Abstract
Background
Inhaled corticosteroids (ICS) are the primary treatment for persistent asthma. Currently available ICS have differing particle size due to both formulation and propellant, and it has been postulated that this may impact patient outcomes. This structured literature review and meta-analysis compared the effect of small and standard particle size ICS on lung function, symptoms, rescue use (when available) and safety in patients with asthma as assessed in head-to-head randomized controlled trials (RCTs).
Methods
A systematic literature search of MEDLINE was performed to identify RCTs (1998–2014) evaluating standard size (fluticasone propionate-containing medications) versus small particle size ICS medication in adults and children with asthma. Efficacy outcomes included forced expiratory volume in 1 s (FEV1), morning peak expiratory flow (PEF), symptom scores, % predicted forced expiratory flow between 25 and 75% of forced vital capacity (FEF25–75%), and rescue medication use. Safety outcomes were also evaluated when available.
Results
Twenty-three independent trials that met the eligibility criteria were identified. Benefit-risk plots did not demonstrate any clinically meaningful differences across the five efficacy endpoints considered and no appreciable differences were noted for most safety endpoints. Meta-analysis results, using a random-effects model, demonstrated no significant difference between standard and small size particle ICS medications in terms of effects on mean change from baseline FEV1 (L) (−0.011, 95% confidence interval [CI]: −0.037, 0.014 [N = 3524]), morning PEF (L/min) (medium/low doses: −3.874, 95% CI: −10.915, 3.166 [N = 1911]; high/high-medium doses: 5.551, 95% CI: −1.948, 13.049 [N = 749]) and FEF25–75% predicted (−2.418, 95% CI: −6.400; 1.564 [N = 115]).
Conclusions
Based on the available literature, no clinically significant differences in efficacy or safety were observed comparing small and standard particle size ICS medications for the treatment of asthma.
Trial registration
GSK Clinical Study Register No: 202012.
Keywords: Inhaled corticosteroids, Particle size, Asthma, Systematic review, Meta-analysis
Background
Asthma is a common chronic lung condition characterized by inflammation of the airways, and defined by episodes of wheezing, chest tightness, shortness of breath, and coughing [1]. Treatment with regular daily inhaled corticosteroids (ICS) is highly effective at reducing symptoms and the risk of asthma exacerbation and is the primary therapy for control of chronic asthma in both adults and children [1]. The clinical effects of daily ICS are recognized in national and international guidelines as they eliminate or reduce chronic symptoms of asthma, prevent exacerbations, maximize lung function, reduce the need for rescue β2-agonist treatment, and enable normal activity with few side effects at low and medium dose [1, 2].
Delivery of drug to the lungs is influenced by a number of factors including inspiratory flow and particle size. Current aerosol delivery systems generally deliver poly-dispersed aerosols with the majority of particles in the range 1–5 μm in diameter [3]. Particles <1 μm are generally exhaled while most particles >5 μm are usually deposited in the upper airways. However, altering the characteristics of the aerosol even within this narrow window of 1–5 μm can alter the pattern of deposition within the lungs. As control of asthma by ICS requires delivery to both small and large airways, the differing particle size of ICS medications could potentially impact both efficacy and safety outcomes [4, 5]. Traditional chlorofluorocarbon (CFC) pressurized metered dose inhalers (pMDIs) were all suspension-based formulations but following the CFC transition and the advent of hydrofluoroalkane (HFA) propellants, a variety of new suspension-based and solution-based formulations have been developed. Solution-based pMDIs differ from traditional suspension-based pMDIs in that the respirable particles are only generated after actuation as the propellant evaporates from the liquid plume [6, 7]. The characteristics of the particles generated with solution-based pMDIs vary from formulation to formulation, with some generating extra-fine particles with mass median aerodynamic diameter (MMADs) of <2 μm while others generate particles with MMADs more comparable with traditional HFA-suspension pMDIs (MMADs of 2–5 μm).
Two of the most widely prescribed ICS treatments are fluticasone propionate (FP) and beclometasone dipropionate (BDP), which are chemically and structurally similar but differ in their pharmacodynamic properties [5]. For patients not controlled on ICS alone, both the United States and European guidelines recommend the additional use of a long-acting β2-agonist (e.g. salmeterol, formoterol, etc.) in a fixed-dose combination device. FP and FP/salmeterol (FP/SAL) are formulated as HFA-suspensions, while BDP, BDP-formoterol (BDP-F), and a more recent ICS, ciclesonide (CIC) are formulated as HFA-solutions which generate extra-fine aerosols [5]. Thus, FP and FP/SAL are considered standard particle size ICS (2–5 μm), while BDP, BDP-F and CIC are considered small particle ICS (<2 μm).
It has been postulated that the use of ICS medications with a smaller particle size may confer additional clinical benefits to patients with asthma compared with medications with particles of a standard size as they are able to access the smaller airways resulting in increased efficacy [8].
The objective of this systematic literature review and meta-analysis was to evaluate the impact of particle size on clinical outcomes of patients with asthma by comparing the effect of small and standard size particle ICS on lung function, symptoms, rescue use (when available) and safety as assessed in head-to-head randomized controlled trials (RCTs).
Methods
Details on the methods of the analysis and inclusion criteria were specified in advance and documented in a protocol (GSK Clinical Study Register ID: 202012, data on file), and are summarized below.
Inclusion criteria, information source, search and study selection
Studies eligible for inclusion in the systematic review were published RCTs comparing FP-containing therapy (standard particle size) with ICS preparations of small particle size in adults and children with asthma. Specifically, treatments evaluated included FP and FP/SAL versus ICS small particle size comparators (BDP, BDP-F or CIC). Abstracts for potential inclusion in the systematic review were identified from the MEDLINE database using the following search terms in PubMed: disease: asthma; exposure: fluticasone, Flovent®, Flixotide®, Advair®, Seretide®. Abstracts in English published between January 1, 1998 and January 13, 2014 were considered.
All identified citations were downloaded and duplicate citations were removed to yield a number of unique hits. Citations were assessed in a multi-stage screening process as outlined in Fig. 1. During Screening Stage One, studies/abstracts were excluded if they included only patients with allergic rhinitis, compared ICS medications other than FP or FP/SAL versus BDP, BDP-F, or CIC, were placebo-controlled, were not a primary epidemiologic/clinical study or were considered ‘gray literature’ (meeting abstracts, letters, websites). Other exclusion criteria included: restricted population (e.g. pregnant women); comparisons of the same ICS at different dosages; no efficacy or safety data. Citations were designated as ‘Exclude’, ‘Include’ or ‘Doubt’ and a record of these decisions was maintained. Abstracts marked as ‘Doubt’ were cross-reviewed by a second epidemiologist. In Screening Stage Two, full text articles of the titles identified as ‘Include’ in Screening Stage One were reviewed and screened against the exclusion criteria listed above. The remaining studies were utilized for extraction.
Fig. 1.
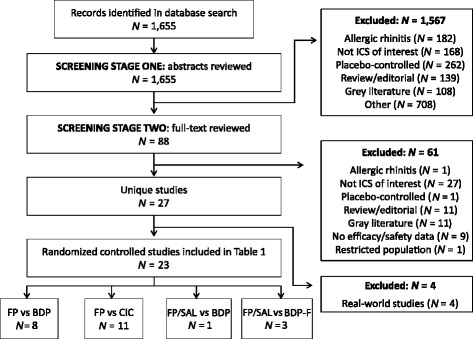
Flow diagram showing implementation of search and screening strategies. BDP, beclometasone dipropionate, BDP-F, beclometasone dipropionate/formoterol fumarate; CIC, ciclesonide; FP, fluticasone propionate; FP/SAL, fluticasone propionate/salmeterol; ICS, inhaled corticosteroid
Data extraction and data items
Study/patient characteristics and interventions were abstracted from the selected studies. Information on the following efficacy outcome measures were also extracted: forced expiratory volume in 1 s (FEV1), morning peak expiratory flow (PEF), asthma symptom scores (on 4–9-point scale where a lower score corresponded to fewer symptoms), % predicted forced expiratory flow between 25 and 75% of forced vital capacity (FVC; FEF25–75%), and rescue medication use per day. In addition to the common lung function measure in asthma studies (FEV1 and PEF), FEF25–75% was chosen as an efficacy measure as it is a more sensitive indicator for small airway obstruction than FEV1 [9], and thus more likely to demonstrate variations in efficacy if the smaller particles were meeting the small airways. To characterize available safety data, the following endpoints were considered: any adverse events (AEs; at least one), local steroid effects (oral candidiasis, hoarseness), upper respiratory tract infections, growth and bone metabolism, and serum cortisol levels to assess adrenal suppression.
Assessment of risk of bias
Funnel plots were used to detect biases in the identification and selection of studies. The funnel plot is a technique used to investigate the possibility of biases in the identification and selection phases. In a funnel plot, the estimated effect size of the intervention from individual studies (mean difference) is plotted on the horizontal axis against the standard error of the intervention effect estimate or sample size on the vertical axis. If there are no biases, the graph will tend to have a symmetrical funnel shape centered on the average effect of the studies. All studies were included and additional sources of bias were not formally assessed.
Planned analysis and statistical methods
Aggregated clinical data from the completed systematic review were summarized in standardized electronic extraction forms, with comparative data also entered into spreadsheets. Clinical statisticians transferred relevant extracted data into SAS (Statistical Analysis Software, Cary, NC) or R (R Foundation for Statistical Computing, Vienna, Austria) for calculation of appropriate statistics and data displays.
The objective of this analysis was to determine if there were any clinically significant differences in the comparative efficacy or safety of FP-containing medications with smaller particle ICS-containing comparators; this was evaluated in the form of a benefit-risk interval plot and/or meta-analysis, when appropriate. The data were extracted on the intent-to-treat (ITT) population as defined in each individual trial. The original publications gave treatment doses as either emitted or delivered; these same doses were reported within this manuscript for consistency.
Treatment comparisons were made using absolute treatment differences between FP-containing formulations and small particle ICS, including 95% confidence intervals (CI). For continuous measures, adjusted mean differences were used, when available. When standard errors (SE) and/or CIs were not directly available, they were estimated using available data [10]. For binary measures, the absolute risk difference and its 95% CI were calculated using the normal approximation to the binomial distribution.
Formal meta-analysis was conducted for efficacy endpoints when there was sufficient sample size and homogeneity across trials. Due to this approach, there was no adjustment for multiple testing. If there was no significant evidence of heterogeneity across the studies, both fixed and random effects models were performed. The statistical heterogeneity of the meta-analysis was assessed by means of the Cochran Q, chi-square test and the I2 statistic with 95% CI. When the assessments such as Cochran Q or chi-square test showed that heterogeneity existed, the results of the random effects model were selected. Results in children (12 years and younger) and adolescents/adults were analyzed separately. Meta regression was used to adjust for differences across studies as appropriate. Additional sensitivity analyses were performed when appropriate.
Where meta-analysis was not feasible, benefit-risk interval plots were produced to visually display the estimated differences between treatments and their 95% CIs for different endpoints on the same graph across studies; irrespective of differences in study designs, endpoints and units.
Results
The search of the PubMed database identified 1655 potentially relevant articles: 1567 were excluded, mainly because they were placebo-controlled, evaluated allergic rhinitis or did not evaluate an ICS of interest; 88 full-text articles were reviewed and 23 RCTs were included in the final analysis (Fig. 1) [4, 11–32].
Eight studies evaluated FP versus BDP, 11 evaluated FP versus CIC, one evaluated FP/SAL versus BDP and three evaluated FP/SAL versus BDP-F (Table 1). No studies evaluating FP versus BDP-F and FP/SAL versus BDP or CIC were identified. Information for children (6 to 15 years in age) was only available in four studies [15, 18, 26, 27]; one of which utilized a spacer in each arm [18]. No other studies (adults or children) were found to use a spacer.
Table 1.
Characteristics of RCTs included in the analysis
| Reference | Study design and treatment duration | Study population | Treatment group | Comparison group | Efficacy outcome measures (as change from baseline at end of treatment unless otherwise indicated) |
Significant results between groups (treatment versus comparison) |
|---|---|---|---|---|---|---|
| Fluticasone propionate (FP) versus beclometasone dipropionate (BDP) | ||||||
| Aubier, 200111 | Open-label, parallel-group 8 weeks | Adults (18–75 years) with ≥4-week clinical history of moderate to severe asthma from 31 medical centers | BDP 800 μg/day via HFA-pMDI (n = 101 ITT) High dose |
FP 1000 μg via HFA-pMDI (n = 97 ITT) High dose |
AM PEF (L/min), PM PEF (L/min), FEV1 (L), patients free from daily asthma symptoms (%), patients using SABA rescue medication (%) | Equivalence demonstrated for AM PEF: CI within ±25 L/min |
| Currie, 200212 | Single-blind, crossover 3 weeks per Tx period | Patients (mean [SE] age = 38 [4] years) with mild to moderate asthma | FP 500 μg/d via HFA-pMDI (n = 20 ITT) Medium dose |
BDP 500 μg/d via HFA-pMDI (n = 20 ITT) Medium dose |
Methacholine PD20, (μg), exhaled tidal NO (ppb), FEV1 (% predicted), AM PEF (L/min) | None |
| FP 1000 μg/d via HFA-pMDI (n = 20 ITT) High dose |
BDP 1000 μg/d via HFA-pMDI (n = 20 ITT) High dose |
Methacholine PD20, (μg), exhaled tidal NO (ppb), FEV1 (% predicted), AM PEF (L/min) | None | |||
| Fairfax, 200113 | Double-blind, double-dummy, parallel-group 6 weeks | Patients 18–65 years with at least a 4-week past history of clinically diagnosed asthma from 30 general practice sites | BDP 400 μg/d via HFA-pMDI (n = 88 ITT) Medium dose |
FP 400 μg/d via CFC-pMDI (84 ITT) Medium dose |
PEFR (L/min), FEV1 (L) | Equivalence demonstrated for AM PEFR: CI within ±25 L/min |
| Ohbayashi, 200814 | Double-crossover (results presented from parallel-group analysis of first stage of study) 3 months per Tx |
Patients (FP group mean age (years): 61.0 ± 17.4; BDP group mean age: 67.9 ± 13.1) with mild to moderate persistent asthma controlled with FP Diskus for ≤6 months | FP dose dependent on patient, via Diskus (n = 25) |
BDP Same dose as FP, via HFA-pMDI (n = 24) |
VC, FVC, FEV1, MEFR, PEF (all % predicted), FEF50% (L/s), FEF75% (L/s) |
None |
| Robroeks, 200815 | Crossover 3 months per Tx |
Children (6–12 years) with moderate persistent asthma from outpatient clinic | BDP 200 μg/d via HFA-pMDI (n = 30) Low dose |
FP 200 μg/d via DPI (n = 30) Low dose |
Absolute measures at end of treatment: FEV1, FVC, MEF50 (all % predicted), FEV1/VC (%) |
None |
| Thongngarm, 200516 | Open-label 3 months |
Patients (≥18 years) with 6-month history of asthma | BDP 320 μg/d via HFA-pMDI (n = 20 ITT) Medium dose |
FP 330 μg/d via CFC-pMDI (n = 10 ITT) Medium dose |
FEV1, FVC, FEF25–75% (all % predicted), CV (L), CV-VC, post-bronchodilator TLC, TGV, RV, FEV1, FVC, FEF25–75% (all % predicted), AM PEF, night-time awakening, shortness of breath, chest tightness, wheezing, cough, phlegm (all symptoms/day), albuterol use (puffs/day) | BDP users had larger improvement in FEF25–75%
(% predicted), CV, CV-VC, phlegm and albuterol use |
| Tunon-de-Lara, 200717 | Open, parallel-group 84 days |
Patients with mild or moderate asthma | BDP 400 μg/d via HFA-pMDI (n = 11 PP) Medium dose |
FP 500 μg/d via Diskus (n = 14 PP) Medium dose |
FEV1, FVC, SVC, SVC-FVC, FEV1/FVC, FEV1/LCV, FEF50% and FEF25–75% (all % predicted) | None |
| Van Aalderen, 200718 | Double-blind, double-dummy, parallel-group 6 weeks |
Patients (5–12 years) with asthma dx ≥ 3 months from 6 sites | BDP 200 μg/d via HFA-pMDI (n = 139 ITT) Low dose |
FP 200 μg/d via CFC-pMDI (n = 140 ITT) Low dose |
AM PEF, PM PEF, FEV1, FVC, FEF25–75% (all % predicted), symptom-free days (%), nights without sleep disturbance (%), β2-agonist therapy,mean (puffs/day) | Noninferior: lower CL > −5 (% predicted) for AM PEF; FP users had greater improvement in FEF25–75% (% predicted) |
| Fluticasone propionate versus extra-fine ciclesonide (CIC) | ||||||
| Bateman, 200819 | Open-label, parallel-group 24 weeks |
Outpatient adults and adolescents (12–75 years) with asthma in Europe | CIC 640 μg/d via HFA-pMDI (n = 255 ITT) High dose |
FP 660 μg/d via HFA-pMDI (n = 273 ITT) High dose |
FEV1 (mL), FVC (L), PEF (L/min), AM PEF (L/min), asthma symptom score, rescue medication use (puffs/day) Absolute measures at end of treatment: Asthma symptom-free days, rescue medication-free days, asthma symptom and rescue medication-free days |
Noninferior: lower CL above −200 mL for FEV1; Noninferior: lower CL above −0.2 L for FVC; Noninferior: lower CL above -25 L/min for PEF and AM PEF |
| Boulet, 200720 | Open-label, parallel-group 12 weeks |
Adult and adolescent (12–75 years) patients with asthma from 59 Tx centers | CIC 320 μg/d via MDI (n = 233 ITT) Medium dose |
FP 400 μg/d via DPI (n = 239 ITT) Medium dose |
FEV1 (mL), FEV1 (% predicted), FVC (L), SVC (L), AM PEF (L/min), PM PEF (L/min), daytime asthma symptom score, total asthma symptom score, rescue medication use (puffs/day) | Noninferior: lower CL above −200 mL for FEV1; Noninferior: lower CL above −0.2 L for FVC; Noninferior: lower CL above −25 L/min for AM PEF and PM PEF |
| Buhl, 200621 | Multicenter, double-blind, double-dummy, parallel-group 12 weeks |
Adult and adolescent (12–75 years) patients with asthma from 57 Tx centers | CIC 160 μg/d (n = 266 ITT) Low dose |
FP 176 μg/d via HFA-pMDI (n = 263 ITT) Low dose |
FEV1 (L), FVC (L), AM PEF (L/min), daytime symptom score, night-time symptom score, total asthma symptom score (day + night score), rescue medication use (puffs/day) | Noninferior: lower CL above -0.2 L for FEV1 and FVC; Noninferior: lower CL above -25 L/min for AM PEF |
| Cohen, 20114 | Double-blind, double-dummy, parallel-group 65 days |
Adult patients with asthma (18–60 years) recruited from outpatient clinics of pulmonology departments in the Netherlands | CIC 160 μg/d via HFA-pMDI (n = 19) Low dose |
FP 200 μg/d via HFA-pMDI (n = 18) Low dose |
FEV1 (% predicted), FVC (L), SVC (L), FVC/SVC (L), FEF50% (L/s), FEF25–75% (% predicted) | FP users had greater improvement in FEV1 and FEF25–75% (% predicted) |
| Dahl, 201022 | Double-blind, double-dummy, 2-arm, parallel-group 24 weeks |
Adult and adolescent patients (12–75 years) with mild to moderate asthma from 48 centers internationally | CIC 80 μg/d via HFA-pMDI (n = 240 ITT) Low dose |
FP 200 μg/d via HFA-pMDI (n = 240 ITT) Low dose |
FEV1 (L), FVC (L), AM PEF (L/min) Absolute measures at end of treatment: Days with asthma control (%), asthma exacerbations requiring Tx (%) |
Noninferior: lower CL above -0.2 L for FEV1 and FVC; Noninferior: lower CL above -25 L/min for AM PEF |
| Lee, 200423 | Double-blind, double-dummy, crossover 4 weeks per Tx |
Non-smoking patients with mild-to-moderate persistent asthma | CIC 400 μg/d via HFA-pMDI (n = 19) High dose |
FP 500 μg/d via HFA-pMDI (n = 19) Medium dose |
NO (ppb), FEV1 (L), FEV1 (% predicted), FEF25–75% (L/s), FEF25–75% (% predicted), methacholine PD20 (mg/ml), AM PEF (L/min), PM PEF (L/min), NO (ppb), AM and PM asthma symptom score, AM and PM rescue medication use (puffs/day) | None |
| Lee, 200524 | Double-blind, double-dummy, crossover 4 weeks per Tx |
Adult patients (mean age 47 years) with moderate, persistent asthma stable for 3 months | CIC 1600 μg/d via HFA-pMDI (n = 14) High dose |
FP 2000 μg/d via HFA-pMDI (n = 14) High dose |
Absolute measures at end of treatment: FEV1 (L), FEV1 (% predicted), FEF25–75% (L/s), FEF25–75% (% predicted), AM PEF (L/min), PM PEF (L/min), AM and PM asthma symptom score, AM and PM rescue meds (puffs/d), exhaled nitric oxide (ppb), methacholine PC20 (mg/mL) | None |
| Magnussen, 200725 | Double-blind, double-dummy, 3-arm, parallel-group 12 weeks |
Patients at least 12 years of age with asthma from 91 sites | CIC 80 μg/d via HFA-pMDI (n = 278 ITT) Low dose |
FP 178 μg/d via HFA-pMDI (n = 259 ITT) Low dose |
FEV1 (mL), FVC (L), PEF (L/min), asthma symptom score, daytime and night-time symptom score, rescue medication use (puffs/day) | Noninferior: lower CL above -200 mL for FEV1; Noninferior: limit NR for FVC and PEF |
| CIC 160 μg/d via HFA-pMDI (n = 270 ITT) Low dose |
FP 178 μg/d via HFA-pMDI (n = 259 ITT) Low dose |
FEV1 (mL), FVC (L), PEF (L/min), asthma symptom score, daytime and night-time symptom score, rescue medication use (puffs/day) | Noninferior: lower CL above -200 mL for FEV1; Noninferior: limit NR for FVC and PEF |
|||
| Pedersen, 200626 | Multicenter, double-blind, double-dummy, 2-arm, parallel-group 12 weeks |
Children (6–15 years) with asthma from 51 sites | CIC 160 μg/d via HFA-pMDI (n = 254 ITT) Low dose |
FP 178 μg/d via HFA-pMDI (n = 257 ITT) Low dose |
FEV1 (L), PEF (L/min), AM PEF (L/min), PM PEF (L/min), asthma symptom score sum, asthma symptom free days, rescue medication-free days | Noninferior: lower CL greater than −0.1 L for FEV1; Not noninferior: lower CL less than −12.5 L/min for PEF, AM PEF and PM PEF |
| Pedersen, 200927 | Double-blind, double-dummy, 3-arm, parallel-group 12 weeks |
Children (6–11 years) with asthma from 50 international centers | CIC 80 μg/d via HFA-pMDI (n = 234 ITT) Low dose |
FP 178 μg/d via HFA-pMDI (n = 245 ITT) Low dose |
FEV1 (L), AM PEF (L/min), asthma symptom score sum, rescue medication use Absolute measures at end of treatment: Asthma exacerbations (%) |
Noninferior: lower CL greater than −0.1 L for FEV1; Not noninferior: lower CL less than −12.5 L/min for AM PEF; Noninferior: upper CL less than 0.30 for asthma symptom score sum |
| CIC 160 μg/d via HFA-pMDI (n = 232 ITT) Low dose |
FP 178 μg/d via HFA-pMDI (n = 245 ITT) Low dose |
Noninferior: lower CL greater than −0.1 L for FEV1; Noninferior: lower CL greater than −12.5 L/min for AM PEF; Noninferior: upper CL less than 0.30 for asthma symptom score sum |
||||
| Van der Molen, 201028 | Multicenter, parallel-group (series of 3 studies) 12–24 weeks |
Health patients with asthma? aged 12–75 years | CIC 320 μg/d via pMDI (n = 224 ITT) Medium dose |
FP 400 μg/d via DPI (n = 228 ITT) Medium dose |
No efficacy outcomes; only safety outcomes available | |
| CIC 640 μg/d via pMDI (n = 244 ITT) High dose |
FP 750 μg/d via pMDI (n = 254 ITT) High dose |
|||||
| CIC 640 μg/d via pMDI (n = 250 ITT) High dose |
FP 1000 μg/d via pMDI (n = 237 ITT) High dose |
|||||
| Fluticasone propionate/salmeterol versus extra-fine beclometasone dipropionate | ||||||
| Fowler, 200229 | Double-blind, double-dummy, parallel-group 8 weeks |
Patients with asthma aged 16–70 years | BDP 400 μg/d via HFA-pMDI (n = 20) Medium dose |
FP/SAL 200/100 μg/d via DPI (n = 19) Low dose |
Absolute measures at end of treatment: Methacholine PD20 (μg), FEV1 (L), FEV1 (% predicted), FEF25–75% (L/s), FEF25–75% (% predicted), AM PEF (L/min), PM PEF (L/min), tidal exhaled NO (ppb), symptom score, reliever use (puffs/d) | FP/SAL users had higher post-study methacholine PD20, FEV1 (L), FEV1 (% predicted), post-study AM/PM PEF |
| Fluticasone propionate/salmeterol versus extra-fine beclometasone dipropionate/formoterol fumarate | ||||||
| Papi, 200730 | Double-blind, 2-arm parallel-group 12 weeks |
Patients with asthma aged 18–65 years from 12 outpatient respiratory clinics in Europe | BDP-F 400/24 μg/d via pMDI (n = 115 ITT) Medium dose |
FP/SAL 500/100 μg/d via pMDI (n = 113 ITT) Medium dose |
AM pre-dose PEF, AM PEF and PM PEF (all L/min), FEV1 (L), FVC (L), Absolute measures at end of treatment: Daytime and night-time symptom score, symptom-free days (%), day time use of rescue medication (puffs) |
Noninferior: lower CL above -20 L/min for AM pre-dose PEF; BDP-F users had greater improvement in FVC |
| Papi, 201231 | Prospective, controlled, 2-arm parallel-group 24 weeks |
Patients with asthma aged 18–65 years from 67 respiratory clinics in Europe | BDP-F 400/24 μg/d via pMDI (n = 206 ITT) Medium dose |
FP/SAL 500/100 μg/d via Diskus DPI (n = 216 ITT) Medium dose |
Absolute measures at end of treatment: AM PEF (L/min), FEV1 (L), FEV1 (% predicted), PEF (L/min), PEF (% predicted), daytime and night-time symptom score, symptom-free days (%), patients with controlled asthma, asthma exacerbations and severe asthma exacerbations (all %) | Equivalent: CI falls within − 20 to +20 L/min for AM PEF |
| Scichilone, 201032 | Double-blind, double-dummy, parallel-group 12 weeks |
Adult patients with asthma (18–50 years) in Italy | BDP-F 400/24 μg/d via HFA-pMDI (n = 15) Medium dose |
FCS 500/100 μg/d via pMDI (n = 15) Medium dose |
Absolute measures at end of treatment: Pre-dose FEV1 (L), FEF25–75% (L/s), PD20 FEV1 methacholine (μg) |
None |
AM, morning; CFC, chlorofluorocarbon; Cl, confidence interval; CL, clearance; CV-VC, closing volume/vital capacity; DPI, dry powder inhaler; dx, diagnosis; ER, emergency room; FEF, forced expiratory flow; FEV 1, forced expiratory volume in 1 sec; FVC, forced vital capacity; HFA-pMDI, hydrofluoroalkane-pressurized metered dose inhaler; ITT, intent-to-treat; NO, nitric oxide; NR, not reached; PEFR, peak expiratory flow rate; PM, evening; PP, per protocol; ppb, part per billion; RV, residual volume; SABA, short-acting beta agonists; SVC, slow vital capacity; TGV, thoracic gas volume; TLC, total lung capacity; Tx, treatment
The main efficacy endpoints evaluated in the studies were FEV1 and PEF (Table 1). The predominant safety endpoints were overall incidence of AEs and urinary cortisol levels.
Fluticasone propionate versus beclometasone dipropionate
In the eight identified trials comparing conventional suspension-based FP pMDIs with the ‘ultra fine’ solution-based BDP formulations similar doses have been used in each arm (Table 1). This is in accordance with GINA guidelines reporting the clinically comparable doses of HFA-FP and HFA-BDP [1]. Hence these comparisons will address the issue of whether differences in particle size results in a change in the efficacy or safety profile.
The majority of the RCTs reported no significant difference in efficacy outcome measures between FP and BDP. Two of the eight RCTs reported significant differences in FEF25–75% between FP and BDP; one demonstrating improvement with FP [18] and the other with BDP [16] (Table 1).
The majority of RCTs reported no significant difference in AEs or other safety markers between the two treatments. Overnight urinary cortisol/creatinine production was suppressed more in patients using BDP compared with patients on FP (1000 μg/day FP versus BDP: geometric mean fold difference 1.97 [95% CI: 1.28, 3.02]; p < 0.05) [12]. No significant difference was found in cortisol levels in three studies, either in levels at the end of the treatment period or in change from baseline [11, 13, 14].
Fluticasone propionate versus ciclesonide
With few exceptions in 10 identified RCTs, CIC was found to be non-inferior or not statistically different from FP for numerous efficacy endpoints (Table 1). Notably, in a trial by Pedersen et al. in children aged 6–11 years, non-inferiority of CIC to FP (88 μg twice daily) was observed with regard to change in FEV1 from baseline over 12 weeks of treatment with 160 μg but not 80 μg once daily [27]. A similar trial in children aged 6–15 years comparing the same doses found that CIC did not show non-inferiority in the change from baseline in morning or afternoon PEF following 12 weeks of treatment [26]. One trial of adult patients, by Cohen et al., found greater improvement in lung function among patients receiving FP compared with patients receiving CIC, specifically in mean ± SD % predicted FEV1 (0.5 ± 4.3 versus −3.0 ± 4.6, respectively; p = 0.021) and FEF25–75% (0.6 ± 5.6 versus −3.6 ± 6.0, respectively; p = 0.034) [4].
Results for differences in urinary cortisol levels (adjusted for creatinine) were variable, with greater adrenal suppression among patients receiving FP (compared with CIC) reported in an ITT analysis restricted to patients with normal creatinine levels (p = 0.006) [26]. In a small crossover study, patients on FP 2000 μg/day had significantly lower mean overnight 10-h urinary cortisol than patients on CIC (CIC versus FP: geometric mean fold difference 1.5 [95% CI: 1.1, 2.0]; p < 0.05) [24]. One trial assessed side effect perception using a 100-point scale, and observed that patients on CIC had either a smaller increase in perceived side effects or a decrease over the treatment period from baseline compared with patients receiving FP (between-treatment least squares mean (±SE) in total Inhaled Corticosteroid Questionnaire Scores: CIC 320 μg once-daily versus FP 200 μg twice-daily (12 weeks): −2.52 ± 0.82, p = 0.0011; CIC 320 μg twice-daily versus FP 500 μg twice-daily (24 weeks): −2.05 ± 0.79, p = 0.0047 [28].
Fluticasone propionate/salmeterol versus beclometasone dipropionate
One trial was identified that compared the efficacy of BDP (400 μg/day) and FP/SAL (200 μg/100 μg/day) as a step-down therapy after high-dose ICS (dry powder inhaler [DPI]-BDP 2000 μg/day) (Table 1) [29]. Lung function measures were compared between treatment groups at the end of the 8-week treatment period instead of comparing the change from baseline in each group. Methacholine PD20, post-study FEV1 (measured and % of predicted), morning and afternoon PEF were all significantly greater in patients on FP/SAL than in patients on BDP (methacholine PD20 (μg): 149.9 [95% CI: 114.3, 196.5] versus 71.2 (95% CI: 54.7, 92.8]; FEV1 (L): 2.46 [95% CI: 2.39, 2.53] versus 2.26 [95% CI: 2.20, 2.33], p < 0.05; FEV1 (% predicted): 77 [95% CI: 75, 79] versus 70 (95% CI: 68, 72], p < 0.05; morning PEF (L/min): 434 [95% CI: 424, 445] versus 402 [95% CI: 391, 411], p < 0.05; evening PEF (L/min): 436 [95% CI: 425, 446] versus 408 [95% CI: 398, 418]; p < 0.05). No differences were found in FEF25–75%, symptom scores, or reliever medication use [29]. No significant differences were found between treatment groups for serum cortisol levels, urinary cortisol/creatinine ratio, or serum osteocalcin [29].
Fluticasone propionate/salmeterol versus beclometasone dipropionate-formoterol
Three RCTs compared the efficacy of FP/SAL with BDP-F (Table 1) [30–32]. One trial in adults with asthma found significantly greater improvement in FVC in patients receiving BDP-F than in those receiving FP/SAL (0.46 ± 0.51 L versus 0.34 ± 0.44 L, respectively; p = 0.040); however, no differences were found for any other efficacy parameters [30].
Two RCTs were identified that compared the safety of BDP-F with FP/SAL; no differences in AEs or urinary cortisol/creatinine ratio were observed between the two treatments [30, 31].
Meta-analysis
Meta-analysis methods could only be applied for the efficacy endpoints of FEV1, PEF, and FEF25–75% (Fig. 2). Other efficacy and safety endpoints were not considered for the meta-analysis due to heterogeneity, potential publication bias, and disparity of endpoint definitions and/or timing of collection. In adults, the random effects models showed no significant differences between small and standard size particle ICS for change in FEV1 (−0.011 L, 95% CI: −0.037, 0.014; p = 0.394), or FEF25–75% (−2.418, 95% CI: −6.400, 1.564; p = 0.234) (Figs. 2a and c).
Fig. 2.
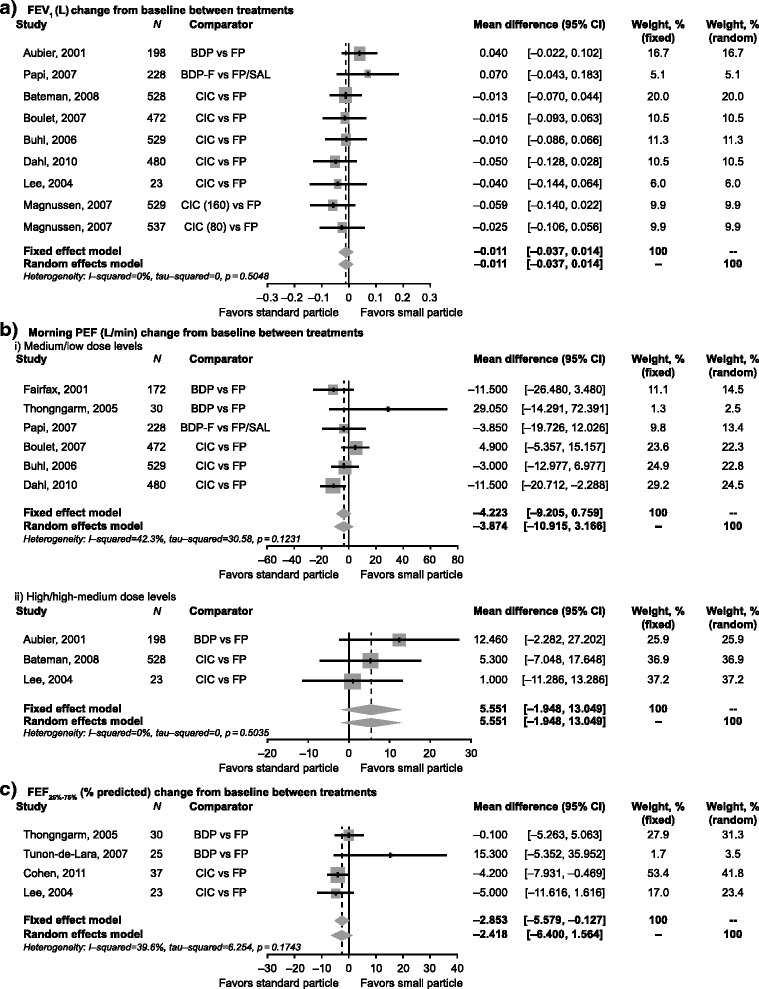
Pooled effects for efficacy endpoints with 95% CI of eligible studies comparing small versus standard size particle ICS medications. Mean difference in change from baseline between treatments for (a) FEV1, (b) morning PEF and (c) FEF25%-75%. BDP, beclometasone dipropionate, BDP-F, beclometasone dipropionate/formoterol fumarate; CI, confidence interval; CIC, ciclesonide; FEF25–75%, % predicted forced expiratory flow between 25% and 75% of forced vital capacity; FEV1, forced expiratory volume in 1 s; FP, fluticasone propionate; FP/SAL, fluticasone propionate/salmeterol; PEF, peak expiratory flow
Meta-regression analysis showed that the only treatment effect modifier present for morning PEF was dose level (high versus low); however, high dose versus medium dose or high hose versus high-medium dose did not show a statistically significant difference. This suggested that it may not be appropriate to use either meta-regression or meta-analysis with all of the data in the final model. Instead, the morning PEF endpoint was analyzed as two separate subgroups (high/high-medium doses and medium/low doses). The random effects models showed no significant differences between small and standard size particle ICS for change in morning PEF (medium/low doses: −3.874 L/min, 95% CI: −10.915, 3.166; high/high-medium doses: 5.551 L/min, 95% CI: −1.948, 13.049) (Figs. 2bi and ii).
For each endpoint, the heterogeneity test showed that there was no between-study variation, suggesting that fixed effects models were also appropriate. The random effects models, which provide a more conservative approach, were retained as primary models. Similar to the random effects model, no significant differences were observed for change in FEV1 and morning PEF using a fixed effects model (p = 0.394 and p = 0.097, respectively). Even though the analysis of both data subgroups for the morning PEF data led to the same conclusion, the results for the mean differences between medium/low (−4.223 L/min) and high/high-medium (5.551 L/min) dose levels appeared to be in opposite directions (Figs. 2bi and ii). Treatment differences in FEF25–75% were found to be significantly in favor of FP using a fixed effects model (−2.853 L/min; 95% CI −5.579, −0.127; p = 0.040), though not in the random effects model (−2.418, 95% CI: −6.400, 1.564; p = 0.234) (Fig. 2c). The heterogeneity test and I2 (p-value = 0.174 and I2 = 39.6%) showed no significant between-study-variation. However, definitive conclusions could not be drawn from these treatment differences due to the small number of studies (N = 4) evaluated, which also explains the wider CIs for the results of the random effects model. In children, the small number of studies with disparate endpoints and results did not allow for meta-analysis.
Sensitivity analyses were performed for FEV1, the high/high-medium dose subgroup data for morning PEF, and FEF25–75%, by excluding trials with crossover design [23] or with multiple arms (only in the case of FEV1) [25]. The results of the sensitivity analyses for FEV1 and the morning PEF were similar to the results of the final model. However, the results of the sensitivity analysis for FEF25–75% (excluding Lee et al. [22]), were found to differ from the final model. There was a statistically significant treatment difference between standard size and small size particles on FEF25–75% in the final fixed effect model (−2.853 L/min; 95% CI −5.579, −0.127; p = 0.040) but not in the sensitivity analysis (−2.414 L/min; 95% CI −5.406, 0.578; p = 0.114). These conflicting conclusions may be due to small patient sample sizes.
Benefit-risk plots
The benefit-risk plots included effect estimates from all individual studies included in the analysis of each efficacy and safety endpoint. For adult/adolescent patients (aged ≥12 years), the effect on total asthma symptom score and rescue medication use was only assessed for FP versus CIC. No clinically meaningful differences were noted across the five efficacy endpoints considered (FEV1, morning PEF, and FEF25–75%, asthma symptoms and rescue medication use) in both adults (Fig. 3) and children (Fig. 4).
Fig. 3.
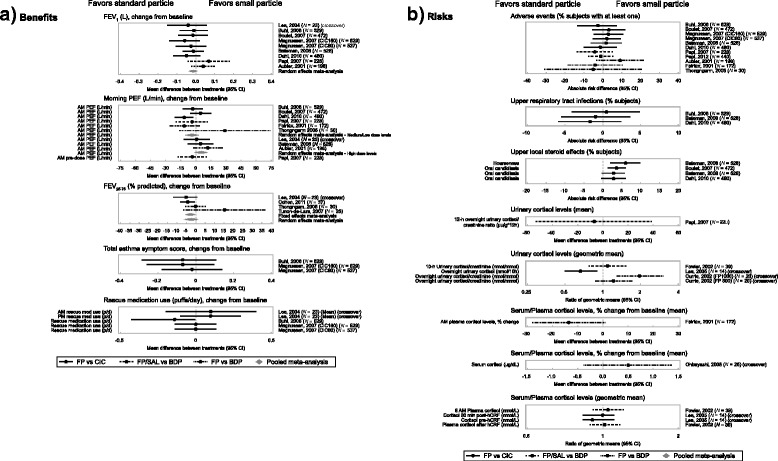
Benefit-risk plot in adolescents and adults by particle size. AM, morning; BDP, beclometasone dipropionate; CI, confidence interval; CIC, ciclesonide; CO, crossover; FEV1, forced expiratory volume in 1 s; FEF25–75%, % predicted forced expiratory flow between 25% and 75% of forced vital capacity; FP, fluticasone propionate; FP/SAL, fluticasone propionate/salmeterol; hCRF, human corticotropin-releasing factor; PEF, peak expiratory flow; PM, evening
Fig. 4.
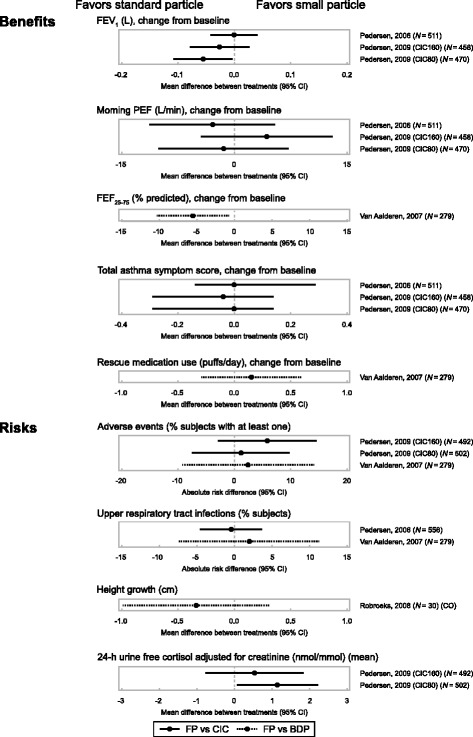
Benefit-risk plot in children by particle size. BDP, beclometasone dipropionate; CIC, ciclesonide; FEF25–75%, % predicted forced expiratory flow between 25% and 75% of forced vital capacity; FEV1, forced expiratory volume in 1 s; FP, fluticasone propionate; PEF, peak expiratory flow
No appreciable differences were noted for most safety endpoints (AEs, local steroid effects, upper respiratory tract infections, growth and bone metabolism, and adrenal suppression) in both adults and children (Figs. 3 and 4); most studies though were not designed to test treatment differences for safety endpoints. Patients receiving FP experienced more local steroid effects at the upper airways than those receiving CIC; this was likely due to the fact that CIC is administered as a pro-drug which is only activated in the lower airways. The cortisol levels data were variable, with no clear differentiation between treatments.
Publication bias
Based on the funnel plots and the test of asymmetry (p-values ranging from 0.225–0.822) (Fig. 5), the FEV1, morning PEF and PEF25–75% data did not exhibit asymmetry, which suggests that there is neither publication bias nor a systematic difference between smaller and larger studies (‘small study effects’).
Fig. 5.
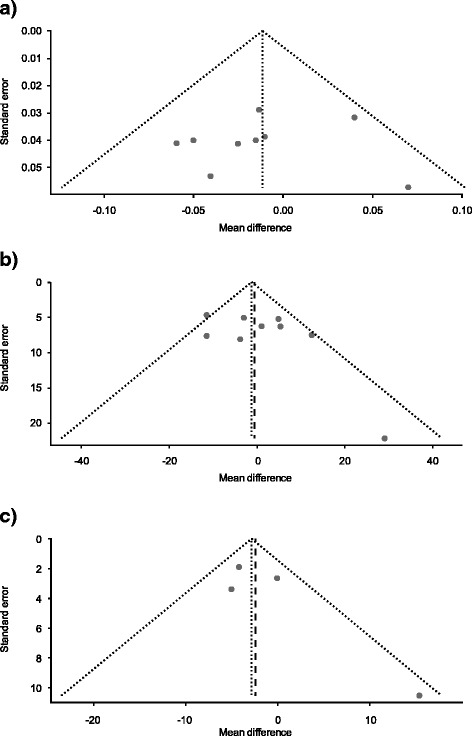
Funnel plots for studies included in the (a) FEV1, (b) morning PEF and (c) FEF25–75% endpoints meta-analyses. a. Linear regression test of funnel plot asymmetry: t = −0.2342, df = 7, p-value = 0.822. b. Linear regression test of funnel plot asymmetry: t = 1.332, df = 7, p-value = 0.225. c. Linear regression test of funnel plot asymmetry t = 1.547, df = 6, p-value = 0.262. FEF25–75%, % predicted forced expiratory flow between 25% and 75% of forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow
Discussion
Summary
In this meta-analysis of studies in adults and adolescents, no significant differences were observed between standard size (FP and FP/SAL) and small size (BDP, CIC and BDP-F) particles ICS for change in FEV1, morning PEF or FEF25–75% using the random effects model. Similarly, no significant differences were observed between standard size and small size particles ICS in the subgroup analysis according to dose for morning PEF. However, it was observed that the result of the medium/low dose analysis for morning PEF was slightly in favor of standard particles (i.e. standard size particles ICS demonstrated better efficacy) versus small particles while the converse was true for high/high-medium dose analysis. This observed difference in the subgroup analyses suggests that dose level may be the effect modifier for morning PEF.
FEF25–75% was chosen as an efficacy endpoint as it is a more sensitive indicator for disease in the small airways than FEV1 [9], and thus more likely to demonstrate variations in efficacy if the smaller particles were meeting the small airways. However, there is ongoing debate as to the role of FEV25–75% values in assessing asthma control and phenotype-driven treatment [33, 34]. Although there were no significant differences in FEF25–75% for different particle sizes using the random effect model (mostly due to the small number of studies), a statistically significant difference in favor of standard particles was seen when the fixed effects model was used because the heterogeneity tests were not significant. However, this statistical difference was not clinically significant.
In terms of specific treatment comparisons, little or no differences in (FEV1 and PEF) were reported for FP versus CIC, FP/SAL versus BDP-F, and FP versus BDP. There were no significant differences in FEF25–75% for FP versus CIC; however, evidence of increased efficacy in FEF25–75% was demonstrated in one trial with FP versus BDP [18] while the opposite was shown in another [16]. The results of this study also showed no significant differences in asthma symptoms and use of rescue medications between small and standard size ICS. However, these results should be treated with caution as these parameters were only evaluated between FP and CIC and not for FP versus BDP.
For the safety endpoints considered, no appreciable differences were observed for most endpoints though it should be noted that the majority of studies were not designed to test treatment differences for safety endpoints. Adult/adolescent patients experienced more local steroid effects with standard particles than small particles. The observation that the local steroid effects at the upper airways favored small particles was likely due to the fact that CIC is administered as a pro-drug which is only activated in the lower airways. Overall, the cortisol data were variable with no clear differentiations between particle sizes.
Particle size, lung deposition, and clinical outcomes
Although there is a relationship between smaller particle size and increased delivery to the distal lung [35, 36], the current study demonstrated that increased deposition in the distal lung does not appear to translate into improved clinical outcomes for patients with asthma. These results are unsurprising for a number of reasons, not least because an increased proportion of the inhaled dose is likely to deposit in the distal respiratory compartment beyond the conducting airways. Based on current understanding, the effect of using an aerosol with smaller particle size or a ‘finer’ aerosol is both to increase the dose reaching the lungs by reducing the oropharyngeal deposition and to increase the proportion of that dose depositing distally. While simplistically this increased distal deposition might be considered to result in increased deposition in the ‘small airways’, it is important to recognize the increased deposition in fact occurs in the respiratory compartment beyond the conducting airways.
In considering the impact of ICS with different particle sizes it is important to understand the factors influencing the pattern of deposition of an aerosol in the complex 3D structure formed by the conducting airways (large and small) and pulmonary/alveolar compartments of the lung. Current mathematical modeling methods combined with 3D imaging suggest that the vast majority of inhaled aerosol (>90%) inhaled during tidal breathing is delivered to the pulmonary/alveolar compartment beyond the conducting airways, and this will increase with the modified inspiratory breath when using a pMDI (with or without a holding chamber) [37, 38]. Hence for a given dose administered to the conducting airways (large, medium, and small), the dose delivered more distally will be relatively greater with the ‘finer’ aerosol. With such a small proportion of the aerosol depositing in the conducting airways it is unlikely that there will be a major change in the concentration of aerosol at the epithelial surface of the conducting airways. This is particularly true of the small conducting airways which have a much greater relative surface area than the more central airways.
Another point of consideration is the conjecture that ‘finer’ aerosols will penetrate more effectively in the face of airways narrowing. However, any perceived advantage of finer aerosols in accessing the blocked/narrowed airways would be transient in nature as it has been shown that the blockage is resolved very rapidly in the vast majority of patients with asthma when they commence ICS treatment [39].
Based on the results of this study and discussions above, it is evident that the key issue should be the evaluation of the ‘therapeutic index’ of different drug-device combinations rather than a comparison of aerosol particle sizes for controlling asthma. Unfortunately, there is no robust method for assessing this. As previously mentioned, apart from particle size, drug deposition within the lung is dependent on other factors such as inhaler device and inhalation technique, which varies between patients [36, 40, 41]. Thus, these factors make it impossible to know what lung doses will be achieved when an individual patient uses a particular drug-device combination, even under controlled conditions [42]. Consequently current guidelines advocate titration of ICS dosages against symptoms and spirometric data [2]. Using the lowest effective dose ensures maximum efficacy and minimizes the risk of side effects. The guidelines do not distinguish between corticosteroids formulations and this approach is supported by this systematic review.
A number of observational studies and historical matched cohort analyses have recently been published comparing the outcomes and cost of treatment with small versus standard size particle ICS in patients with asthma [43–46]. In general, the studies found that asthma treatment outcomes were similar or better with small size particle ICS (BDP) compared with standard size particle ICS (FP). However, such studies are usually confounded by variables that are not present in RCTs. For example, observational studies often rely on prescription data (which does not necessarily translate to actual dosage taken) or are not able to quantify past exposure of some drugs. Furthermore, control for asthma severity in these studies was often indirect via rescue medication and hospitalizations. Another factor that might confound the results of these studies is the lack of patient randomization.
Most of the individual clinical studies included in this review may not have been powered to detect clinically meaningful differences but statistically significant differences. This meta-analysis offered the opportunity to increase the sample size and power to calculate pooled estimates for treatment differences. Despite this increased power to detect statistical differences, the relevance and clinical meaningfulness of these results must be determined beyond the results showing statistical significance.
A potential limitation of literature reviews/meta-analyses pertains to publication bias. Searches of databases such as PubMed or EMBASE yield long lists of studies that have been published. Such searches are unlikely to yield a representative sample because studies that show a ‘positive’ result are more likely to be published than those that do not. However, based on the funnel plots and the test of asymmetry, the FEV1, morning PEF and FEF25–75% data did not exhibit asymmetry, which suggests that publication bias is not likely to be a limiting factor in this study.
Another potential limitation is that the present study did not explore whether other parameters of inflammation such as fractional exhaled nitric oxide [47] were differentially affected by particle size. Similarly, endpoints such as asthma exacerbations, which together with lung function and asthma symptoms indicate sub-optimal asthma control [1], was also not assessed in the present study.
Conclusion
In summary, the results of this systematic review do not support the suggestion that smaller size particle ICS are intrinsically more ‘effective’ than larger standard size particle ICS on the endpoints of lung function, asthma symptoms and rescue medication use. Markers of inflammation and asthma exacerbation were not assessed in this meta-analysis and so the ability of small particle treatments to differentially affect these outcomes were not possible to ascertain. No study to date has clearly addressed the key issue of the relative therapeutic index of the different drug-delivery combinations though there are robust data that regular (>80% of doses) use of these treatments at licenced doses is effective and well tolerated.
Acknowledgments
All authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the following individuals for their critical review during the development of the outlines and first draft of this manuscript: David Hinds, Daniel Parks, and Michael Gibbs (all employees of GSK). The authors also acknowledge Maggie Davis (GSK employee) for editorial input, data review, and coordination of author review cycles. Initial drafting of the outline was conducted by Tracy Taylor Sandell of TaylorWrit Communications Ltd, UK, funded by GSK. Editorial support in the form of editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, and referencing, was provided by Karen Yee PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Funding
Financial support for the conduct of this study and preparation of the article was provided by GSK. Staff from GSK were involved in the study design, analysis and interpretation of data, in the preparation of the article, and in the decision to submit the article for publication.
Availability of data and materials
The dataset supporting the conclusions of this article is available on the GSK Study Register http://www.gsk-clinicalstudyregister.com/study/202012#rs.
Authors’ contributions
RDS participated in study conception and design, data acquisition and interpretation. CEB participated in study conception and design, data acquisition (involved in meta-analyses), analysis and interpretation. RA-C contributed to the study concept and design, and data acquisition (conducted meta-analyses). EAS participated in data acquisition (planned and conducted literature review, wrote literature review report, performed preliminary data extraction, critically reviewed material). DS participated in study conception and design; data acquisition, analysis and interpretation. MLE contributed to data analysis and interpretation. NB participated in study conception and design; data acquisition, analysis and interpretation. All authors read and approved the final manuscript.
Competing interests
CEB was contracted by GSK during the conduct of this analysis. RDS was a full-time employee of GSK during the conduct of this analysis and is currently a shareholder. RA-C, DS, and NB are full-time employees and shareholders of GSK. MLE previously received honoraria for presentations/attending advisory boards as well as support for attending conferences from a number of companies producing inhaled corticosteroid products including GSK, AstraZeneca, 3 M, Orion, and Chiesi. EAS does not have any conflict of interest to declare.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- AE
Adverse event
- AM
Morning
- BDP
Beclometasone dipropionate
- BDP-F
Beclometasone dipropionate-formoterol
- CFC
Chlorofluorocarbon
- CI
Confidence interval
- CIC
Ciclesonide
- CL
Clearance
- CO
crossover
- CV-VC
Closing volume/vital capacity
- DPI
Dry powder inhaler
- dx
Diagnosis
- ER
Emergency room
- FEF
Forced expiratory flow
- FEV1
Forced expiratory volume in 1 s
- FP
Fluticasone propionate
- FP/SAL
Fluticasone propionate-salmeterol combination
- FVC
Forced vital capacity
- hCRF
human corticotropin-releasing factor
- HFA
Hydrofluoroalkane
- HFA-pMDI
Hydrofluoroalkane-pressurized metered dose inhaler
- ICS
Inhaled corticosteroids
- ITT
Intent-to-treat
- MMAD
Mass median aerodynamic diameter
- NO
Nitric oxide
- NR
Not reached
- PEF
Peak expiratory flow
- PEFR
Peak expiratory flow rate
- PM
Evening
- PMDI
Pressurized metered dose inhalers
- PP
Per protocol
- ppb
Part per billion
- RCT
Randomized controlled trial
- RV
Residual volume
- SABA
Short-acting beta agonists
- SE
Standard error
- SVC
Slow vital capacity
- TGV
Thoracic gas volume
- TLC
Total lung capacity
- Tx
Treatment
Contributor Information
Céline El Baou, Phone: 44(0)2071837062, Email: celine.elbaou@phastar.com.
Rachael L. Di Santostefano, Email: rdisantostefano@gmail.com
Rafael Alfonso-Cristancho, Email: rafael.x.alfonso@gsk.com.
Elizabeth A Suarez, Email: easuarez4@gmail.com.
David Stempel, Email: david.a.stempel@gsk.com.
Mark L Everard, Email: mark.everard@uwa.edu.au.
Neil Barnes, Email: neil.c.barnes@gsk.com.
References
- 1.Global Strategy for Asthma Management and Prevention. Available from: http://ginasthma.org/gina-reports/. Accessed May 2016.
- 2.British guideline on the management of asthma. Available from: https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/. Accessed May 2016.
- 3.Usmani OS. Small-airway disease in asthma: pharmacological considerations. Curr Opin Pulm Med. 2015;21(1):55–67. doi: 10.1097/MCP.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J, Postma DS, Douma WR, et al. Particle size matters: diagnostics and treatment of small airways involvement in asthma. Eur Respir J. 2011;37(3):532–40. doi: 10.1183/09031936.00204109. [DOI] [PubMed] [Google Scholar]
- 5.Dyer MJ, Halpin DM, Stein K. Inhaled ciclesonide versus inhaled budesonide or inhaled beclomethasone or inhaled fluticasone for chronic asthma in adults: a systematic review. BMC Fam Pract. 2006;7:34. doi: 10.1186/1471-2296-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grainger CI, Saunders M, Buttini F, et al. Critical characteristics for corticosteroid solution metered dose inhaler bioequivalence. Mol Pharm. 2012;9(3):563–9. doi: 10.1021/mp200415g. [DOI] [PubMed] [Google Scholar]
- 7.Buttini F, Miozzi M, Balducci AG, et al. Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers. Int J Pharm. 2014;465(1–2):42–51. doi: 10.1016/j.ijpharm.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Gentile DA, Skoner DP. New asthma drugs: small molecule inhaled corticosteroids. Curr Opin Pharmacol. 2010;10(3):260–5. doi: 10.1016/j.coph.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Lipworth B. Targeting the small airways asthma phenotype: if we can reach it, should we treat it? Ann Allergy Asthma Immunol. 2013;110(4):233–9. doi: 10.1016/j.anai.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: http://community.cochrane.org/handbook. Accessed May 2016.
- 11.Aubier M, Wettenger R, Gans SJ. Efficacy of HFA-beclomethasone dipropionate extra-fine aerosol (800 microg day(−1)) versus HFA-fluticasone propionate (1000 microg day(−1)) in patients with asthma. Respir Med. 2001;95(3):212–20. doi: 10.1053/rmed.2000.1025. [DOI] [PubMed] [Google Scholar]
- 12.Currie GP, Fowler SJ, Wilson AM, et al. Airway and systemic effects of hydrofluoroalkane fluticasone and beclomethasone in patients with asthma. Thorax. 2002;57(10):865–8. doi: 10.1136/thorax.57.10.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairfax A, Hall I, Spelman R. A randomized, double-blind comparison of beclomethasone dipropionate extrafine aerosol and fluticasone propionate. Ann Allergy Asthma Immunol. 2001;86(5):575–82. doi: 10.1016/S1081-1206(10)62907-9. [DOI] [PubMed] [Google Scholar]
- 14.Ohbayashi H, Adachi M. Hydrofluoroalkane-beclomethasone dipropionate effectively improves airway eosinophilic inflammation including the distal airways of patients with mild to moderate persistent asthma as compared with fluticasone propionate in a randomized open double-cross study. Allergol Int. 2008;57(3):231–9. doi: 10.2332/allergolint.O-07-522. [DOI] [PubMed] [Google Scholar]
- 15.Robroeks CM, van de Kant KD, van Vliet D, et al. Comparison of the anti-inflammatory effects of extra-fine hydrofluoroalkane-beclomethasone vs fluticasone dry powder inhaler on exhaled inflammatory markers in childhood asthma. Ann Allergy Asthma Immunol. 2008;100(6):601–7. doi: 10.1016/S1081-1206(10)60052-X. [DOI] [PubMed] [Google Scholar]
- 16.Thongngarm T, Silkoff PE, Kossack WS, et al. Hydrofluoroalkane-134A beclomethasone or chlorofluorocarbon fluticasone: effect on small airways in poorly controlled asthma. J Asthma. 2005;42(4):257–63. doi: 10.1081/JAS-200057888. [DOI] [PubMed] [Google Scholar]
- 17.Tunon-de-Lara JM, Laurent F, Giraud V, et al. Air trapping in mild and moderate asthma: effect of inhaled corticosteroids. J Allergy Clin Immunol. 2007;119(3):583–90. doi: 10.1016/j.jaci.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 18.van Aalderen WM, Price D, De Baets FM, et al. Beclometasone dipropionate extrafine aerosol versus fluticasone propionate in children with asthma. Respir Med. 2007;101(7):1585–93. doi: 10.1016/j.rmed.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Bateman ED, Linnhof AE, Homik L, et al. Comparison of twice-daily inhaled ciclesonide and fluticasone propionate in patients with moderate-to-severe persistent asthma. Pulm Pharmacol Ther. 2008;21(2):264–75. doi: 10.1016/j.pupt.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Boulet LP, Bateman ED, Voves R, et al. A randomized study comparing ciclesonide and fluticasone propionate in patients with moderate persistent asthma. Respir Med. 2007;101(8):1677–86. doi: 10.1016/j.rmed.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Buhl R, Vinkler I, Magyar P, et al. Comparable efficacy of ciclesonide once daily versus fluticasone propionate twice daily in asthma. Pulm Pharmacol Ther. 2006;19(6):404–12. doi: 10.1016/j.pupt.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Dahl R, Engelstatter R, Trebas-Pietras E, et al. A 24-week comparison of low-dose ciclesonide and fluticasone propionate in mild to moderate asthma. Respir Med. 2010;104(8):1121–30. doi: 10.1016/j.rmed.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Lee DK, Haggart K, Currie GP, et al. Effects of hydrofluoroalkane formulations of ciclesonide 400 microg once daily vs fluticasone 250 microg twice daily on methacholine hyper-responsiveness in mild-to-moderate persistent asthma. Br J Clin Pharmacol. 2004;58(1):26–33. doi: 10.1111/j.1365-2125.2004.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DK, Fardon TC, Bates CE, et al. Airway and systemic effects of hydrofluoroalkane formulations of high-dose ciclesonide and fluticasone in moderate persistent asthma. Chest. 2005;127(3):851–60. doi: 10.1378/chest.127.3.851. [DOI] [PubMed] [Google Scholar]
- 25.Magnussen H, Hofman J, Staneta P, et al. Similar efficacy of ciclesonide once daily versus fluticasone propionate twice daily in patients with persistent asthma. J Asthma. 2007;44(7):555–63. doi: 10.1080/02770900701537081. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen S, Garcia Garcia ML, Manjra A, et al. A comparative study of inhaled ciclesonide 160 microg/day and fluticasone propionate 176 microg/day in children with asthma. Pediatr Pulmonol. 2006;41(10):954–61. doi: 10.1002/ppul.20474. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen S, Engelstatter R, Weber HJ, et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulm Pharmacol Ther. 2009;22(3):214–20. doi: 10.1016/j.pupt.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 28.van der Molen T, Foster JM, Caeser M, et al. Difference between patient-reported side effects of ciclesonide versus fluticasone propionate. Respir Med. 2010;104(12):1825–33. doi: 10.1016/j.rmed.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Fowler SJ, Currie GP, Lipworth BJ. Step-down therapy with low-dose fluticasone-salmeterol combination or medium-dose hydrofluoroalkane 134a-beclomethasone alone. J Allergy Clin Immunol. 2002;109(6):929–35. doi: 10.1067/mai.2002.123869. [DOI] [PubMed] [Google Scholar]
- 30.Papi A, Paggiaro P, Nicolini G, et al. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy. 2007;62(10):1182–8. doi: 10.1111/j.1398-9995.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 31.Papi A, Nicolini G, Crimi N, et al. Step-down from high dose fixed combination therapy in asthma patients: a randomized controlled trial. Respir Res. 2012;13:54. doi: 10.1186/1465-9921-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scichilone N, Battaglia S, Sorino C, et al. Effects of extra-fine inhaled beclomethasone/formoterol on both large and small airways in asthma. Allergy. 2010;65(7):897–902. doi: 10.1111/j.1398-9995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 33.Ciprandi G, Cirillo I, Pasotti F, Luigi F, Ricciardolo M. FEF25–75: A marker for small airways and asthma control. Ann Allerg Asthma Im. 2013;111(3):233. doi: 10.1016/j.anai.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Lipworth B. Author response. Ann Allerg Asthma Im. 2013;111(3):234. doi: 10.1016/j.anai.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 35.van den Berge M, ten Hacken NH, Cohen J, et al. Small airway disease in asthma and COPD: clinical implications. Chest. 2011;139(2):412–23. doi: 10.1378/chest.10-1210. [DOI] [PubMed] [Google Scholar]
- 36.Usmani OS, Barnes PJ. Assessing and treating small airways disease in asthma and chronic obstructive pulmonary disease. Ann Med. 2012;44(2):146–56. doi: 10.3109/07853890.2011.585656. [DOI] [PubMed] [Google Scholar]
- 37.Tossici-Bolt L, Fleming JS, Conway JH, et al. An analytical technique to recover the third dimension in planar imaging of inhaled aerosols--2 estimation of the deposition per airway generation. J Aerosol Med. 2007;20(2):127–40. doi: 10.1089/jam.2007.0577. [DOI] [PubMed] [Google Scholar]
- 38.Majoral C, Fleming J, Conway J, et al. Controlled, parametric, individualized, 2D and 3D imaging measurements of aerosol deposition in the respiratory tract of healthy human volunteers: in vivo data analysis. J Aerosol Med Pulm Drug Deliv. 2014;27(5):349–62. doi: 10.1089/jamp.2013.1065. [DOI] [PubMed] [Google Scholar]
- 39.Brown HM, Storey G, George WH. Beclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthma. Br Med J. 1972;1(5800):585–90. doi: 10.1136/bmj.1.5800.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haughney J, Price D, Barnes NC, et al. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010;104(9):1237–45. doi: 10.1016/j.rmed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Scheuch G, Kohlhaeufl MJ, Brand P, et al. Clinical perspectives on pulmonary systemic and macromolecular delivery. Adv Drug Deliv Rev. 2006;58(9–10):996–1008. doi: 10.1016/j.addr.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Everard ML. CFC transition: the emperor’s new clothes. Each class of drug deserves a delivery system that meets its own requirements. Thorax. 2000;55(10):811–4. doi: 10.1136/thorax.55.10.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colice G, Martin RJ, Israel E, et al. Asthma outcomes and costs of therapy with extrafine beclomethasone and fluticasone. J Allergy Clin Immunol. 2013;132(1):45–54. doi: 10.1016/j.jaci.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 44.van Aalderen WMC, Grigg J, Guilbert TW, et al. Small-particle inhaled corticosteroid as first-line or step-up controller therapy in childhood asthma. J Allergy Clin Immunol Pract. 2015;3(5):721–31. doi: 10.1016/j.jaip.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Price D, Martin RJ, Barnes N, et al. Prescribing practices and asthma control with hydrofluoroalkane-beclomethasone and fluticasone: a real-world observational study. J Allergy Clin Immunol. 2010;126(3):511–8. doi: 10.1016/j.jaci.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 46.Lage MJ, Gross GN, Brewster C, et al. Outcomes and costs of patients with persistent asthma treated with beclomethasone dipropionate hydrofluoroalkane or fluticasone propionate. Adv Ther. 2009;26(8):762–75. doi: 10.1007/s12325-009-0056-z. [DOI] [PubMed] [Google Scholar]
- 47.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is available on the GSK Study Register http://www.gsk-clinicalstudyregister.com/study/202012#rs.


