Abstract
The mammalian immune system has evolved over many millennia to be best equipped to protect the host from pathogen infection. In many cases, host and pathogen have coevolved, each acquiring sophisticated ways of inducing or protecting from disease. Epstein-Barr virus (EBV) is a human herpes virus that infects >90% of individuals. Despite its ubiquity, infection by EBV is often subclinical; this invariably reflects the necessity of the virus to preserve its host, balanced with sophisticated host immune mechanisms that maintain viral latency. However, EBV infection can result in various, and often fatal, clinical sequelae, including fulminant infectious mononucleosis, hemophagocytic lymphohistiocytosis, lymphoproliferative disease, organomegaly, and/or malignancy. Such clinical outcomes are typically observed in immunosuppressed individuals, with the most extreme cases being Mendelian primary immunodeficiencies (PIDs). Although these conditions are rare, they have provided critical insight into the cellular, biochemical, and molecular requirements for robust and long-lasting immunity against EBV infection. Here, we review the virology of EBV, mechanisms underlying disease pathogenesis in PIDs, and developments in immune cell–mediated therapy to treat disorders associated with or induced by EBV infection.
Introduction
EBV is one of eight human herpesviruses that establish life-long persistent infection in humans. It is estimated that >90% of the global population are seropositive. It was the first described oncogenic virus, and to date, seven different malignancies are associated with EBV infection. Although primary infection in childhood is generally asymptomatic, acquisition in healthy adolescents can cause infectious mononucleosis (IM). In addition, EBV can induce lymphoproliferation, lymphoma, and hemophagocytic lymphohistiocytosis (HLH) in immunocompromised patients, suggesting continuous immune surveillance is critical for virus–host homeostasis (Young and Rickinson, 2004; Hislop et al., 2007; Rickinson et al., 2014; Cohen, 2015a; Taylor et al., 2015).
EBV virology, infection, and immunology
Although EBV is initially transmitted through saliva, the early events of infection are poorly understood. During primary infection, there is a high degree of viral shedding from the throat. However, the cellular source of these infectious virions remains contentious. Circumstantial evidence implicates oral epithelial cells and possibly some infiltrating B cells at mucosal surfaces as sites for primary viral replication. A possible role for oral epithelial cells gained momentum when full lytic virus replication was observed in lingual epithelium from patients coinfected with HIV (Hutt-Fletcher, 2017). However, subsequent studies of postmortem biopsies of normal lingual tissues indicated that such replication was infrequent (Herrmann et al., 2002; Frangou et al., 2005). Similarly, whether B cells are required for initial viral infection and the mode of viral entry into B cells remain unclear. Nevertheless, viral glycoproteins (gp’s) facilitate entry and internalization of EBV into host cells: gp350 initiates binding to B cells through interactions with the complement receptor CD21, and fusion with the cell membrane and internalization are triggered by gp42 binding MHC class II and then mediated by the core fusion complex gH/gL/gp42. As epithelial cells lack both MHC class II and CD21, the mechanisms by which EBV infects epithelial cells is distinct from B cells but appears to involve interactions between viral gH and several αV integrins (see Young and Rickinson, 2004; Kutok and Wang, 2006; Hislop et al., 2007; Shannon-Lowe and Rowe, 2011; Rickinson et al., 2014; Cohen, 2015a; Taylor et al., 2015; Hutt-Fletcher, 2017).
Binding of EBV to B cells may also facilitate formation of B cell–epithelial cell conjugates, which allows epithelial cell entry (Kutok and Wang, 2006; Shannon-Lowe and Rowe, 2011). The replicative cycle that follows viral entry results in the sequential expression of >80 lytic proteins involved in producing new viral particles and/or immune evasion. Among these, at least three early lytic proteins—BNLF2A, BILF1, and BGLF5—interfere with antigen (Ag) processing and presentation to CD8+ T cells. BZLF1, another immediate early lytic protein, down-regulates MHC class II, whereas some EBV micro-RNAs reduce expression of ligands for NK cell–activating receptors (Rowe and Zuo, 2010). Together, these immune evasion proteins provide a window of opportunity for the virus to establish infection.
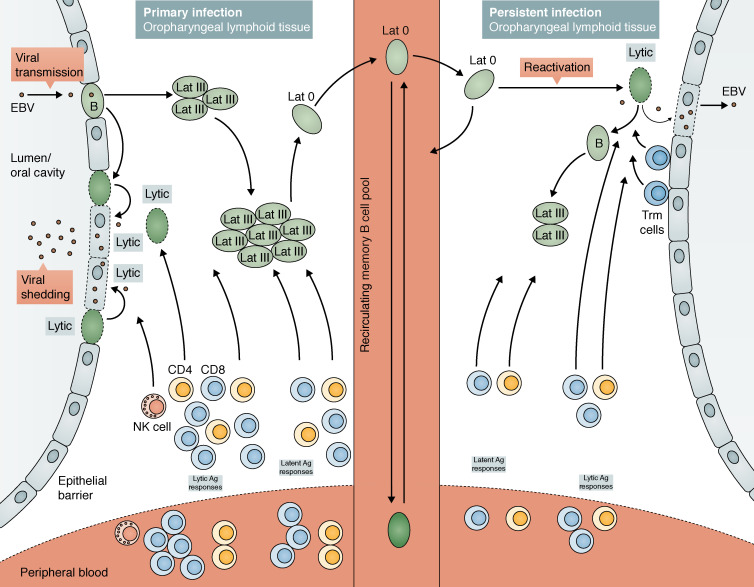
Then, EBV switches to latent infection of B cells, which is critical for colonization of the host. Several models have been proposed for how EBV persists as a latent infection in B cells, including selective infection of memory B cells (Thorley-Lawson, 2015) or, alternatively, infection of naive or activated B cells which traverse through a germinal center reaction to become EBV+ memory B cells (Küppers, 2003). Irrespective of the exact mechanism, latency can take one of at least three forms. Initially, EBV establishes a growth-transforming latent infection of B cells where expression of the full array of latent proteins (EBV nuclear Ags [EBNA] 1, 2, 3A, 3B, 3C, and LP; latent membrane proteins [LMPs] 1 and 2) along with several small noncoding RNAs, various micro-RNAs, and EBV-encoded small RNAs can be detected. This is classed as latency III and is similar to that observed in EBV-transformed B cell lymphoblastoid cell lines (LCLs) in vitro. Among the latent proteins, LMP1 and LMP2 mimic signaling through CD40 and the B cell receptor, respectively. Consequently, latently infected B cells expand rapidly in extrafollicular areas of oropharyngeal lymphoid tissues such as the tonsils, and large numbers of infected B cells can be found in the blood. Most of these infected cells are cleared by the immune system; however, EBV then enters a more restricted form of latency to escape such immune control. In the latency II program, only EBNA1 and LMPs are expressed, whereas only EBNA1 is expressed in latency I. In fact, viral persistence is achieved largely through silent infection of memory B cells where expression of viral Ags is extinguished (latency 0); in healthy carriers, EBV is exclusively found within this population in the blood. These long-lived memory B cells are proposed to recirculate continuously between blood and oropharyngeal lymphoid tissues. Viral reactivation likely occurs when these cells enter oropharyngeal lymphoid tissues, resulting in viral replication at the barrier surface to seed foci of new infection and viral shedding (Fig. 1; Küppers, 2003; Kutok and Wang, 2006; Hislop et al., 2007; Rickinson et al., 2014; Cohen, 2015a; Taylor et al., 2015; Thorley-Lawson, 2015).
Figure 1.
EBV infection and viral persistence in the tonsils of immunocompetent hosts. Early events of infection are poorly understood. However, there is accumulating evidence for a potential role for oral epithelial cells and/or infiltrating B cells. Orally transmitted virus is believed to establish lytic infection within the lymphoepithelium of the oropharynx, resulting in shedding of high levels of virus in the throat. Then, the virus switches to a growth-transforming latent infection (Lat III) of B cells within the local lymphoid tissues leading to proliferation of infected cells. However, viral persistence is largely achieved through the silent infection of memory B cells, where viral DNA is maintained in a circular form and no viral proteins are expressed. These virally infected memory B cells are believed to constantly recirculate between blood and oropharyngeal lymphoid tissues. Occasional reactivation of the virus within these lymphoid tissues may result in the production of new viral particles that could seed new foci of new infection of local B cells and also result in viral shedding from the throat. In immunocompetent hosts, tight immune control maintains virus during both lytic phase and latency. During primary infection as seen in IM, the vast array of antigenically rich EBV-lytic and -latent proteins induce priming and expansion of EBV-specific CD4+ and CD8+ T cells, as well as activation of NK cells. These effective immune responses bring the infection under control. Upon disease resolution, contraction of the T cell pool results in a small population of the memory T cell pool that persists both as tissue-resident memory cells (Trm cells) and circulating memory cells. Continuous immune surveillance by these resident and circulating memory cells is important for viral control. Black arrows denote movement of infected B cells.
The clinical manifestations of primary EBV infection depend on the age and immunocompetent state of the host. In children, EBV is mostly acquired asymptomatically. However, 10% of infected adolescents develop IM, a febrile disease characterized by sore throat, swollen lymph nodes, body aches, and fatigue. Rare complications can lead to chronic active EBV infection, where severe symptoms persist for >6 mo (Crawford et al., 2006; Kutok and Wang, 2006; Hislop et al., 2007; Balfour et al., 2013; Rickinson et al., 2014; Cohen, 2015a; Taylor et al., 2015).
Role of innate lymphocytes in EBV immunity
Most data pertaining to the immunological events surrounding primary EBV infection is derived from studying IM patients. Innate immune responses, mediated by NK and NKT cells, may be involved during primary EBV acquisition. Studies have shown both direct and inverse correlations between NK cell frequencies, severity of symptoms, and EBV load in IM patients, suggesting NK cells may play a role in either protection against EBV infection or pathogenesis of IM (Williams et al., 2005; Balfour et al., 2013). These disparate findings may reflect the dynamics of responses of specific subsets of NK cells during EBV infection (Azzi et al., 2014; Chijioke et al., 2016).
Despite these opposing findings, numerous studies support a role for NK cells in anti-EBV immunity. NK cells can reduce EBV-mediated growth transformation of B cells (Strowig et al., 2008; Azzi et al., 2014; Chijioke et al., 2016), probably because of their ability to be potently activated by EBV-induced ligands on infected B cells (Lanier, 2015). In vivo depletion of NK cells from humanized mice caused more severe symptoms and a greater incidence of EBV-induced tumorigenesis than in NK-sufficient hosts (Chijioke et al., 2013, 2016). Thus, a failure of early viral control by NK cells could lead to IM. A role for NKT cells in immune-mediated control of EBV infection has also been posited, as these cells can limit EBV transformation of B cells in vitro (Chung et al., 2013) and are absent from some individuals who are susceptible to EBV-induced disease (Palendira and Rickinson, 2015). The possible contributions of NK and NKT cells to host defense against EBV are discussed in The relative importance of innate versus adaptive immune cells in responses against EBV infection section.
Role of adaptive immunity in EBV immunity
Adaptive immunity is unequivocally required for robust anti-EBV immunity, as shown by severe EBV-induced disease in individuals with broad defects in T cell development or function (Palendira and Rickinson, 2015; Picard et al., 2015; Taylor et al., 2015). During primary EBV infection in IM patients, the absolute number of peripheral blood CD8+ T cells increases 5–10-fold compared with asymptomatic individuals (Callan et al., 2000; Taylor et al., 2015). The majority of activated CD8+ T cells are specific for lytic Ag, with responses to individual epitopes comprising up to 50% of the total CD8+ T cell pool, whereas responses to latent Ag are typically 10-fold lower in magnitude (Callan et al., 2000; Leen et al., 2001; Long et al., 2005; Taylor et al., 2015). Coinciding with the expansion of activated CD8+ T cells are elevated serum levels of proinflammatory and immunoregulatory cytokines (IFN-γ, TNF, IL-6, IL-10, and TGF-β; Callan et al., 2000; Panikkar et al., 2015a). In comparison, there is a modest increase in proportions of EBV-specific CD8+ T cells in asymptomatic patients, accounting for up to 15% of all CD8+ T cells, contracting to ∼2–5% of memory CD8+ T cells upon viral control (Hislop et al., 2007).
EBV-specific CD4+ T cells can comprise up to 1% of all circulating CD4+ T cells in IM patients, contracting to ∼0.1% upon infection resolution (Amyes et al., 2003; Long et al., 2013). CD4+ T cell responses are directed toward a broader repertoire of viral Ags, with latent-specific responses dominating over lytic-specific responses; these cells not only secrete cytokines, but also can be cytotoxic (Amyes et al., 2003; Long et al., 2005; Hislop et al., 2007; Ning et al., 2011; Mautner and Bornkamm, 2012; Rickinson et al., 2014; Taylor et al., 2015). These observations suggest that although numerically small, CD4+ T cell responses may also be important in controlling EBV infection (Amyes et al., 2003; Long et al., 2013). This is consistent with HIV/AIDS patients having low CD4+ T cell counts, high EBV viral loads, commonly developing EBV-associated lymphomas (Petrara et al., 2013; Cohen, 2015a), and improved outcomes of adoptive T cell therapy for EBV-driven posttransplant lymphoproliferative disease (PTLD) when EBV-specific CD4+ T cells are transferred with CD8+ T cells (Rooney et al., 1998; Haque et al., 2007).
Antibody (Ab)-mediated immune responses are typically required to successfully generate protective immunity against vaccines and many natural pathogen infections (Tangye and Tarlinton, 2009). Patients with IM have lower levels of gp350-specific neutralizing Ab than healthy carriers (Panikkar et al., 2015b), and although vaccination of seronegative adults with a gp350 vaccine did not prevent EBV infection, symptoms of IM were reduced in these individuals (Sokal et al., 2007; Cohen, 2015a). These findings suggest that although specific Abs are generated after EBV infection, these Abs may preferentially influence disease severity rather than prevent viral infection. This may be one explanation for the challenges in generating a successful and durable EBV vaccine (Cohen, 2015a).
Primary immunodeficiencies (PIDs) reveal cell types and signaling pathways critical for host defense against EBV infection and disease
PIDs are generally caused by monogenic mutations that compromise immune cell development, differentiation, or function. Consequently, affected individuals are highly susceptible to infection by a diverse array of pathogens. Whereas some immunodeficient individuals exhibit susceptibility to a broad range of bacterial, viral, and fungal infections, others are vulnerable to a surprisingly narrow range of pathogens (Picard et al., 2015). Thus, the study of PIDs not only identifies genetic defects underlying infectious disease, but also provides mechanistic insight into nonredundant cellular, molecular, and biochemical pathways required for host defense against specific pathogens.
Several PIDs have been described in which chronic EBV viremia and/or EBV-induced disease (e.g., fulminant IM, HLH, lymphoproliferation, lymphoma, and dysgammaglobulinemia) are common features of the clinical presentation of affected individuals (Parvaneh et al., 2013; Cohen, 2015b; Palendira and Rickinson, 2015). Studies of these patients have provided great insight into the functional requirements for efficacious immune responses against EBV. Although up to 18 different genetic mutations can predispose affected individuals to EBV-induced disease (Parvaneh et al., 2013; Cohen, 2015b; Palendira and Rickinson, 2015), we will focus on those PIDs where EBV is predominantly responsible for the most severe clinical features of these conditions. We will also summarize recently described EBV-susceptible PIDs (Table 1).
Table 1. Human PIDs manifesting as severe EBV-induced disease.
| Mutated gene | Clinical features | Infections | Outcomes | References |
|---|---|---|---|---|
| SH2D1A (XLP-1); n > 100 | age of onset: 0.5–40 yr; EBV viremia (65%); severe FIM/HLH (∼50–60%); hypogammaglobulinemia (40–50%); B lymphoma (Burkitt’s/NHL; often EBV−) ∼25% | EBV | ∼30–75% mortality (depends on study in relation to discovery of SH2D1A gene mutation in 1998); successfully treated with HSCT | Coffey et al., 1998; Nichols et al., 1998; Sayos et al., 1998; Sumegi et al., 2000; Booth et al., 2011; Pachlopnik Schmid et al., 2011; Tangye, 2014 |
| XIAP (XLP-2); n > 100 | age of onset: 0.5–40 yr; EBV viremia (50–60%); severe FIM/HLH (∼50–90%); splenomegaly/hepatitis (50–80%); inflammatory bowel disease (10–25%), cytopenias; transient hypogammaglobulinemia (10%) | EBV; CMV, HHV6 | ∼40–80% mortality; poor outcomes after HSCT | Rigaud et al., 2006; Filipovich et al., 2010; Marsh et al., 2010; Pachlopnik Schmid et al., 2011; Speckmann et al., 2013; Aguilar and Latour, 2015 |
| ITK; n = 13 | age of onset: 3–13 yr; EBV viremia (100%); EBV-induced lymphoproliferation (∼70%); EBV+ B cell lymphoma (mostly Hodgkin’s; 1 NHL; 1 Burkitt’s lymphoma; ∼70%); CD4+ T cell lymphopenia (∼65%); splenomegaly (∼60%); progressive hypogammaglobulinemia (>50%); autoimmunity (infrequent; ∼20%) | EBV; CMV, VZV | ∼60–65% mortality; 2/3 patients successfully treated with HSCT | Huck et al., 2009; Linka et al., 2012; Mansouri et al., 2012; Ghosh et al., 2014; Bienemann et al., 2015; Cipe et al., 2015; Çağdaş et al., 2017 |
| MAGT1; (X-MEN) n = 11 | age of onset: 3–45 yr; chronic EBV viremia (100%); EBV-induced lymphoproliferation (not HLH); EBV+ B lymphoma (Burkitt’s/Hodgkin’s/ DLBCL; 70%); CD4+ T cell lymphopenia (∼50%); splenomegaly; progressive hypogammaglobulinemia (>50%) | EBV; VZV, HSV, CMV, KSHV, molluscum contagiosum | ∼30% mortality; poor outcomes after HSCT | Li et al., 2011; Chaigne-Delalande et al., 2013; Dhalla et al., 2015; Patiroglu et al., 2015; Brigida et al., 2016 |
| CD27; n = 17 | age of onset: 1–22 yr; EBV viremia (∼90%); severe HLH (∼25%); EBV-induced lymphoproliferation (∼50%); EBV+ B lymphoma (Hodgkin’s, DLBCL; ∼50%); hypogammaglobulinemia (∼70%) | EBV; VZV, CMV (<15%); recurrent bacterial/viral infections | ∼30% mortality; 3/3 patients successfully treated with HSCT | van Montfrans et al., 2012; Salzer et al., 2013; Alkhairy et al., 2015 |
| CD70; n = 5 | age of onset: 1–5 yr; EBV viremia (100%); EBV-lymphoproliferation (80%); EBV+ B lymphoma (Hodgkin’s; 80%); hypogammaglobulinemia (60–80%) | EBV; VZV, CMV (50%); recurrent bacterial/viral infections | all patients currently alive | Abolhassani et al., 2017; Izawa et al., 2017 |
| NFKB1; n = 2 | age of onset: 14–15 yr; EBV viremia; lymphoproliferation, hepatosplenomegaly; hypogammaglobulinemia | EBV; recurrent viral/ bacterial/fungal infections | alive and awaiting HSCT | Boztug et al., 2016; Schipp et al., 2016 |
| RASGRP1; n = 1 | age of onset: 12 yr; EBV+ B lymphoma; CD4+ T cell lymphopenia | EBV; recurrent bacterial/viral infections | successful HSCT | Salzer et al., 2016 |
Abbreviations used: DLBCL, diffuse large B cell lymphoma; FIM, fulminant IM; HHV6, human herpesvirus 6; KSHV, Kaposi’s sarcoma herpes virus; NHL, non-Hodgkin’s lymphoma.
X-linked lymphoproliferative disease (XLP) 1: mutations in SH2D1A
XLP-1 was initially reported in 1974–1975 in 13 otherwise healthy males from three unrelated families who developed an often-fatal immune-deficient condition precipitated by EBV infection (Bar et al., 1974; Provisor et al., 1975; Purtilo et al., 1975). Remarkably, XLP-1 patients have normal responses to other childhood viruses including other herpesviruses (HSV, varicella zoster virus [VZV], and CMV; Cohen, 2015b; Palendira and Rickinson, 2015). This established that individuals with XLP-1 are uniquely sensitive to diseases caused by EBV, which otherwise runs a fairly benign course in most healthy individuals.
XLP-1 is caused by mutations in SH2D1A, encoding signaling lymphocytic activation molecule (SLAM)–associated protein (SAP). SAP is an SH2 domain–containing intracellular adaptor protein that regulates signaling downstream of the SLAM family of surface receptors expressed on hematopoietic cells (Coffey et al., 1998; Nichols et al., 1998; Sayos et al., 1998). Analyses of >150 patients revealed that the most common clinical features of XLP-1 are EBV-induced fulminant IM/HLH (∼45–70%), B cell lymphoma (∼25%), and hypogammaglobulinemia (∼50%). Lymphoma and Ab defects occur equally in EBV-naive and EBV+ XLP patients (Sumegi et al., 2000; Booth et al., 2011; Pachlopnik Schmid et al., 2011). The current survival rate in XLP-1 is 71.4%, a marked improvement over the 25% reported in the 1980s (Table 1; Booth et al., 2011; Pachlopnik Schmid et al., 2011). This reflects improved detection of affected individuals and patient management in the time since the molecular lesion underlying XLP-1 was identified in 1998. It is important to note that XLP-1 is unique among all currently identified PIDs, as it is the only condition where affected individuals are susceptible to severe disease caused by only EBV infection and not other pathogens. This indicated that SLAM/SAP signaling is critical for protective immunity against EBV infection, but it is redundant for immunity against other infections.
XLP-2: mutations in XIAP
In 2006, Rigaud et al. (2006) reported 12 males presenting with EBV-induced HLH. These individuals had inactivating mutations in XIAP, encoding X-linked inhibitor of apoptosis (Rigaud et al., 2006). The commonality of EBV-induced disease caused by mutations in SH2D1A and XIAP (affecting ∼60% of cases and triggering HLH in >80% of cases) led to the naming of these conditions as XLP-1 and XLP-2 (Filipovich et al., 2010; Pachlopnik Schmid et al., 2011). Over the past decade, >100 patients with XIAP deficiency have been identified, and their detailed clinical descriptions have clearly revealed marked differences in disease pathogenesis, severity, and outcomes for XLP-1 and XLP-2. Thus, splenomegaly, cytopenias, fever, recurrent HLH, and inflammatory bowel disease are common in XIAP but not SAP deficiency. Unlike XLP-1, B lymphoma rarely occurs in XIAP deficiency, and when hypogammaglobulinemia is noted in XLP-2, it is usually transient (Filipovich et al., 2010; Marsh et al., 2010; Pachlopnik Schmid et al., 2011; Speckmann et al., 2013; Aguilar and Latour, 2015). Mortality caused by XIAP deficiency is ∼40%, with approximately one third of deaths resulting from HLH (Marsh et al., 2010; Pachlopnik Schmid et al., 2011). However, a study of patients identified since 2010 indicated that >95% of patients were alive (Speckmann et al., 2013), suggesting that, like XLP-1, survival has greatly improved since the discovery of the molecular lesion causing this condition (Table 1).
IL-2–inducible T cell kinase (ITK) deficiency
ITK encodes ITK, a member of the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family of nonreceptor kinases that is expressed by hematopoietic cells involved in proximal TCR signaling. By regulating PLCγ1 phosphorylation, ITK coordinates T cell activation and function (Readinger et al., 2009). 13 individuals from eight unrelated families have been identified with loss-of-function biallelic mutations in ITK, 12 of whom presented with EBV viremia, EBV-induced lymphoproliferation that frequently developed into lymphoma, CD4+ T cell lymphopenia, hepatosplenomegaly, and progressive hypogammaglobulinemia (Huck et al., 2009; Stepensky et al., 2011; Linka et al., 2012; Mansouri et al., 2012; Ghosh et al., 2014; Bienemann et al., 2015; Cipe et al., 2015; Çağdaş et al., 2017). Interestingly, one ITK-deficient patient was identified who presented at the age of 6 yr with recurrent respiratory tract infections, CD4+ T cell lymphopenia, and progressive hypogammaglobulinemia but remained EBV naive until 17 yr of age (Serwas et al., 2014). This infers that CD4+ T lymphopenia and hypogammaglobulinemia occurs in ITK deficiency independently of EBV infection. Of the 13 reported ITK-deficient patients, eight died, whereas two of three survived hematopoietic stem cell transplantation (HSCT; Table 1; Huck et al., 2009; Stepensky et al., 2011; Linka et al., 2012; Mansouri et al., 2012; Ghosh et al., 2014; Bienemann et al., 2015; Cipe et al., 2015; Çağdaş et al., 2017).
X-MEN disease: MAGT1 mutations
A syndrome of chronic EBV viremia, EBV+ B lymphoma, and CD4+ T cell lymphopenia in seven males was first reported in 2011 (Li et al., 2011). Because of the clinical features of this condition, it was named X-MEN disease (X-linked immunodeficiency with magnesium defect EBV and neoplasia). Some individuals also presented with recurrent infections with pathogens other than EBV (e.g., HSV and VZV). Molecular studies identified mutations in MAGT1, encoding a ubiquitously expressed magnesium transporter controlling basal levels of intracellular Mg2+, in all affected individuals (Li et al., 2011). Since the initial identification of X-MEN patients, four additional individuals have been reported (Dhalla et al., 2015; Patiroglu et al., 2015; Brigida et al., 2016). All 11 patients experienced extremely high levels of EBV viremia, with EBV-induced B cell lymphoproliferation or malignancy occurring in 70% of cases (Li et al., 2011; Dhalla et al., 2015; Patiroglu et al., 2015). Three patients died of complications of EBV infection (Table 1). The discovery of mutations in MAGT1 revealed an unappreciated and nonredundant role for magnesium as a second messenger, upstream of Ca2+, in TCR-mediated activation and immunity against EBV-induced infectious disease and malignancy.
CD27 and CD70 deficiency
In 2012 and 2013, two papers reported 10 patients from four kindreds presenting with a combined immunodeficiency characterized by EBV viremia, lymphoproliferative disease, HLH, EBV+ B cell lymphoma, hypogammaglobulinemia, and impaired Ab responses (van Montfrans et al., 2012; Salzer et al., 2013). Molecular analysis revealed homozygous mutations in CD27 (van Montfrans et al., 2012; Salzer et al., 2013), a TNFR superfamily member expressed by naive and central memory T cells, germinal center and memory B cells, plasma cells, and some NK cells (Hamann et al., 1997; Lens et al., 1998; Jung et al., 2000; Borst et al., 2005; Vossen et al., 2008; Tangye and Tarlinton, 2009). A total of 16 patients from eight unrelated families have now been reported with biallelic loss-of-function mutations in CD27 (van Montfrans et al., 2012; Salzer et al., 2013; Alkhairy et al., 2015). One individual with a heterozygous mutation has also been identified (Alkhairy et al., 2015). As heterozygous parents and siblings of patients with homozygous CD27 mutations are unaffected, this patient probably has an unidentified mutation on the other CD27 allele. EBV-induced disease was evident in 88% (15/17) of patients, manifesting as HLH (23.5%), lymphoproliferative disease (53%), and B cell lymphoma (47%), and was fatal in 30% of cases (Table 1). A few CD27-deficient patients were infected with other herpesviruses (CMV and VZV), and several had recurrent bacterial infections (van Montfrans et al., 2012; Salzer et al., 2013; Alkhairy et al., 2015), possibly caused by humoral immune defects, as engaging CD27 promotes human B cell differentiation (Borst et al., 2005; Tangye and Tarlinton, 2009). Thus, CD27 deficiency is a PID characterized by extreme susceptibility to EBV infection and impaired Ab responses.
The counter-structure of CD27 is CD70 (Lens et al., 1998; Borst et al., 2005). CD70 is largely absent from naive or resting leukocytes but is rapidly induced after activation of B cells, myeloid cells, and to a lesser extent T cells (Hintzen et al., 1994; Tesselaar et al., 1997, 2003; Lens et al., 1998; Borst et al., 2005; Izawa et al., 2017). Thus, CD70 is predominantly expressed by activated APCs, with highest levels on B cells. Recently, two groups identified five individuals from three unrelated families with homozygous mutations in CD70, affecting either expression of CD70 or its ability to bind CD27 (Abolhassani et al., 2017; Izawa et al., 2017). The clinical features of CD70 deficiency resembled those of CD27 deficiency, with all patients experiencing EBV viremia and most developing EBV-associated lymphoproliferation or B cell malignancy, hypogammaglobulinemia, and impaired Ag-specific Ab responses; infection with other herpesviruses also occurred in a few patients. However, unlike CD27 deficiency, all patients with CD70 deficiency are currently alive (Table 1; Abolhassani et al., 2017; Izawa et al., 2017), suggesting mutations in CD27 cause more severe disease than those in CD70. Although this seems paradoxical, as CD70 is the only ligand for CD27 (Lens et al., 1998; Borst et al., 2005), this may reflect a ligand-independent function of CD27. Other members of the TNFR superfamily, such as CD95, TNF-RI, CD40, and TACI (transmembrane activator and CAML interactor), form homotrimers independently of interacting with cognate ligands (Chan et al., 2000; Siegel et al., 2000; Garibyan et al., 2007). Thus, preassembled trimers may provide a qualitatively different signal from that initiated by ligand-induced receptor multimerization (Golstein, 2000). Although this has not been formally established, one possibility is that, in the absence of CD70, only ligand-dependent signals are compromised, whereas in the absence of CD27, both ligand-dependent and -independent signals are impaired, thus resulting in a more severe phenotype. The identification of additional CD70-deficient patients will facilitate investigating this hypothesis.
Mechanisms underlying susceptibility to EBV-induced disease in PIDs
Analyses of defects in these PID patients have not only revealed mechanisms of disease pathogenesis, but also have defined unique requirements for host defense against EBV and identified pathways that could be targeted to improve anti-EBV immunity in immunocompromised hosts.
XLP-1
Early studies of XLP-1 reported defects in NK cell cytotoxicity (Sullivan et al., 1980). Subsequent studies revealed that the ability of SAP-associating receptors 2B4 and NTB-A to activate NK cell cytotoxicity was abolished in the absence of SAP (Nakajima et al., 2000; Parolini et al., 2000; Tangye et al., 2000; Bottino et al., 2001). Activation of Ag-specific CD8+ T cells by B cells, but not other APCs (monocytes, DCs, and fibroblasts), was also impaired by SAP deficiency (Dupré et al., 2005; Hislop et al., 2010; Palendira et al., 2011). Consistent with these findings, SAP-deficient T and NK cells exhibited intact responses when activated through SAP-independent receptors (Nakajima et al., 2000; Parolini et al., 2000; Tangye et al., 2000; Bottino et al., 2001; Dupré et al., 2005). Thus, successful engagement of CD8+ T cells by cognate B cells requires interactions between SLAM receptors and SAP-dependent signaling in responding CD8+ T cells.
As EBV is a B cell tropic–virus, these findings explain the (a) selectivity of XLP-1 patients to infection with EBV but not human herpesviruses with other cell tropisms, (b) increased incidence of B cell lymphoma in XLP-1, and (c) EBV independence of lymphoma development in these patients; i.e., SAP-deficient, lymphoma-specific CD8+ T cells would be unresponsive to activating signals provided by B cell lymphomas via SLAM/SAP complexes. Interestingly, ligands of 2B4 (CD48) and NTB-A (NTB-A itself) are highly expressed on EBV-infected B cells (Thorley-Lawson et al., 1982; Parolini et al., 2000). This suggests that EBV-induced up-regulation of CD48 and NTB-A on B cells acts as a signal to activate NK cells as a first line of defense against viral infection and, subsequently, functions to activate EBV-specific CD8+ T cells.
SAP-deficient T cells are also resistant to reactivation-induced cell death, a process considered to regulate T cell contraction during responses to specific Ags (Snow et al., 2009). Enhanced survival of SAP-deficient cells was NTB-A dependent and resulted from impaired induction of proapoptotic Bim and Fas ligand (Snow et al., 2009). SAP-deficient human CD8+ T cells also exhibited enhanced proliferation to polyclonal stimulation relative to SAP+ CD8+ T cells (Palendira et al., 2012). Sustained proliferation and persistence of highly activated CD8+ T cells may contribute to HLH in XLP-1 (Filipovich et al., 2010; Marsh et al., 2010).
XLP-2
In contrast to XLP-1, functional responses of cytolytic lymphocytes from XIAP-deficient patients are normal (Rigaud et al., 2006; Aguilar and Latour, 2015). XIAP prevents apoptosis by binding and inactivating caspases (Aguilar and Latour, 2015). Thus, T cells with XIAP mutations exhibit increased death after TCR stimulation (Rigaud et al., 2006; Filipovich et al., 2010; Speckmann et al., 2013). It is possible that activation-induced loss of effector T cells compromises effective control of viral infection, thereby predisposing XIAP-deficient individuals to EBV infection and HLH. However, the exact mechanisms underlying disease in XIAP deficiency remain unclear, particularly as SAP deficiency protects T cells from restimulation-induced cell death allowing prolonged proliferation (Snow et al., 2009; Palendira et al., 2012), with HLH characterized by dysregulated proliferation of cytotoxic lymphocytes (Filipovich et al., 2010; Marsh et al., 2010).
X-MEN
MAGT1 mutations abrogate TCR-mediated PLCγ1 activation and subsequent Ca2+ flux, thereby impairing T cell function (Li et al., 2011). However, it was unclear how a global defect in TCR-induced activation would render affected individuals susceptible to EBV infection. Insight into this came from the finding that MAGT1-deficient NK and CD8+ T cells failed to express NKG2D (Chaigne-Delalande et al., 2013; Dhalla et al., 2015; Patiroglu et al., 2015; Brigida et al., 2016), a C-type lectin that potently activates effector function of cytotoxic lymphocytes (Lanier, 2015). Thus, despite individuals with MAGT1 mutations being able to generate EBV-specific CD8+ T cells, these cells could not kill autologous, EBV-transformed B cells (Chaigne-Delalande et al., 2013).
The functional requirement for NKG2D on MAGT1-deficient NK and CD8+ T cells was elegantly demonstrated by the ability to restore both expression of NKG2D and NKG2D-dependent cytotoxicity by in vitro treatment with magnesium (Chaigne-Delalande et al., 2013). Remarkably, in vivo administration of magnesium to X-MEN patients restored NKG2D expression on CD8+ T cells, and this correlated with reductions in proportions of EBV-infected B cells (Chaigne-Delalande et al., 2013). Thus, recognition of EBV-infected B cells by NK and CD8+ T cells critically depends on interactions between NKG2D on cytolytic lymphocytes and its cognate ligands on EBV-B cells.
CD27/CD70 deficiency
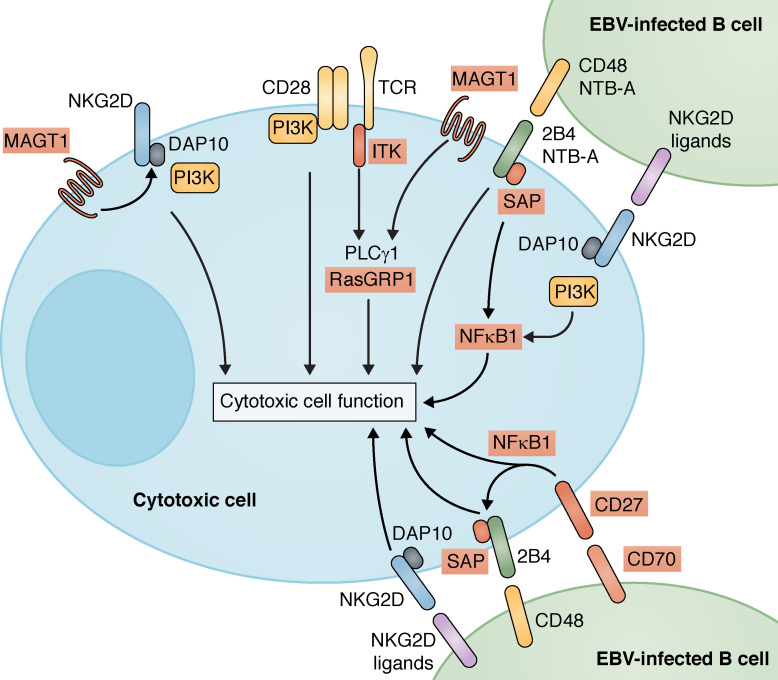
Analysis of CD8+ T cells from patients with CD70 mutations also revealed an impaired ability to kill autologous EBV-infected B cells. Interestingly, CD27/CD70 interactions were critical for initial priming and expansion of EBV-specific CD8+ T cells, rather than activating cytotoxic function (Abolhassani et al., 2017; Izawa et al., 2017). Importantly, cytotoxicity of effector CD8+ T cells from CD70-deficient individuals against autologous EBV-B cells could be greatly improved by restoring CD70 expression on the target B cells (Izawa et al., 2017). Thus, consistent with CD70 being more prominently expressed on APCs than on T cells, CD27 delivers the activating signal to T cells for their effector function, with CD70 on APCs—predominantly B cells—acting as the ligand in this interaction (Lens et al., 1998; Borst et al., 2005; Izawa et al., 2017). Notably, memory and EBV-specific CD8+ T cells from CD70-deficient individuals failed to up-regulate expression of NKG2D and 2B4 (Abolhassani et al., 2017). Thus, mutations in CD70, and by inference CD27, compromise the ability of CD8+ T cells to be activated by EBV-infected B cells by at least two pathways—directly via CD27 and indirectly by crippling 2B4- and NKG2D-induced cytotoxicity (Fig. 2).
Figure 2.
Signaling pathways responsible for generating cell-mediated EBV-specific immunity. The scheme highlights the key cell surface receptor-induced signaling pathways that, when mutated, manifest as PIDs characterized by increased susceptibility to EBV-induced disease. Genes found to be mutated in these conditions (SAP/SH12D1A, MAGT1, ITK, CD27, CD70, NFKB1, and RASGRP1) are highlighted by red boxes. XIAP is not included here, as the mechanism by which mutations in XIAP cause disease remains unknown.
Converging mechanisms underlying EBV susceptibility in genetically distinct PIDs
There are several points of intersection in the molecular pathogenesis of severe EBV-induced disease caused by mutations in SH2D1A, ITK, MAGT1, and CD27/CD70, despite these molecules functioning in distinct signaling pathways (Fig. 2). First, phosphorylation of PLCγ1, a substrate of ITK, induces the intracellular Ca2+ flux necessary for TCR-mediated T cell activation (Readinger et al., 2009; Ghosh et al., 2014). Mutations in ITK or MAGT1 largely abolished TCR-induced PLCγ1 activation (Readinger et al., 2009; Li et al., 2011; Linka et al., 2012; Brigida et al., 2016). Thus, ITK can function downstream of the TCR and MAGT1 signaling pathways, thereby explaining common clinical outcomes of mutations in MAGT1 or ITK (Table 1).
Second, a key feature of X-MEN patients is a lack of NKG2D expression on cytolytic cells, which compromises their ability to lyse EBV-infected B cells (Chaigne-Delalande et al., 2013; Dhalla et al., 2015; Patiroglu et al., 2015). In contrast, SAP is required for 2B4- and NTB-A–mediated activation of NK and T cells by B cells (Hislop et al., 2010; Palendira et al., 2011). Thus, reduced expression of NKG2D and 2B4 on CD8+ T cells from CD70-deficient individuals likely contributes to their impaired responsiveness to EBV-infected B cells, akin to that observed for MAGT1- and SAP-deficient CD8+ T cells in X-MEN disease and XLP-1, respectively (Fig. 2). Third, co-engagement of 2B4 and NKG2D on human NK cells yields a greater response than engaging either receptor alone (Fig. 2; Kwon et al., 2016). Such synergy between 2B4 and NKG2D potentially explains previous puzzling observations of why an inability to signal through 2B4 in the absence of SAP could not be compensated by other pathways, including NKG2D, and vice versa. It is likely that co-signaling through NKG2D and SLAM receptors is required to induce activation of Ag-specific CD8+ T cells and acquisition of cytotoxic function to lyse EBV-infected B cells. Fourth, it is striking that expression of ligands of CD27, 2B4, NTB-A, and NKG2D are greatest on EBV-infected B cells (Thorley-Lawson et al., 1982; Lens et al., 1998; Bottino et al., 2001; Lanier, 2015; Izawa et al., 2017). These observations further underscore the concept that individuals with mutations in SH2D1A, MAGT1, CD27/CD70, or ITK are particularly susceptible to disease caused by EBV simply because of the B cell tropism of EBV and subsequent compromised ability of CD8+ T cells from these individuals to be optimally activated by Ag-presenting B cells (Fig. 2).
Identifying these points of overlap and cooperativity in signaling pathways defective in PIDs arising from mutations in SH2D1A, ITK, MAGT1, CD27, or CD70 also potentially explains susceptibility to EBV-induced disease innovel PIDs. Two unrelated patients with lymphoproliferation, severe EBV viremia, and heterozygous mutations in NFKB1 (Boztug et al., 2016; Schipp et al., 2016) and one patient with homozygous mutations in RASGRP1 with a combined immunodeficiency, CD4+ T cell lymphopenia, and EBV+ B cell lymphoma (Salzer et al., 2016) were recently identified (Table 1). NKG2D, 2B4, or CD27 can all activate NF-κB1 (Lens et al., 1998; Kwon et al., 2016), and co-engagement of NKG2D and 2B4 results in a robust NF-κB1 response (Kwon et al., 2016). RasGRP1 is a guanine nucleotide exchange factor activated by diacylglycerol, a second messenger produced by cleavage of phosphatidylinositol-4,5-bisphosphate by PLCγ1 downstream of ITK (Readinger et al., 2009). Thus, these two new genetic etiologies probably cause EBV-induced disease by compromising lymphocyte activation induced via TCR/ITK/PLCγ1 signaling (RasGRP1) or NF-κB1–activating receptors (2B4, NKG2D, and CD27; Fig. 2). In light of these findings, it will be important to determine whether engagement of these receptors can activate other signaling pathways implicated in EBV-susceptible PIDs, such as Ca2+ or Mg2+ flux or PLCγ1 activation.
The relative importance of innate versus adaptive immune cells in responses against EBV infection
Detailed studies of PIDs allow identification of redundant and nonredundant roles of particular cell types in anti-EBV immunity. A common feature of individuals with mutations in SH2D1A, XIAP, ITK, and CD27 is a paucity of NKT cells (Nichols et al., 2005; Pasquier et al., 2005; Rigaud et al., 2006; Huck et al., 2009; Stepensky et al., 2011; Salzer et al., 2013; Ghosh et al., 2014; Serwas et al., 2014). This, together with additional experimental evidence (Chung et al., 2013), suggests that NKT cells may play a fundamental role in anti-EBV immunity. However, although this is a tempting hypothesis, it has important caveats. First, assessment of EBV-naive XIAP-deficient patients revealed normal frequencies of NKT cells, with studies indicating that the loss of NKT cells in these individuals was secondary to EBV infection (Filipovich et al., 2010; Marsh et al., 2010; Speckmann et al., 2013). Thus, although a diminution in the frequency of NKT cells after EBV infection may compromise host defense, EBV-induced disease in XLP-2 does not result per se from a primary absence of NKT cells. Second, individuals with mutations in RORC completely lack NKT cells and are susceptible to infection with mycobacteria and candida but not EBV (Okada et al., 2015), arguing against a central role for NKT cells in anti-EBV immunity. Despite these observations, it cannot be entirely ruled out that a deficiency of NKT cells may contribute to disease pathogenesis in these PIDs. Indeed, NKT cells are polyfunctional and can influence the behavior of numerous cell types, including DCs, granulocytes, macrophages, B cells, and T cells (Brennan et al., 2013). Thus, it is possible that a lack of NKT cell–mediated activation of these immune cells underlies some of the additional clinical features typical of PIDs resulting from SH2D1A, XIAP, ITK, or CD27 mutations.
Similar arguments have been made for essential roles of NK cells in protection against EBV. Many examples exist of PIDs characterized by compromised development or function of NK cells and overwhelming infections with viruses, including EBV (Orange, 2013), consistent with a role for NK cells in EBV immunity (Chijioke et al., 2013, 2016; Orange, 2013). However, most PIDs characterized by defects in NK cell development or function usually have defects in other cell lineages, such as T cells (Orange, 2013; Palendira and Rickinson, 2015). Thus, susceptibility to infection in these settings probably results from the combined effect of impaired T and NK cell–mediated immunity. Indeed, a recent study reported that the T cell, but not NK cell, compartment is restored in individuals with SCID because of mutations in IL2RG or JAK3 after HSCT, thus rendering them NK cell deficient (Vély et al., 2016). Remarkably, NK cell deficiency in these transplanted individuals did not result in any adverse clinical phenotype with respect to increased frequency of pathogen infection, including with herpesviruses (Vély et al., 2016). These data suggest T cells are predominantly responsible for long-term protection against EBV infection, and NK cells play a complementary, rather than exclusive, role in host defense. This is consistent with the finding that, in XLP-1 patients, somatic reversion of SH2D1A, resulting in expression of functional SAP, is largely restricted to memory CD8+ T cells and is infrequently detected in NK cells (Palendira et al., 2012), inferring that the pressure exerted on the immune system by EBV preferentially drives responses by CD8+ T cells. A predominant role of CD8+ T cells in host defense against EBV is also evident by a lack of severe EBV-induced disease in individuals with mutations in RFXANK, which dramatically reduces expression of MHC class II causing severe CD4+ T cell lymphopenia (Ouederni et al., 2011).
Cellular therapy for treatment of EBV-induced disease
Studies of patients with EBV-associated pathologies, including those with specific PIDs, have demonstrated the requirement for intact cellular immunity in controlling EBV. This has facilitated the development of treatment protocols using EBV-targeted cellular therapy.
PTLD
Allogeneic HSCT and solid organ transplantation (SOT) can treat a myriad of human conditions. However, the success of these treatments can be curtailed by susceptibility of transplant recipients to viral infections after immunosuppression (Moss and Rickinson, 2005). Recipients are particularly vulnerable to complications associated with primary EBV infection or reactivation of latent infection, either arising naturally or transmitted from the donor graft (Green, 2013). During immunosuppression, surveillance of EBV-infected B cells is compromised resulting in EBV viremia and uncontrolled lymphoproliferation. This manifests as PTLD, a potentially life-threatening complication in ∼1–30% of transplant recipients (Rooney et al., 1995, 1998; Lucas et al., 1998; Gustafsson et al., 2000; Nalesnik, 2002; van Esser et al., 2002).
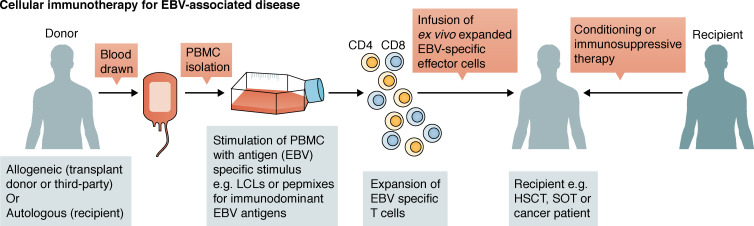
EBV-induced PTLD can be treated by combination chemotherapy (Heslop, 2009) or B cell depletion (Kuehnle et al., 2000; van Esser et al., 2002). However, because most PTLD lesions are EBV+ (Bollard and Heslop, 2016), cellular immunotherapy targeting EBV Ags expressed by infected B cells has become an attractive strategy for prophylaxis and treatment (Fig. 3). Indeed, >75% of PTLD patients undergoing adoptive cellular therapy using EBV-specific CD8+ T cells achieved complete remission (Heslop et al., 1996, 2010; Gustafsson et al., 2000; Doubrovina et al., 2012) including some with rituximab-resistant disease (Tables 2 and 3; Comoli et al., 2007). Furthermore, when administered prophylactically, none of the patients developed PTLD compared with 11.5% of HSCT recipients not receiving T cell immunotherapy (Heslop et al., 2010). Thus, adoptive cell therapy is a viable means of controlling EBV-driven lymphoproliferation in immunosuppressed individuals.
Figure 3.
Schematic representation of the generation of EBV-specific T cells to treat EBV-associated disease. PBMCs are isolated from donors and stimulated using different approaches to generate EBV-specific T cells, which are then infused into patients after conditioning or immunosuppressive therapy.
Table 2. Prophylaxis for EBV-induced PTLD (or treatment for increased EBV DNA levels).
| EBV-specific T cell activator | T cell source | Prophylaxis for | EBV-specific pathology | References |
|---|---|---|---|---|
| LCL | Transplant donor derived | Post-HSCT PTLD | 0/101 developed PTLD | Rooney et al., 1995, 1998; Heslop et al., 1996, 2010 |
| 5/6 exhibited a decrease in EBV DNA | Gustafsson et al., 2000 | |||
| 1/4 decrease in EBV DNA | Comoli et al., 2007 | |||
| Allogeneic | Post-HSCT PTLD | 0/1 showed evidence of viral activation (EBV, CMV, ADV) | Naik et al., 2016 | |
| DCs + LCLs transduced with ADV | Transplant donor derived | Post-HSCT PTLD | 0/1 showed evidence of viral activation (EBV, CMV, ADV) | |
| DCs + LCLs transduced with ADV + CMV pp65 | Transplant donor derived | Post-HSCT PTLD | 0/2 showed evidence of viral activation (EBV, CMV, ADV) | |
| Allogeneic | Post-HSCT PTLD | 0/3 showed evidence of viral activation (EBV, CMV, ADV) | ||
| Viral Ags from EBV, CMV, ADV | Transplant donor derived | Post-HSCT PTLD | 0/2 showed evidence of viral activation (EBV, CMV, ADV) |
Abbreviations used: ADV, adenovirus.
Table 3. Treatment for EBV-associated malignancies.
| EBV-specific T cell activator | T cell source | Disease | CR, PR, remission, or disease free | References |
|---|---|---|---|---|
| LCL | Transplant donor derived | Post-HSCT PTLD | 11/13 | Rooney et al., 1995, 1998; Heslop et al., 1996, 2010 |
| 10/14 | Doubrovina et al., 2012 | |||
| 1/1 | Lucas et al., 1998 | |||
| 3/4 | Comoli et al., 2007 | |||
| Allogeneic | Post-HSCT PTLD | 4/5 | Doubrovina et al., 2012 | |
| Post-HSCT viremia or EBV-LPD | 9/10 | Naik et al., 2016 | ||
| Post-SOT PTLD | 15/31 | Haque et al., 2007 | ||
| Autologous | Post-SOT PTLD | 1/1 | Khanna et al., 1999 | |
| Viral Ags from EBV, CMV, and ADV | Transplant donor derived | Post-HSCT PTLD | 2/2 | Naik et al., 2016 |
| Allogeneic | Post-HSCT viremia or EBV-LPD | 1/2 | Naik et al., 2016 | |
| APCs transduced with ADV vector encoding LMP1 and/or LMP2 | Autologous or third party | Active EBV+ lymphoma; EBV+ lymphoma (in remission) | 13/21; 27/29 | Bollard et al., 2014 |
Abbreviations used: ADV, adenovirus; CR, complete response; LPD, lymphoproliferative disease; PR, partial response.
Combining HSCT and adoptive cellular therapy in PIDs
HSCT can cure ∼70–90% of patients with PIDs (Slatter and Cant, 2011). However, HSCT often fails to provide rapid protection against viral infections (Moss and Rickinson, 2005). For this reason, HSCT has been combined with adoptive cellular therapy to enhance immune-mediated control of infection by EBV and other viruses. A retrospective study of 36 high-risk PID patients who received prophylactic virus-specific T cells to treat viral infections either before or after HSCT found that 81% remained free of EBV, CMV, and adenovirus. Although the remaining 19% exhibited reactivation of EBV or CMV, their infection resolved within 2 mo of onset. Overall, 76% of the patients treated therapeutically for active EBV infection exhibited a complete or partial response to cellular therapy (Table 2Naik et al., 2016).
SOT
Outcomes of SOT patients receiving autologous EBV-specific T cells to treat high EBV loads or active PTLD have been promising, with >50% showing a complete or partial response (Table 3; Khanna et al., 1999; Savoldo et al., 2005; Haque et al., 2007). The requirement for continued immunosuppression likely reflects the lower response rate compared with HSCT (56% versus 75%, respectively). The demonstration that CD8+ T cells specific for latent rather than lytic EBV Ag were necessary for the effective treatment of PTLD (Sherritt et al., 2003) will aid efficient Ag targeting for the treatment of these patients. Impaired generation of autologous T cells as well as potential alloreactivity as a result of intense immunosuppression and/or seronegativity of the recipient represent major drawbacks in the treatment of SOT recipients (Khanna et al., 1999; Savoldo et al., 2005). However, the continuing evolution of the field, most prominently the availability of banked allogeneic T cell products, will potentially circumvent these issues.
EBV+ B cell malignancies
Adoptive cellular therapy has also been trialled in a subset of patients with EBV+ B cell lymphoma including Hodgkin’s lymphoma. Here, 56% of patients infused with EBV-specific CD8+ T cells were in remission up to 4 yr after treatment, and a further 26% showed clinical responses for relapsed or refractory disease (Tables 2 and 3; Bollard et al., 2014). These outcomes are particularly promising because a subset of EBV-PID patients present with or develop EBV+ B lymphomas during the clinical course of their disease (∼50–80%; Table 1)
Developments aimed at broadening the EBV Ags that are targeted by CD8+ T cells, streamlining the manufacture, and improving strategies to expand EBV-specific T cells will further improve the efficacy of adoptive cellular therapy to control EBV-induced disease. It is likely that adoptive cellular therapy will be used as combinatorial treatments for EBV-associated disease. Ongoing development of an EBV vaccine will further improve our ability to combat EBV-induced disease.
Conclusions and perspectives
EBV infection is a remarkable example of host–pathogen evolution as the majority of the adult population has been exposed to EBV, yet only a minor proportion develops severe disease. Consequently, EBV infection can manifest in a variety of ways: asymptomatic/mild infection, IM, HLH, or a variety of malignancies. These clinical features are most prominent in immunocompromised individuals resulting from coincident infections, iatrogenic therapies, or gene mutations. Studies of monogenic immunodeficient patients with a predisposition to EBV-induced disease have been extremely instructive in revealing the cellular, biochemical, and molecular requirements for robust immunity against EBV infection. These analyses have also highlighted redundancies in immune control of EBV. Exploiting such findings, combined with further investigation into the etiology of EBV infection, immunity, and disease, will pave the way for ongoing development of improved methodologies for treating EBV-induced disease, such as adoptive cellular therapy, and the realization of a successful prophylactic vaccine. The study of EBV-induced disease in PIDs is a stark illustration of how insights from basic and clinical investigations of rare individuals can yield substantial outcomes for the treatment of disease resulting from a persistent virus in the general population.
Acknowledgments
We thank Kaan Boztug, Alain Fischer, and Sylvain Latour for providing preprints of manuscripts before publication. S.G. Tangye thanks Jean-Laurent Casanova for ongoing support, inspiration, and scientific discussions.
The Tangye laboratory is supported by a program grant and a Principal Research Fellowship from the National Health and Medical Research Council of Australia (grants 1016953 and 1042925, respectively) and the Susan and John Freeman Project Grant from the Cancer Council NSW (RG16-11).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Ab
- antibody
- Ag
- antigen
- HLH
- hemophagocytic lymphohistiocytosis
- HSCT
- hematopoietic stem cell transplantation
- IM
- infectious mononucleosis
- ITK
- IL-2–inducible T cell kinase
- LCL
- lymphoblastoid cell line
- LMP
- latent membrane protein
- PID
- primary immunodeficiency
- PTLD
- posttransplant lymphoproliferative disease
- SAP
- SLAM-associated protein
- SLAM
- signaling lymphocytic activation molecule
- SOT
- solid organ transplantation
- VZV
- varicella zoster virus
- XLP
- X-linked lymphoproliferative disease
References
- Abolhassani, H., Edwards E.S.J., Ikinciogullari A., Jing H., Borte S., Buggert M., Du L., Matsuda-Lennikov M., Romano R., Caridha R., et al. . 2017. Combined immunodeficiency and Epstein-Barr virus–induced B cell malignancy in humans with inherited CD70 deficiency. J. Exp. Med. 214:91–106. 10.1084/jem.20160849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, C., and Latour S.. 2015. X-linked inhibitor of apoptosis protein deficiency: more than an X-linked lymphoproliferative syndrome. J. Clin. Immunol. 35:331–338. 10.1007/s10875-015-0141-9 [DOI] [PubMed] [Google Scholar]
- Alkhairy, O.K., Perez-Becker R., Driessen G.J., Abolhassani H., van Montfrans J., Borte S., Choo S., Wang N., Tesselaar K., Fang M., et al. . 2015. Novel mutations in TNFRSF7/CD27: Clinical, immunologic, and genetic characterization of human CD27 deficiency. J. Allergy Clin. Immunol. 136:703–712.e10. 10.1016/j.jaci.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Amyes, E., Hatton C., Montamat-Sicotte D., Gudgeon N., Rickinson A.B., McMichael A.J., and Callan M.F.. 2003. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J. Exp. Med. 198:903–911. 10.1084/jem.20022058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi, T., Lünemann A., Murer A., Ueda S., Béziat V., Malmberg K.J., Staubli G., Gysin C., Berger C., Münz C., et al. . 2014. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 124:2533–2543. 10.1182/blood-2014-01-553024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour, H.H. Jr., Odumade O.A., Schmeling D.O., Mullan B.D., Ed J.A., Knight J.A., Vezina H.E., Thomas W., and Hogquist K.A.. 2013. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J. Infect. Dis. 207:80–88. 10.1093/infdis/jis646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar, R.S., DeLor C.J., Clausen K.P., Hurtubise P., Henle W., and Hewetson J.F.. 1974. Fatal infectious mononucleosis in a family. N. Engl. J. Med. 290:363–367. 10.1056/NEJM197402142900704 [DOI] [PubMed] [Google Scholar]
- Bienemann, K., Borkhardt A., Klapper W., and Oschlies I.. 2015. High incidence of Epstein-Barr virus (EBV)-positive Hodgkin lymphoma and Hodgkin lymphoma-like B-cell lymphoproliferations with EBV latency profile 2 in children with interleukin-2-inducible T-cell kinase deficiency. Histopathology. 67:607–616. 10.1111/his.12677 [DOI] [PubMed] [Google Scholar]
- Bollard, C.M., and Heslop H.E.. 2016. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 127:3331–3340. 10.1182/blood-2016-01-628982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard, C.M., Gottschalk S., Torrano V., Diouf O., Ku S., Hazrat Y., Carrum G., Ramos C., Fayad L., Shpall E.J., et al. . 2014. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 32:798–808. 10.1200/JCO.2013.51.5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, C., Gilmour K.C., Veys P., Gennery A.R., Slatter M.A., Chapel H., Heath P.T., Steward C.G., Smith O., O’Meara A., et al. . 2011. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood. 117:53–62. 10.1182/blood-2010-06-284935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst, J., Hendriks J., and Xiao Y.. 2005. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 17:275–281. 10.1016/j.coi.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Bottino, C., Falco M., Parolini S., Marcenaro E., Augugliaro R., Sivori S., Landi E., Biassoni R., Notarangelo L.D., Moretta L., and Moretta A.. 2001. NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus–infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 194:235–246. 10.1084/jem.194.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boztug, H., Hirschmugl T., Holter W., Lakatos K., Kager L., Trapin D., Pickl W., Förster-Waldl E., and Boztug K.. 2016. NF-κB1 haploinsufficiency causing immunodeficiency and EBV-driven lymphoproliferation. J. Clin. Immunol. 36:533–540. 10.1007/s10875-016-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, P.J., Brigl M., and Brenner M.B.. 2013. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13:101–117. 10.1038/nri3369 [DOI] [PubMed] [Google Scholar]
- Brigida, I., Chiriaco M., Di Cesare S., Cittaro D., Di Matteo G., Giannelli S., Lazarevic D., Zoccolillo M., Stupka E., Jenkner A., et al. . 2016. Large deletion of MAGT1 gene in a patient with classic kaposi sarcoma, CD4 lymphopenia, and EBV infection. J. Clin. Immunol. 1:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çağdaş, D., Erman B., Hanoğlu D., Tavil B., Kuşkonmaz B., Aydın B., Akyüz C., Uçkan D., Sanal Ö., and Tezcan I.. 2017. Course of IL-2-inducible T-cell kinase deficiency in a family: lymphomatoid granulomatosis, lymphoma and allogeneic bone marrow transplantation in one sibling; and death in the other. Bone Marrow Transplant. 52:126–129. 10.1038/bmt.2016.185 [DOI] [PubMed] [Google Scholar]
- Callan, M.F., Fazou C., Yang H., Rostron T., Poon K., Hatton C., and McMichael A.J.. 2000. CD8+ T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Invest. 106:1251–1261. 10.1172/JCI10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigne-Delalande, B., Li F.Y., O’Connor G.M., Lukacs M.J., Jiang P., Zheng L., Shatzer A., Biancalana M., Pittaluga S., Matthews H.F., et al. . 2013. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 341:186–191. 10.1126/science.1240094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, F.K., Chun H.J., Zheng L., Siegel R.M., Bui K.L., and Lenardo M.J.. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 288:2351–2354. 10.1126/science.288.5475.2351 [DOI] [PubMed] [Google Scholar]
- Chijioke, O., Müller A., Feederle R., Barros M.H.M., Krieg C., Emmel V., Marcenaro E., Leung C.S., Antsiferova O., Landtwing V., et al. . 2013. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Reports. 5:1489–1498. 10.1016/j.celrep.2013.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijioke, O., Landtwing V., and Münz C.. 2016. NK cell influence on the outcome of primary Epstein-Barr virus infection. Front. Immunol. 7:323. 10.3389/fimmu.2016.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, B.K., Tsai K., Allan L.L., Zheng D.J., Nie J.C., Biggs C.M., Hasan M.R., Kozak F.K., van den Elzen P., Priatel J.J., and Tan R.. 2013. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood. 122:2600–2608. 10.1182/blood-2013-01-480665 [DOI] [PubMed] [Google Scholar]
- Cipe, F.E., Aydogmus C., Serwas N.K., Tuğcu D., Demirkaya M., Biçici F.A., Hocaoglu A.B., Doğu F., and Boztuğ K.. 2015. ITK deficiency: How can EBV be treated before lymphoma? Pediatr. Blood Cancer. 62:2247–2248. 10.1002/pbc.25648 [DOI] [PubMed] [Google Scholar]
- Coffey, A.J., Brooksbank R.A., Brandau O., Oohashi T., Howell G.R., Bye J.M., Cahn A.P., Durham J., Heath P., Wray P., et al. . 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20:129–135. 10.1038/2424 [DOI] [PubMed] [Google Scholar]
- Cohen, J.I. 2015a. Epstein-barr virus vaccines. Clin. Transl. Immunology. 4:e32. 10.1038/cti.2014.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J.I. 2015b. Primary immunodeficiencies associated with EBV disease. Curr. Top. Microbiol. Immunol. 390:241–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli, P., Basso S., Zecca M., Pagliara D., Baldanti F., Bernardo M.E., Barberi W., Moretta A., Labirio M., Paulli M., et al. . 2007. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am. J. Transplant. 7:1648–1655. 10.1111/j.1600-6143.2007.01823.x [DOI] [PubMed] [Google Scholar]
- Crawford, D.H., Macsween K.F., Higgins C.D., Thomas R., McAulay K., Williams H., Harrison N., Reid S., Conacher M., Douglas J., and Swerdlow A.J.. 2006. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin. Infect. Dis. 43:276–282. 10.1086/505400 [DOI] [PubMed] [Google Scholar]
- Dhalla, F., Murray S., Sadler R., Chaigne-Delalande B., Sadaoka T., Soilleux E., Uzel G., Miller J., Collins G.P., Hatton C.S., et al. . 2015. Identification of a novel mutation in MAGT1 and progressive multifocal leucoencephalopathy in a 58-year-old man with XMEN disease. J. Clin. Immunol. 35:112–118. 10.1007/s10875-014-0116-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovina, E., Oflaz-Sozmen B., Prockop S.E., Kernan N.A., Abramson S., Teruya-Feldstein J., Hedvat C., Chou J.F., Heller G., Barker J.N., et al. . 2012. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 119:2644–2656. 10.1182/blood-2011-08-371971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré, L., Andolfi G., Tangye S.G., Clementi R., Locatelli F., Aricò M., Aiuti A., and Roncarolo M.G.. 2005. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 105:4383–4389. 10.1182/blood-2004-08-3269 [DOI] [PubMed] [Google Scholar]
- Filipovich, A.H., Zhang K., Snow A.L., and Marsh R.A.. 2010. X-linked lymphoproliferative syndromes: brothers or distant cousins? Blood. 116:3398–3408. 10.1182/blood-2010-03-275909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou, P., Buettner M., and Niedobitek G.. 2005. Epstein-Barr virus (EBV) infection in epithelial cells in vivo: rare detection of EBV replication in tongue mucosa but not in salivary glands. J. Infect. Dis. 191:238–242. 10.1086/426823 [DOI] [PubMed] [Google Scholar]
- Garibyan, L., Lobito A.A., Siegel R.M., Call M.E., Wucherpfennig K.W., and Geha R.S.. 2007. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID). J. Clin. Invest. 117:1550–1557. 10.1172/JCI31023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S., Bienemann K., Boztug K., and Borkhardt A.. 2014. Interleukin-2-inducible T-cell kinase (ITK) deficiency - clinical and molecular aspects. J. Clin. Immunol. 34:892–899. 10.1007/s10875-014-0110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein, P. 2000. Signal transduction. FasL binds preassembled Fas. Science. 288:2328–2329. 10.1126/science.288.5475.2328 [DOI] [PubMed] [Google Scholar]
- Green, M. 2013. Introduction: Infections in solid organ transplantation. Am. J. Transplant. 13:3–8. 10.1111/ajt.12093 [DOI] [PubMed] [Google Scholar]
- Gustafsson, A., Levitsky V., Zou J.Z., Frisan T., Dalianis T., Ljungman P., Ringden O., Winiarski J., Ernberg I., and Masucci M.G.. 2000. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 95:807–814. [PubMed] [Google Scholar]
- Hamann, D., Baars P.A., Rep M.H., Hooibrink B., Kerkhof-Garde S.R., Klein M.R., and van Lier R.A.. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418. 10.1084/jem.186.9.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, T., Wilkie G.M., Jones M.M., Higgins C.D., Urquhart G., Wingate P., Burns D., McAulay K., Turner M., Bellamy C., et al. . 2007. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 110:1123–1131. 10.1182/blood-2006-12-063008 [DOI] [PubMed] [Google Scholar]
- Herrmann, K., Frangou P., Middeldorp J., and Niedobitek G.. 2002. Epstein-Barr virus replication in tongue epithelial cells. J. Gen. Virol. 83:2995–2998. 10.1099/0022-1317-83-12-2995 [DOI] [PubMed] [Google Scholar]
- Heslop, H.E. 2009. How I treat EBV lymphoproliferation. Blood. 114:4002–4008. 10.1182/blood-2009-07-143545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop, H.E., Ng C.Y., Li C., Smith C.A., Loftin S.K., Krance R.A., Brenner M.K., and Rooney C.M.. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2:551–555. 10.1038/nm0596-551 [DOI] [PubMed] [Google Scholar]
- Heslop, H.E., Slobod K.S., Pule M.A., Hale G.A., Rousseau A., Smith C.A., Bollard C.M., Liu H., Wu M.F., Rochester R.J., et al. . 2010. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 115:925–935. 10.1182/blood-2009-08-239186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzen, R.Q., Lens S.M., Beckmann M.P., Goodwin R.G., Lynch D., and van Lier R.A.. 1994. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J. Immunol. 152:1762–1773. [PubMed] [Google Scholar]
- Hislop, A.D., Taylor G.S., Sauce D., and Rickinson A.B.. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617. 10.1146/annurev.immunol.25.022106.141553 [DOI] [PubMed] [Google Scholar]
- Hislop, A.D., Palendira U., Leese A.M., Arkwright P.D., Rohrlich P.S., Tangye S.G., Gaspar H.B., Lankester A.C., Moretta A., and Rickinson A.B.. 2010. Impaired Epstein-Barr virus-specific CD8+ T-cell function in X-linked lymphoproliferative disease is restricted to SLAM family-positive B-cell targets. Blood. 116:3249–3257. 10.1182/blood-2009-09-238832 [DOI] [PubMed] [Google Scholar]
- Huck, K., Feyen O., Niehues T., Rüschendorf F., Hübner N., Laws H.J., Telieps T., Knapp S., Wacker H.H., Meindl A., et al. . 2009. Girls homozygous for an IL-2–inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J. Clin. Invest. 119:1350–1358. 10.1172/JCI37901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher, L.M. 2017. The long and complicated relationship between Epstein-Barr virus and epithelial cells. J. Virol. 91:e01677-16. 10.1128/JVI.01677-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa, K., Martin E., Soudais C., Bruneau J., Boutboul D., Rodriguez R., Lenoir C., Hislop A.D., Besson C., Touzot F., et al. . 2017. Inherited CD70 deficiency in humans reveals a critical role for the CD70–CD27 pathway in immunity to Epstein-Barr virus infection. J. Exp. Med. 214:73–89. 10.1084/jem.20160784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J., Choe J., Li L., and Choi Y.S.. 2000. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur. J. Immunol. 30:2437–2443. [DOI] [PubMed] [Google Scholar]
- Khanna, R., Bell S., Sherritt M., Galbraith A., Burrows S.R., Rafter L., Clarke B., Slaughter R., Falk M.C., Douglass J., et al. . 1999. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc. Natl. Acad. Sci. USA. 96:10391–10396. 10.1073/pnas.96.18.10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehnle, I., Huls M.H., Liu Z., Semmelmann M., Krance R.A., Brenner M.K., Rooney C.M., and Heslop H.E.. 2000. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 95:1502–1505. [PubMed] [Google Scholar]
- Küppers, R. 2003. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 3:801–812. 10.1038/nri1201 [DOI] [PubMed] [Google Scholar]
- Kutok, J.L., and Wang F.. 2006. Spectrum of Epstein-Barr virus–associated diseases. Annu. Rev. Pathol. 1:375–404. 10.1146/annurev.pathol.1.110304.100209 [DOI] [PubMed] [Google Scholar]
- Kwon, H.J., Choi G.E., Ryu S., Kwon S.J., Kim S.C., Booth C., Nichols K.E., and Kim H.S.. 2016. Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nat. Commun. 7:11686. 10.1038/ncomms11686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier, L.L. 2015. NKG2D receptor and its ligands in host defense. Cancer Immunol. Res. 3:575–582. 10.1158/2326-6066.CIR-15-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen, A., Meij P., Redchenko I., Middeldorp J., Bloemena E., Rickinson A., and Blake N.. 2001. Differential immunogenicity of Epstein-Barr virus latent-cycle proteins for human CD4+ T-helper 1 responses. J. Virol. 75:8649–8659. 10.1128/JVI.75.18.8649-8659.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens, S.M., Tesselaar K., van Oers M.H., and van Lier R.A.. 1998. Control of lymphocyte function through CD27–CD70 interactions. Semin. Immunol. 10:491–499. 10.1006/smim.1998.0154 [DOI] [PubMed] [Google Scholar]
- Li, F.Y., Chaigne-Delalande B., Kanellopoulou C., Davis J.C., Matthews H.F., Douek D.C., Cohen J.I., Uzel G., Su H.C., and Lenardo M.J.. 2011. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 475:471–476. 10.1038/nature10246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka, R.M., Risse S.L., Bienemann K., Werner M., Linka Y., Krux F., Synaeve C., Deenen R., Ginzel S., Dvorsky R., et al. . 2012. Loss-of-function mutations within the IL-2 inducible kinase ITK in patients with EBV-associated lymphoproliferative diseases. Leukemia. 26:963–971. 10.1038/leu.2011.371 [DOI] [PubMed] [Google Scholar]
- Long, H.M., Haigh T.A., Gudgeon N.H., Leen A.M., Tsang C.W., Brooks J., Landais E., Houssaint E., Lee S.P., Rickinson A.B., and Taylor G.S.. 2005. CD4+ T-cell responses to Epstein-Barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. J. Virol. 79:4896–4907. 10.1128/JVI.79.8.4896-4907.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, H.M., Chagoury O.L., Leese A.M., Ryan G.B., James E., Morton L.T., Abbott R.J., Sabbah S., Kwok W., and Rickinson A.B.. 2013. MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1 response. J. Exp. Med. 210:933–949. 10.1084/jem.20121437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, K.G., Burton R.L., Zimmerman S.E., Wang J., Cornetta K.G., Robertson K.A., Lee C.H., and Emanuel D.J.. 1998. Semiquantitative Epstein-Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood. 91:3654–3661. [PubMed] [Google Scholar]
- Mansouri, D., Mahdaviani S.A., Khalilzadeh S., Mohajerani S.A., Hasanzad M., Sadr S., Nadji S.A., Karimi S., Droodinia A., Rezaei N., et al. . 2012. IL-2-inducible T-cell kinase deficiency with pulmonary manifestations due to disseminated Epstein-Barr virus infection. Int. Arch. Allergy Immunol. 158:418–422. 10.1159/000333472 [DOI] [PubMed] [Google Scholar]
- Marsh, R.A., Madden L., Kitchen B.J., Mody R., McClimon B., Jordan M.B., Bleesing J.J., Zhang K., and Filipovich A.H.. 2010. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 116:1079–1082. 10.1182/blood-2010-01-256099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautner, J., and Bornkamm G.W.. 2012. The role of virus-specific CD4+ T cells in the control of Epstein-Barr virus infection. Eur. J. Cell Biol. 91:31–35. 10.1016/j.ejcb.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Moss, P., and Rickinson A.. 2005. Cellular immunotherapy for viral infection after HSC transplantation. Nat. Rev. Immunol. 5:9–20. 10.1038/nri1526 [DOI] [PubMed] [Google Scholar]
- Naik, S., Nicholas S.K., Martinez C.A., Leen A.M., Hanley P.J., Gottschalk S.M., Rooney C.M., Hanson I.C., Krance R.A., Shpall E.J., et al. . 2016. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J. Allergy Clin. Immunol. 137:1498–1505.e1. 10.1016/j.jaci.2015.12.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, H., Cella M., Bouchon A., Grierson H.L., Lewis J., Duckett C.S., Cohen J.I., and Colonna M.. 2000. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur. J. Immunol. 30:3309–3318. [DOI] [PubMed] [Google Scholar]
- Nalesnik, M.A. 2002. Clinicopathologic characteristics of post-transplant lymphoproliferative disorders. Recent Results Cancer Res. 159:9–18. 10.1007/978-3-642-56352-2_2 [DOI] [PubMed] [Google Scholar]
- Nichols, K.E., Harkin D.P., Levitz S., Krainer M., Kolquist K.A., Genovese C., Bernard A., Ferguson M., Zuo L., Snyder E., et al. . 1998. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA. 95:13765–13770. 10.1073/pnas.95.23.13765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, K.E., Hom J., Gong S.Y., Ganguly A., Ma C.S., Cannons J.L., Tangye S.G., Schwartzberg P.L., Koretzky G.A., and Stein P.L.. 2005. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 11:340–345. 10.1038/nm1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning, R.J., Xu X.Q., Chan K.H., and Chiang A.K.. 2011. Long-term carriers generate Epstein-Barr virus (EBV)-specific CD4+ and CD8+ polyfunctional T-cell responses which show immunodominance hierarchies of EBV proteins. Immunology. 134:161–171. 10.1111/j.1365-2567.2011.03476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, S., Markle J.G., Deenick E.K., Mele F., Averbuch D., Lagos M., Alzahrani M., Al-Muhsen S., Halwani R., Ma C.S., et al. . 2015. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 349:606–613. 10.1126/science.aaa4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, J.S. 2013. Natural killer cell deficiency. J. Allergy Clin. Immunol. 132:515–525. 10.1016/j.jaci.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouederni, M., Vincent Q.B., Frange P., Touzot F., Scerra S., Bejaoui M., Bousfiha A., Levy Y., Lisowska-Grospierre B., Canioni D., et al. . 2011. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood. 118:5108–5118. 10.1182/blood-2011-05-352716 [DOI] [PubMed] [Google Scholar]
- Pachlopnik Schmid, J., Canioni D., Moshous D., Touzot F., Mahlaoui N., Hauck F., Kanegane H., Lopez-Granados E., Mejstrikova E., Pellier I., et al. . 2011. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood. 117:1522–1529. 10.1182/blood-2010-07-298372 [DOI] [PubMed] [Google Scholar]
- Palendira, U., and Rickinson A.B.. 2015. Primary immunodeficiencies and the control of Epstein-Barr virus infection. Ann. N. Y. Acad. Sci. 1356:22–44. 10.1111/nyas.12937 [DOI] [PubMed] [Google Scholar]
- Palendira, U., Low C., Chan A., Hislop A.D., Ho E., Phan T.G., Deenick E., Cook M.C., Riminton D.S., Choo S., et al. . 2011. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 9:e1001187. 10.1371/journal.pbio.1001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palendira, U., Low C., Bell A.I., Ma C.S., Abbott R.J., Phan T.G., Riminton D.S., Choo S., Smart J.M., Lougaris V., et al. . 2012. Expansion of somatically reverted memory CD8+ T cells in patients with X-linked lymphoproliferative disease caused by selective pressure from Epstein-Barr virus. J. Exp. Med. 209:913–924. 10.1084/jem.20112391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikkar, A., Smith C., Hislop A., Tellam N., Dasari V., Hogquist K.A., Wykes M., Moss D.J., Rickinson A., Balfour H.H. Jr., and Khanna R.. 2015a. Cytokine-mediated loss of blood dendritic cells during Epstein-Barr virus–associated acute infectious mononucleosis: Implication for immune dysregulation. J. Infect. Dis. 212:1957–1961. 10.1093/infdis/jiv340 [DOI] [PubMed] [Google Scholar]
- Panikkar, A., Smith C., Hislop A., Tellam N., Dasari V., Hogquist K.A., Wykes M., Moss D.J., Rickinson A., Balfour H.H. Jr., and Khanna R.. 2015b. Impaired Epstein-Barr virus-specific neutralizing antibody response during acute infectious mononucleosis is coincident with global B-cell dysfunction. J. Virol. 89:9137–9141. 10.1128/JVI.01293-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini, S., Bottino C., Falco M., Augugliaro R., Giliani S., Franceschini R., Ochs H.D., Wolf H., Bonnefoy J.Y., Biassoni R., et al. . 2000. X-linked lymphoproliferative disease. J. Exp. Med. 192:337–346. 10.1084/jem.192.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaneh, N., Filipovich A.H., and Borkhardt A.. 2013. Primary immunodeficiencies predisposed to Epstein-Barr virus-driven haematological diseases. Br. J. Haematol. 162:573–586. 10.1111/bjh.12422 [DOI] [PubMed] [Google Scholar]
- Pasquier, B., Yin L., Fondanèche M.C., Relouzat F., Bloch-Queyrat C., Lambert N., Fischer A., de Saint-Basile G., and Latour S.. 2005. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 201:695–701. 10.1084/jem.20042432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patiroglu, T., Haluk Akar H., Gilmour K., Unal E., Akif Ozdemir M., Bibi S., Burns S., Chiang S.C., Schlums H., Bryceson Y.T., and Karakukcu M.. 2015. A case of XMEN syndrome presented with severe auto-immune disorders mimicking autoimmune lymphoproliferative disease. Clin. Immunol. 159:58–62. 10.1016/j.clim.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Petrara, M.R., Freguja R., Gianesin K., Zanchetta M., and De Rossi A.. 2013. Epstein-Barr virus-driven lymphomagenesis in the context of human immunodeficiency virus type 1 infection. Front. Microbiol. 4:311. 10.3389/fmicb.2013.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, C., Al-Herz W., Bousfiha A., Casanova J.L., Chatila T., Conley M.E., Cunningham-Rundles C., Etzioni A., Holland S.M., Klein C., et al. . 2015. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency 2015. J. Clin. Immunol. 35:696–726. 10.1007/s10875-015-0201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provisor, A.J., Iacuone J.J., Chilcote R.R., Neiburger R.G., and Crussi F.G.. 1975. Acquired agammaglobulinemia after a life-threatening illness with clinical and laboratory features of infectious mononucleosis in three related male children. N. Engl. J. Med. 293:62–65. 10.1056/NEJM197507102930202 [DOI] [PubMed] [Google Scholar]
- Purtilo, D.T., Yang J.P., Cassel C.K., Harper R., Stephenson S.R., Landing B.H., and Vawter G.F.. 1975. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease). Lancet. 305:935–941. 10.1016/S0140-6736(75)92004-8 [DOI] [PubMed] [Google Scholar]
- Readinger, J.A., Mueller K.L., Venegas A.M., Horai R., and Schwartzberg P.L.. 2009. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 228:93–114. 10.1111/j.1600-065X.2008.00757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson, A.B., Long H.M., Palendira U., Münz C., and Hislop A.D.. 2014. Cellular immune controls over Epstein-Barr virus infection: new lessons from the clinic and the laboratory. Trends Immunol. 35:159–169. 10.1016/j.it.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Rigaud, S., Fondanèche M.C., Lambert N., Pasquier B., Mateo V., Soulas P., Galicier L., Le Deist F., Rieux-Laucat F., Revy P., et al. . 2006. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 444:110–114. 10.1038/nature05257 [DOI] [PubMed] [Google Scholar]
- Rooney, C.M., Smith C.A., Ng C.Y., Loftin S., Li C., Krance R.A., Brenner M.K., and Heslop H.E.. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 345:9–13. 10.1016/S0140-6736(95)91150-2 [DOI] [PubMed] [Google Scholar]
- Rooney, C.M., Smith C.A., Ng C.Y., Loftin S.K., Sixbey J.W., Gan Y., Srivastava D.K., Bowman L.C., Krance R.A., Brenner M.K., and Heslop H.E.. 1998. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 92:1549–1555. [PubMed] [Google Scholar]
- Rowe, M., and Zuo J.. 2010. Immune responses to Epstein-Barr virus: molecular interactions in the virus evasion of CD8+ T cell immunity. Microbes Infect. 12:173–181. 10.1016/j.micinf.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer, E., Daschkey S., Choo S., Gombert M., Santos-Valente E., Ginzel S., Schwendinger M., Haas O.A., Fritsch G., Pickl W.F., et al. . 2013. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica. 98:473–478. 10.3324/haematol.2012.068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer, E., Cagdas D., Hons M., Mace E.M., Garncarz W., Petronczki O.Y., Platzer R., Pfajfer L., Bilic I., Ban S.A., et al. . 2016. RASGRP1 deficiency causes immunodeficiency with impaired cytoskeletal dynamics. Nat. Immunol. 17:1352–1360. 10.1038/ni.3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo, B., Rooney C.M., Quiros-Tejeira R.E., Caldwell Y., Wagner H.J., Lee T., Finegold M.J., Dotti G., Heslop H.E., and Goss J.A.. 2005. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am. J. Transplant. 5:566–572. 10.1111/j.1600-6143.2004.00693.x [DOI] [PubMed] [Google Scholar]
- Sayos, J., Wu C., Morra M., Wang N., Zhang X., Allen D., van Schaik S., Notarangelo L., Geha R., Roncarolo M.G., et al. . 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 395:462–469. 10.1038/26683 [DOI] [PubMed] [Google Scholar]
- Schipp, C., Nabhani S., Bienemann K., Simanovsky N., Kfir-Erenfeld S., Assayag-Asherie N., Oommen P.T., Revel-Vilk S., Hönscheid A., Gombert M., et al. . 2016. Specific antibody deficiency and autoinflammatory disease extend the clinical and immunological spectrum of heterozygous NFKB1 loss-of-function mutations in humans. Haematologica. 101:e392–e396. 10.3324/haematol.2016.145136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwas, N.K., Cagdas D., Ban S.A., Bienemann K., Salzer E., Tezcan I., Borkhardt A., Sanal O., and Boztug K.. 2014. Identification of ITK deficiency as a novel genetic cause of idiopathic CD4+ T-cell lymphopenia. Blood. 124:655–657. 10.1182/blood-2014-03-564930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon-Lowe, C., and Rowe M.. 2011. Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PLoS Pathog. 7:e1001338. 10.1371/journal.ppat.1001338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherritt, M.A., Bharadwaj M., Burrows J.M., Morrison L.E., Elliott S.L., Davis J.E., Kear L.M., Slaughter R.E., Bell S.C., Galbraith A.J., et al. . 2003. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation. 75:1556–1560. 10.1097/01.TP.0000058745.02123.6F [DOI] [PubMed] [Google Scholar]
- Siegel, R.M., Frederiksen J.K., Zacharias D.A., Chan F.K., Johnson M., Lynch D., Tsien R.Y., and Lenardo M.J.. 2000. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 288:2354–2357. 10.1126/science.288.5475.2354 [DOI] [PubMed] [Google Scholar]
- Slatter, M.A., and Cant A.J.. 2011. Hematopoietic stem cell transplantation for primary immunodeficiency diseases. Ann. N. Y. Acad. Sci. 1238:122–131. 10.1111/j.1749-6632.2011.06243.x [DOI] [PubMed] [Google Scholar]
- Snow, A.L., Marsh R.A., Krummey S.M., Roehrs P., Young L.R., Zhang K., van Hoff J., Dhar D., Nichols K.E., Filipovich A.H., et al. . 2009. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J. Clin. Invest. 119:2976–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal, E.M., Hoppenbrouwers K., Vandermeulen C., Moutschen M., Léonard P., Moreels A., Haumont M., Bollen A., Smets F., and Denis M.. 2007. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 196:1749–1753. 10.1086/523813 [DOI] [PubMed] [Google Scholar]
- Speckmann, C., Lehmberg K., Albert M.H., Damgaard R.B., Fritsch M., Gyrd-Hansen M., Rensing-Ehl A., Vraetz T., Grimbacher B., Salzer U., et al. . 2013. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin. Immunol. 149:133–141. 10.1016/j.clim.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Stepensky, P., Weintraub M., Yanir A., Revel-Vilk S., Krux F., Huck K., Linka R.M., Shaag A., Elpeleg O., Borkhardt A., and Resnick I.B.. 2011. IL-2-inducible T-cell kinase deficiency: clinical presentation and therapeutic approach. Haematologica. 96:472–476. 10.3324/haematol.2010.033910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig, T., Brilot F., Arrey F., Bougras G., Thomas D., Muller W.A., and Münz C.. 2008. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-γ. PLoS Pathog. 4:e27. 10.1371/journal.ppat.0040027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, J.L., Byron K.S., Brewster F.E., and Purtilo D.T.. 1980. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 210:543–545. 10.1126/science.6158759 [DOI] [PubMed] [Google Scholar]
- Sumegi, J., Huang D., Lanyi A., Davis J.D., Seemayer T.A., Maeda A., Klein G., Seri M., Wakiguchi H., Purtilo D.T., and Gross T.G.. 2000. Correlation of mutations of the SH2D1A gene and epstein-barr virus infection with clinical phenotype and outcome in X-linked lymphoproliferative disease. Blood. 96:3118–3125. [PubMed] [Google Scholar]
- Tangye, S.G. 2014. XLP: clinical features and molecular etiology due to mutations in SH2D1A encoding SAP. J. Clin. Immunol. 34:772–779. 10.1007/s10875-014-0083-7 [DOI] [PubMed] [Google Scholar]
- Tangye, S.G., and Tarlinton D.M.. 2009. Memory B cells: effectors of long-lived immune responses. Eur. J. Immunol. 39:2065–2075. 10.1002/eji.200939531 [DOI] [PubMed] [Google Scholar]
- Tangye, S.G., Phillips J.H., Lanier L.L., and Nichols K.E.. 2000. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J. Immunol. 165:2932–2936. 10.4049/jimmunol.165.6.2932 [DOI] [PubMed] [Google Scholar]
- Taylor, G.S., Long H.M., Brooks J.M., Rickinson A.B., and Hislop A.D.. 2015. The immunology of Epstein-Barr virus–induced disease. Annu. Rev. Immunol. 33:787–821. 10.1146/annurev-immunol-032414-112326 [DOI] [PubMed] [Google Scholar]
- Tesselaar, K., Gravestein L.A., van Schijndel G.M., Borst J., and van Lier R.A.. 1997. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J. Immunol. 159:4959–4965. [PubMed] [Google Scholar]
- Tesselaar, K., Xiao Y., Arens R., van Schijndel G.M., Schuurhuis D.H., Mebius R.E., Borst J., and van Lier R.A.. 2003. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J. Immunol. 170:33–40. 10.4049/jimmunol.170.1.33 [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson, D.A. 2015. EBV Persistence—Introducing the Virus. Curr. Top. Microbiol. Immunol. 390:151–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson, D.A., Schooley R.T., Bhan A.K., and Nadler L.M.. 1982. Epstein-Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell. 30:415–425. 10.1016/0092-8674(82)90239-2 [DOI] [PubMed] [Google Scholar]
- van Esser, J.W., Niesters H.G., van der Holt B., Meijer E., Osterhaus A.D., Gratama J.W., Verdonck L.F., Löwenberg B., and Cornelissen J.J.. 2002. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 99:4364–4369. 10.1182/blood.V99.12.4364 [DOI] [PubMed] [Google Scholar]
- van Montfrans, J.M., Hoepelman A.I., Otto S., van Gijn M., van de Corput L., de Weger R.A., Monaco-Shawver L., Banerjee P.P., Sanders E.A., Jol-van der Zijde C.M., et al. . 2012. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J. Allergy Clin. Immunol. 129:787–793.e6. 10.1016/j.jaci.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vély, F., Barlogis V., Vallentin B., Neven B., Piperoglou C., Ebbo M., Perchet T., Petit M., Yessaad N., Touzot F., et al. . 2016. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol. 17:1291–1299. 10.1038/ni.3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen, M.T., Matmati M., Hertoghs K.M., Baars P.A., Gent M.R., Leclercq G., Hamann J., Kuijpers T.W., and van Lier R.A.. 2008. CD27 defines phenotypically and functionally different human NK cell subsets. J. Immunol. 180:3739–3745. 10.4049/jimmunol.180.6.3739 [DOI] [PubMed] [Google Scholar]
- Williams, H., McAulay K., Macsween K.F., Gallacher N.J., Higgins C.D., Harrison N., Swerdlow A.J., and Crawford D.H.. 2005. The immune response to primary EBV infection: a role for natural killer cells. Br. J. Haematol. 129:266–274. 10.1111/j.1365-2141.2005.05452.x [DOI] [PubMed] [Google Scholar]
- Young, L.S., and Rickinson A.B.. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer. 4:757–768. 10.1038/nrc1452 [DOI] [PubMed] [Google Scholar]