Introduction
Autoinflammatory syndromes are characterized by recurrent episodes of sterile inflammation in the absence of circulating autoantibodies or autoreactive T cells. PASH syndrome is a recently identified hereditary autoinflammatory syndrome consisting of multiple neutrophilic dermatoses including pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS). The triad of PG, acne, and HS occurs in extremely rare instances. To our knowledge, the clinical tetrad of PG, acne, HS, and vasculitis has not been reported.
Case
A 36-year-old man presented with abscesses increasing in size on the buttocks and axillae. His medical history included recurrent PG on the lower extremities, generalized severe acne, and leukocytoclastic vasculitis (LCV) that developed while on 100 mg daily prednisone for a large PG lesion (Fig 1). He self-reported no history of arthritis. Review of systems was otherwise negative. He was previously treated with prednisone, dapsone, colchicine, mycophenolate mofetil, and cyclosporine.
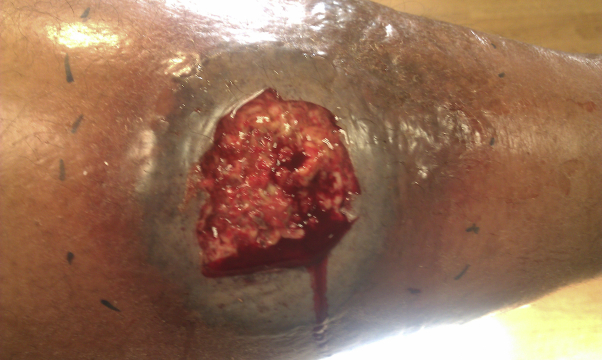
Fig 1.
Acute pyoderma gangrenosum on the lower leg.
Physical examination revealed cysts, papules, and pustules with hyperpigmentation and scarring on the back and chest (Fig 2). Multiple inflammatory abscesses with sinus tracts and scarring were present on the lower back and buttocks (Fig 3). A 14-cm ulcer with violaceous, undermined borders was present on the lower leg (Fig 4). Scattered petechiae with surrounding palpable purpura and hemorrhagic patches developed extensively on the lower extremities (Fig 5, Fig 6, Fig 7). This eruption presented on multiple occasions while on high-dose prednisone for treatment of PG, and resolved without developing into PG-like lesions.
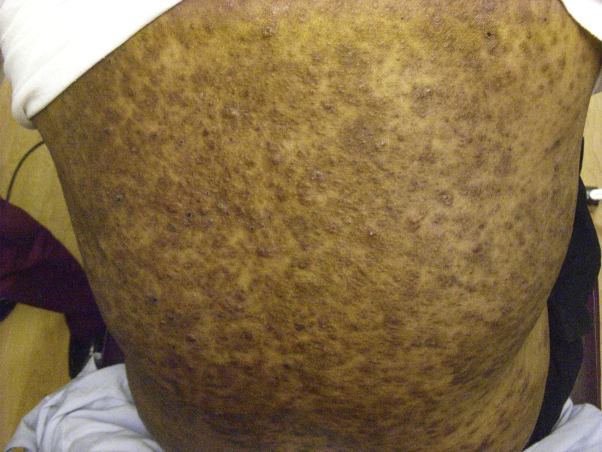
Fig 2.
Generalized acne on the back.
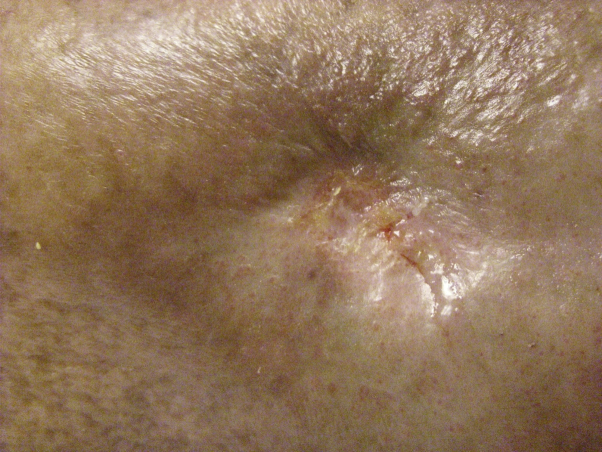
Fig 3.
Scarring on the lower back from hidradenitis suppurativa.
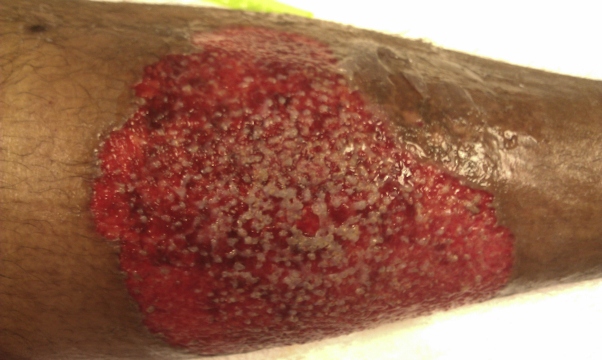
Fig 4.
Pyoderma gangrenosum ulcer stage.
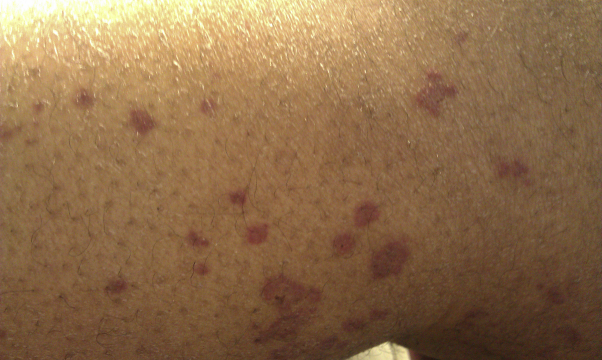
Fig 5.
Petechiae on right lower extremity.
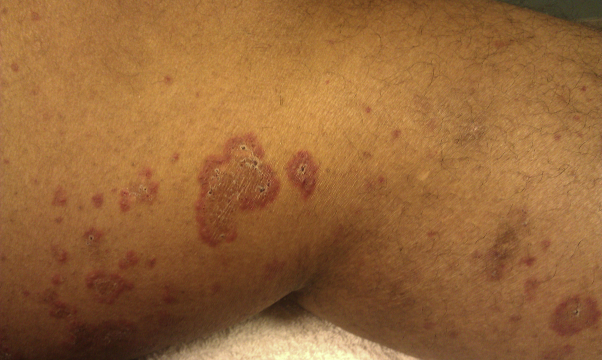
Fig 6.
Hemorrhagic papules and plaques on left lower extremity.
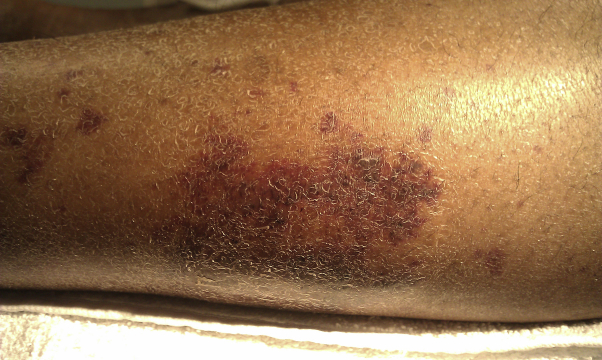
Fig 7.
Resolving palpable purpura on left lower extremity.
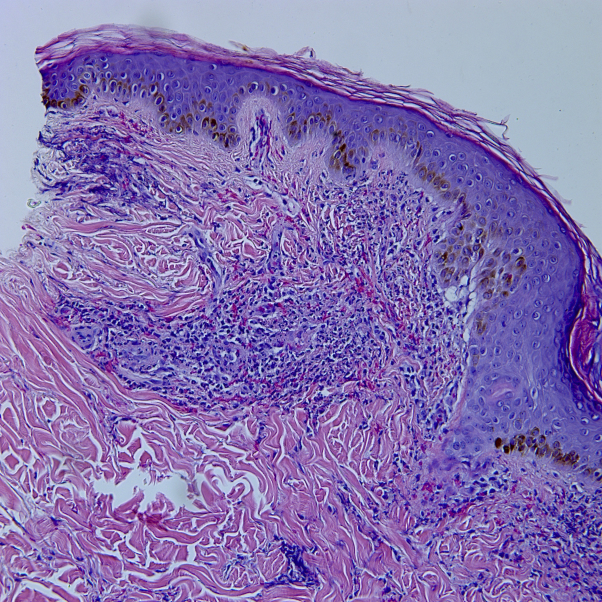
Laboratory evaluation had previously shown a bimodal IgG and IgA monoclonal gammopathy. Other serologies that were negative or normal included antinuclear antibodies; hepatitis A, B, and C; rheumatoid factor; HLA-B27; lactate dehydrogenase; peripheral smear; cryoglobulin; cryofibrinogen; and CD4:CD8 ratio. Genetic testing of the proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1) gene revealed no mutations. A skin biopsy was taken of the palpable purpura on the left lateral thigh; histochemical staining revealed a perivascular, interstitial infiltrate composed of neutrophils, demonstrating prominent leukocytoclasis, extravasation of red blood cells, and focal fibrin deposition within small vessel walls (Fig 8).
Fig 8.
Perivascular and interstitial infiltrate of neutrophils with leukocytoclasis, extravasation of red blood cells, and focal fibrin deposition within small vessel walls. (Hematoxylin-eosin stain; original magnification: ×10.)
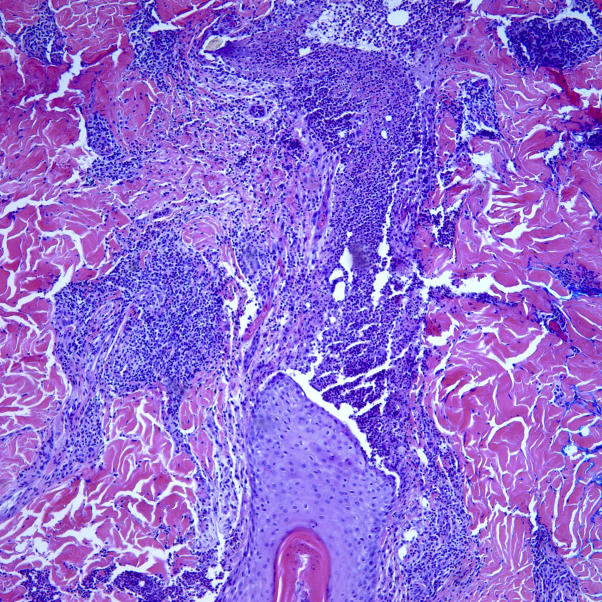
Direct immunofluorescence studies were performed and revealed mild granular perivascular deposition of C3 in small vessel walls that were negative for IgG, IgM, and IgA. No other cutaneous or internal evidence of vasculitis was found. A skin biopsy of the lower back was taken, which revealed a ruptured follicle containing a dense neutrophilic infiltrate extending into the surrounding dermis with a patchy perivascular lymphocytic infiltrate (Fig 9). Biopsy sites healed well and lacked a positive pathergy sign. Dapsone up to 300 mg daily was added to the prednisone treatment, and the PG lesion and LCV lesions resolved.
Fig 9.
Ruptured follicle containing a dense neutrophilic infiltrate extending into the surrounding dermis with a patchy perivascular lymphocytic infiltrate. (Hematoxylin-eosin stain; original magnification: ×20.)
The patient is currently disease free on only dapsone 100 mg daily for treatment of neutrophilic processes and chlorhexidine wash and topical clindamycin solution for HS prophylaxis.1, 2
Discussion
PASH syndrome is a rare autoinflammatory syndrome characterized by the triad of PG, acne, and HS. Mutations in the promoter region of the PSTPIP1 gene have been identified in patients. Mutations in PSTPIP1, via increased binding affinity to pyrin, are responsible for the activation of an inflammasome.1, 2, 3 In turn, caspase-1 is activated and cleaves pro-interleukin (IL)-1ß to its active isoform IL-1ß. The overproduction of IL-1ß leads to uncontrolled release of pro-inflammatory cytokines, particularly IL-17, which direct recruitment and activation of neutrophils.3, 4 This corresponds to neutrophil-mediated inflammation of the skin clinically as PG, HS, and acne. Our patient had a steroid resistant vasculitis that developed while other PASH symptoms were also active. No new medications had been introduced during this time frame, which allowed us to exclude the diagnosis of a reaction to medication. Steroid therapy is a common treatment for vasculitis; therefore, development and persistence in our patient is unique. LCV has not been reported as a feature of any of the autoinflammatory syndromes that include symptoms of PG and acne (ie, PAPA, PAPASH, PASH) despite being a common complication of systemic autoimmune diseases, like lupus and rheumatoid arthritis.
Mutations in the PSTPIP1 gene can be found in multiple PG-associated syndromes, which should be included in the differential diagnosis of PASH syndrome.3 PAPA syndrome is characterized by pyogenic arthritis, PG, and acne. PAPASH syndrome is a tetrad of pyogenic arthritis, PG, acne, and HS. PsAPASH syndrome includes psoriatic arthritis, PG, acne, and HS. Aseptic abscess syndrome shares the abnormality in the PSTPIP1 promoter region seen in PASH syndrome, specifically the increased number of the CCTG microsatellite repeat.1, 2, 3 None of these are noted to have vasculitis as a feature, despite the predominant neutrophilic infiltrates these all share.
Our patient fits the PASH phenotype but had no detected PSTPIP1 gene mutations, including a lack of increased CCTG microsatellite repeat of the promoter region. This is either a simulant of this syndrome or has some other genetic defect that can give similar symptoms. Other genes involved in PASH syndrome have been reported, including a mutation in NCSTN.2, 3, 4
Several treatments for PASH syndrome exist with varying degrees of efficacy,2, 3, 4 but no treatment has been accepted as the standard therapy. Prolonged antibiotic therapy, topical tacrolimus ointment, and surgical ablation of abscesses and sinus tracts related to HS lesions have all been described. Systemic therapy with tumor necrosis factor–α inhibitors, IL-1 receptor antagonists, corticosteroids, cyclosporine, or dapsone (on which our patient is now controlled) is often necessary, given the chronic and relapsing nature of the disease. This case underscores that neutrophilic dermatoses can simulate genetic autoinflammatory syndromes and that other neutrophilic disorders (ie, LCV) might complicate the management of the disease.
Footnotes
Funding source: None.
Conflicts of interest: None declared.
References
- 1.Braun-Falco M., Kovnerystyy O., Lohse P. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)-a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409–415. doi: 10.1016/j.jaad.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Join-Lambert O., Duchatelet S., Delage M. Remission of refractory pyoderma gangrenosum, severe acne, and hidradenitis suppurativa (PASH) syndrome using targeted antibiotic therapy in 4 patients. J Am Acad Dermatol. 2015;73:S66–S69. doi: 10.1016/j.jaad.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Marzano A.V., Ceccherini I., Gattorno M. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore) 2015;94(8):1. doi: 10.1097/01.md.0000464213.86167.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzano A.V., Borghi A., Meroni P.L. Pyoderma gangrenosum and its syndromic forms: evidence for a link with autoinflammation. Br J Dermatol. 2016;175(5):882–891. doi: 10.1111/bjd.14691. [DOI] [PubMed] [Google Scholar]