Abstract
Whole-exome sequencing (WES) has increasingly enabled new pathogenic gene variant identification for undiagnosed neurodevelopmental disorders and provided insights into both gene function and disease biology. Here, we describe seven children with a neurodevelopmental disorder characterized by microcephaly, profound developmental delays and/or intellectual disability, cataracts, severe epilepsy including infantile spasms, irritability, failure to thrive, and stereotypic hand movements. Brain imaging in these individuals reveals delay in myelination and cerebral atrophy. We observe an identical recurrent de novo heterozygous c.892C>T (p.Arg298Trp) variant in the nucleus accumbens associated 1 (NACC1) gene in seven affected individuals. One of the seven individuals is mosaic for this variant. NACC1 encodes a transcriptional repressor implicated in gene expression and has not previously been associated with germline disorders. The probability of finding the same missense NACC1 variant by chance in 7 out of 17,228 individuals who underwent WES for diagnoses of neurodevelopmental phenotypes is extremely small and achieves genome-wide significance (p = 1.25 × 10−14). Selective constraint against missense variants in NACC1 makes this excess of an identical missense variant in all seven individuals more remarkable. Our findings are consistent with a germline recurrent mutational hotspot associated with an allele-specific neurodevelopmental phenotype in NACC1.
Keywords: NACC1, cataracts, microcephaly, epilepsy, whole-exome sequencing, developmental/intellectual disabilities, stereotypy, irritability
Main Text
Advances in massively parallel, next generation sequencing such as whole-exome sequencing (WES) have led to improved molecular diagnostic rates for rare and undiagnosed Mendelian disorders, identification of new pathogenic gene variants, better treatment options, and accurate prediction of recurrence risk.1 WES in large cohorts of neurologic phenotypes including autism spectrum disorders,2, 3 intellectual disability,4, 5, 6 and epilepsy7, 8 have demonstrated a major contribution of rare, de novo variants to disease phenotypes.9, 10 However, since many of these de novo variants are often seen in a singleton setting, it can be challenging to conclusively infer causality. Moreover, the extent to which the phenotype includes findings in addition to the developmental delay may not be initially appreciated.11 This can be resolved by ascertaining a number of affected individuals with similar phenotypes who harbor the same recurrent de novo, potentially pathogenic variant, as seen in this series of seven individuals all with the same missense variant in the nucleus accumbens associated 1 gene (NACC1 [MIM: 610672]). NACC1 is a member of the BTB/POZ domain-containing gene family and encodes for NAC1, a protein that functions as a transcriptional regulator.12 Although NACC1 has not previously been associated with human Mendelian disease, its function makes it a plausible disease-associated gene, since it has been increasingly shown that a number of neurodevelopmental disorders are caused by misregulated gene expression.13
The seven individuals we report here (Figure 1) have a clinical constellation of severe to profound developmental delay and/or intellectual disability (HP: 0012736), epilepsy (HP: 0001250), feeding difficulties and/or feeding intolerance (HP: 0011968), irritability (HP: 0000737), and in the majority, postnatal microcephaly (HP: 0005484), bilateral cataracts (HP: 0000519), sleep disorder (HP: 0002360), and stereotypic motor behaviors (HP: 0000733). Detailed medical summaries and WES results are available in the Supplemental Data. Consent for publication was obtained from parents of all subjects, and procedures were followed in accordance with guidelines specified by Institutional Review Boards and Ethics Committees of each institution. Experienced pediatricians, geneticists, and neurologists clinically assessed the individuals.
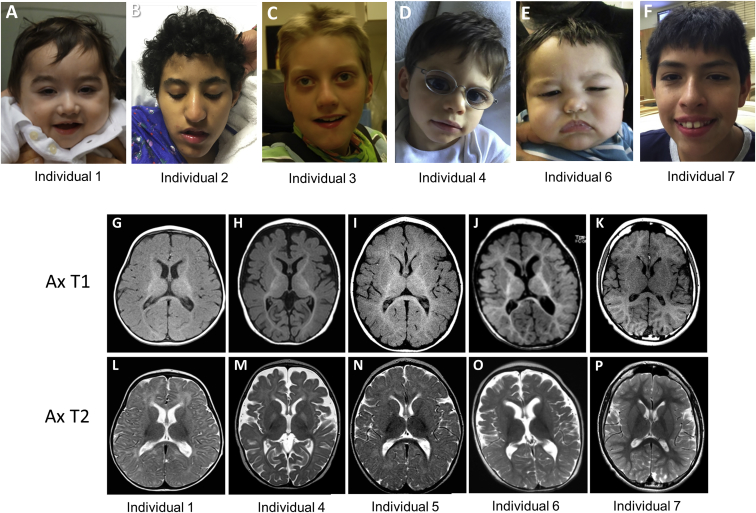
Figure 1.
Clinical and Neuroradiologic Characteristics of the Seven Probands
(A) Individual 1 at 20 months of age.
(B) Individual 2 at 12 years of age.
(C) Individual 3 at 9 years of age.
(D) Individual 4 at 3.5 years of age.
(E) Individual 6 at 13 months of age.
(F) Individual 7 at 12 years of age.
(G–P) Axial T1 and T2 brain MRI images demonstrating mildly delayed myelination in individual 1 at 9 months old (G, L); severely delayed myelination and volume loss in individual 4 at 3 years old (H, M); severely delayed myelination and minimal volume loss in individual 5 at 10 years old (I, N); volume loss with normal myelination in individual 6 at 12 months old (J, O); and normal myelination and brain volume in individual 7 at 10 years old (K, P).
The key clinical phenotypes are summarized in Table 1. Most individuals had profound developmental impairment such that ambulation and speech were absent or greatly impaired. Infantile epilepsy had been noted in all seven individuals, with infantile spasms in four. Overall seizure control had been variable in the seven individuals but generally required multiple anti-epileptics and sub-specialized neurologic care. Bilateral cataracts requiring surgical extraction were present in five out of seven individuals, with age of onset ranging from birth to 10 years, with variability in the type of cataracts. All individuals had a history of feeding difficulties or intolerance, with four out of seven requiring feeding by gastrostomy tube. Postnatal microcephaly was noted in five out of seven individuals, being evident prior to the onset of seizures in some individuals. All individuals were described as being irritable or having bouts of severe irritability. Hypotonia, sleep disorders, breath-holding spells, and repetitive or choreiform movements, hand flapping, or Rett-like hand automatisms were present in the majority of individuals. Interestingly, individuals presented here were consistently evaluated for severe infantile epilepsy disorders such as CDKL5 (MIM: 300203)- and ARX (MIM: 300382)-related diseases, in addition to MECP2 (MIM: 300005) and other Rett-like disorders due to the repetitive hand movements. Brain MR imaging showed delayed myelination in four individuals, decreased brain volume in six individuals, and focal cortical dysplasia in one individual (Figure 1). The appearance of a broad nasal tip was the only consistent facial dysmorphic feature noted in these individuals.
Table 1.
Phenotypic Features in Individuals with NACC1 c.892C>T (p.Arg298Trp) Variant
| Individual 1 | Individual 2 | Individual 3 | Individual 4 | Individual 5 | Individual 6 | Individual 7 | Total | |
|---|---|---|---|---|---|---|---|---|
| Age | 20 months | 12 years | 18 years | 3 years | 9 years | 13 months | 12 years | |
| Gender | M | F | M | M | F | M | M | |
| NACC1 c.892C>T (Arg298Trp) variant | constitutional, de novo | constitutional, de novo | constitutional, de novo | constitutional, de novo | constitutional, de novo | constitutional, de novo | mosaic, de novo | 7/7 |
| Postnatal microcephaly | yes | yes | yes | yes | yes | no | no | 5/7 |
| Intellectual/developmental disability | profound | profound | profound | profound | profound | profound | severe | 7/7 |
| Bilateral cataracts | lamellar, diagnosed at 7 months old | diagnosed at 10 and 11 years old | diagnosed at 6 weeks old | anterior subcapsular, congenital | nuclear, diagnosed at 11 and 12 months old | no | no | 5/7 |
| GI problems/feeding difficulties | feeding intolerance, failure to thrive, gastrostomy at 9 months | dysphagia | severe feeding problems, PEG at 6 years old | feeding intolerance, failure to thrive, gastrostomy at 9 months | feeding intolerance, cyclic vomiting, gastrostomy at 2.5 years old | dysphagia, failure to thrive | feeding difficulties, GERD | 7/7 |
| Hypotonia | present | present | present | present | present | present | no | 6/7 |
| Seizures | infantile spasms | focal seizures | generalized tonic | infantile spasms | infantile spasms, mixed generalized and multifocal epilepsy | infantile spasms, tonic seizures | single febrile seizure, nocturnal seizures | 7/7 |
| Irritability | very fussy, breath-holding spells | yes | tactile aversion, unable to tolerate being held, periods of inconsolability | bouts of irritability, breath-holding spells | cyclical bouts of severe irritability, breath-holding spells | yes | yes | 7/7 |
| Sleep disorder | yes | – | yes | yes | episodes of dysauto-nomia (tachycardia and insomnia) | – | obstructive sleep apnea | 5/7 |
| Stereotypic movements | repetitive movement bringing hands to midline and pulling shirt down | autistic features, Rett-like hand auto-matisms; hands always in her mouth | Rett-like stereotypic hand movements | repetitive hand movements | random choreiform movements of the hands | no | hand flapping, autistic features | 6/7 |
| Brain MRI findings | delayed myelination, mildly decreased brain volume, slight enlargement of ventricles | brain atrophy, temporal arachnoid cyst, MRS showed decreased NAA over basal ganglia | reduced white matter, delayed and incomplete myelination with temporal lobes most affected, mildly decreased brain volume, MRS showed decreased NAA levels | delayed myelination and volume loss | minimal volume loss, delayed myelination | volume loss | scattered areas of subcortical white matter T2/FLAIR hyperintensity | 7/7 |
Individuals 1, 2, 6, and 7 had trio exome sequencing, and individuals 3–5 had proband exome sequencing followed by Sanger confirmation (Figure 2). Detailed WES platform14, 15, 16 and coverage for the seven individuals are listed in Table S1. The WES data on individual 1 were reanalyzed after he was enrolled into the Undiagnosed Diseases Network (UDN). GeneMatcher,17 a web-based tool for researchers and clinicians with interest in common genes, connected researchers and clinicians involved with individuals 1 and 2. Individual 3 was identified from the Baylor-Hopkins Center for Mendelian Genomics Database (BHCMG) database,14 and individuals 4 and 5 were identified from clinical exome data from the Baylor Genetics laboratory.15 Finally, individuals 6 and 7 were connected through a UDN webpage designed to connect individuals with overlapping clinical features and candidate genes.
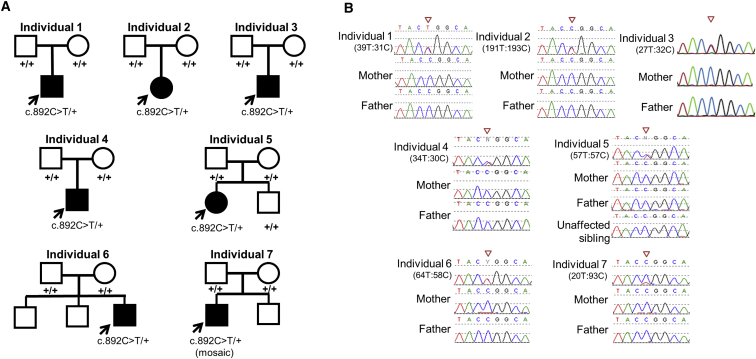
Figure 2.
Recurrent De Novo c.892C>T (p.Arg298Trp) Missense Change in NACC1 Observed in All Seven Probands
(A) Family pedigrees for individuals 1–7 with de novo events in each affected proband. Note that unaffected sibling of individual 5 was also sequenced and found not to have the de novo variant.
(B) Sanger sequencing traces for individuals 1–7 showing the heterozygous de novo C-T transition at the c.892 CGG codon encoding arginine, resulting in a TGG codon encoding tryptophan. Individual 7 was found to be mosaic for this variant in peripheral blood. Numbers below each individual represent allele count of alternative to reference alleles from exome sequencing.
WES in all seven individuals identified a recurrent, heterozygous, de novo variant c.892C>T (p.Arg298Trp) in NACC1 (GenBank: NM_052876.3) (Figures 2A and 2B). Sanger sequencing of this variant in all families confirmed de novo inheritance. Low alternative allele fraction observed in the exome data (93 reference allele and 20 alternate allele) and Sanger sequencing for individual 7 suggested NACC1 mosaicism. The significance of the allele balance bias observed at c.892C>T was evaluated by binomial test of the observed alternate allele counts and overall allele coverage of all well-covered (>10×) heterozygous positions in the affected individual’s exome. After adjusting p values using the Benjamini-Hochberg method with an FDR of 0.05, the NACC1 variant was confirmed mosaic (p < 0.0001). Parentage of six of the seven trio families was confirmed by trio exome sequencing (individuals 1, 2, 6, 7) or Sanger sequencing of rare variants (individuals 4 and 5) (Table S2).
The c.892C>T transition occurs at a CpG dinucleotide within an arginine codon, a hypermutable CpG pattern reported in association with de novo events at numerous loci in the setting of advanced paternal age.18, 19, 20 However, advanced paternal age was not a common feature of this cohort. Additional variants identified through exome sequencing in these individuals are summarized in Table S2.
Further investigation of this gene in the ExAC database revealed a single loss-of-function (LoF) variant (probability of LoF intolerance [pLI] = 0.96) and fewer than expected number of missense variants (z = 5.23) despite relatively good coverage of the gene, suggesting that mutations in NACC1 are subject to selection and that rare, predicted pathogenic variants may confer risk for human diseases. Additionally, the residual variation intolerance score (RVIS) of NACC1 is 14, with a raw score of −0.7,21 indicating that it is a gene that does not tolerate functional variants. The p.Arg298Trp variant is not observed in the ExAC or gnomAD databases, although a missense change from arginine to leucine of the same amino acid (p.Arg298Leu) has been observed in one individual in the ExAC database and in four individuals in gnomAD.22 This suggests that the p.Arg298Trp change that we report is likely to exert a specific functional effect on the protein and may lead to the distinct disease phenotype presented in this report. Extensive search of somatic databases identified a single case in metastatic breast cancer with the p.Arg298Trp variant.23 The combined annotation dependent depletion (CADD) score,24 which ranks deleteriousness of single-nucleotide variants within the human genome, is 25.8 for this variant, indicating that it is predicted to be in the top 0.14% most damaging in the genome. The Arg298 residue is extremely conserved from human to zebrafish with a GERP++ RS score of 4.89 (Figure 3A) and this variant is predicted to be deleterious by several prediction algorithms including align GVGD, PolyPhen, SIFT, and MutationTaster. These in silico analysis tools support the contention that the p.Arg298Trp change is likely to exert a specific functional effect on the protein.
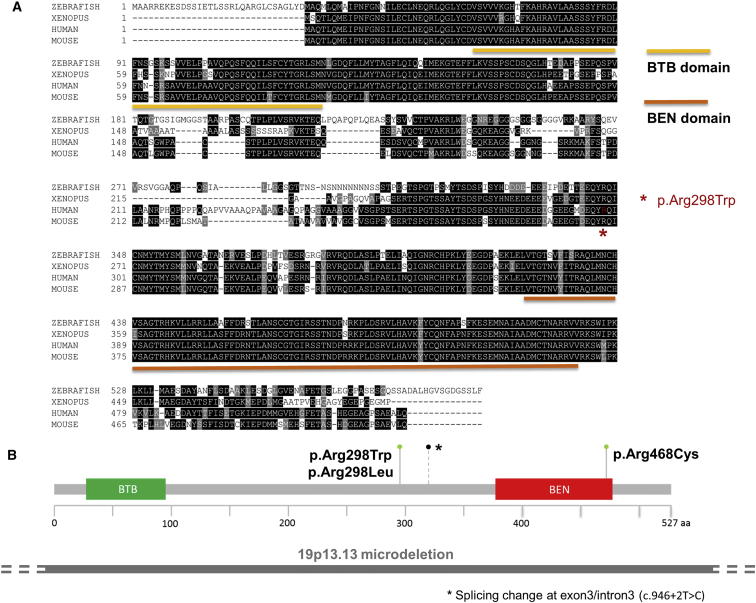
Figure 3.
Predicted Protein Change for the De Novo NACC1 Variant in Context of Protein Domains
(A) Amino acid sequence alignment of human NAC1 with zebrafish, Xenopus, and mouse. The BEN and BTB domains are shown, and the location of the p.Arg298Trp missense change is depicted by a red star. The amino acid residue is conserved in all four species and is within a stretch of identical residues.
(B) The Arg298 residue occurs within NAC1 between the BTB domain and BEN domain, closer to the BEN domain. A single p.Arg468Cys change (gray-green lollipop) has been noted in a large cohort study of intellectual disability,6 while the c.946+2T>C (A316 splice) change (black lollipop with asterisk to indicate the position of the splice change) has been noted in a cohort of patients with autism spectrum disorder. Interestingly, a variant involving the same amino acid, converting from Arg to Leu (c.893G>T [p.Arg298Leu]), has been observed once in the ExAC database and in four individuals in gnomAD, with undefined clinical information. Spanning this region is a microdeletion, shown as a gray bar under the gene domain structure.
Furthermore, we calculated the estimated probability of observing seven de novo NACC1 variants among the 17,228 individuals who underwent exome sequencing for neurodevelopmental/neurologic disorders across the different clinical testing laboratories and research studies that tested the seven individuals (GeneDx, 6,478; BHCMG, 3,400; Baylor, 6,250; UCLA, 1,100). The significance of this finding was evaluated using a binomial test, with the mutation rate as the probability of success, the number of individuals who underwent exome sequencing as the number of trials, and the number of probands who were found to have this mutation as the number of successes.25 The probability of a chance occurrence is small and remains highly significant when Bonferonni correction is applied for the 3 billion base pairs in the genome (p = 1.25 × 10−14). Thus, our findings implicate NACC1 as a genome-wide significant disease-associated gene.
Although NACC1 has not previously been associated with a Mendelian phenotype, a link between NACC1 and intellectual disability has been suggested, with apparent de novo variants being noted in single individuals within large sequencing studies of intellectual disability5, 6 and autism spectrum disorder2 cohorts (Table S3). One female with intellectual disability (IQ of ∼45), autism, and schizo-affective disorder has been noted with a de novo missense allele c.1402C>T (p.Arg468Cys) in NACC1, located in a different region of the gene, the BEN domain (Figure 3A).5, 6 In another exome-sequencing effort from the Simons Simplex Collection, one individual with autism spectrum disorder was identified to have a de novo splicing variant (presumably LoF), c.946+2T>C, in NACC1.2 These individuals do not appear to have, or at least were not reported as having, other key manifestations seen in our individuals, such as cataracts, epilepsy, irritability, and microcephaly. Contiguous gene deletions involving NACC1 are seen in 18 individuals in the DECIPHER database, ranging in size from 83 kb to 2.39 Mb, and duplications involving NACC1 are seen in 12 individuals, ranging in size from 211 kb to 58.83 Mb (Table S3), and thus the relative contribution of the loss of NACC1 in these individuals is difficult to infer. A known microdeletion/microduplication syndrome involving chromosome 19p13.13 includes most of NACC1 along with ∼16 other genes and is characterized by macrocephaly, overgrowth, and intellectual disability, but it has been speculated that NFIX is the critical gene in the region.26, 27 There are no copy losses involving NACC1 in non-disease individuals in the Database of Genomic Variants.28 Taken together, none of the previous studies involving NACC1 aberrations report the syndromic phenotype we delineate here in the seven individuals carrying a de novo p.Arg298Trp variant, strengthening the phenotypic association specific to this missense change. Individual 7 had a milder clinical presentation compared to the other individuals reported here; at 12 years of age he had not developed cataracts, had normal tone and head size, and was ambulatory with limited speech. This may be explained by the likely mosaicism of the recurrent c.892C>T variant in peripheral blood, demonstrated by atypical allele balance (Figures 2A and 2B). Although the exact contribution of the mosaic NACC1 variant to the individual’s milder phenotype is difficult to ascertain from exome data alone and requires further functional studies, previous studies suggest that mosaic mutations frequently occur in individuals diagnosed with intellectual disability and autism spectrum disorders.29, 30
NAC1 has a documented role in cancer. NAC1 knockdown or overexpression of an NAC1 mutant containing only the BTB/POZ domain promotes cell apoptosis and senescence, inhibits cytokinesis, and prevents tumor formation.31, 32, 33 NAC1 is upregulated in several types of neoplasms, especially ovarian serous carcinomas, either at the transcriptional level or through DNA amplification and overexpression. This is associated with cell proliferation, migration and invasion, chemotherapy resistance, tumor recurrence, and poor prognosis.31, 32, 34, 35, 36, 37 While an oncogenic role has been proposed for NAC1’s involvement in cancers,32 we do not currently know whether or not our individuals with the recurrent p.Arg298Trp variant are at risk for cancers. Certainly, there is precedence for many genes in which germline variants cause neurodevelopmental disorders and somatic variants can be associated with cancers.38, 39, 40
Previous studies have shown that NAC1 forms a homodimer or heterodimers with other binding partners through the BTB/POZ domain and functions as a transcriptional repressor through recruitment of histone deacetylase.12, 41 The p.Arg298Trp variant is located outside the BTB/POZ domain that is important for cancer progression and the BEN domain in which one missense variant associated with intellectual disability has been reported6 (Figure 3B). A dominant-negative function (especially since the protein dimerizes) or a gain-of-function (GoF) is possible for the p.Arg298Trp variant, since the prior reported LoF alleles in NACC1 result in an overlapping but less severe phenotype of intellectual disability and autism,2, 6 and it is well known that LoF and GoF variants in the same gene can result in heterogeneous phenotypes with overlapping features.25, 42, 43 Future studies are required to elucidate the mechanism of disease related to this NACC1 variant.
NAC1 is known to have other biological functions, including involvement in psychomotor response to cocaine administration in rats,30, 31, 32 vertebral patterning in mice,44 interaction with Parkin suggestive of a role in Parkinson disease,45 and involvement with TDP-43, which is implicated in individuals with amyotrophic lateral sclerosis.46 The role of NAC1 in normal neurologic function is highlighted by its role in mitigating protein turnover in dendritic cells and in maintaining synaptic plasticity.40 As a transcriptional repressor and protein binding factor, NAC1 probably functions in regulating neural development and network establishment by altering expression, localization, and degradation of many downstream nervous system genes. Given the characteristic phenotype associated with the p.Arg298Trp variant, future transcriptional investigations may further delineate the impact of this rare, recurrent, highly penetrant allele and could potentially lead to targeted therapies.
The constellation of cataracts, severe epilepsy, profound intellectual disability, irritability, microcephaly, and stereotypical movements is unusual and given the consistent occurrence of these phenotypes across our seven individuals, the allele-specific variant that we report in NACC1 should be considered strongly in individuals with similar features.
Consortia
The members of the UCLA Clinical Genomics Center Contributor List are Stanley F. Nelson, Wayne W. Grody, Hane Lee, Samuel P. Strom, Eric Vilain, Joshua Deignan, Fabiola Quintero-Rivera, Sibel Kantarci, Naghmeh Dorrani, Sureni Mullegama, Sung-Hae Kang, and Szabolcs Szelinger.
The members of the Undiagnosed Diseases Network are as follows: Mercedes E. Alejandro, Carlos A. Bacino, Ashok Balasubramanyam, Lindsay C. Burrage, Gary D. Clark, William J. Craigen, Shweta U. Dhar, Lisa T. Emrick, Brett H. Graham, Neil A. Hanchard, Mahim Jain, Seema R. Lalani, Brendan H. Lee, Richard A. Lewis, Azamian S. Mashid, Paolo M. Moretti, Sarah K. Nicholas, Jordan S. Orange, Jennifer E. Posey, Lorraine Potocki, Jill A. Rosenfeld, Daryl A. Scott, Alyssa A. Tran, Hugo J. Bellen, Michael F. Wangler, Shinya Yamamoto, Christine M. Eng, Donna M. Muzny, Patricia A. Ward, Yaping Yang, Andrea L. Gropman, David B. Goldstein, Nicholas Stong, Yong-hui Jiang, Allyn McConkie-Rosell, Loren D.M. Pena, Kelly Schoch, Vandana Shashi, Rebecca C. Spillmann, Jennifer A. Sullivan, Nicole M. Walley, Alan H. Beggs, Lauren C. Briere, Cynthia M. Cooper, Laurel A. Donnell-Fink, Elizabeth L. Krieg, Joel B. Krier, Sharyn A. Lincoln, Joseph Loscalzo, Richard L. Maas, Calum A. MacRae, J. Carl Pallais, Lance H. Rodan, Edwin K. Silverman, Joan M. Stoler, David A. Sweetser, Chris A. Walsh, Cecilia Esteves, Ingrid A. Holm, Isaac S. Kohane, Paul Mazur, Alexa T. McCray, Matthew Might, Rachel B. Ramoni, Kimberly Splinter, David P. Bick, Camille L. Birch, Braden E. Boone, Donna M. Brown, Dan C. Dorset, Lori H. Handley, Howard J. Jacob, Angela L. Jones, Jozef Lazar, Shawn E. Levy, J. Scott Newberry, Molly C. Schroeder, Kimberly A. Strong, Elizabeth A. Worthey, Jyoti G. Dayal, David J. Eckstein, Sarah E. Gould, Ellen M. Howerton, Donna M. Krasnewich, Carson R. Loomis, Laura A. Mamounas, Teri A. Manolio, John J. Mulvihill, Anastasia L. Wise, Ariane G. Soldatos, Matthew Brush, Jean-Philippe F. Gourdine, Melissa Haendel, David M. Koeller, Jennifer E. Kyle, Thomas O. Metz, Katrina M. Waters, Bobbie-Jo M. Webb-Robertson, Euan A. Ashley, Jonathan A. Bernstein, Annika M. Dries, Paul G. Fisher, Jennefer N. Kohler, Daryl M. Waggott, Matt T. Wheeler, Patricia A. Zornio, Patrick Allard, Hayk Barseghyan, Esteban C. Dell’Angelica, Katrina M. Dipple, Naghmeh Dorrani, Matthew R. Herzog, Hane Lee, Stan F. Nelson, Christina G.S. Palmer, Jeanette C. Papp, Janet S. Sinsheimer, Eric Vilain, Christopher J. Adams, Elizabeth A. Burke, Katherine R. Chao, Mariska Davids, David D. Draper, Tyra Estwick, Trevor S. Frisby, Kate Frost, Valerie Gartner, Rena A. Godfrey, Mitchell Goheen, Gretchen A. Golas, Mary “Gracie” G. Gordon, Catherine A. Groden, Mary E. Hackbarth, Isabel Hardee, Jean M. Johnston, Alanna E. Koehler, Lea Latham, Yvonne L. Latour, C. Christopher Lau, Denise J. Levy, Adam P. Liebendorder, Ellen F. Macnamara, Valerie V. Maduro, Thomas C. Markello, Alexandra J. McCarty, Jennifer L. Murphy, Michele E. Nehrebecky, Donna Novacic, Barbara N. Pusey, Sarah Sadozai, Katherine E. Schaffer, Prashant Sharma, Sara P. Thomas, Nathanial J. Tolman, Camilo Toro, Zaheer M. Valivullah, Colleen E. Wahl, Mike Warburton, Alec A. Weech, Guoyun Yu, David R. Adams, William A. Gahl, May Christine V. Malicdan, Cynthia J. Tifft, Lynne A. Wolfe, Paul R. Lee, John H. Postlethwait, Monte Westerfield, Anna Bican, Rizwan Hamid, John H. Newman, John A. Phillips III, Amy K. Robertson, and Joy D. Cogan.
Acknowledgments
We are grateful to the individuals and their families who contributed to this study and allowed us to publish their information and pictures. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from molecular testing offered at the Baylor Genetics Laboratories. D.B.G. has received consultancy fees and has equity ownership in Pairnomix, LLC. S.B. is the founder and F.M. is an employee of GeneDx. J.R.L. has stock ownership in 23 and Me, is a paid consultant for Regeneron Pharmaceuticals, has stock options in Lasergen, Inc., and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. This work was supported by Duke Undiagnosed Diseases Network (1U01HG007672-03 to V.S. and D.B.G.), Simons Foundation Functional Screen (368479 to M.F.W.), the National Human Genome Research Institute and National Heart Lung and Blood Institute (UM1 HG006542 to J.R.L.), Common Fund (U54NS093793 to M.F.W. and others), March of Dimes (grant# 6-FY12-324 to J.A.M.-A.), UCLA Children’s Discovery Institute, UCLA CART (NIH/NICHD grant# P50-HD-055784 to J.A.M.-A.), NIH/NCATS UCLA CTSI (grant# UL1TR000124 to J.A.M.-A.), and Ting Tsung and Wei Fong Chao Foundation (to J.E.P.).
Published: January 26, 2017
Footnotes
Supplemental Data include case reports and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.12.013.
Contributor Information
Michael F. Wangler, Email: michael.wangler@bcm.edu.
Vandana Shashi, Email: vandana.shashi@duke.edu.
UCLA Clinical Genomics Center:
Stanley F. Nelson, Wayne W. Grody, Hane Lee, Samuel P. Strom, Eric Vilain, Joshua Deignan, Fabiola Quintero-Rivera, Sibel Kantarci, Naghmeh Dorrani, Sureni Mullegama, Sung-Hae Kang, and Szabolcs Szelinger
Web Resources
DECIPHER, http://decipher.sanger.ac.uk/
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
GeneMatcher, https://genematcher.org/
gnomAD Browser, http://gnomad.broadinstitute.org/
Human Phenotype Ontology (HPO), http://www.human-phenotype-ontology.org/
MutationTaster, http://www.mutationtaster.org/
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
The Human Protein Atlas, http://www.proteinatlas.org/
Supplemental Data
References
- 1.Gonzaga-Jauregui C., Lupski J.R., Gibbs R.A. Human genome sequencing in health and disease. Annu. Rev. Med. 2012;63:35–61. doi: 10.1146/annurev-med-051010-162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iossifov I., Ronemus M., Levy D., Wang Z., Hakker I., Rosenbaum J., Yamrom B., Lee Y.H., Narzisi G., Leotta A. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ligt J., Veltman J.A., Vissers L.E. Point mutations as a source of de novo genetic disease. Curr. Opin. Genet. Dev. 2013;23:257–263. doi: 10.1016/j.gde.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 5.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 6.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 7.Ohba C., Kato M., Takahashi N., Osaka H., Shiihara T., Tohyama J., Nabatame S., Azuma J., Fujii Y., Hara M. De novo KCNT1 mutations in early-onset epileptic encephalopathy. Epilepsia. 2015;56:e121–e128. doi: 10.1111/epi.13072. [DOI] [PubMed] [Google Scholar]
- 8.Rivière J.B., van Bon B.W., Hoischen A., Kholmanskikh S.S., O’Roak B.J., Gilissen C., Gijsen S., Sullivan C.T., Christian S.L., Abdul-Rahman O.A. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat. Genet. 2012;44:440–444. doi: 10.1038/ng.1091. S1–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veltman J.A., Brunner H.G. De novo mutations in human genetic disease. Nat. Rev. Genet. 2012;13:565–575. doi: 10.1038/nrg3241. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier J., Rouleau G.A. De novo mutations in neurological and psychiatric disorders: effects, diagnosis and prevention. Genome Med. 2012;4:71. doi: 10.1186/gm372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White J., Beck C.R., Harel T., Posey J.E., Jhangiani S.N., Tang S., Farwell K.D., Powis Z., Mendelsohn N.J., Baker J.A. POGZ truncating alleles cause syndromic intellectual disability. Genome Med. 2016;8:3. doi: 10.1186/s13073-015-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korutla L., Wang P.J., Mackler S.A. The POZ/BTB protein NAC1 interacts with two different histone deacetylases in neuronal-like cultures. J. Neurochem. 2005;94:786–793. doi: 10.1111/j.1471-4159.2005.03206.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee T.I., Young R.A. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–1251. doi: 10.1016/j.cell.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong J.X., Buckingham K.J., Jhangiani S.N., Boehm C., Sobreira N., Smith J.D., Harrell T.M., McMillin M.J., Wiszniewski W., Gambin T., Centers for Mendelian Genomics The genetic basis of Mendelian phenotypes: discoveries, challenges, and opportunities. Am. J. Hum. Genet. 2015;97:199–215. doi: 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y., Muzny D.M., Xia F., Niu Z., Person R., Ding Y., Ward P., Braxton A., Wang M., Buhay C. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H., Deignan J.L., Dorrani N., Strom S.P., Kantarci S., Quintero-Rivera F., Das K., Toy T., Harry B., Yourshaw M. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312:1880–1887. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krkljus S., Abernathy C.R., Johnson J.S., Williams C.A., Driscoll D.J., Zori R., Stalker H.J., Rasmussen S.A., Collins F.S., Kousseff B.G. Analysis of CpG C-to-T mutations in neurofibromatosis type 1. Mutations in brief no. 129. Online. Hum. Mutat. 1998;11:411. doi: 10.1002/(SICI)1098-1004(1998)11:5<411::AID-HUMU11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Francioli L.C., Polak P.P., Koren A., Menelaou A., Chun S., Renkens I., van Duijn C.M., Swertz M., Wijmenga C., van Ommen G., Genome of the Netherlands Consortium Genome-wide patterns and properties of de novo mutations in humans. Nat. Genet. 2015;47:822–826. doi: 10.1038/ng.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper D.N., Mort M., Stenson P.D., Ball E.V., Chuzhanova N.A. Methylation-mediated deamination of 5-methylcytosine appears to give rise to mutations causing human inherited disease in CpNpG trinucleotides, as well as in CpG dinucleotides. Hum. Genomics. 2010;4:406–410. doi: 10.1186/1479-7364-4-6-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrovski S., Gussow A.B., Wang Q., Halvorsen M., Han Y., Weir W.H., Allen A.S., Goldstein D.B. The intolerance of regulatory sequence to genetic variation predicts gene dosage sensitivity. PLoS Genet. 2015;11:e1005492. doi: 10.1371/journal.pgen.1005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson D.R., Wu Y.M., Vats P., Su F., Lonigro R.J., Cao X., Kalyana-Sundaram S., Wang R., Ning Y., Hodges L. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., Epstein M.P., Glauser T., Goldstein D.B., Han Y., Epi4K Consortium. Epilepsy Phenome/Genome Project De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorge R., Silva C., Águeda S., Dória S., Leão M. Intellectual disability and overgrowth-A new case of 19p13.13 microdeletion syndrome with digital abnormalities. Am. J. Med. Genet. A. 2015;167A:2839–2843. doi: 10.1002/ajmg.a.37280. [DOI] [PubMed] [Google Scholar]
- 27.Nimmakayalu M., Horton V.K., Darbro B., Patil S.R., Alsayouf H., Keppler-Noreuil K., Shchelochkov O.A. Apparent germline mosaicism for a novel 19p13.13 deletion disrupting NFIX and CACNA1A. Am. J. Med. Genet. A. 2013;161A:1105–1109. doi: 10.1002/ajmg.a.35790. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald J.R., Ziman R., Yuen R.K., Feuk L., Scherer S.W. The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42:D986–D992. doi: 10.1093/nar/gkt958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freed D., Pevsner J. The contribution of mosaic variants to autism spectrum disorder. PLoS Genet. 2016;12:e1006245. doi: 10.1371/journal.pgen.1006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acuna-Hidalgo R., Bo T., Kwint M.P., van de Vorst M., Pinelli M., Veltman J.A., Hoischen A., Vissers L.E., Gilissen C. Post-zygotic point mutations are an underrecognized source of de novo genomic variation. Am. J. Hum. Genet. 2015;97:67–74. doi: 10.1016/j.ajhg.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa M., Nakayama K., Yeasmin S., Katagiri A., Iida K., Nakayama N., Miyazaki K. NAC1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int. J. Oncol. 2010;36:1097–1103. doi: 10.3892/ijo_00000591. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama K., Nakayama N., Davidson B., Sheu J.J., Jinawath N., Santillan A., Salani R., Bristow R.E., Morin P.J., Kurman R.J. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc. Natl. Acad. Sci. USA. 2006;103:18739–18744. doi: 10.1073/pnas.0604083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap K.L., Fraley S.I., Thiaville M.M., Jinawath N., Nakayama K., Wang J., Wang T.L., Wirtz D., Shih IeM. NAC1 is an actin-binding protein that is essential for effective cytokinesis in cancer cells. Cancer Res. 2012;72:4085–4096. doi: 10.1158/0008-5472.CAN-12-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishibashi M., Nakayama K., Yeasmin S., Katagiri A., Iida K., Nakayama N., Miyazaki K. Expression of a BTB/POZ protein, NAC1, is essential for the proliferation of normal cyclic endometrial glandular cells and is up-regulated by estrogen. Clin. Cancer Res. 2009;15:804–811. doi: 10.1158/1078-0432.CCR-08-2134. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama K., Rahman M.T., Rahman M., Yeasmin S., Ishikawa M., Katagiri A., Iida K., Nakayama N., Miyazaki K. Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol. Oncol. 2010;119:469–478. doi: 10.1016/j.ygyno.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Shih IeM., Nakayama K., Wu G., Nakayama N., Zhang J., Wang T.L. Amplification of the ch19p13.2 NACC1 locus in ovarian high-grade serous carcinoma. Mod. Pathol. 2011;24:638–645. doi: 10.1038/modpathol.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeasmin S., Nakayama K., Rahman M.T., Rahman M., Ishikawa M., Katagiri A., Iida K., Nakayama N., Otuski Y., Kobayashi H. Biological and clinical significance of NAC1 expression in cervical carcinomas: a comparative study between squamous cell carcinomas and adenocarcinomas/adenosquamous carcinomas. Hum. Pathol. 2012;43:506–519. doi: 10.1016/j.humpath.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Hernández-Porras I., Schuhmacher A.J., Garcia-Medina R., Jiménez B., Cañamero M., de Martino A., Guerra C. K-Ras(V14I) -induced Noonan syndrome predisposes to tumour development in mice. J. Pathol. 2016;239:206–217. doi: 10.1002/path.4719. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson D.A., Schill L., Schoyer L., Andresen B.S., Bakker A., Bayrak-Toydemir P., Burkitt-Wright E., Chatfield K., Elefteriou F., Elgersma Y. The Fourth International Symposium on Genetic Disorders of the Ras/MAPK pathway. Am. J. Med. Genet. A. 2016;170:1959–1966. doi: 10.1002/ajmg.a.37723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shashi V., Pena L.D., Kim K., Burton B., Hempel M., Schoch K., Walkiewicz M., McLaughlin H.M., Cho M., Stong N., Undiagnosed Diseases Network De novo truncating variants in ASXL2 are associated with a unique and recognizable clinical phenotype. Am. J. Hum. Genet. 2016;99:991–999. doi: 10.1016/j.ajhg.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korutla L., Wang P., Jackson T.G., Mackler S.A. NAC1, a POZ/BTB protein that functions as a corepressor. Neurochem. Int. 2009;54:245–252. doi: 10.1016/j.neuint.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Blanchard M.G., Willemsen M.H., Walker J.B., Dib-Hajj S.D., Waxman S.G., Jongmans M.C., Kleefstra T., van de Warrenburg B.P., Praamstra P., Nicolai J. De novo gain-of-function and loss-of-function mutations of SCN8A in patients with intellectual disabilities and epilepsy. J. Med. Genet. 2015;52:330–337. doi: 10.1136/jmedgenet-2014-102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endele S., Rosenberger G., Geider K., Popp B., Tamer C., Stefanova I., Milh M., Kortüm F., Fritsch A., Pientka F.K. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010;42:1021–1026. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 44.Yap K.L., Sysa-Shah P., Bolon B., Wu R.C., Gao M., Herlinger A.L., Wang F., Faiola F., Huso D., Gabrielson K. Loss of NAC1 expression is associated with defective bony patterning in the murine vertebral axis. PLoS ONE. 2013;8:e69099. doi: 10.1371/journal.pone.0069099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korutla L., Furlong H.A., 4th, Mackler S.A. NAC1, A POZ/BTB protein interacts with Parkin and may contribute to Parkinson’s disease. Neuroscience. 2014;257:86–95. doi: 10.1016/j.neuroscience.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Scofield M.D., Korutla L., Jackson T.G., Kalivas P.W., Mackler S.A. Nucleus Accumbens 1, a Pox virus and Zinc finger/Bric-a-brac Tramtrack Broad protein binds to TAR DNA-binding protein 43 and has a potential role in amyotrophic lateral sclerosis. Neuroscience. 2012;227:44–54. doi: 10.1016/j.neuroscience.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.