86)81141-3. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Goldsmith P., Rossiter K., Carter A., Simonds W., Unson C. G., Vinitsky R., Spiegel A. M. Identification of the GTP-binding protein encoded by Gi3 complementary DNA. J Biol Chem. 1988 May 15;263(14):6476–6479. [PubMed] [Google Scholar]
- Graziano M. P., Gilman A. G. Synthesis in Escherichia coli of GTPase-deficient mutants of Gs alpha. J Biol Chem. 1989 Sep 15;264(26):15475–15482. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Imamura K., Dianoux A., Nakamura T., Kufe D. Colony-stimulating factor 1 activates protein kinase C in human monocytes. EMBO J. 1990 Aug;9(8):2423-8, 2389. doi: 10.1002/j.1460-2075.1990.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lowndes J. M., Gupta S. K., Osawa S., Johnson G. L. GTPase-deficient G alpha i2 oncogene gip2 inhibits adenylylcyclase and attenuates receptor-stimulated phospholipase A2 activity. J Biol Chem. 1991 Aug 5;266(22):14193–14197. [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Miller R. T., Chi M. H., Chang F. H., Beiderman B., Lopez N. G., Bourne H. R. Mutations in the GTP-binding site of GS alpha alter stimulation of adenylyl cyclase. J Biol Chem. 1989 Sep 15;264(26):15467–15474. [PubMed] [Google Scholar]
- Miller R. T., Masters S. B., Sullivan K. A., Beiderman B., Bourne H. R. A mutation that prevents GTP-dependent activation of the alpha chain of Gs. Nature. 1988 Aug 25;334(6184):712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Phosphatidic acid may stimulate membrane receptors mediating adenylate cyclase inhibition and phospholipid breakdown in 3T3 fibroblasts. J Biol Chem. 1987 Apr 25;262(12):5522–5529. [PubMed] [Google Scholar]
- Murayama T., Ui M. Possible involvement of a GTP-binding protein, the substrate of islet-activating protein, in receptor-mediated signaling responsible for cell proliferation. J Biol Chem. 1987 Sep 15;262(26):12463–12467. [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Murayama Y., Okamoto T., Ogata E., Asano T., Iiri T., Katada T., Ui M., Grubb J. H., Sly W. S., Nishimoto I. Distinctive regulation of the functional linkage between the human cation-independent mannose 6-phosphate receptor and GTP-binding proteins by insulin-like growth factor II and mannose 6-phosphate. J Biol Chem. 1990 Oct 15;265(29):17456–17462. [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Dhanasekaran N., Woon C. W., Johnson G. L. G alpha i-G alpha s chimeras define the function of alpha chain domains in control of G protein activation and beta gamma subunit complex interactions. Cell. 1990 Nov 16;63(4):697–706. doi: 10.1016/0092-8674(90)90136-3. [DOI] [PubMed] [Google Scholar]
- Osawa S., Heasley L. E., Dhanasekaran N., Gupta S. K., Woon C. W., Berlot C., Johnson G. L. Mutation of the Gs protein alpha subunit NH2 terminus relieves an attenuator function, resulting in constitutive adenylyl cyclase stimulation. Mol Cell Biol. 1990 Jun;10(6):2931–2940. doi: 10.1128/mcb.10.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Johnson G. L. A dominant negative G alpha s mutant is rescued by secondary mutation of the alpha chain amino terminus. J Biol Chem. 1991 Mar 15;266(8):4673–4676. [PubMed] [Google Scholar]
- Pace A. M., Wong Y. H., Bourne H. R. A mutant alpha subunit of Gi2 induces neoplastic transformation of Rat-1 cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7031–7035. doi: 10.1073/pnas.88.16.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Collins R. M., Spiegel A. M., Brann M. R. Membrane attachment of recombinant G-protein alpha-subunits in excess of beta gamma subunits in a eukaryotic expression system. Biochem Biophys Res Commun. 1989 Oct 16;164(1):46–53. doi: 10.1016/0006-291x(89)91680-x. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Woodard C. J., Unson C. G., Spiegel A. M. Receptor and effector interactions of Gs. Functional studies with antibodies to the alpha s carboxyl-terminal decapeptide. FEBS Lett. 1989 Jun 5;249(2):189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Kanaho Y., Hewlett E. L., Moss J. Effects of guanyl nucleotides and rhodopsin on ADP-ribosylation of the inhibitory GTP-binding component of adenylate cyclase by pertussis toxin. J Biol Chem. 1984 Dec 25;259(24):15320–15323. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wong Y. H., Federman A., Pace A. M., Zachary I., Evans T., Pouysségur J., Bourne H. R. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature. 1991 May 2;351(6321):63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- Zachary I., Masters S. B., Bourne H. R. Increased mitogenic responsiveness of Swiss 3T3 cells expressing constitutively active Gs alpha. Biochem Biophys Res Commun. 1990 May 16;168(3):1184–1193. doi: 10.1016/0006-291x(90)91154-k. [DOI] [PubMed] [Google Scholar]