Abstract
YB-1 (Y-box binding protein 1) is a multifunctional cold-shock protein that has been implicated in all hallmarks of cancer. Elevated YB-1 protein level was associated with poor prognosis in several types of cancers, including breast cancer (BC), where it is a marker of decreased overall survival (OS) and distant metastasis-free survival across all subtypes. YB-1 is also secreted by different cell types and may act as an extracellular mitogen; however the pathological implications of the secreted form of YB-1 (sYB-1) are unknown. Our purpose was to retrospectively evaluate the association between YB-1 measured by ELISA in serum and disease characteristics and outcomes in patients with BC and bone metastases (BM). In our cohort, sYB-1 was detected in the serum of 22 (50%) patients, and was associated with the presence of extra-bone metastases (p=0.044). Positive sYB-1 was also associated with faster bone disease progression (HR 3.1, 95% CI 1.09–8.95, P=0.033), but no significant differences were observed concerning OS, and time to development of skeletal-related events. Moreover, patients with positive sYB-1 also had higher levels of IL-6, a known osteoclastogenic inducer. Therefore, detection of sYB-1 in patients with BC and BM may indicate a higher tumor burden, in bone and extra-bone locations, and is a biomarker of faster bone disease progression.
Abbreviations: BC, breast cancer; BM, bone metastases; BPs, bisphosphonates; CSD, cold shock domain; CT, computed tomography; CTCs, circulating tumor cells; CV, coefficient of variation; EMT, epithelial-to-mesenchymal transition; HCC, hepatocellular carcinoma; IL-6, interleukin 6; IQR, interquartile range; LPS, lipopolysaccharide; NTX, N-terminal telopeptide; OS, overall survival; SREs, skeletal related events; sYB-1, secreted/serum YB-1; TAMs, tumor-associated macrophages; TTBP, time to bone progression; TTSRE, time to first skeletal-related event;; YB-1, Y-box binding protein 1
Keywords: Breast cancer, Bone metastases, Y-box binding protein 1, Prognostic factor, Serum biomarker
1. Introduction
Y box binding protein 1 (YBX1 or YB-1) is one of the three members of the YBX family of transcription factors that contain a conserved cold shock domain (CSD) [1], and its features and functions were deeply reviewed by Eliseeva et al. [2].
YB-1 is a recognized oncoprotein, overexpressed in different types of cancers including breast cancer (BC). YB-1 plays an important role in tumor cell proliferation and progression, and is not only a marker of poor prognosis, but is also emerging as a putative molecular target for the development of new therapeutic strategies, as recently reviewed by Kosnopfel et al. [3]. In BC, YB-1 is a prognostic marker of disease aggressiveness and tumor resistance to chemotherapy, across all tumor sub-types, although an association with clinicopathological characteristics was not always found [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. A seminal paper has established that YB-1 expression is related to epithelial-to-mesenchymal transition (EMT) induction and enhanced metastatic potential and reduced proliferation rates of mammary epithelial cells [14]. This effect is due to ability of YB-1 to directly activate cap-independent translation of messenger RNAs encoding transcription factors implicated in EMT.
Beside its intracellular role, YB-1 can also be secreted through a non-classical vesicle-mediated pathway, from mesangial and monocytic cells under lipopolysaccharide (LPS)-induced inflammatory stress [15]. In this case, secreted YB-1 showed mitogenic as well as promigratory effects in cell line models. However, despite the correlation of sYB-1 with infectious response and lysosomal metabolism [16], its role in the setting of cancer is still largely unknown. In this setting, using an in vitro model of BC it was demonstrated that addition of recombinant YB-1 increased the proliferation of MCF-7 cells [17]. In addition, in the clinical arena, it was shown that YB-1/p18 is detectable in plasma samples by immunoblotting, and that it was present in 78% of cases from a small cohort of patients with different malignancies. Furthermore, in this cohort that included ten patients with BC, the detection of sYB-1 was independent of the tumor origin. Of note, sYB-1 concentrations altered during therapeutic interventions, but sYB-1 did not predicted prognosis when combining all tumor types. At the same time, other groups are exploring the role of sYB-1 in the diagnosis of hepatocellular carcinoma (HCC) [18], and epithelial ovarian cancer [19].
Interestingly, it was also found in in vitro studies of BC, that YB-1 interacts with other mediators, in specific interleukin 6 (IL-6) creating a positive feed-forward loop driving epithelial to mesenchymal (EMT)-like metastatic features during cancer progression [20]. IL-6 is known for its strong pro-tumorigenic activity due to its multiple effects on bone metabolism, tumor cell proliferation and survival, angiogenesis, and inflammation. In addition, IL-6 is known to promote BC metastasis, and to be associated with poor prognosis in BC patients [21]. Finally, IL-6 has an important role in the development of bone metastases (BM), as reviewed by Ara and DeClerck [22]. Therefore, we hypothesize that levels of sYB-1 in patients with BM may be associated with disease characteristics and/or patients’ outcomes.
In the present study we performed a retrospective analysis of the prognostic value of sYB-1 in patients with BC and BM, and assessed the correlation between sYB-1 and serum IL-6 (sIL-6).
2. Material and methods
2.1. Study population and design
In this retrospective cohort study we included 44 consecutive female patients followed at the Oncology Division of Santa Maria Hospital (HSM), Lisbon, Portugal. All the eligible patients had the diagnosis of BC with BM (either de novo or after recurrence) between 1998 and 2014, were started on bisphosphonates (BPs; zoledronic acid), agreed to participate in a prospective collection of biological specimens, and had available peripheral blood collected at the time of first treatment with BPs (following BM diagnosis).
Cancer treatment was provided according to patient-physician description. Demographic and clinicopathological information was retrospectively collected, namely: age at diagnosis of primary BC and BM; primary BC histology, hormone receptors and HER2 status; TNM staging; sites of metastatic disease at BC presentation, if metastatic, or at time of recurrence; radiographic pattern of BM; date and site of disease recurrence; date of bone progression; date of skeletal related events (SREs); and survival status. Progression in bone was defined as date of new or increased bone lesions on imaging (CT scan or bone scan), or attending physician indication on medical records. SREs were defined as any of the following events: pathological bone fracture, need for radiation therapy or surgery to the bone (due to pain or impending fracture), or spinal cord compression.
This study was ethically approved by local Institutional Review Board, and complies with all national regulations. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines.
2.2. YB-1 and IL-6 quantification
Peripheral blood was collected in serum separation tubes. Serum was separated by centrifugation at 2000g for 10 min at 4 °C, aliquoted and stored at −80 °C until usage. Freeze and thaw cycles were avoided. YB-1 was quantified in serum using Human YBX1/YB1 Sandwich ELISA kit (LSBio, LifeSpan BioSciences, Inc.), and IL-6 using Human IL-6 Quantikine ELISA kit (R&D Systems), according to manufacturer's instructions. Absorbance at 450 nm was measured in an InfiniteM200 Plate Reader (Tecan) and protein concentration was calculated based on standard curve. All determinations were performed in duplicate. YB-1 assay detection range: 0.156–10 ng/ml; assay sensitivity: 0.064 ng/ml; estimated intra-assay coefficient of variation (CV) <10%. IL-6 assay minimum detectable dose 0.70 pg/ml; estimated intra-assay coefficient of variation (CV)<5%. For subsequent analysis and patient stratification sYB-1 was considered to be negative (undetectable) or positive, except for correlation analysis with IL-6, where it was analyzed as a continuous variable. IL-6 was always analyzed as a continuous variable.
2.3. Statistical analysis
Demographic and clinicopathological characteristics were tabulated according to the full cohort and to sYB-1 status. Frequencies for categorical variables and central tendency, dispersion and range for continuous variables were calculated. Univariate association with sYB-1 status is presented. Survival and cumulative incidence plots were built using Kaplan–Meier methods. Univariate and multivariate differences between survival rates were tested using the log-rank test or Cox proportional hazards models, respectively. For time dependent variables, study follow-up was performed until August 2014. Overall survival (OS), if not otherwise specified, was defined as time from bone-disease recurrence to death from any cause. Time to first skeletal-related event (TTSRE) and time to bone progression (TTBP) were defined as time from bone-disease recurrence to first SRE or bone-disease progression, respectively (as reported by assistant physician). All patients with missing data in relevant variables were excluded from the multivariate analysis. Analyses were performed using Stata 13.1 software (StataCorp LP).
3. Results
3.1. Patient characteristics
Patients’ demographic and clinicopathological characteristics are presented in Table 1. On the whole cohort, median age at diagnosis of BC was 54.3 (interquartile range [IQR] 44.0–62.3) years. The majority of patients had locorregional disease at BC diagnosis (n=33, 80.5%). In these patients, disease relapsed at distant sites after a median interval of 66 months (IQR 38.5–126.1), with bone-specific recurrence after a median interval of 75.9 months (IQR 45.4–130.8). As expected, the radiographic pattern of bone disease was mostly lytic (n=24, 60.0%). Finally, the majority of tumors were hormone receptor-positive (n=32, 76.2%) and HER2-negative (n=29, 80.6%).
Table 1.
Patients’ demographic, clinical and pathological characteristics according to secreted YB1 (sYB1) status.
| Variable list | Full cohort (n=44) | Positive YB-1 (n=22) | Negative YB-1 (n=22) | p-value |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 54.3 | 48.4 | 55.2 | 0.639 |
| IQR | 44.0 – 62.3 | 40.9 – 62.1 | 44.5–62.4 | |
| Age (years), n (%) | ||||
| < 35 | 1 (2.3) | 1 (4.6) | 0 (0) | 0.245 |
| 35–49 | 19 (43.2) | 11 (50.0) | 8 (36.4) | |
| 50–69 | 18 (40.9) | 6 (27.3) | 12 (54.6) | |
| ≥ 70 | 6 (13.6) | 4 (18.2) | 2 (9.1) | |
| Histology, n (%) | ||||
| Ductal carcinomas | 34 (82.9) | 17 (85.0) | 17 (81.0) | 1.000 |
| Lobular carcinomas | 3 (7.3) | 1 (5.0) | 2 (9.5) | |
| Other | 4 (9.8) | 2 (10.0) | 2 (9.5) | |
| Missing | 3 (6.8) | 2 (9.1) | 1 (4.6) | |
| Hormone receptor status, n (%) | ||||
| ER or PR positive | 32 (76.2) | 18 (85.7) | 14 (66.7) | 0.277 |
| ER and PR negative | 10 (23.8) | 3 (14.3) | 7 (33.3) | |
| Missing | 2 (4.6) | 1 (4.6) | 1 (4.6) | |
| HER2 receptor status, n (%) | ||||
| Positive | 7 (19.4) | 4 (22.2) | 3 (16.7) | 1.000 |
| Negative | 29 (80.6) | 14 (77.8) | 15 (83.3) | |
| Missing | 8 (18.2) | 4 (18.2) | 4 (18.2) | |
| Metastatic at diagnosis, n (%) | ||||
| Yes | 8 (19.5) | 4 (18.2) | 4 (21.1) | 1.000 |
| No | 33 (80.5) | 18 (81.8) | 15 (79.0) | |
| Missing | 3 (6.8) | 0 (0) | 3 (13.6) | |
| Age at diagnosis of bone involvement | ||||
| Median | 61.3 | 59.1 | 61.3 | 0.706 |
| P25 – P75 | 49.6–68.1 | 46.8–75.9 | 52.8–67.0 | |
| Missing, n (%) | 2 (4.5) | 0 (0) | 2 (9.1) | |
| Radiographic pattern of bone lesions, n (%) | ||||
| Lytic | 24 (60.0) | 15 (71.4) | 9 (47.4) | 0.064 |
| Blastic | 6 (15.0) | 4 (19.1) | 2 (10.5) | |
| Mixed | 10 (25.0) | 2 (9.5) | 8 (42.1) | |
| Missing | 4 (9.1) | 1 (4.6) | 3 (13.6) | |
| Metastatic disease outside bone, n (%) | ||||
| Yes | 28 (65.1) | 18 (81.8) | 10 (47.6) | 0.019 |
| No | 15 (34.9) | 4 (18.2) | 11 (52.4) | |
| Missing, n (%) | 1 (2.3) | 0 (0) | 1 (4.6) | |
| Time to disease recurrence in patients stage I-III | ||||
| Median | 66.0 | 61.6 | 71.3 | 0.715 |
| P25-P75 | 38.5–126.1 | 38.5 – 126.1 | 34.7–130.8 | |
| Time to bone-disease recurrence in patients stage I-III | ||||
| Median | 75.9 | 73.1 | 76.2 | 1.000 |
| P25-P75 | 45.4–130.8 | 39.9–137.9 | 59.8–130.8 | |
| Overall survival follow-up from date of bone recurrence, months | ||||
| Median | 34.0 | 29.4 | 57.9 | 0.060 |
| P25-P75 | 21.1–74.5 | 17.6–50.3 | 21.1–107.6 | |
3.2. sYB-1 levels and association with clinical features and outcomes
In the whole cohort, median sYB-1 was 4.94±7.34 pg/ml (range 0.0–43.61 pg/ml), while median sYB-1 was 9.25±7.23 pg/ml for the group of patients with positive sYB-1 (range 3.49–43.61 pg/ml) and 0 for the negative sYB-1 group. When comparing YB-1 levels to relevant clinicopathological characteristics, patients with positive sYB-1 presented more frequently with metastases outside the bone (n=18, 81.8% vs. n=10, 47.6% in patients with negative sYB-1; p=0.019). Moreover, despite not significantly different, patients with positive sYB-1 tended to be slightly younger, a fact mostly dependent on the strata 35–49 and 50–69 (n=11, 50% of sYB-1 positive had 35–49 at date of bone disease diagnosis, while n=12, 54.6% of sYB-1 patients of the sYB-1 group were 50–69 at date of bone disease), a cut-off closely related to menopausal status. Finally, we further observed that patients with positive sYB-1 tended more frequently to present bone lesions with a lytic radiography pattern, while those with negative sYB-1 had a more balanced distribution of radiographic patterns (lytic lesions n=15, 71.4% vs. n=9, 47.4% in patients with negative sYB-1; p=0.064).
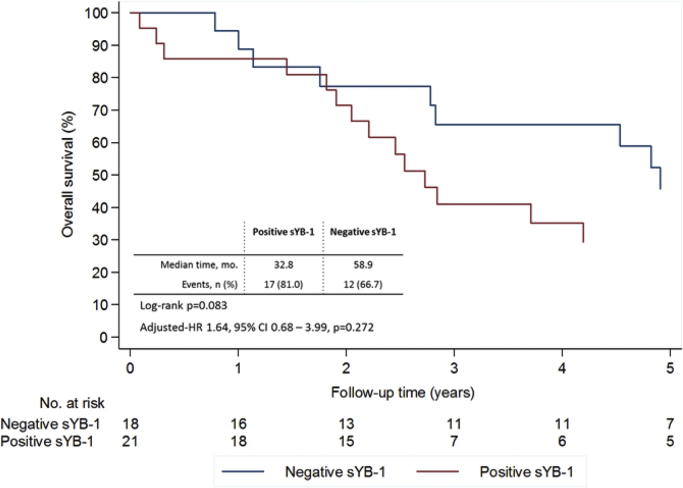
After a median follow-up of 34.0 (IQR 21.1–74.5) months 30 patients (68.2%) died: 17 (77.3%) vs. 13 (59.1%) in the positive and negative sYB-1 groups, respectively. Patients with negative sYB-1 tended to live longer, with a median survival of 58.9 months, compared to a median survival of 32.8 months of those patients with positive sYB-1 (Fig. 1). Despite this difference, only a statistical trend was noted favoring those patients with negative sYB-1, both in the univariate (p=0.083) and multivariate analysis (controlling for age at diagnosis, hormone receptor status and presence of extra-bone metastases; HR 1.64, 95% CI 0.68–3.99, p=0.272).
Fig. 1.
Overall survival according to sYB1 at baseline from date of bone recurrence. Multivariate analysis controlling for age at diagnosis, hormone receptor status, and extra-bone metastases.
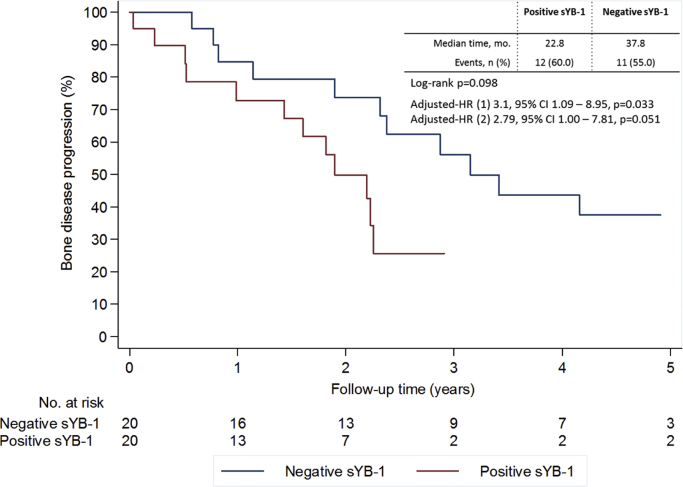
When analyzing TTBP, patients with positive sYB-1 showed a shorter median TTBP (22.8 vs. 37.8 months for negative sYB-1 patients). This difference was however not significant in the univariate analysis (p=0.098), but when controlling for relevant clinical and pathologic variables a significant association was noted (model 1: adjusted HR 3.1, 95% CI 1.09–8.95, p=0.033, controlling for age at diagnosis, hormone receptor status, extra-bone metastases status and radiographic pattern of BM; model 2: adjusted HR 2.79, 95% CI 1.00–7.81, p=0.051 controlling for the same variables except age) (Fig. 2).
Fig. 2.
Time to bone disease progression according to sYB1 at baseline. Multivariate analysis (1) controlling for age at diagnosis, hormone receptor status, extra-bone metastases and radiographic pattern of bone metastases or (2) controlling for hormone receptor status, extra-bone metastases and radiographic pattern of bone metastases.
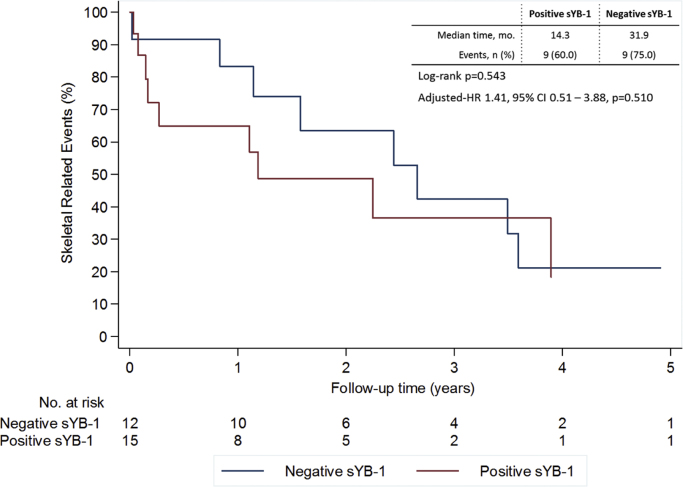
When analyzing TTSRE, patients with sYB-1 positive tumors had a shorter median TTSRE (14.3 vs. 31.9 months for negative sYB-1 patients). No statistically significant differences were however observed both in the univariate (p=0.543) of multivariate analysis (HR 1.41, 95% CI 0.51–3.88, p=0.510 controlling for hormone receptor status and radiographic pattern of BM) (Fig. 3).
Fig. 3.
Skeletal related events according to sYB1 at baseline. Multivariate analysis controlling for hormone receptor status and radiographic pattern of bone metastases.
3.3. Association of serum YB-1 and IL-6
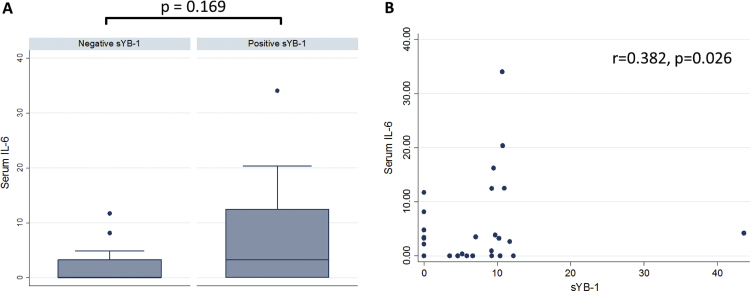
In the overall cohort 34 (77.3%) patients had available samples for IL-6 measurement Median sIL-6 was 0.65±7.43 pg/ml (range 0.0–34.03 pg/ml) for the whole cohort, and 4.04±8.58 pg/ml for the group of patients with positive sYB-1 (range 0.37–34.03 pg/ml). sIL-6 concentration was higher in patients with positive sYB-1 (20/34) (Fig. 4a), with a weak but significant direct correlation between sYB-1 and sIL-6 (r=0.324, p=0.026) (Fig. 4b).
Fig. 4.
Association between serum YB-1 and IL-6. A) Levels of serum IL-6 according to sYB1, B) correlation between sYB-1 and sIL-6.
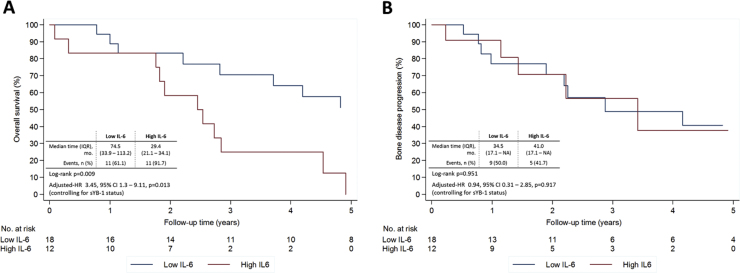
We subsequently tested the prognostic value of sIL-6, and observed that high levels of sIL-6 were strongly associated with lower OS, both in the univariate (p=0.009) and multivariate analysis controlling for sYB-1 status (adjusted HR 3.45, 95% CI 1.3–9.11, p=0.013) (Fig. 5a). On the contrary, we did not observe an association of sIL-6 with bone disease progression (Fig. 5b).
Fig. 5.
Clinical outcomes according to serum IL-6 at baseline. A) overall survival, B) bone disease progression, from date of bisphosphonates introduction Multivariate analysis controlling for sYB-1.
4. Discussion
In this exploratory study we aimed to test the association sYB-1 with clinical and pathological variables, as well as its prognostic relevance in patients with BC and BM. Thus, our cohort included patients with BC metastatic to the bone, which had peripheral blood collected at the time of first treatment with BPs (shortly after BM diagnosis). In our cohort we observed that the secreted form of the oncoprotein YB-1 is detected in the serum of 50% of patients (22/44). Furthermore, sYB-1 positivity was significantly associated with the presence of extra-bone metastases and faster bone disease progression, although OS and TTSRE were identical between groups.
YB-1 is a widely known marker of poor prognosis across different types of cancer; however, the prognostic role of its secreted form (sYB-1) is still poorly characterized. The significance of YB-1 is explained by its transversal role in several cellular processes that account for most hallmarks of cancer [3]. Most studies on the prognostic utility of YB-1 were based on YB-1 determination by IHC on tissue samples. However, the detection of YB-1 in serum (sYB-1) using immunobloting was also performed in a small pan-cancer cohort showing that sYB-1 is present in 70% of metastatic BC (7/10). This marker was also able to discriminate between healthy and cancer patients in the overall cohort; however, no association with clinical outcomes was noted [16]. In our cohort, sYB-1 was present in a relatively smaller proportion of patients; of note, all patients in this cohort had BM. In agreement with the study by Tacke et al., sYB-1 positivity was not associated with OS. In addition, it was not associated with TTSRE, but a faster bone disease progression was noted. It is intriguing to note however a statistically non-significant trend towards worst survival and decreased time to SRE in sYB-1 patients. A larger and more definitive study could clarify the clinical relevance of these results.
In our cohort of patients with BM, sYB-1 positivity was associated with the presence of extra-skeletal metastases. We thus admit that sYB-1 positivity might select for patients with higher tumor burden and more aggressive clinical behavior, further justifying the faster bone disease progression. Moreover, as we have previously shown, patients with BMs plus extraskeletal metastases receiving bisphosphonates have an erratic bone resorption normalization pattern, as assessed by urinary NTX [23], thus highlighting the possible interactions between bone and extra-bone disease. sYB-1 could be a putative candidate in the group of mediators of such an interaction.
Previous studies using an in vitro model of BC found that YB-1 and IL-6 create a positive feed-forward loop that is associated with tumor invasiveness [20]; furthermore, IL-6 plays a cornerstone role in bone resorption [24]. We thus interrogated whether sYB-1 could be associated with sIL-6 levels. In our cohort we show a positive, but weak correlation between sYB-1 and IL-6, hence patients with positive sYB-1 tend to have higher levels of sIL-6. IL-6 is produced in the bone marrow microenvironment and exerts a strong pro-tumorigenic activity, by affecting not only bone metabolism, but also tumor cell proliferation and survival, angiogenesis, and inflammation. Elevated sIL-6 in patients with BC and BM has been associated with poor survival in patients with metastatic BC and was associated with the extent of disease [25]. In this study patients with visceral disease had significantly higher values compared to those with BM only. Overexpression of IL-6 in other organs than bone and even at primary tumor site is associated with the recruitment of circulating tumor cells (CTCs) to these locations [22].
It is therefore important to further explore the origin and the biological role of sYB-1, as well as the contribution of sYB-1 to the YB-1/IL-6 loop. It has been demonstrated that sYB-1 is produced by mesangial and monocytic cells under inflammatory stress [15]. At the same time, it is widely accepted that inflammatory cells that infiltrate various types of tumors affect the intrinsic characteristics of the tumors and that tumor-associated macrophages (TAMs) are the most common inflammatory cells in BC, enriching the tumor microenvironment with hormones, growth factors and cytokines [26]. Moreover, several studies have associated TAMs with poor prognosis in BC patients [27], [28]. Therefore, it is of paramount importance to clarify whether there is a connection between BC, TAMs, sYB-1 and poor prognosis. Concurrently, sIL-6 is also produced by monocytes and macrophages; hence it will be important to dissect a possible connection between YB-1 and IL-6 in these cells.
This study presents several limitations. It is a small exploratory retrospective single center study. Moreover, despite our efforts in assuring data accuracy, the outcomes bone progression and SRE are intrinsically difficult to assess in the context of regular clinical practice (and even in the context of clinical trials). With this in mind, researchers were blinded for sYB-1 status, thus we admit that any residual bias in the accurate assessment of these variables may be balanced between arms. Finally, given the retrospective nature of the study and the fact that some patients were diagnosed close to two decades ago, missing data exists for some variables (see Table 1) and not all patients had biological specimens to test for sIL-6.
In conclusion, our study provides the first evidence that sYB-1 is related with tumors that are clinically more aggressive (visceral involvement and with faster bone progression) in patients with BC and BM. It is important to further validate this study in an independent and larger cohort, which could also extend the analysis of sYB-1 relevance in early BC. Although we could not find an association with OS and TTSRE, an intriguing trend for worse outcomes was noted, hence a larger cohort could also help to interpret these findings. Side with these questions, the mechanistic underpinnings of these clinical manifestations will also require further studies.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was partially supported by the Oncology Research Grant Terry Fox 2014/2015, Liga Portuguesa Contra o Cancro – Núcleo Regional do Sul, Portugal.
References
- 1.Didier D.K., Schiffenbauer J., Woulfe S.L., Zacheis M., Schwartz B.D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc. Natl. Acad. Sci. USA. 1988;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eliseeva I.A., Kim E.R., Guryanov S.G., Ovchinnikov L.P., Lyabin D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochem. Biokhimiia. 2011;76(13):1402–1433. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- 3.Kosnopfel C., Sinnberg T., Schittek B. Y-box binding protein 1--a prognostic marker and target in tumour therapy. Eur. J. Cell Biol. 2014;93(1–2):61–70. doi: 10.1016/j.ejcb.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bargou R.C., Jurchott K., Wagener C., Bergmann S., Metzner S., Bommert K., Mapara M.Y., Winzer K.J., Dietel M., Dorken B., Royer H.D. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 1997;3(4):447–450. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- 5.Janz M., Harbeck N., Dettmar P., Berger U., Schmidt A., Jurchott K., Schmitt M., Royer H.D. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1, J. Int. du Cancer. 2002;97(3):278–282. doi: 10.1002/ijc.1610. (International journal of cancer) [DOI] [PubMed] [Google Scholar]
- 6.Dahl E., En-Nia A., Wiesmann F., Krings R., Djudjaj S., Breuer E., Fuchs T., Wild P.J., Hartmann A., Dunn S.E., Mertens P.R. Nuclear detection of Y-box protein-1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer. 2009;9:410. doi: 10.1186/1471-2407-9-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J., Lee C., Yokom D., Jiang H., Cheang M.C., Yorida E., Turbin D., Berquin I.M., Mertens P.R., Iftner T., Gilks C.B., Dunn S.E. Disruption of the Y-box binding protein-1 results in suppression of the epidermal growth factor receptor and HER-2. Cancer Res. 2006;66(9):4872–4879. doi: 10.1158/0008-5472.CAN-05-3561. [DOI] [PubMed] [Google Scholar]
- 8.Habibi G., Leung S., Law J.H., Gelmon K., Masoudi H., Turbin D., Pollak M., Nielsen T.O., Huntsman D., Dunn S.E. Redefining prognostic factors for breast cancer: yb-1 is a stronger predictor of relapse and disease-specific survival than estrogen receptor or HER-2 across all tumor subtypes. Breast Cancer Res.: BCR. 2008;10(5):R86. doi: 10.1186/bcr2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluz O., Mengele K., Schmitt M., Kates R., Diallo-Danebrock R., Neff F., Royer H.D., Eckstein N., Mohrmann S., Ting E., Kiechle M., Poremba C., Nitz U., Harbeck N. Y-box-binding protein YB-1 identifies high-risk patients with primary breast cancer benefiting from rapidly cycled tandem high-dose adjuvant chemotherapy. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2009;27(36):6144–6151. doi: 10.1200/JCO.2008.19.6261. [DOI] [PubMed] [Google Scholar]
- 10.Lee A., Woo J., Park H., Sung S.H., Seoh J.Y., Lim W., Moon B.I. The value of cytoplasmic Y-box-binding protein 1 as a prognostic marker for breast cancer in Korean. Breast Cancer. 2015 doi: 10.1007/s12282-015-0625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maciejczyk A., Szelachowska J., Ekiert M., Matkowski R., Halon A., Lage H., Surowiak P. Elevated nuclear YB1 expression is associated with poor survival of patients with early breast cancer. Anticancer Res. 2012;32(8):3177–3184. [PubMed] [Google Scholar]
- 12.Mylona E., Melissaris S., Giannopoulou I., Theohari I., Papadimitriou C., Keramopoulos A., Nakopoulou L. Y-box-binding protein 1 (YB1) in breast carcinomas: relation to aggressive tumor phenotype and identification of patients at high risk for relapse. Eur. J. Surg. Oncol.: J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014;40(3):289–296. doi: 10.1016/j.ejso.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Guo X.B., Shen X.C., Zhou H., Wan D.W., Xue X.F., Han Y., Yuan B., Zhou J., Zhao H., Zhi Q.M., Kuang Y.T. Prognostic role of YB-1 expression in breast cancer: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8(2):1780–1791. [PMC free article] [PubMed] [Google Scholar]
- 14.Evdokimova V., Tognon C., Ng T., Ruzanov P., Melnyk N., Fink D., Sorokin A., Ovchinnikov L.P., Davicioni E., Triche T.J., Sorensen P.H. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell. 2009;15(5):402–415. doi: 10.1016/j.ccr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Frye B.C., Halfter S., Djudjaj S., Muehlenberg P., Weber S., Raffetseder U., En-Nia A., Knott H., Baron J.M., Dooley S., Bernhagen J., Mertens P.R. Y-box protein-1 is actively secreted through a non-classical pathway and acts as an extracellular mitogen. EMBO Rep. 2009;10(7):783–789. doi: 10.1038/embor.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacke F., Galm O., Kanig N., Yagmur E., Brandt S., Lindquist J.A., Eberhardt C.S., Raffetseder U., Mertens P.R. High prevalence of Y-box protein-1/p18 fragment in plasma of patients with malignancies of different origin. BMC Cancer. 2014;14:33. doi: 10.1186/1471-2407-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moiseeva N.I., Stromskaya T.P., Rybalkina E.Y., Vaiman A.V., Malyshkina M.A., Kim E.R., Eliseeva I.A., Kulakovskiy L.P., Ovchinnikov L.P., Stavroskaya A.A. Effects of extracellular YB1 protein on cultured cells of human breast cancer. Biochem. (Mosc.) Suppl. Ser. A: Membr. Cell Biol. 2013;7(1):7. [Google Scholar]
- 18.Pu L., Jing S., Bianqin G., Ping L., Qindong L., Chenggui L., Feng C., Wenbin K., Qin W., Jinyu D., Qianfeng X., Yu L., Zhiguang T. Development of a chemiluminescence immunoassay for serum YB-1 and its clinical application as a potential diagnostic marker for Hepatocellular Carcinoma. Hepat. Mon. 2013;13(7):e8918. doi: 10.5812/hepatmon.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohr I., Braicu E.I., En-Nia A., Heinrich M., Richter R., Chekerov R., Dechend R., Heidecke H., Dragun D., Schafer R., Gorny X., Lindquist J.A., Brandt S., Sehouli J., Mertens P.R. Y-box protein-1/p18 as novel serum marker for ovarian cancer diagnosis: a study by the Tumor Bank Ovarian Cancer (TOC) Cytokine. 2016;85:157–164. doi: 10.1016/j.cyto.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Castellana B., Aasen T., Moreno-Bueno G., Dunn S.E., Ramon y Cajal S. Interplay between YB-1 and IL-6 promotes the metastatic phenotype in breast cancer cells. Oncotarget. 2015;6(35):38239–38256. doi: 10.18632/oncotarget.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knupfer H., Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res. Treat. 2007;102(2):129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 22.Ara T., Declerck Y.A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer. 2010;46(7):1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira A.R., Alho I., Shan N., Matias M., Faria M., Casimiro S., Leitzel K., Ali S., Lipton A., Costa L. N-Telopeptide of type I Collagen long-term dynamics in breast cancer patients with bone metastases: clinical outcomes and influence of extraskeletal metastases. oncologist. 2016 doi: 10.1634/theoncologist.2015-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishimi Y., Miyaura C., Jin C.H., Akatsu T., Abe E., Nakamura Y., Yamaguchi A., Yoshiki S., Matsuda T., Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1990;145(10):3297–3303. [PubMed] [Google Scholar]
- 25.Salgado R., Junius S., Benoy I., Van Dam P., Vermeulen P., Van Marck E., Huget P., Dirix L.Y. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int. J. Cancer J. Int. du Cancer. 2003;103(5):642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 26.Pollard J.W. Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 2008;84(3):623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud S.M., Lee A.H., Paish E.C., Macmillan R.D., Ellis I.O., Green A.R. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J. Clin. Pathol. 2012;65(2):159–163. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- 28.Medrek C., Ponten F., Jirstrom K., Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]