Schuldiner and Zalckvar discuss the identification of a peroxisome–ER tether in human cells by Costello et al. and Hua et al.
Abstract
Peroxisomes are tiny organelles that control important and diverse metabolic processes via their interplay with other organelles, including the endoplasmic reticulum (ER). In this issue, Costello et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201607055) and Hua et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201608128) identify a peroxisome–ER contact site in human cells held together by a tethering complex of VAPA/B (vesicle-associated membrane protein–associated proteins A/B) and ACBD5 (acyl Co-A binding protein 5).
Peroxisomes are organelles enclosed by a single membrane that exist in most eukaryotic organisms and cells and are involved in various metabolic, as well as nonmetabolic, cellular functions (Smith and Aitchison, 2013). Peroxisomes communicate with other organelles to mediate these functions via signal transduction pathways, by vesicular trafficking, and by physical membrane contact sites (Shai et al., 2016). In human cells, the interplay between peroxisomes and the ER is important for different metabolic pathways, including the biosynthesis of sterols, unsaturated fatty acids, and ether-phosholipids, and defects in these functions lead to devastating diseases (Schrader et al., 2013). The ER is important for peroxisome biogenesis (Raychaudhuri and Prinz, 2008), and physical contact sites between peroxisomes and the ER were recently identified in yeast (David et al., 2013; Knoblach et al., 2013). Although the close proximity of peroxisomes and the ER was observed already a long time ago in mammalian cells (Shai et al., 2016), the presence of a bona fide peroxisome–ER contact site had not been demonstrated.
In this issue, Costello et al. and Hua et al. used different experimental approaches that independently identified a peroxisome–ER contact site in human cells. The starting point for both groups was a search for proteins that interact with known peroxisomal proteins. Costello et al. (2017) sought proteins that coimmunoprecipitate with ACBD5, which is predominantly found in peroxisomes, and identified VAPB and VAPA as candidate interactors. Hua et al. (2017) sought proteins that bind PEX16, which can shuttle proteins from the ER to peroxisomes and is required for peroxisome biogenesis, and identified both the VAPA and VAPB proteins as components of PEX16 complexes. Because VAP proteins have long been known to be residents of contact sites and to mediate membrane association through their major sperm protein (MSP) domain (Wyles and Ridgway, 2004), they were exciting candidates for tethers. The MSP domain of VAPs interacts with proteins that contain two phenylalanines (FF) in an acidic tract (FFAT) motif. When the MSP and FFAT motifs are located in proteins that are anchored to opposing organelle membranes, the MSP–FFAT interaction zippers up the contact. Hua et al. (2017) looked among their PEX16-binding candidates for proteins with a FFAT domain and identified ACBD5. The observation that peroxisomal ACBD5 contained a FFAT domain and could be found in the same protein complex as the ER tethering proteins VAPA and VAPB led both groups to investigate whether or not these proteins play a role in peroxisome–ER tethering.
We have recently put in place a suggestion to term a protein a tether only if it abides by three criteria (Eisenberg-Bord et al., 2016). Together, the studies by Costello et al. (2017) and Hua et al. (2017) fulfill all of the necessary criteria to define VAP–ACBD5 as a real tether complex.
1. Defined localization uniquely to contact sites or enrichments in contacts. Hua et al. (2017) use structured illumination superresolution microscopy to show that the VAP proteins, although found on ER membranes, are enriched in puncta that are localized in proximity to peroxisomes.
2. Structural capacity to tether to opposing membranes. The VAPs and ACBD5 are anchored to opposing organelles and bind each other through defined motifs, enabling tethering. Both studies demonstrate that the ability of VAPB to bind ACBD5 requires the FFAT domain. Costello et al. (2017) use transmission electron microscopy to show that overexpression of VAPB or ACBD5 increases peroxisomal–ER contact in a FFAT-dependent manner, whereas depletion of ACBD5 reduces the amount of close contact between peroxisomal and ER membranes.
3. Functional activity. The VAPs and ACBD5 affect peroxisome migration and contact site function. Both groups confirm that depletion of ACBD5 leads to an increase in the motility of peroxisomes, presumably as they lose their tethering to the ER. Likewise, both studies demonstrate that loss of peroxisome–ER tethering prevents the elongation and growth of the peroxisome membrane, which suggests that transfer of membrane lipids from the ER to peroxisomes is one of the functions of the physical contact between these organelles. Hua et al. (2017) also show the potential involvement of this tether in the biosynthesis of plasmalogens and cholesterol homeostasis.
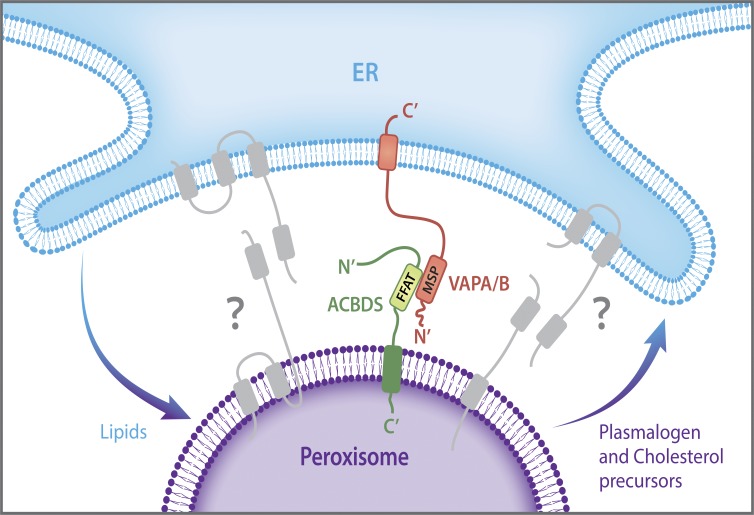
Therefore, the peroxisome–ER tether complex is composed of the peroxisomal protein ACBD5 and the VAPA and VAPB ER proteins. ACBD5 and the VAP proteins are anchored to the peroxisome or to the ER membrane, respectively, through their transmembrane domains that are localized at their C′ termini, while their N′ termini face the cytosol (Fig. 1). Similar to other VAP-interacting proteins, ACBD5 contains a FFAT-like motif that is important for the interaction with the VAP proteins and for the ability to mediate peroxisome–ER proximity. ACBD5 was previously suggested to be a lipid transfer protein for very-long-chain fatty acids (Ferdinandusse et al., 2016). The fact that a lipid transfer protein acts as a tether is in line with tethers identified at other contact sites, which seem to often couple functional transfer of small molecules with a tethering capacity.
Figure 1.
The peroxisomal ACBD5 protein and the VAPA/B ER proteins mediate a physical peroxisome–ER contact in humans. ACBD5 and the VAPA and VAPB proteins are anchored to the peroxisome or to the ER membrane, respectively, through a transmembrane domain localized at their C’ termini, whereas their N’ termini face the cytosol. The VAP–ACBD5 interaction is mediated through the FFAT motif of ACBD5 and the MSP domain of the VAP proteins. Together the proteins form a tether complex that holds the peroxisome and ER membranes in close proximity. The physical contact is suggested to enable ER-to-peroxisome transport of lipids, required for elongation and growth of the peroxisome membrane, as well as peroxisome-to-ER transport of plasmalogen and cholesterol precursors. Because other cellular contacts are held by more than a single tether, additional tethering complexes (proteins marked in gray) might be discovered in the future.
The identification of a bona fide peroxisome–ER tether complex by Costello et al. (2017) and Hua et al. (2017) builds a new, exciting direction in the field of contact sites and, as is always the case in science, opens up many intriguing questions. For example, it would be interesting to further characterize the peroxisome–ER tether complex. The results of Hua et al. (2017) imply that manipulating both VAPA and VAPB entails more severe effects on the peroxisome–ER contact than a single manipulation, but it is not clear if the VAP proteins interact simultaneously with ACBD5 in the same tethering complex or if there are two types of complexes (VAPA–ACBD5 and VAPB–ACBD5). Another interesting direction would be to identify the other proteins that are localized to the peroxisome–ER contact site, which will shed light on the functions and regulation of the physical interaction between the organelles. All other contact sites studied to date contain more than one single tethering complex (Gatta and Levine, 2016). Therefore, additional tethering complexes may be discovered within the peroxisome–ER contact site, as well as molecules that mediate the transfer of additional lipid species and other metabolites such as glutathione disulfide. Moreover, it would be fascinating to study if defects in the peroxisome–ER contact affect the etiology of different diseases as mutations in ACBD5 and VAPB are linked to neuropathological disorders. Mutations in VAPB cause an inherited amyotrophic lateral sclerosis (ALS) and can lead to increased cholesterol levels (Marques et al., 2006), whereas ACBD5 mutations were recently shown to exist in patients with impaired metabolism of very-long-chain fatty acids (Ferdinandusse et al., 2016; Yagita et al., 2016). Indeed, Hua et al. (2017) show that overexpression of an ALS-associated VAPB mutant protein leads to clustering of peroxisomes in an ACBD5-dependent manner. Hence, it will be interesting to examine how the mutations identified in ACBD5 and VAPB affect the peroxisome–ER contact and its functions, and how these changes lead to the pathology of the diseases.
Contact sites are now being extensively studied, and new contacts, tether complexes, regulators, and functions are discovered on a monthly basis. These new observations provide invaluable pieces of the contact site puzzle that promote our understanding of how the complicated cellular machine works as one coherent, interlaced, and highly communicative unit.
Acknowledgments
This work was supported by the H2020 European Research Council (ERC) (Consolidator grant Peroxisystem 646604). M. Schuldiner is an incumbent of the Dr. Gilbert Omenn and Martha Darling Professorial Chair in Molecular Genetics.
The authors declare no competing financial interests.
References
- Costello J.L., Castro I.G., Hacker C., Schrader T.A., Metz J., Zeuschner D., Azadi A.S., Godinho L.F., Costina V., Findeisen P., et al. . 2017. ACBD5 and VAPB mediate membrane association between peroxisomes and the ER. J. Cell Biol. 10.1083/jcb.201607055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C., Koch J., Oeljeklaus S., Laernsack A., Melchior S., Wiese S., Schummer A., Erdmann R., Warscheid B., and Brocard C.. 2013. A combined approach of quantitative interaction proteomics and live-cell imaging reveals a regulatory role for endoplasmic reticulum (ER) reticulon homology proteins in peroxisome biogenesis. Mol. Cell. Proteomics. 12:2408–2425. 10.1074/mcp.M112.017830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg-Bord M., Shai N., Schuldiner M., and Bohnert M.. 2016. A tether is a tether is a tether: Tethering at membrane contact sites. Dev. Cell. 39:395–409. 10.1016/j.devcel.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Ferdinandusse S., Falkenberg K.D., Koster J., Mooyer P.A., Jones R., van Roermund C.W., Pizzino A., Schrader M., Wanders R.J., Vanderver A., and Waterham H.R.. 2016. ACBD5 deficiency causes a defect in peroxisomal very long-chain fatty acid metabolism. J. Med. Genet. 10.1136/jmedgenet-2016-104132 10.1136/jmedgenet-2016-104132 [DOI] [PubMed] [Google Scholar]
- Gatta A.T., and Levine T.P.. 2016. Piecing together the patchwork of contact sites. Trends Cell Biol. 10.1016/j.tcb.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Hua R., Cheng D., Coyaud E., Freeman S., Di Pietro E., Wang Y., Vissa A., Yip C.M., Fairn G.D., Braverman N., et al. . 2017. VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J. Cell Biol. 10.1083/jcb.201608128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach B., Sun X., Coquelle N., Fagarasanu A., Poirier R.L., and Rachubinski R.A.. 2013. An ER-peroxisome tether exerts peroxisome population control in yeast. EMBO J. 32:2439–2453. 10.1038/emboj.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques V.D., Barreira A.A., Davis M.B., Abou-Sleiman P.M., Silva W.A. Jr., Zago M.A., Sobreira C., Fazan V., and Marques W. Jr. 2006. Expanding the phenotypes of the Pro56Ser VAPB mutation: proximal SMA with dysautonomia. Muscle Nerve. 34:731–739. 10.1002/mus.20657 [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S., and Prinz W.A.. 2008. Nonvesicular phospholipid transfer between peroxisomes and the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 105:15785–15790. 10.1073/pnas.0808321105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M., Grille S., Fahimi H.D., and Islinger M.. 2013. Peroxisome interactions and cross-talk with other subcellular compartments in animal cells. Subcell. Biochem. 69:1–22. 10.1007/978-94-007-6889-5_1 [DOI] [PubMed] [Google Scholar]
- Shai N., Schuldiner M., and Zalckvar E.. 2016. No peroxisome is an island—Peroxisome contact sites. Biochim. Biophys. Acta. 1863:1061–1069. 10.1016/j.bbamcr.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J., and Aitchison J.D.. 2013. Peroxisomes take shape. Nat. Rev. Mol. Cell Biol. 14:803–817. 10.1038/nrm3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyles J.P., and Ridgway N.D.. 2004. VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus. Exp. Cell Res. 297:533–547. 10.1016/j.yexcr.2004.03.052 [DOI] [PubMed] [Google Scholar]
- Yagita Y., Shinohara K., Abe Y., Nakagawa K., Al-Owain M., Alkuraya F.S., and Fujiki Y.. 2016. Deficiency of a retinal dystrophy protein ACBD5 impairs peroxisomal β-oxidation of very-long-chain fatty acids. J. Biol. Chem.:jbc.M116.760090 10.1074/jbc.M116.760090 [DOI] [PMC free article] [PubMed] [Google Scholar]